Do No Harm: Efficacy of a Single Herbicide Application to Control an Invasive Shrub While Minimizing Collateral Damage to Native Species
Abstract
1. Introduction
2. Results
2.1. Site Characteristics
2.2. Treatment Effects on Lespedeza Cuneata
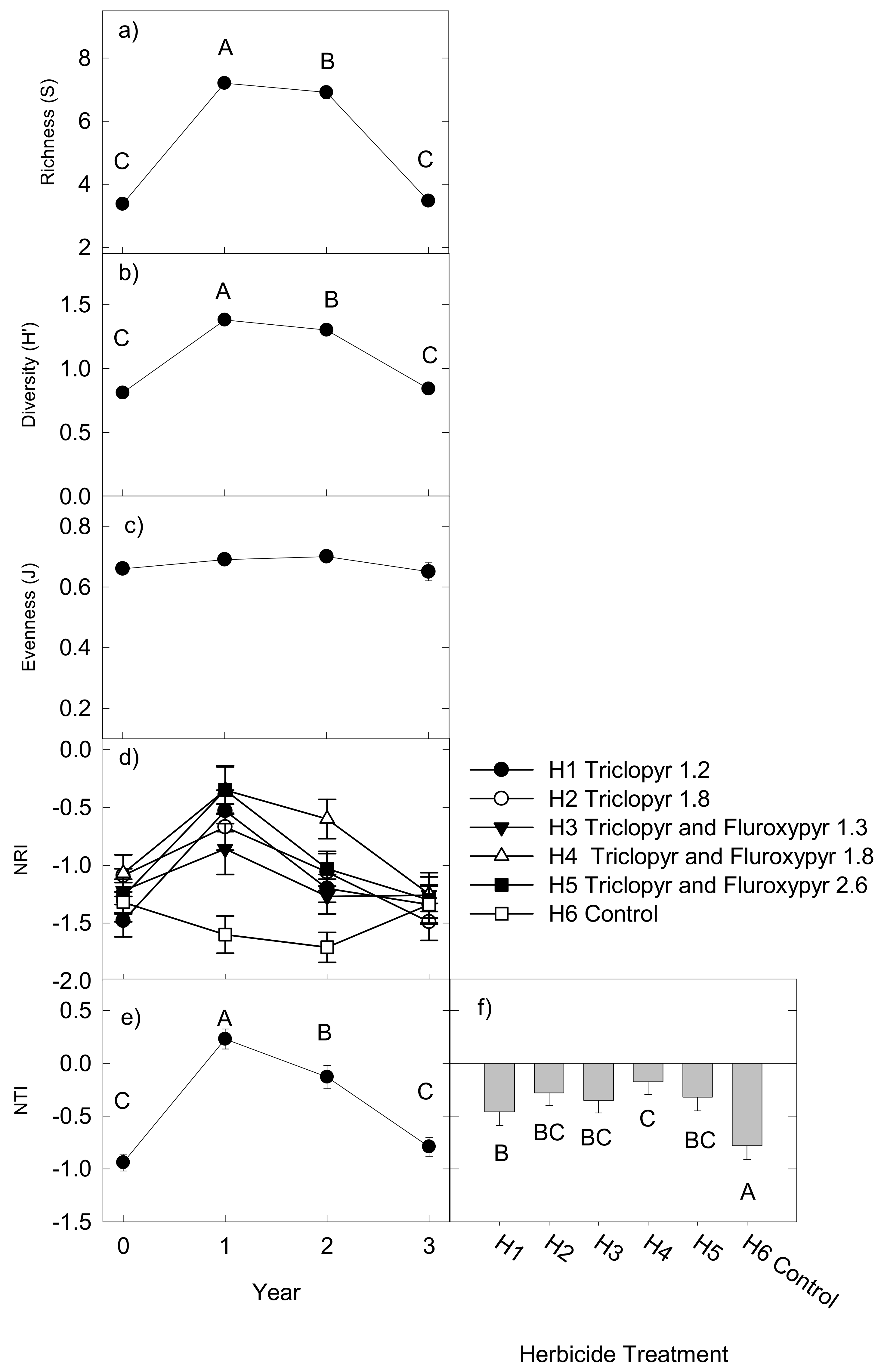
2.3. Taxonomic and Phylogenetic Diversity
2.4. Effects on Functional Groups
2.5. Species Composition
3. Discussion
4. Materials and Methods
4.1. Study Species
4.2. Sites
4.3. Experimental Design and Treatments
4.4. Data Collection
4.5. Statistical Analyses
4.5.1. Phylogenetic Analysis and Tree Construction
4.5.2. Species Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baer, S.G.; Engle, D.; Knops, J.M.H.; Langeland, K.A.; Maxwell, B.D.; Menalled, F.D.; Symstad, A. Vulnerability of rehabilitated agricultural production systems to invasion by nontarget plant species. Environ. Manag. 2009, 43, 189–196. [Google Scholar] [CrossRef][Green Version]
- D’Antonio, C.; Meyerson, L.A. Exotic plant species as problems and solutions in ecological restoration: A synthesis. Restor. Ecol. 2002, 10, 703–713. [Google Scholar] [CrossRef]
- United Nations. Report of the United Nations Conference on Environment and Development: Annex I: Rio Declaration on Environment and Development. Available online: http://www.un.org/documents/ga/conf151/aconf15126-1annex1.htm (accessed on 23 February 2018).
- Van Rijssen, F.W.J.; Eloff, J.N.; Jane Morris, E. The precautionary principle: Making managerial decisions on GMOs is difficult. S. Afr. J. Sci. 2015, 111, 1–9. [Google Scholar]
- Solecki, M.K. Controlling invasive plants. In The Tallgrass Restoration Handbook, 2nd ed.; Packard, S., Mutel, C.F., Eds.; Island Press: Washington, DC, USA, 2005; pp. 251–278. [Google Scholar]
- Wagner, V.; Antunes, P.M.; Irvine, M.; Nelson, C.R. Herbicide usage for invasive non-native plant management in wildland areas of North America. J. Appl. Ecol. 2017, 54, 198–204. [Google Scholar] [CrossRef]
- Crone, E.E.; Marler, M.; Pearson, D.E. Non-target effects of broadleaf herbicide on a native perennial forb: A demographic framework for assessing and minimizing impacts. J. Appl. Ecol. 2009, 46, 673–682. [Google Scholar] [CrossRef]
- Martinez, K.A.; Gibson, D.J.; Middleton, B.A. Core-satellite species hypothesis and native versus exotic species in secondary succession. Plant Ecol. 2015, 216, 419–427. [Google Scholar] [CrossRef]
- Guo, Q. Temporal changes in native-exotic richness correlations during early post-fire succession. Acta Oecologia 2017, 80, 47–50. [Google Scholar] [CrossRef]
- Tognetti, P.M.; Chaneton, E.J. Invasive exotic grasses and seed arrival limit native species establishment in an old-field grassland succession. Biol. Invasions 2012, 14, 2531–2544. [Google Scholar] [CrossRef]
- McManamen, C.; Nelson, C.R.; Wagner, V. Timing of seeding after herbicide application influences rates of germination and seedling biomass of native plants used for grassland restoration. Restor. Ecol. 2018, 26, 1137–1148. [Google Scholar] [CrossRef]
- Johnson, D.P.; Catford, J.A.; Driscoll, D.A.; Gibbons, P. Seed addition and biomass removal key to restoring native forbs in degraded temperate grassland. Appl. Veg. Sci. 2018, 21, 219–228. [Google Scholar] [CrossRef]
- Hipp, A.L.; Larkin, D.J.; Barak, R.S.; Bowles, M.L.; Cadotte, M.W.; Jacobi, S.K.; Lonsdorf, E.; Scharenbroch, B.C.; Williams, E.; Weiher, E. Phylogeny in the service of ecological restoration. Am. J. Bot. 2015, 102, 647–648. [Google Scholar] [CrossRef] [PubMed]
- Gerhold, P.; Cahill, J.F.; Winter, M.; Bartish, I.V.; Prinzing, A. Phylogenetic patterns are not proxies of community assembly mechanisms (they are far better). Funct. Ecol. 2015, 29, 600–614. [Google Scholar] [CrossRef]
- Lean, C.; Maclaurin, J. The value of phylogenetic diversity. In Biodiversity Conservation and Phylogenetic Systematics: Preserving our evolutionary heritage in an extinction crisis; Pellens, R., Grandcolas, P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 19–37. [Google Scholar] [CrossRef]
- Barber, N.A.; Farrell, A.K.; Blackburn, R.C.; Bauer, J.T.; Groves, A.M.; Brudvig, L.A.; Jones, H.P. Grassland restoration characteristics influence phylogenetic and taxonomic structure of plant communities and suggest assembly mechanisms. J. Ecol. 2019, 107, 2105–2120. [Google Scholar] [CrossRef]
- Khalil, M.; Gibson, D.J.; Bear, S.G. Phylogenetic diversity reveals hidden patterns related to population source in a grassland restoration. J. Appl. Ecol. 2017, 54, 91–100. [Google Scholar] [CrossRef]
- Woods, T.M.; Hartnett, D.C.; Ferguson, C.J. High propagule production and reproductive fitness homeostasis contribute to the invasiveness of Lespedeza cuneata (Fabaceae). Biol. Invasions 2009, 11, 1913–1927. [Google Scholar] [CrossRef]
- Koger, C.H.; Stritzke, J.F.; Cummings, D.C. Control of Sericea Lespedeza (Lespedeza cuneata) with Triclopyr, Fluroxypyr, and Metsulfuron. Weed Technol. 2012, 16, 893–900. [Google Scholar] [CrossRef]
- Weed Science Society of America. Herbicide Handbook, 10th ed.; Shaner, D.L., Ed.; Weed Science Society of America: Lawrence, KS, USA, 2014. [Google Scholar]
- Brooke, J.M.; Harper, C.A. Herbicides are effective for reducing dense native warm-season grass and controlling a common invasive species, Sericea Lespedeza. J. Southeastern Assoc. Fish Wildlife Agencies 2016, 3, 178–184. [Google Scholar]
- Wong, B.M.; Houseman, G.R.; Hinman, S.E.; Foster, B.L. Targeting vulnerable life-stages of Sericea Lespedeza (Lespedeza cuneata) with prescribed burns. Invasive Plant Sci. Manag. 2017, 5, 487–493. [Google Scholar] [CrossRef]
- Brandon, A.L.; Gibson, D.J.; Middleton, B.A. Mechanisms for dominance in an early successional old field by the invasive non-native Lespedeza cuneata (Dum. Cours.) G. Don. Biol. Invasions 2004, 6, 483–493. [Google Scholar] [CrossRef]
- Reed, H.E.; Seastedt, T.R.; Blair, J.M. Ecological Consequences of C4 grass invasion of a C4 grassland: A dilemma for management. Ecol. Appl. 2005, 15, 1560–1569. [Google Scholar] [CrossRef]
- Simmons, M.T.; Windhager, S.; Power, P.; Lott, J.; Lyons, R.K.; Schwope, C. Selective and non-selective control of invasive plants: The short-term effects of growing-season prescribed fire, herbicide, and mowing in two Texas prairies. Restor. Ecol. 2007, 15, 662–669. [Google Scholar] [CrossRef]
- Havill, S.; Schwinning, S.; Lyons, K.G. Fire effects on invasive and native warm-season grass species in a North American grassland at a time of extreme drought. Appl. Veg. Sci. 2015, 18, 637–649. [Google Scholar] [CrossRef]
- Myers, J.A.; Harms, K.E. Seed arrival, ecological filters, and plant species richness: A meta-analysis. Ecol. Lett. 2009, 12, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Endress, B.A.; Parks, C.G.; Naylor, B.J.; Radosevich, S.R.; Porter, M. Grassland response to herbicides and seeding of native grasses 6 years posttreatment. Invasive Plant Sci. Manag. 2017, 5, 311–316. [Google Scholar] [CrossRef]
- Khalil, M.I.; Gibson, D.J.; Baer, S.G.; Willand, J.E. Functional diversity is more sensitive to biotic filters than phylogenetic diversity during community assembly. Ecosphere 2018, 9, e02164. [Google Scholar] [CrossRef]
- Barber, N.A.; Jones, H.P.; Duvall, M.R.; Wysocki, W.P.; Hansen, M.J.; Gibson, D.J. Phylogenetic diversity is maintained despite richness losses over time in restored tallgrass prairie plant communities. J. Appl. Ecol. 2017, 54, 137–144. [Google Scholar] [CrossRef]
- Jones, H.P.; Barber, N.A.; Gibson, D.J. Is phylogenetic and functional trait diversity a driver or a consequence of grassland community assembly? J. Ecol. 2019, 107, 2027–2032. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Carboni, M.; Si, X.; Tatsumi, S. Do traits and phylogeny support congruent community diversity patterns and assembly inferences? J. Ecol. 2019, 107, 2065–2077. [Google Scholar] [CrossRef]
- Loiola, P.P.; Bello, F.; Chytrý, M.; Götzenberger, L.; Carmona, C.P.; Pyšek, P.; Lososová, Z. Invaders among locals: Alien species decrease phylogenetic and functional diversity while increasing dissimilarity among native community members. J. Ecol. 2018, 106, 2230–2241. [Google Scholar] [CrossRef]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef]
- Chai, Y.; Yue, M.; Liu, X.; Guo, Y.; Wang, M.; Xu, J.; Zhang, C.; Chen, Y.; Zhang, L.; Zhang, R. Patterns of taxonomic, phylogenetic diversity during a long-term succession of forest on the Loess Plateau, China: Insights into assembly process. Sci. Rep. 2016, 6, 27087. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, S.; Cadotte, M.W.; Mori, A.S. Individual-based models of community assembly: Neighbourhood competition drives phylogenetic community structure. J. Ecol. 2018, 107, 735–746. [Google Scholar] [CrossRef]
- Pellissier, L.; Wisz, M.S.; Strandberg, B.; Damgaard, C. Herbicide and fertilizers promote analogous phylogenetic responses but opposite functional responses in plant communities. Environ. Res. Lett. 2014, 9, 024016. [Google Scholar] [CrossRef]
- Carter, D.L.; Blair, J.M. High richness and dense seeding enhance grassland restoration establishment but have little effect on drought response. Ecol. Appl. 2012, 22, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Holling, C.S. Resilience and stability of ecological systems. Ann. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef]
- Gucker, C. Lespedeza cuneata. In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). Available online: http://www.fs.fed.us/database/feis plants/forb/lescun/all.html (accessed on 16 October 2019).
- Allred, B.W.; Fuhlendorf, S.D.; Monaco, T.A.; Will, R.E. Morphological and physiological traits in the success of the invasive plant Lespedeza cuneata. Biol. Invasions 2010, 12, 739–749. [Google Scholar] [CrossRef]
- Sollenberger, L.E.; Collins, M. Legumes for Southern Areas. In Forages: An Introduction to Grassland Agriculture, 6th ed.; Barnes, R.F., Nelson, C.J., Collins, M., Moore, K.J., Eds.; Iowa State Press: Ames, IA, USA, 2003; Volume 1, pp. 191–213. [Google Scholar]
- Powell, M.C.; Muntifering, R.B.; Lin, J.C.; Chappelka, A.H. Yield and nutritive quality of sericea lespedeza (Lespedeza cuneata) and little bluestem (Schizachyrium scoparium) exposed to ground-level ozone. Environ. Pollut. 2003, 122, 313–322. [Google Scholar] [CrossRef]
- Clark, I.D.; Frey, R.W. Seasonal variation in tannin content of Lespedeza sericea. J. Agric. Res. 1939, 58, 131–139. [Google Scholar]
- Scasta, J.D.; Engle, D.M.; Fuhlendorf, S.D.; Redfearn, D.D.; Bidwell, T.G. Meta-Analysis of exotic forages as invasive plants in complex multi-functioning landscapes. Invasive Plant Sci. Manag. 2017, 8, 292–306. [Google Scholar] [CrossRef]
- Coykendall, K.E.; Houseman, G.R. Lespedeza cuneata invasion alters soils facilitating its own growth. Biol. Invasions 2014, 16, 1735–1742. [Google Scholar] [CrossRef]
- Dudley, D.M.; Fick, W.H. Effects of sericea lespedeza residues on selected tallgrass prairie grasses. Trans. Kansas Acad. Sci. 2003, 106, 166–170. [Google Scholar] [CrossRef]
- Crawford, K.M.; Knight, T.M. Competition overwhelms the positive plant–soil feedback generated by an invasive plant. Oecologia 2017, 183, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Garten, C.T.; Classen, A.T.; Norby, R.J.; Brice, D.J.; Weltzin, J.F.; Souza, L. Role of N2-fixation in constructed old-field communities under different regimes of [CO2], temperature, and water availability. Ecosystems 2008, 11, 125–137. [Google Scholar] [CrossRef]
- Dow AgroSciences. Sericea Lespedeza. Available online: http://www.dowagro.com/en-us/range/weedbrush/biennials/s/sericea-lespedeza (accessed on 15 February 2018).
- Alton, J.V.; Stritzke, J.F.; Weeks, D.L. Sericea lespedeza (Lespedeza cuneata) control with selected post-emergence herbicides. Weed Technol. 1992, 6, 575–576. [Google Scholar]
- Diboll, N. Designing seed mixes. In The Tallgrass Restoration Handbook; Packard, S., Mutel, C.F., Eds.; Island Press: Washington, DC, USA, 2005; pp. 135–150. [Google Scholar]
- Abrams, M.D.; Hulbert, L.C. Effect of topographic position and fire on species in tallgrass prairie in northeast Kansas. Am. Midl. Nat. 1987, 117, 442–445. [Google Scholar] [CrossRef]
- SAS Institute Inc. The SAS System for Windows, Version 9.4; SAS institute Inc.: Cary, NC, USA, 2002–2012.
- Qian, H.; Jin, Y. An updated megaphylogeny of plants, a tool for generating plant phylogenies and an analysis of phylogenetic community structure. J. Plant Ecol. 2016, 9, 233–239. [Google Scholar] [CrossRef]
- Zanne, A.E.; Tank, D.C.; Cornwell, W.K.; Eastman, J.M.; Smith, S.A.; FitzJohn, R.G.; McGlinn, D.J.; O’Meara, B.C.; Moles, A.T.; Reich, P.B.; et al. Three keys to the radiation of angiosperms into freezing environments. Nature 2013, 506, 89. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Swenson, N. Functional and Phylogenetic Ecology in R; Springer: New York, NY, USA, 2014. [Google Scholar]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and community ecology. Ann. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Martinez Arbizu, P. pairwise.Adonis: Pairwise Multilevel Comparison Using Adonis. R Package Version 0.0.1. Available online: https://github.com/pmartinezarbizu/pairwiseAdonis (accessed on 16 October 2019 ).
- Oksanen, J.; Guillaume Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlin, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, O.; et al. Vegan: Community Ecology Package. R package version 2.3-2. Available online: https://CRAN.R-project.org/package=vegan (accessed on 16 October 2019).


| Treatment | S | H | E | NRI | NTI |
|---|---|---|---|---|---|
| Year (Y) | 338.763, 367*** | 145.273, 364*** | 2.003, 370 | 14.883, 361*** | 35.903, 374*** |
| Herbicide (H) | 0.905, 55.2 | 0.515, 55 | 0.235, 57 | 6.195, 49.3*** | 4.115, 54** |
| Seeding (S) | 3.841, 11.3 | 6.981, 11.2* | 4.001, 10.6 | 0.901, 11 | 1.481, 10.8 |
| Y*H | 1.4015, 374 | 0.9915, 370 | 0.6615, 376 | 2.2815, 365** | 1.2315, 378 |
| Y*S | 2.093, 368 | 1.383, 367 | 4.273, 372 | 0.863, 361 | 0.433, 375 |
| H*S | 1.755, 55.4 | 1.725, 54.4 | 1.095, 53.9 | 0.755, 52.8 | 1.995, 54.8 |
| Y*H*S | 0.6315, 375 | 0.6715, 372 | 0.7515, 377 | 0.8815, 365 | 0.7215, 378 |
| Factor | ||||
|---|---|---|---|---|
| df | F | R2 | P | |
| Sites | 1 | 49.59 | 0.06 | 0.001 |
| Blocks | 10 | 20.44 | 0.25 | 0.001 |
| Y | 1 | 11.81 | 0.01 | 0.001 |
| H | 5 | 4.38 | 0.03 | 0.001 |
| S | 1 | 1.94 | 0.01 | 0.102 |
| Y*H | 5 | 0.25 | <0.01 | 1.000 |
| Y*S | 1 | 2.88 | <0.01 | 0.004 |
| H*S | 5 | 1.21 | <0.01 | 0.104 |
| Y*H*S | 5 | 0.63 | <0.01 | 0.071 |
| Residuals | 504 | 0.62 | ||
| total | 538 | 1.00 |
| Treatment | Herbicide Brand Name | Active Ingredient (s) | Rate (L. ha-1) |
|---|---|---|---|
| H1 | Garlon 4 Ultra® | Triclopyr | 1.17 |
| H2 | Garlon 4 Ultra® | Triclopyr | 1.76 |
| H3 | Pastureguard® HL | Triclopyr and Fluroxypyr | 1.29 |
| H4 | Pastureguard® HL | Triclopyr and Fluroxypyr | 1.76 |
| H5 | Pastureguard® HL | Triclopyr and Fluroxypyr | 2.57 |
| H6 | Control | N/A | N/A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibson, D.J.; Shupert, L.A.; Liu, X. Do No Harm: Efficacy of a Single Herbicide Application to Control an Invasive Shrub While Minimizing Collateral Damage to Native Species. Plants 2019, 8, 426. https://doi.org/10.3390/plants8100426
Gibson DJ, Shupert LA, Liu X. Do No Harm: Efficacy of a Single Herbicide Application to Control an Invasive Shrub While Minimizing Collateral Damage to Native Species. Plants. 2019; 8(10):426. https://doi.org/10.3390/plants8100426
Chicago/Turabian StyleGibson, David J., Lindsay A. Shupert, and Xian Liu. 2019. "Do No Harm: Efficacy of a Single Herbicide Application to Control an Invasive Shrub While Minimizing Collateral Damage to Native Species" Plants 8, no. 10: 426. https://doi.org/10.3390/plants8100426
APA StyleGibson, D. J., Shupert, L. A., & Liu, X. (2019). Do No Harm: Efficacy of a Single Herbicide Application to Control an Invasive Shrub While Minimizing Collateral Damage to Native Species. Plants, 8(10), 426. https://doi.org/10.3390/plants8100426






