Biological Control of Alternaria Leaf Spot Caused by Alternaria spp. in Jerusalem Artichoke (Helianthus tuberosus L.) under Two Fertilization Regimes
Abstract
1. Introduction
2. Results
2.1. Disease Parameters
2.2. Monitoring of Trichoderma Soil Populations and Plant Colonization
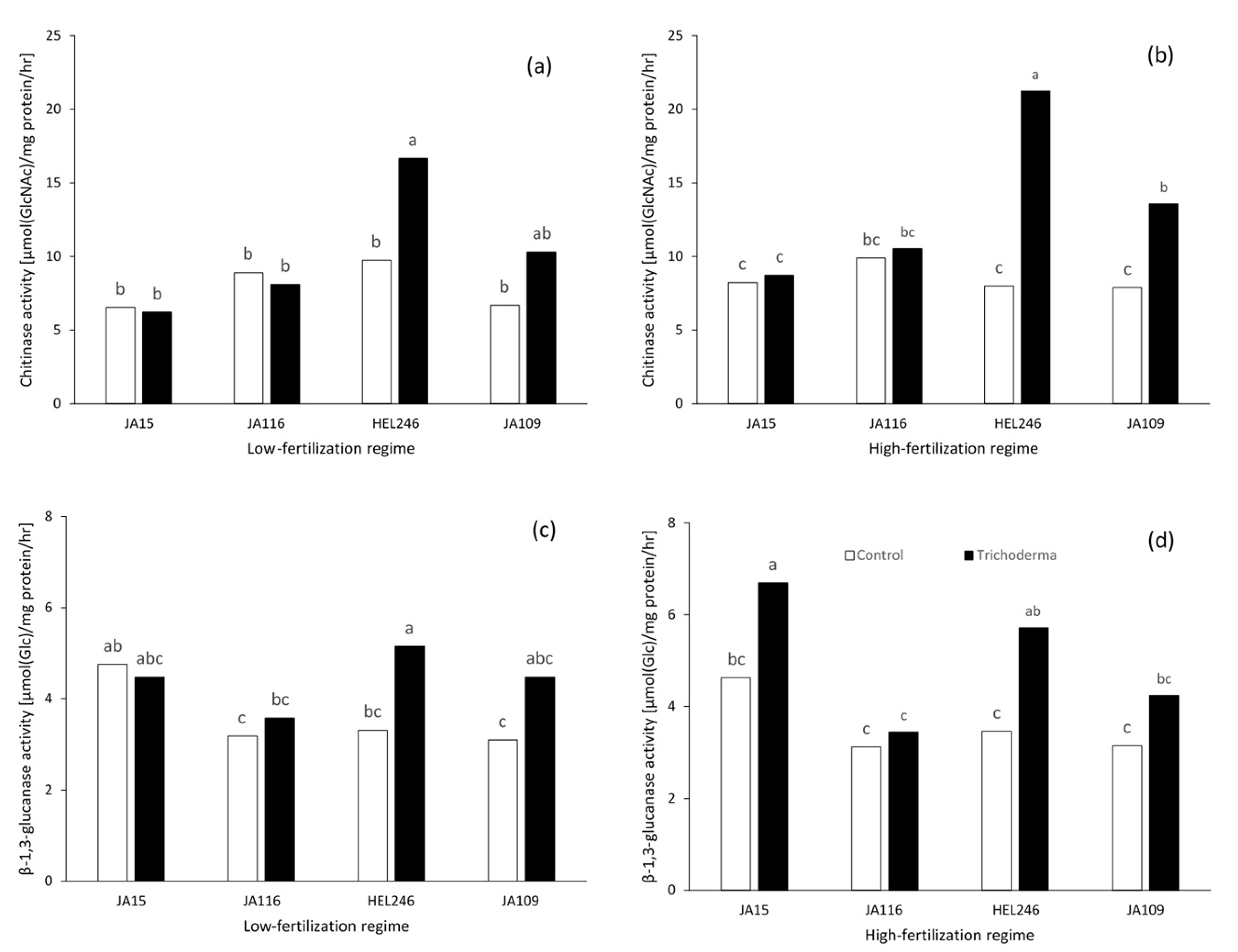
2.3. Enzyme Activity
2.3.1. Chitinase Activity
2.3.2. β-1,3-Glucanase Activity
2.4. Yield and Yield Components
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Treatment
4.2. Preparation of T. harzianum T9 Inoculum
4.3. Plant Material Preparation and Experimental Management
4.4. Monitoring of T. harzianum Propagules in Soil and Colonization of Roots and Leaves
4.5. Enzyme Activity Analysis
4.6. Disease Assessment
4.7. Yield and Yield Components of Jerusalem Artichoke
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Puttha, R.; Jogloy, S.; Wangsomnuk, P.P.; Srijaranai, S.; Kesmala, T.; Patanothai, A. Genotypic variability and genotype by environment interactions for inulin content of Jerusalem artichoke germplasm. Euphytica 2012, 183, 119–131. [Google Scholar] [CrossRef]
- Kays, S.J.; Nottingham, S.F. Biology and Chemistry of Jerusalem Artichoke Helianthus tuberosus L.; Taylor & Francis: Boca Raton, FL, USA, 2008. [Google Scholar]
- Hidaka, H.; Adachi, T.; Hirayama, M. Development and beneficial effects of fructo-oligosaccharides (Neosugar). In Advdnced Dietary Fibre Technology; McCleary, B.V., Prosky, L., Eds.; Blackwell Science: Oxford, UK, 2001; pp. 471–479. [Google Scholar]
- Ruttanaprasert, R.; Jogloy, S.; Vorasoot, N.; Kesmala, T.; Kanwar, R.S.; Holbrook, C.C.; Patanothai, A. Root responses of Jerusalem artichoke genotypes to different water regimes. Biomass Bioenergy 2015, 81, 369–377. [Google Scholar] [CrossRef]
- Viriyasuthee, W.; Saksirirat, W.; Saepaisan, S.; Gleason, M.L.; Jogloy, S. Variability of Alternaria leaf spot resistance in Jerusalem artichoke (Helianthus tuberosus L.) accessions grown in a humid tropical region. Agromomy 2019, 9, 268. [Google Scholar] [CrossRef]
- Dudhe, M.Y.; Bharsakle, S.S. Screening of germplasm line for resistance to Alterneria blight and necrosis disease in sunflower. Crop. Prot. Prod. 2005, 1, 84–87. [Google Scholar]
- Horsfield, A.; Wicks, T.; Davies, K.; Wilson, D.; Paton, S. Effect of fungicide use strategies on the control of early blight (Alternaria solani) and potato yield. Australas. Plant Pathol. 2010, 39, 368–375. [Google Scholar] [CrossRef]
- Gohel, N.M.; Solanky, K.U. In-vitro and In-vivo evaluation of fungicides against Alternaria alternata causing leaf spot and fruit rot disease of chilli. Green Farm. 2012, 3, 84–86. [Google Scholar]
- Mesta, R.K.; Benagi, V.I.; Kulkarni, S.; Basavarajappa, M.P. Management of Alternaria blight of sunflower through fungicides. Karnataka J. Agric. Sci. 2011, 24, 149–152. [Google Scholar]
- Figueirêdo, G.S.D.; Figueirêdo, L.C.D.; Cavalcanti, F.C.N.; Santos, A.C.D.; Antonio Felix da Costa, A.F.D.; Oliveira, N.T.D. Biological and Chemical Control of Sclerotinia sclerotiorum using Trichoderma spp. and Ulocladium atrum and Pathogenicity to Bean Plants. Braz. Arch. Biol. Technol. 2010, 53, 1–9. [Google Scholar] [CrossRef]
- Gremillion, S.K.; Culbreath, A.K.; Gorbet, D.W.; Mullinix, B.G.; Pittman, R.N.; Stevenson, K.L.; Todd, J.W.; Escobar, R.E.; Condori, M.M. Field evaluations of leaf spot resistance and yield in peanut genotypes in the United States and Bolivia. Plant Dis. 2011, 95, 263–268. [Google Scholar] [CrossRef]
- Reddy, C.V.C.M.; Reddy, A.V.V.; Sinha, B.; Lakshmi, M.S. Screening of sunflower genotypes for resistance against Alternaria blight. Asian J. Plant. Sci. 2006, 5, 511–515. [Google Scholar] [CrossRef][Green Version]
- Vijayalakshmi, G.; Karuna, K.; Mahadevaswamy, G. Evaluation of Microbial Biocontrol Agents and Fungicides against Alternaria helianthi Causing Leaf Blight of Sunflower. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 2726–2730. [Google Scholar] [CrossRef]
- Ghosh, R.; Barman, S.; Khatun, J.; Mandal, N.C. Biological control of Alternaria alternata causing leaf spot disease of Aloe vera using two strains of rhizobacteria. Biol. Control. 2016, 97, 102–108. [Google Scholar] [CrossRef]
- Ajayabhai, C.D.; Nath, K.; Bekriwala, T.; Bala, M. Management of Alternaria leaf blight of groundnut caused by Alternaria alternata. Indian Phytopathol. 2018, 71, 543–548. [Google Scholar] [CrossRef]
- Begum, M.F.; Rahman, M.A.; Alam, M.F. Biological control of Alternaria fruit rot of chili by Trichoderma species under field conditions. Mycobiology 2010, 38, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Sennoi, R.; Jogloy, S.; Saksirirat, W.; Kesmala, T.; Patanothai, A. Genotypic variation of resistance to southern stem rot of Jerusalem artichoke caused by Sclerotium Rolfsii. Euphytica 2013, 190, 415–424. [Google Scholar] [CrossRef]
- Charirak, P.; Saksirirat, W.; Jogloy, S.; Saepaisan, S. Application of microorganisms for induced resistance in Jerusalem artichoke (Helianthus tuberosus L.) against stem rot caused by Sclerotium Rolfsii Sacc. J. Pure Appl. Microbiol. 2016, 10, 853–863. [Google Scholar]
- Wright, P.R. Research into early senescence syndrome in cotton. Better Crops Int. 1998, 12, 14–16. [Google Scholar]
- Zhao, J.; Zhao, F.; Jian, G.; Ye, Y.; Zhang, W.; Li, J.; Qi, F. Intensified Alternaria spot disease under potassium deficiency conditions results in acceleration of cotton (Gossypium hirsutum L.) leaf senescence. AJCS 2013, 7, 241–248. [Google Scholar]
- MacDonald, W.; Peters, R.D.; Coffin, R.H.; Lacroix, C. Effect of strobilurin fungicides on control of early blight (Alternaria solani) and yield of potatoes grown under two N fertility regimes. Phytoprotection 2007, 28, 9–15. [Google Scholar] [CrossRef]
- Castillo, A.G.; Puig, C.G.; Cumagun, C.J.R. Non-synergistic effect of Trichoderma harzianum and Glomus spp. in reducing infection of Fusarium wilt in banana. Pathogens 2019, 8, 43. [Google Scholar] [CrossRef]
- Suriyagamon, S.; Phonkerd, N.; Bunyatratchata, W.; Riddech, N.; Mongkolthanaruk, W. Compost seed of Trichoderma harzianum UD12-102 in controlling collar and stem rot of tomato caused by Sclerotium Rolfsii. Environ. Nat. Resour. J. 2018, 16, 20–28. [Google Scholar]
- Abd-El-Kareem, F. Induced resistance in bean plants against root rot and Alternaria leaf spot diseases using biotic and abiotic inducers under field conditions. Res. J. Agric. Biol. Sci. 2007, 3, 767–774. [Google Scholar]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codón, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar] [PubMed]
- Zeilinger, S.; Omann, M. Trichoderma biocontrol: Signal transduction pathways involved in host sensing and mycoparasitism. Gene Regul. Syst. Biol. 2007, 1, 227–234. [Google Scholar] [CrossRef]
- Boller, T. Antimicrobial functions of the plant hydrolases chitinase and β-1,3-glucanase. In Mechanisms of Plant Defense Responses; Fritig, B., Legrand, M., Eds.; Springer: Dordrecht, The Netherlands, 1993; Volume 2, pp. 391–400. [Google Scholar]
- Bigirimana, J.; De Meyer, G.; Poppe, J.; Elad, Y.; Hofte, M. Induction of systemic resistance on bean (Phaseolus vulgaris) by Trichoderma harzianum. Med. Fac. Landbouww. Univ. Gent 1997, 62, 1001–1007. [Google Scholar]
- Yedidia, I.; Shoresh, M.; Kerem, Z.; Benhamou, N.; Kapulnik, Y.; Chet, I. Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in cucumber by Trichoderma asperellum (T-203) and the accumulation of phytoalexins. Appl. Environ. Microbiol. 2003, 69, 7343–7353. [Google Scholar] [CrossRef]
- Kumar, M.; Brar, A.; Yadav, M.; Chawade, A.; Vivekanand, V.; Pareek, N. Chitinases—Potential candidates for enhanced plant resistance towards fungal pathogens. Agriculture 2018, 8, 88. [Google Scholar] [CrossRef]
- Gupta, R.C. Veterinary Toxicology; Elsevier: San Diego, CA, USA, 2018. [Google Scholar]
- Dordas, C. Role of nutrients in controlling plant disease in sustainable agriculture. Agron. Sustain. Dev. 2008, 28, 33–46. [Google Scholar] [CrossRef]
- Blachinski, D.; Shtienberg, D.; Dinoor, A.; Kafkafi, U.; Sujkowski, L.S.; Zitter, T.A.; Fry, W.E. Influence of foliar application of nitrogen and potassium on Alternaria diseases in potato, tomato and cotton. Phytoparasitica 1996, 24, 281–292. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis Second Edition; Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Walkley, A.A. Critical examination of rapid method for determining organic carbon in soils: Effect of variation in digestion conditions and inorganic soil constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Bremner, J.M. Total nitrogen. Agronomy 1965, 9, 1149–1178. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total organic and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Johnson, L.F.; Curl, E.A. Methods for Research on the Ecology of Soil Borne Plant Pathogens; Burgess Publishing Co.: Minneapolis, MN, USA, 1972; p. 247. [Google Scholar]
- Slusarski, C.; Pietr, S.J. Combined application of dazomet and Trichoderma asperellum as an efficient alternative to methyl bromide in controlling the soil-borne disease complex of bell pepper. Crop Prot. 2009, 28, 668–674. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Saksirirat, W.; Hoppe, H.H. Secretion of extracellular enzyme by Verticillium psalliotae Treschow and Verticillium lecanii (Zimm,) Viegas during growth on uredospore of the soybean rust fungus (Phakopsora pachyrhizi Syd) in liquid cultures. J. Phytopathol. 1991, 131, 161–173. [Google Scholar] [CrossRef]
- Somogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 95, 19–23. [Google Scholar]
- Mayee, C.D.; Datar, V.V. Sunflower Diseases. Phytopathometry—Tech. Bull. 1; Marathwad Agricultural University: Parabhan, India, 1986. [Google Scholar]
- Anfok, G.H. Benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester induces systemic resistance in tomato (Lycopersicon esculentum. Mill cv. Vollendung) to cucumber mosaic virus. Crop Prot. 2000, 19, 401–405. [Google Scholar] [CrossRef]
- Ojiambo, P.S.; Narla, R.D.; Ayiecho, P.O.; Nyabundi, J.O. Effect of infection level of sesame (Sesamum indicum L.) seed by Alternaria sesame on severity of Alternaria leaf spot. Tare 1998, 1, 125–130. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- Hoshmand, A.R. Design of Experiments for Agriculture and the Natural Sciences; Chapman & Hall: Boca Raton, FL, USA, 2006. [Google Scholar]
- Statistix8: Analytical Software User’s Manual; Analytical Software: Tallahassee, FL, USA, 2003.
| Genotypes | Disease Incidence (%) 1 | Disease Severity (%) 1 | |||||||
| Non-Inoculated Control | Trichoderma | Fungicide | Mean | Non-Inoculated Control | Trichoderma | Fungicide | Mean | ||
| JA15 | 100 a | 100 a | 100 a | 100 A | 39 d | 33 e | 33 e | 35 D | |
| JA86 | 67 b | 0 c | 50 b | 39 B | 7 fg | 0 h | 6 g | 4 F | |
| JA116 | 100 a | 100 a | 100 a | 100 A | 11 f | 11 f | 11 f | 11 E | |
| HEL246 | 100 a | 100 a | 100 a | 100 A | 78 a | 69 b | 68 b | 72 B | |
| HEL293 | 100 a | 100 a | 100 a | 100 A | 78 a | 75 a | 75 a | 76 A | |
| JA109 | 100 a | 100 a | 100 a | 100 A | 56 c | 56 c | 56 c | 56 C | |
| Mean | 94 A | 83 B | 92 A | 45 A | 41 B | 42 B | |||
| Genotypes | Areas under Disease Progress Curve for Disease Incidence | Areas under Disease Progress Curve for Disease Severity | |||||||
| Non-Inoculated Control | Trichoderma | Fungicide | Mean | Non-Inoculated Control | Trichoderma | Fungicide | Mean | ||
| JA15 | 2606 c | 2575 c | 2550 c | 2577 C | 485 e | 453 e | 448 e | 462 C | |
| JA86 | 413 ef | 0 g | 230 fg | 214 E | 46 f | 0 f | 26 f | 24 D | |
| JA116 | 700 d | 525 de | 579 de | 601 D | 78 f | 58 f | 64 f | 67 D | |
| HEL246 | 5506 a | 5528 a | 5416 a | 5483 A | 1840 a | 1534 bc | 1501 c | 1625 A | |
| HEL293 | 5581 a | 5559 a | 5341 a | 5494 A | 1811 a | 1523 c | 1609 b | 1648 A | |
| JA109 | 3950 b | 3944 b | 3769 b | 3888 B | 1189 d | 1160 d | 1138 d | 1163 B | |
| Mean | 3126 A | 3022 B | 2981 B | 908 A | 788 B | 798 B | |||
| Genotypes | Disease Incidence (%) 1 | Disease Severity (%) 1 | |||||||
| Non-Inoculated Control | Trichoderma | Fungicide | Mean | Non-Inoculated Control | Trichoderma | Fungicide | Mean | ||
| JA15 | 100 a | 100 a | 100 a | 100 A | 33 e | 33 e | 33 e | 33 C | |
| JA86 | 100 a | 63 b | 100 a | 88 B | 15 f | 7 h | 11 g | 11 E | |
| JA116 | 100 a | 100 a | 100 a | 100 A | 16 f | 11 g | 11 g | 13 D | |
| HEL246 | 100 a | 100 a | 100 a | 100 A | 70 a | 64 bc | 66 b | 67 A | |
| HEL293 | 100 a | 100 a | 100 a | 100 A | 71 a | 61 c | 66 b | 66 A | |
| JA109 | 100 a | 100 a | 100 a | 100 A | 56 d | 56 d | 56 d | 56 B | |
| Mean | 100 A | 94 B | 100 A | 44 A | 39 C | 40 B | |||
| Genotypes | Areas under Disease Progress Curve for Disease Incidence | Areas under Disease Progress Curve for Disease Severity | |||||||
| Non-Inoculated Control | Trichoderma | Fungicide | Mean | Non-Inoculated Control | Trichoderma | Fungicide | Mean | ||
| JA15 | 2150 f | 2006 f | 2150 f | 2102 D | 402 f | 374 f | 397 f | 391 D | |
| JA86 | 820 g | 225 i | 641 h | 562 E | 97 g | 25 g | 71 g | 64 E | |
| JA116 | 627 h | 519 h | 532 h | 559 E | 77 g | 58 g | 59 g | 65 E | |
| HEL246 | 5469 a | 5444 ab | 5281 b | 5398 A | 1864 a | 1453 bc | 1576 b | 1631 A | |
| HEL293 | 5453 ab | 5459 a | 4958 c | 5290 B | 1801 a | 1383 c | 1410 c | 1532 B | |
| JA109 | 4213 d | 3956 e | 3981 e | 4050 C | 1208 d | 1067 e | 1050 e | 1108 C | |
| Mean | 3122 A | 2935 B | 2924 B | 908 A | 727 B | 761 B | |||
| Factors | Low-Fertilization Regime | High-Fertilization Regime | ||
|---|---|---|---|---|
| Genotypes | ||||
| JA15 | 182 bc | 273 b | ||
| JA86 | 243 a | 278 ab | ||
| JA116 | 107 d | 150 c | ||
| HEL246 | 189 bc | 330 a | ||
| HEL293 | 222 ab | 321 ab | ||
| JA109 | 150 cd | 171 c | ||
| Treatments | Across Fertilization Regimes | |||
| Control (non-inoculated) | 213 a | |||
| Trichoderma | 219 a | |||
| Fungicide | 221 a | |||
| Factors | Number of Tubers/Plant | Tuber Size (g/tuber) | |
|---|---|---|---|
| Genotypes | |||
| JA15 | 15.7 b | 14.4 b | |
| JA86 | 8.9 c | 30.2 a | |
| JA116 | 16.5 b | 7.7 c | |
| HEL246 | 16.3 b | 15.8 b | |
| HEL293 | 17.6 b | 15.4 b | |
| JA109 | 20.7 a | 7.8 c | |
| Treatments | |||
| Control (non-inoculated) | 16.3 a | 14.5 a | |
| Trichoderma | 15.0 a | 15.9 a | |
| Fungicide | 16.5 a | 15.3 a | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viriyasuthee, W.; Jogloy, S.; Saksirirat, W.; Saepaisan, S.; Gleason, M.L.; Chen, R.S. Biological Control of Alternaria Leaf Spot Caused by Alternaria spp. in Jerusalem Artichoke (Helianthus tuberosus L.) under Two Fertilization Regimes. Plants 2019, 8, 463. https://doi.org/10.3390/plants8110463
Viriyasuthee W, Jogloy S, Saksirirat W, Saepaisan S, Gleason ML, Chen RS. Biological Control of Alternaria Leaf Spot Caused by Alternaria spp. in Jerusalem Artichoke (Helianthus tuberosus L.) under Two Fertilization Regimes. Plants. 2019; 8(11):463. https://doi.org/10.3390/plants8110463
Chicago/Turabian StyleViriyasuthee, Wanalai, Sanun Jogloy, Weerasak Saksirirat, Suwita Saepaisan, Mark L. Gleason, and Ruey Shyang Chen. 2019. "Biological Control of Alternaria Leaf Spot Caused by Alternaria spp. in Jerusalem Artichoke (Helianthus tuberosus L.) under Two Fertilization Regimes" Plants 8, no. 11: 463. https://doi.org/10.3390/plants8110463
APA StyleViriyasuthee, W., Jogloy, S., Saksirirat, W., Saepaisan, S., Gleason, M. L., & Chen, R. S. (2019). Biological Control of Alternaria Leaf Spot Caused by Alternaria spp. in Jerusalem Artichoke (Helianthus tuberosus L.) under Two Fertilization Regimes. Plants, 8(11), 463. https://doi.org/10.3390/plants8110463





