Phytochemical Screening, Antibacterial Activity and Heavy Metal Analysis of Ethnomedicinal Recipes and Their Sources Used Against Infectious Diseases
Abstract
1. Introduction
2. Results
2.1. Phytochemical Determination, Antibacterial Activity and Heavy Metals Analysis of Recipe 1 and Its Individual Plants
2.2. Phytochemical Classes Determination, Antibacterial Activity and Heavy Metal Analysis of Recipe 2 and Its Individual Plants
3. Discussion
3.1. Comparison of Recipe 1 and Its Individual Plants
3.2. Comparison of Recipe 2 and Its Individual Plants
4. Materials and Methods
4.1. Plant Parts Collection and Identification
4.2. Plants Parts Grinding and Preparation of Herbal Recipes
4.3. Qualitative Phytochemical Screening
4.4. Quantitative Phytochemical Screening
4.4.1. Alkaloids Determinations
4.4.2. Flavonoids Determination
4.4.3. Saponins Determination
4.4.4. Antibacterial Activity of Medicinal Plants and Their Recipes
4.4.5. Determination of Minimum Inhibitory Concentrations (MICs) by Broth Dilution Method
4.4.6. Heavy Metals Analysis of Medicinal Plants and Their Recipes
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhat, R.S.; Aldaihan, S. Antimicrobial activity of Garcinia mangostana using different solvents extracts. Intern. J. Biosci. 2013, 3, 262. [Google Scholar]
- Ahmad, M.; Khan, M.A.; Rashid, U.; Zafar, M.; Arshad, M.; Sultana, S. Quality assurance of herbal drug valerian by chemotaxonomic markers. Afr. J. Biotechnol. 2009, 8, 1148. [Google Scholar]
- Jain, S.K. Manual of Ethnobotany; Scientific Publishers: Rajasthan, India, 2010. [Google Scholar]
- Sheng-Ji, P. Ethnobotanical Approaches of Traditional Medicine Studies: Some Experiences from Asia. Pharm. Boil. 2001, 39, 74–79. [Google Scholar]
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.A.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied Sci. 2012, 4, 10–20. [Google Scholar] [CrossRef]
- Wink, M. Medicinal Plants: A Source of Anti-Parasitic Secondary Metabolites. Molecules 2012, 17, 12771–12791. [Google Scholar] [CrossRef]
- Ahmad, M.; Khan, M.A.; Zafar, M.; Arshad, M.; Sultana, S.; Abbasi, B.H. Use of chemotaxonomic markers for misidentified medicinal plants used in traditional medicines. J. Med. Plants Res. 2010, 4, 1244–1252. [Google Scholar]
- Casciaro, B.; Calcaterra, A.; Cappiello, F.; Mori, M.; Loffredo, M.R.; Ghirga, F.; Mangoni, M.L.; Botta, B.; Quaglio, D. Nigritanine as a New Potential Antimicrobial Alkaloid for the Treatment of Staphylococcus aureus-Induced Infections. Toxins 2019, 11, 511. [Google Scholar] [CrossRef]
- Khan, R.; Islam, B.; Akram, M. Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. Molecules 2009, 14, 586–597. [Google Scholar] [CrossRef]
- Acharya, S.; Dash, G.K.; Mondal, S.; Dash, S.K. Antioxidative and antimicrobial study of Spondias mangifera willd root. Int. J. Pharm. Pharm. Sci. 2010, 2, 68. [Google Scholar]
- Hussain, J.; Khan, A.L.; ur Rehman, N.; Hamayun, M.; Shinwari, Z.K.; Ullah, W.; Lee, I.J. Assessment of herbal products and their composite medicinal plants through proximate and micronutrients analysis. J. Med. Plants Res. 2009, 3, 1072. [Google Scholar]
- Ernst, E. Toxic heavy metals and undeclared drugs in Asian herbal medicines. Trends Pharmacol. Sci. 2002, 23, 136–139. [Google Scholar] [CrossRef]
- Hamayun, M.; Khan, S.A.; Iqbal, I.; Rehman, G.; Hayat, T.; Khan, M.A. Ethnobotanical profile of Utror and Gabral valleys, District Swat, Pakistan. Ethnobot. Leafl. 2005, 2005, 9. [Google Scholar]
- Shinwari, Z.K. Medicinal plants research in Pakistan. J. Med. Plants Res. 2010, 4, 161. [Google Scholar]
- Williams, J.T.; Ahmad, Z. Priorities for Medicinal Plants Research and Development in Pakistan; International Development Research Centre IDRC: Ottawa, ON, Canada, 2004; p. 15. [Google Scholar]
- Walter, C.; Shinwari, Z.K.; Afzal, I.; Malik, R.N. Antibacterial activity in herbal products used in Pakistan. Pak. J. Bot. 2011, 43, 155. [Google Scholar]
- Haq, I. Antimicrobial agents in Islamic medicine. Ham Medi 1997, 11.2, 496–499. [Google Scholar]
- Khan, N.; Abbasi, A.M.; Dastagir, G.; Nazir, A.; Shah, G.M.; Shah, M.M.; Shah, M.H. Ethnobotanical and antimicrobial study of some selected medicinal plants used in Khyber Pakhtunkhwa (KPK) as a potential source to cure infectious diseases. BMC Complement. Altern. Med. 2014, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, P.J.; Kurowska, E.; Freeman, D.J.; Chambers, A.F.; Koropatnick, D.J. A flavonoid fraction from cranberry extract inhibits proliferation of human tumor cell lines. J. Nutr. 2004, 134, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Kandaswami, C.; Kanadaswami, C.; Lee, L.T.; Lee, P.P.H.; Hwang, J.J.; Ke, F.C.; Huang, Y.T.; Lee, M.T. The antitumor activities of flavonoids. In Vivo 2005, 19, 895. [Google Scholar]
- Ferguson, L.R. Antimutagens as cancer chemopreventive agents in the diet. Mutat. Res. Mol. Mech. Mutagen. 1994, 307, 395–410. [Google Scholar] [CrossRef]
- Taraphdar, A.K.; Roy, M.; Bhattacharya, R.K. Natural products as inducers of apoptosis: Implication for cancer therapy and prevention. Curr. Sci. 2001, 80, 1387. [Google Scholar]
- Sparg, S.; Light, M.; Van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Akinpelu, D.A.; Onakoya, T.M. Antimicrobial activities of medicinal plants used in folklore remedies in South-western Nigeria. Afr. J. Biotechnol. 2006, 5, 1078. [Google Scholar]
- Padulosi, S.; Caruso, T.; Barone, E. Taxonomy, distribution, conservation and uses of Pistacia genetic resources. Int. Plant Genet. Resour. Inst. 1996, 29–30. [Google Scholar]
- Kalia, A.N. Text Book of Industrial Pharmacognosy; Oscar Publications: New Delhi, India, 2005; ISBN 8123912404. [Google Scholar]
- Saetung, A.; Itharat, A.; Dechsukum, C.; Wattanapiromsakul, C.; Keawpradub, N.; Ratanasuwan, P. Cytotoxic activity of Thai medicinal plants for cancer treatment. J. Sci. Technol. 2005, 27, 469. [Google Scholar]
- Pistelli, L.; Bertoli, A.; Zucconelli, S.; Morelli, I.; Panizzi, L.; Menichini, F. Antimicrobial activity of crude extracts and pure compounds of Hypericum hircinum. Fitoterapia 2000, 71, S138–S140. [Google Scholar] [CrossRef]
- Hunt, J.R. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am. J. Clin. Nutr. 2003, 78, 633S–639S. [Google Scholar] [CrossRef]
- World Health Organization. Basic Tests for Drugs: Pharmaceutical Substances, Medicinal Plant Materials and Dosage Forms; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- World Health Organization. Quality Control Methods for Medicinal Plant Materials; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Hassan, Z.; Anwar, Z.; Khattak, K.U.; Islam, M.; Khan, R.U.; Khattak, J.Z. Civic Pollution and Its Effect on Water Quality of River Toi at District Kohat, NWFP. Res. J. Environ. Earth. Sci. 2012, 4, 334. [Google Scholar]
- Pendias, A.K.; Pendias, H. Trace Elements in Soil and Plants, 2nd ed.; CRC Press: Baca Raton, FL, USA, 1992; p. 365. [Google Scholar]
- McGrath, S.P.; Smith, S. Chromium and Nickel, in Heavy Metals in Soils; Alloway, B.J., Ed.; Blackie: Glasgow, UK, 1990; p. 125. [Google Scholar]
- Ullah, R. Investigation of macro and micro-nutrients in selected medicinal plants. Afr. J. Pharm. Pharmacol. 2012, 6, 6. [Google Scholar]
- Rehman, A.; Ullah, H.; Khan, R.U.; Ahmad, I. Population based study of heavy metals in medicinal plant Capparis decidua. Int. J. Pharm. Pharm. Sci. 2013, 5, 108. [Google Scholar]
- Jabeen, S.; Shah, M.T.; Khan, S.; Hayat, M.Q. Determination of major and trace elements in ten important folk therapeutic plants of Haripur basin, Pakistan. J. Med. Plants Res. 2010, 4, 559. [Google Scholar]
- Adelekan, B.A.; Abegunde, K.D. Heavy metals contaminationof soil and ground water at automobile mechanic villages inIbadan, Nigeria. Int. J. Phy. Sci. 2011, 6, 1045. [Google Scholar]
- Shah, A.; Niaz, A.; Ullah, N.; Rehman, A.; Akhlaq, M.; Zakir, M.; Khan, M.S. Comparative Study of Heavy Metals in Soil and Selected Medicinal Plants. J. Chem. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Chishti, K.A.; Khan, F.A.; Shah, S.M.H.; Asif Khan, M.; Khan, J.; Shah, S.M.M.; Hussain, I. Estimation of heavy metals in the seeds of blue and white capitulum’s of sil ybum marianum grown in various districts of Pakistan. J. Basic. Appl. Sci. 2011, 7, 45–49. [Google Scholar]
- Khan, S.A.; Khan, L.; Hussain, I.; Marwat, K.B.; Akhtar, N. Profile of heavy metals in selected medicinal plants. J. Weed Sci. Res. 2008, 14, 101. [Google Scholar]
- Gupta, U. Copper in the Environment; John Wiley and Sons: New York, NY, USA, 1975; p. 255. [Google Scholar]
- Abourashed, E.A.; El-Alfy, A.T.; Khan, I.A.; Walker, L. Ephedra in perspective—A current review. Phytother. Res. 2003, 17, 703–712. [Google Scholar] [CrossRef]
- Goyer, R.A. Handbook on Toxicity of Inorganic Compounds; Marcel Dekker: New York, NY, USA, 1988. [Google Scholar]
- Baye, H.; Hymete, A. Lead and cadmium accumulation in medicinal plants collected from environmentally different sites. Bull. Environ. Contam. Toxicol. 2010, 84, 197–201. [Google Scholar] [CrossRef]
- Wendakoon, C.; Calderon, P.; Gagnon, D. Evaluation of selected medicinal plants extracted in different ethanol concentrations for antibacterial activity against human pathogens. J. Med. Act. Plants 2012, 1, 60. [Google Scholar]
- Yaseen, G.; Ahmad, M.; Potter, D.; Zafar, M.; Sultana, S.; Mir, S. Ethnobotany of Medicinal Plants for Livelihood and Community Health in Deserts of Sindh-Pakistan. In Plant and Human Health, Volume 1; Springer: Cham, Germany, 2018; Volume 1, pp. 767–792. [Google Scholar]
- Khan, R.U.; Wazir, S.M.; Arayne, S.U.K.; Ullah, R. Methods for the Preparation of Recipes and its Uses for Curing Different Diseases Reported from District Bannu. Can. J. Appl. Sci. 2014, 4, 51. [Google Scholar] [CrossRef]
- Williams, J.T.; Ahmad, Z. Priorities for Medicinal Plants Research and Development in Pakistan; Medicinal and Aromatic Plants Program in Asia (MAPPA): New Delhi, India, 1999. [Google Scholar]
- Tyler, V.E. Phytomedicines in Western Europe: Potential Impact on Herbal Medicine in the United States; ACS Publications: Washington, DC, USA, 1993. [Google Scholar]
- Harborne, J.B. Phytochemical Methods; Chapman and Hall: London, UK, 1973; p. 49. [Google Scholar]
- Obadoni, B.O.; Ochuko, P.O. Phytochemical studies and Comparative efficacy of the crude extracts of some homeostatic plants in Edo and Delta States of Nigeria. Glob. J. Pure. Appl. Sci. 2002, 8, 203. [Google Scholar]
- Mattila, P.; Hellström, J. Phenolic acids in potatoes, vegetables, and some of their products. J. Food Compos. Anal. 2007, 20, 152–160. [Google Scholar] [CrossRef]
- Boham, A.B.; Kouipai, A.C.; Muhammad, R. Flavonoid and condensed tannins from Leaves of Hawaiian vaccinium reticulum and v. calycinum. Pac. Sci. 1994, 48, 458. [Google Scholar]
- Krishnaiah, D.; Devi, T.; Bono, A.S.; Sarbatly, R. Studies on phytochemical constituents of six Malaysian medicinal plants. J. Med. Plants Res. 2009, 3, 67. [Google Scholar]
- Kirby, W.M.; Yoshihara, G.M.; Sundsted, K.S.; Warren, J.H. Clinical usefulness of a single disc method for antibiotic sensitivity testing. Antibiot. Annu. 1956, 1, 892. [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect 2003, 9, ix–xv. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Duke, J.A.; Ayensu, E.S. Medicinal Plants of China; Reference Publications, Inc.: Algonac, Michigan, US, 1987; p. 90. [Google Scholar] [CrossRef]
| S.No | Recipes/Plants (Part Used) | Alkaloids (%) | Flavonoids (%) | Saponins (%) |
|---|---|---|---|---|
| 1 | Recipe 1 | 10.60 | 28.50 | 10.90 |
| 2 | N. sativa (seeds) | 1.30 | 13.00 | 1.15 |
| 3 | B. campestris(seeds) | 3.22 | 10.00 | 0.35 |
| 4 | T. foenum-graecum (seeds) | 3.23 | 13.80 | 2.45 |
| 5 | P. integerrima (galls) | 30.00 | 41.20 | 3.60 |
| 6 | Recipe 2 | 7.80 | 12.70 | 1.00 |
| 7 | L. usitatissimum(seeds) | 3.21 | 6.10 | 0.65 |
| 8 | H. officinalis(flowers) | 5.00 | 8.10 | 1.05 |
| 9 | E. vulgaris (dry branches) | 10.40 | 24.70 | 2.20 |
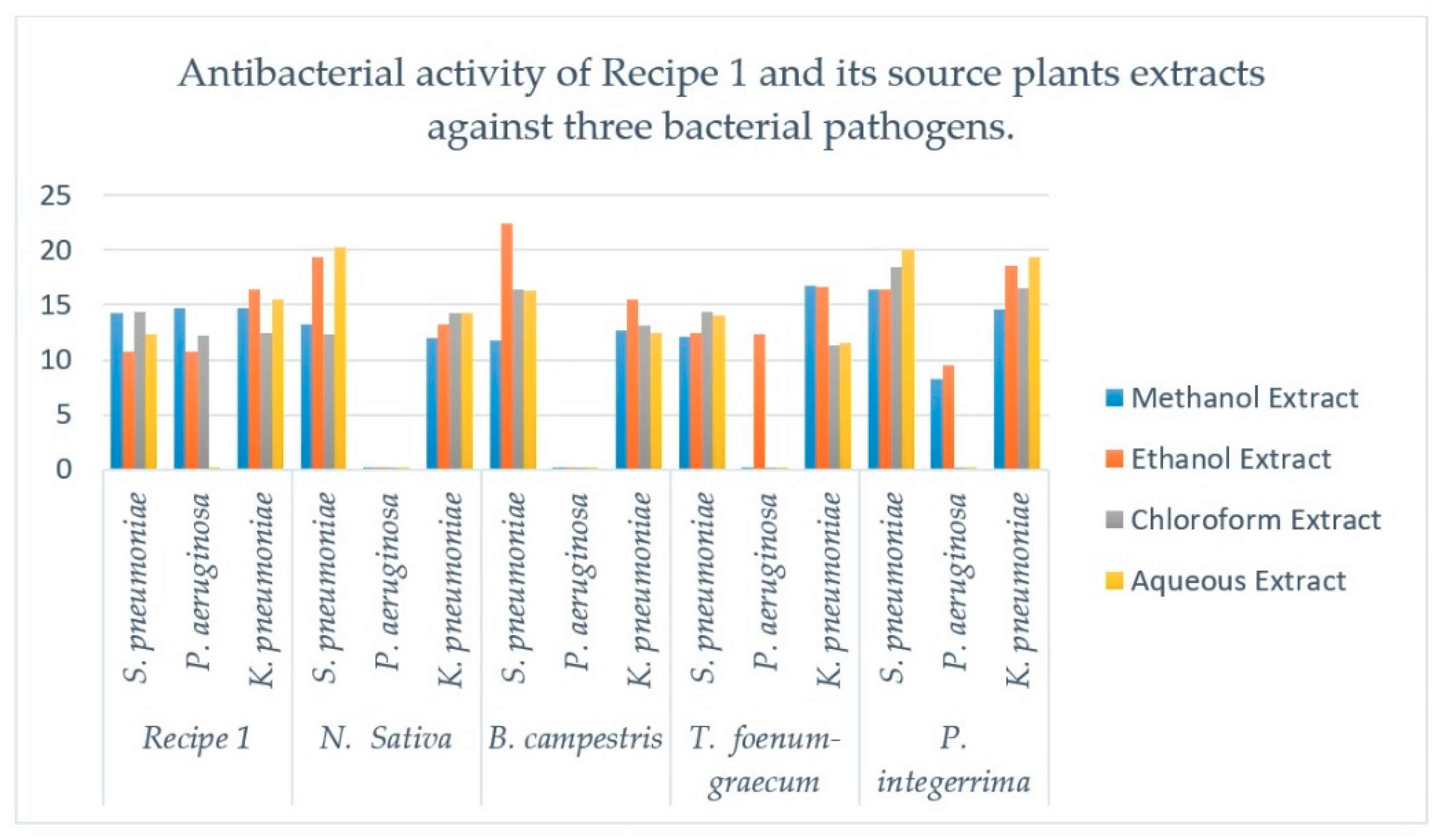
| Recipe/Plant (parts used) | Bacteria | Methanol Extract (mm) | Ethanol Extract (mm) | Chloroform Extract (mm) | Aqueous Extract (mm) | Cipr (mm) | DMSO (mm) |
|---|---|---|---|---|---|---|---|
| Recipe 1 | S. pneumonia | 14.23 ± 0.20 | 10.70 ± 0.16 | 14.43 ± 0.30 | 12.33 ± 0.28 | 28 | - |
| P. aeruginosa | 14.70 ± 0.16 | 10.70 ± 0.16 | 12.26 ± 0.20 | 0 | 0 | - | |
| K. pneumoniae | 14.66 ± 0.16 | 16.46 ± 0.12 | 12.46 ± 0.16 | 15.50 ± 0.16 | 32 | - | |
| N. sativa | S. pneumonia | 13.21 ± 0.16 | 19.40 ± 0.08 | 12.36 ± 0.28 | 20.23 ± 0.16 | 33 | - |
| P. aeruginosa | 0 | 0 | 0 | 0 | 0 | - | |
| K. pneumoniae | 12.03 ± 0.16 | 13.20 ± 0.12 | 14.26 ± 0.20 | 14.30 ± 0.16 | 30 | - | |
| B. campestris | S. Pneumoniae | 11.76 ± 0.12 | 22.40 ± 0.24 | 16.36 ± 0.28 | 16.26 ± 0.20 | 32 | - |
| P. aeruginosa | 0 | 0 | 0 | 0 | 0 | - | |
| K. pneumoniae | 12.70 ± 0.16 | 15.46 ± 0.16 | 13.16 ± 0.16 | 12.43 ± 0.20 | 29 | - | |
| T. foenum-graecum | S. pneumonia | 12.13 ± 0.12 | 12.50 ± 0.16 | 14.40 ± 0.29 | 14.03 ± 0.12 | 31 | - |
| P. aeruginosa | 0 | 12.30 ± 0.16 | 0 | 0 | 0 | - | |
| K. pneumoniae | 16.70 ± 0.16 | 16.63 ± 0.09 | 11.30 ± 0.24 | 11.53 ± 0.28 | 28 | - | |
| P. integerrima | S. pneumonia | 16.36 ± 0.20 | 16.43 ± 0.24 | 18.43 ± 0.36 | 20.06 ± 0.16 | 33 | - |
| P. aeruginosa | 8.30 ± 0.16 | 9.53 ± 0.16 | 0 | 0 | 0 | - | |
| K. pneumoniae | 14.60 ± 0.24 | 18.56 ± 0.24 | 16.50 ± 0.32 | 19.36 ± 0.32 | 34 | - |
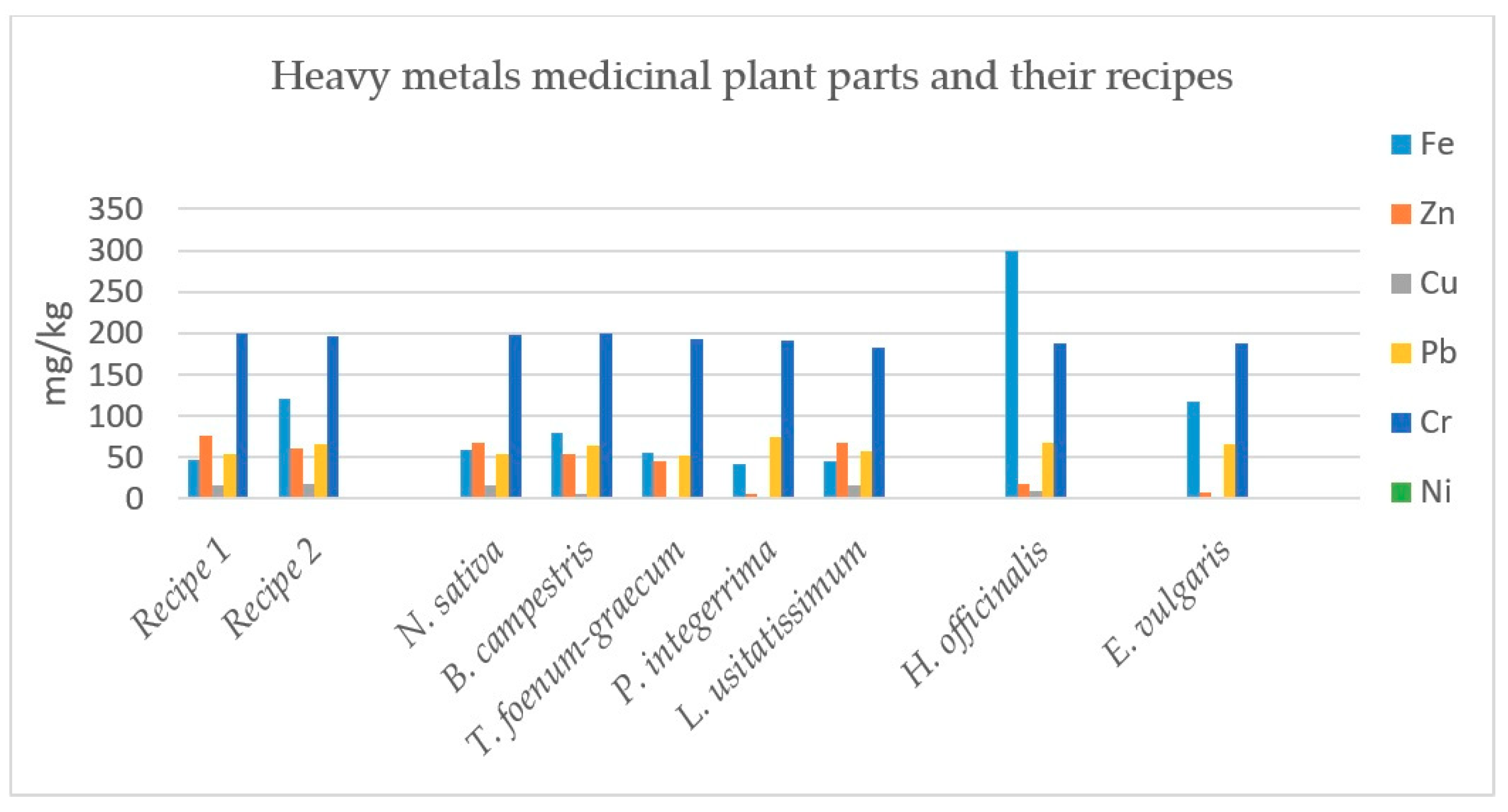
| Recipe/Plant (parts used) | Cd (mg/kg) | Fe (mg/kg) | Zn (mg/kg) | Cu (mg/kg) | Pb (mg/kg) | Cr (mg/kg) | Ni (mg/kg) |
|---|---|---|---|---|---|---|---|
| Recipe 1 | BDL | 47.22 ± 0.010 | 76.98 ± 0.019 | 16.62 ± 0.043 | 53.22 ± 0.178 | 199.92 ± 0.218 | BDL |
| Nigella sativa | BDL | 59.61 ± 0.023 | 68.52 ± 0.027 | 16.77 ± 0.009 | 53.79 ± 0.577 | 197.91 ± 0.216 | 0.69 ± 0.014 |
| Brassica campestris | BDL | 80.43 ± 0.060 | 53.94 ± 0.019 | 6.3 ± 0.019 | 65.1 ± 0.062 | 200.07 ± 0.152 | 0.63 ± 0.015 |
| Trigonellafoenum-graecum | BDL | 55.92 ± 0.023 | 45.24 ± 0.023 | 12.24 ± 0.017 | 52.8 ± 0.738 | 192.72 ± 0.316 | BDL |
| Pistacia integerrima | BDL | 42.03 ± 0.030 | 6.66 ± 0.009 | 5.1 ± 0.011 | 74.46 ± 0.165 | 191.97 ± 0.502 | BDL |
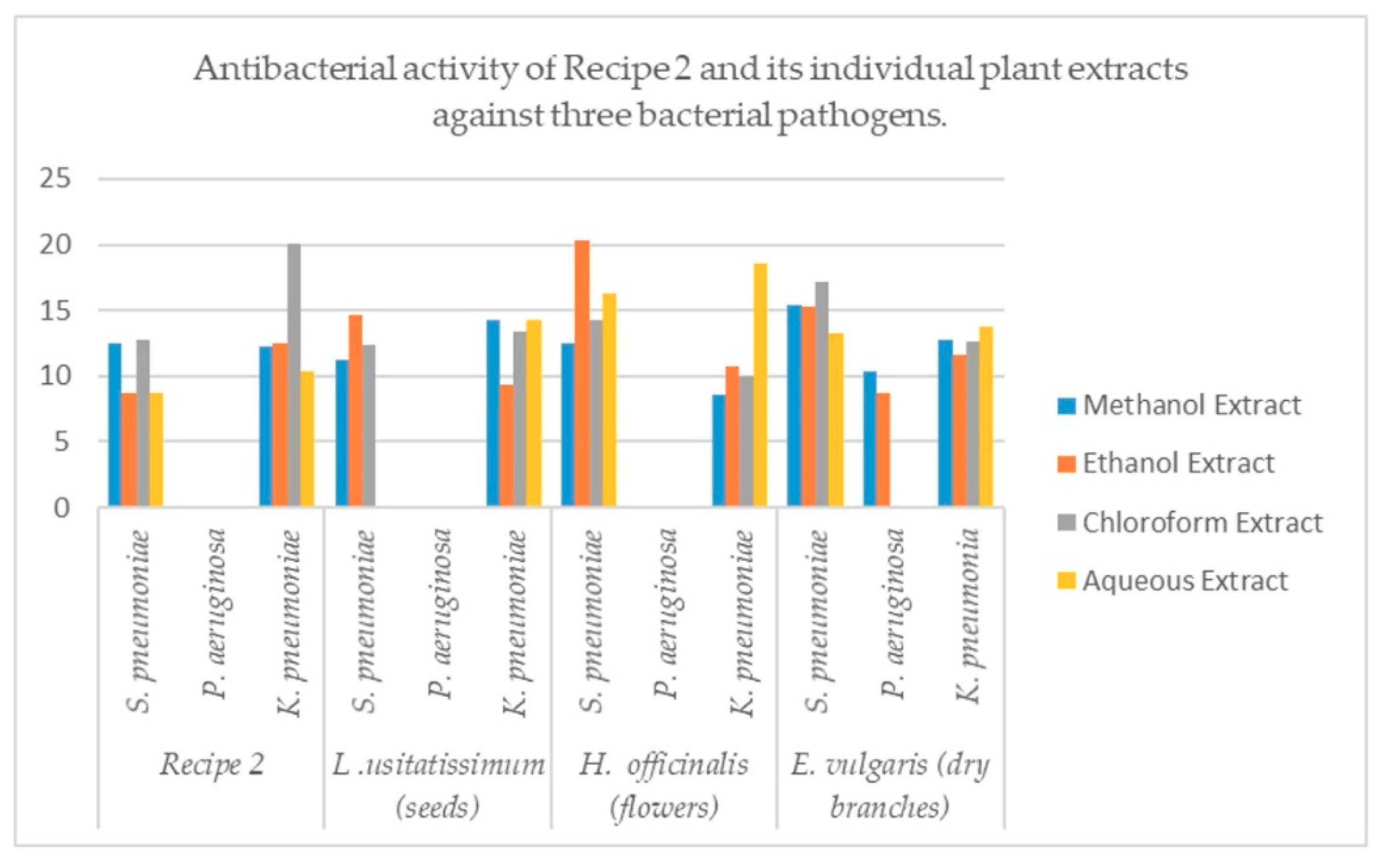
| Recipe/Plant (parts used) | Bacteria | Methanol Extract (mm) | Ethanol Extract (mm) | Chloroform Extract (mm) | Aqueous Extract (mm) | Cipr (mm) | DMSO (mm) |
|---|---|---|---|---|---|---|---|
| Recipe 2 | S. pneumoniae | 12.46 ± 0.32 | 8.70 ± 0.16 | 12.73 ± 0.12 | 8.66 ± 0.16 | 20 | - |
| P. aeruginosa | 0 | 0 | 0 | 0 | 0 | - | |
| K. pneumoniae | 12.30 ± 0.16 | 12.50 ± 0.16 | 20.06 ± 0.16 | 10.30 ± 0.16 | 33 | - | |
| L. usitatissimum (seeds) | S. pneumoniae | 11.23 ± 0.16 | 14.60 ± 0.08 | 12.40 ± 0.08 | 0 | 31 | - |
| P. aeruginosa | 0 | 0 | 0 | 0 | 0 | - | |
| K. pneumoniae | 14.30 ± 0.16 | 9.30 ± 0.16 | 13.43 ± 0.16 | 14.2 ± 0.16 | 32 | - | |
| H. officinalis (flowers) | S. pneumoniae | 12.46 ± 0.12 | 20.30 ± 0.16 | 14.30 ± 0.24 | 16.26 ± 0.20 | 32 | - |
| P. aeruginosa | 0 | 0 | 0 | 0 | 0 | - | |
| K. pneumoniae | 8.56 ± 0.40 | 10.70 ± 0.16 | 10.03 ± 0.12 | 18.56 ± 0.24 | 33 | - | |
| E. vulgaris (dry branches) | S. pneumoniae | 15.36 ± 0.24 | 15.30 ± 0.16 | 17.16 ± 0.16 | 13.26 ± 0.16 | 33 | - |
| P. aeruginosa | 10.36 ± 0.24 | 8.70 ± 0.16 | 0 | 0 | 0 | - | |
| K. pneumonia | 12.70 ± 0.16 | 11.60 ± 0.08 | 12.63 ± 0.20 | 13.70 ± 0.16 | 34 | - |
| Recipe/Plant (parts used) | Fe (mg/kg) | Zn (mg/kg) | Cu (mg/kg) | Pb (mg/kg) | Cr (mg/kg) | Ni (mg/kg) |
|---|---|---|---|---|---|---|
| Recipe 2 | 121.11 ± 0.680 | 60.81 ± 0.021 | 17.70 ± 0.024 | 65.37 ± 0.343 | 196.56 ± 0.960 | 0.93 ± 0.016 |
| Linum usitatissimum | 45.03 ± 0.045 | 67.59 ± 0.016 | 16.17 ± 0.039 | 57.93 ± 0.255 | 182.91 ± 0.244 | 0.54 ± 0.025 |
| Hyssopus officinalis | 299.79 ± 0.084 | 17.19 ± 0.013 | 8.82 ± 0.031 | 68.16 ± 0.088 | 188.07 ± 0.371 | BDL |
| Ephedra vulgaris | 117.51 ± 0.008 | 7.89 ± 0.004 | 3.33 ± 0.024 | 66.81 ± 0.343 | 188.07 ± 0.249 | 0.57 ± 0.003 |
| S. No | Bacteria | Methanol Extracts (mg/L) | Aqueous Extracts (mg/L) | Chloroform Extracts (mg/L) |
|---|---|---|---|---|
| 1 | S. Pneumoniae | 9000 | 10,500 | 9000 |
| 2 | P. aeruginosa | 10,000 | N/A | 12,000 |
| 3 | K. pneumonia | 11,500 | 12,000 | 14,000 |
| S. No | Bacteria | Methanol Extracts (mg/L) | Aqueous Extracts (mg/L) | Chloroform Extracts (mg/L) |
|---|---|---|---|---|
| 1 | S. pneumoniae | 11,000 | 2000 | 13,000 |
| 2 | P. aeruginosa | N/A | N/A | N/A |
| 3 | K. pneumonia | 11,500 | 13,500 | 8000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, N.; Nazir, R.; Khan, M.; Iqbal, R.; Adnan, M.; Ullah, M.; Yang, H. Phytochemical Screening, Antibacterial Activity and Heavy Metal Analysis of Ethnomedicinal Recipes and Their Sources Used Against Infectious Diseases. Plants 2019, 8, 454. https://doi.org/10.3390/plants8110454
Mahmood N, Nazir R, Khan M, Iqbal R, Adnan M, Ullah M, Yang H. Phytochemical Screening, Antibacterial Activity and Heavy Metal Analysis of Ethnomedicinal Recipes and Their Sources Used Against Infectious Diseases. Plants. 2019; 8(11):454. https://doi.org/10.3390/plants8110454
Chicago/Turabian StyleMahmood, Nasir, Ruqia Nazir, Muslim Khan, Rashid Iqbal, Muhammad Adnan, Mohib Ullah, and Hongyi Yang. 2019. "Phytochemical Screening, Antibacterial Activity and Heavy Metal Analysis of Ethnomedicinal Recipes and Their Sources Used Against Infectious Diseases" Plants 8, no. 11: 454. https://doi.org/10.3390/plants8110454
APA StyleMahmood, N., Nazir, R., Khan, M., Iqbal, R., Adnan, M., Ullah, M., & Yang, H. (2019). Phytochemical Screening, Antibacterial Activity and Heavy Metal Analysis of Ethnomedicinal Recipes and Their Sources Used Against Infectious Diseases. Plants, 8(11), 454. https://doi.org/10.3390/plants8110454





