Exploration of Floral Volatile Organic Compounds in Six Typical Lycoris taxa by GC-MS
Abstract
1. Introduction
2. Results
2.1. Identification of VOCs
2.2. Chemometric Analysis of VOCs
2.3. Determination of Key VOCs
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Sample Pretreatment
4.3. GC-MS Analysis
4.4. Identification and Quantification
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ang, S.; Liu, X.-M.; Huang, X.-J.; Zhang, D.-M.; Zhang, W.; Wang, L.; Ye, W.-C. Four New Amaryllidaceae Alkaloids from Lycoris radiata and Their Cytotoxicity. Planta Med. 2015, 81, 1712–1718. [Google Scholar] [CrossRef]
- Tsi, Z.H.; Meerow, A.W. Amaryllidaceae. In Flora of China; Wu, C.Y., Raven, P.H., Eds.; Beijing Science Press & Missouri Botanical Garden Press: Beijing, China, 2000; Volume 24, p. 264. [Google Scholar]
- Takos, A.M.; Rook, F. Towards a Molecular Understanding of the Biosynthesis of Amaryllidaceae Alkaloids in Support of Their Expanding Medical Use. Int. J. Mol. Sci. 2013, 14, 11713–11741. [Google Scholar] [CrossRef]
- Yan, H.; Xie, N.; Zhong, C.; Su, A.; Hui, X.; Zhang, X.; Jin, Z.; Li, Z.; Feng, J.; He, J. Aphicidal activities of Amaryllidaceae alkaloids from bulbs of Lycoris radiata against Aphis citricola. Ind. Crop. Prod. 2018, 123, 372–378. [Google Scholar] [CrossRef]
- Li, A.; Du, Z.; Liao, M.; Feng, Y.; Ruan, H.; Jiang, H. Discovery and characterisation of lycorine-type alkaloids in Lycoris spp. (Amaryllidaceae) using UHPLC-QTOF-MS. Phytochem. Anal. 2019, 30, 268–277. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Xu, X.-L.; Yang, L.-J.; Jiang, J.-G. Identification of narciclasine from Lycoris radiata (L’Her.) Herb. and its inhibitory effect on LPS-induced inflammatory responses in macrophages. Food Chem. Toxicol. 2019, 125, 605–613. [Google Scholar] [CrossRef]
- Sun, B.; Wang, P.; Wang, R.; Li, Y.; Xu, S. Molecular Cloning and Characterization of a meta/para-O-Methyltransferase from Lycoris aurea. Int. J. Mol. Sci. 2018, 19, 1911. [Google Scholar] [CrossRef]
- Meng, W.; Zhang, D.; Qin, H.; Wang, L.; Zheng, L.; Xia, Q.; Liu, K. Hybrid Origin of Lycoris shaanxiensis Revealed by Karyotype Survey. Cytologia 2018, 83, 133–136. [Google Scholar] [CrossRef]
- Chen, I.-J.; Shii, C.-T.; Chang, T.-L.; Hwu, K.-K. Development of 17 novel microsatellite markers for Lycoris aurea and L. radiata (Amaryllidaceae) using next-generation sequencing. Appl. Plant Sci. 2018, 6, e01198. [Google Scholar] [CrossRef]
- He, Q.; Shen, Y.; Wang, M.; Huang, M.; Yang, R.; Zhu, S.; Wang, L.; Xu, Y.; Wu, R. Natural Variation in Petal Color in Lycoris longituba Revealed by Anthocyanin Components. PLoS ONE 2011, 6, e22098. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Borghi, M.; Fernie, A.R.; Schiestl, F.P.; Bouwmeester, H.J. The Sexual Advantage of Looking, Smelling, and Tasting Good: The Metabolic Network that Produces Signals for Pollinators. Trends Plant Sci. 2017, 22, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, F.P. Ecology and evolution of floral volatile-mediated information transfer in plants. New Phytol. 2015, 206, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Whitehead, M.R. Floral odour chemistry defines species boundaries and underpins strong reproductive isolation in sexually deceptive orchids. Ann. Bot. 2013, 113, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.A.; Mahnert, A.; Müller, H.; Berg, C. The plant is crucial: Specific composition and function of the phyllosphere microbiome of indoor ornamentals. FEMS Microbiol. Ecol. 2016, 92, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, F.P. The evolution of floral scent and insect chemical communication. Ecol. Lett. 2010, 13, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Liu, J.; Shi, T.; Chen, M.; Li, Y.; Du, J.; Jiang, H.; Yang, X.; Hu, H.; Wang, L. Integrating Transcriptomic and GC-MS Metabolomic Analysis to Characterize Color and Aroma Formation during Tepal Development in Lycoris longituba. Plants 2019, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, B.; Li, M.; Gao, X.; Fang, Q.; Liu, C.; Ding, H.; Wang, L.; Gao, X. Identification and characterization of terpene synthase genes accounting for volatile terpene emissions in flowers of Freesia x hybrida. J. Exp. Bot. 2018, 69, 4249–4265. [Google Scholar] [CrossRef]
- Kaya, M.; Merdivan, M.; Tashakkori, P.; Erdem, P.; Anderson, J.L. Analysis of Echinacea flower volatile constituents by HS-SPME-GC/MS using laboratory-prepared and commercial SPME fibers. J. Essent. Oil Res. 2019, 31, 91–98. [Google Scholar] [CrossRef]
- Kutty, N.N.; Mitra, A. Profiling of volatile and non-volatile metabolites in Polianthes tuberosa L. flowers reveals intraspecific variation among cultivars. Phytochemistry 2019, 162, 10–20. [Google Scholar] [CrossRef]
- Zhu, Y.; Shao, C.-Y.; Lv, H.-P.; Zhang, Y.; Dai, W.-D.; Guo, L.; Tan, J.-F.; Peng, Q.-H.; Lin, Z. Enantiomeric and quantitative analysis of volatile terpenoids in different teas (Camellia sinensis). J. Chromatogr. A 2017, 1490, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeng, Z.; Zhao, C.; Kong, H.; Lü, X.; Xu, G. A comparative study of volatile components in green, oolong and black teas by using comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry and multivariate data analysis. J. Chromatogr. A 2013, 1313, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Liu, X.; Wang, S.; Zhang, X.; Ma, L. Classification of Different Dried Vine Fruit Varieties in China by HS-SPME-GC-MS Combined with Chemometrics. Food Anal. Methods 2017, 10, 2856–2867. [Google Scholar] [CrossRef]
- Giannetti, V.; Mariani, M.B.; Mannino, P.; Marini, F. Volatile fraction analysis by HS-SPME/GC-MS and chemometric modeling for traceability of apples cultivated in the Northeast Italy. Food Control. 2017, 78, 215–221. [Google Scholar] [CrossRef]
- Farres, M.; Platikanov, S.; Tsakovski, S.; Tauler, R. Comparison of the variable importance in projection (VIP) and of the selectivity ratio (SR) methods for variable selection and interpretation. J. Chemom. 2015, 29, 528–536. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemometr. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Lubes, G.; Goodarzi, M. Analysis of Volatile Compounds by Advanced Analytical Techniques and Multivariate Chemometrics. Chem. Rev. 2017, 117, 6399–6422. [Google Scholar] [CrossRef]
- Kong, Y.; Sun, M.; Pan, H.; Zhang, Q.; Ai, C.; Wang, Y. Floral scent composition of Lilium sulphureum. Chem. Nat. Compd. 2013, 49, 362–364. [Google Scholar] [CrossRef]
- Bera, P.; Mukherjee, C.; Mitra, A. Enzymatic production and emission of floral scent volatiles in Jasminum sambac. Plant Sci. 2017, 256, 25–38. [Google Scholar] [CrossRef]
- Yang, X.; Yue, Y.; Li, H.; Ding, W.; Chen, G.; Shi, T.; Chen, J.; Park, M.S.; Chen, F.; Wang, L. The chromosome-level quality genome provides insights into the evolution of the biosynthesis genes for aroma compounds of Osmanthus fragrans. Hortic. Res. 2018, 5, 72. [Google Scholar] [CrossRef]
- Kong, Y.; Sun, M.; Pan, H.-T.; Zhang, Q.-X. Composition and Emission Rhythm of Floral Scent Volatiles from Eight Lily Cut Flowers. J. Am. Soc. Hortic. Sci. 2012, 137, 376–382. [Google Scholar] [CrossRef]
- Li, X.; Tang, D.; Shi, Y. Volatile compounds in perianth and corona of Narcissus Pseudonarcissus cultivars. Nat. Prod. Res. 2019, 33, 2281–2284. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tang, B.; Wu, Q.; Zheng, J.; Leng, P.; Zhang, K. Transcriptome Sequencing Analysis Reveals a Difference in Monoterpene Biosynthesis between Scented Lilium ‘Siberia’ and Unscented Lilium ‘Novano’. Front. Plant Sci. 2017, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.P.; Ma, Z.Y.; Zhao, K.G.; Zhang, J.; Xiang, L.; Chen, L.Q. Transcriptomic and proteomic approaches to explore the differences in monoterpene and benzenoid biosynthesis between scented and unscented genotypes of wintersweet. Physiol. Plant. 2019, 166, 478–493. [Google Scholar] [CrossRef]
- Chun, J.-H.; Jang, I.H.; Arasu, M.V.; Al-Dhabi, N.A.; Duraipandiyan, V.; Lee, D.-H.; Lee, S.; Kim, S.-J. Isolation and identification of alkaloids and anthocyanins from flower and bulb of Lycoris radiata using HPLC and LC-ESI-MS. J. Agric. Chem. Environ. 2013, 2, 22–26. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, C.; Guo, M. Comparative Analysis of Amaryllidaceae Alkaloids from Three Lycoris Species. Molecules 2015, 20, 21854–21869. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.-Y.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. Structural and physicochemical characteristics of lycoris starch treated with different physical methods. Food Chem. 2019, 275, 8–14. [Google Scholar] [CrossRef]
- Ruíz-Ramón, F.; Águila, D.J.; Egea-Cortines, M.; Weiss, J. Optimization of fragrance extraction: Daytime and flower age affect scent emission in simple and double narcissi. Ind. Crop. Prod. 2014, 52, 671–678. [Google Scholar] [CrossRef]
- Raguso, R.A.; Levin, R.A.; Foose, S.E.; Holmberg, M.W.; McDade, L.A. Fragrance chemistry, nocturnal rhythms and pollination “syndromes” in Nicotiana. Phytochemistry 2003, 63, 265–284. [Google Scholar] [CrossRef]
- Shi, S.; Qiu, Y.; Wu, L.; Fu, C. Interspecific relationships of Lycoris (Amaryllidaceae) inferred from inter-simple sequence repeat data. Sci. Hortic. 2006, 110, 285–291. [Google Scholar] [CrossRef]
- Shi, S.; Qiu, Y.; Li, E.; Wu, L.; Fu, C. Phylogenetic Relationships and Possible Hybrid Origin of Lycoris Species (Amaryllidaceae) Revealed by ITS Sequences. Biochem. Genet. 2006, 44, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Sun, Y.; Wei, L.; Lei, X.; Cameron, K.M.; Fu, C. Plastid DNA sequence data help to clarify phylogenetic relationships and reticulate evolution in Lycoris (Amaryllidaceae). Bot. J. Linn. Soc. 2014, 176, 115–126. [Google Scholar]
- Klahre, U.; Gurba, A.; Hermann, K.; Saxenhofer, M.; Bossolini, E.; Guerin, P.M.; Kuhlemeier, C. Pollinator Choice in Petunia Depends on Two Major Genetic Loci for Floral Scent Production. Curr. Boil. 2011, 21, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Farré-Armengol, G.; Filella, I.; Llusià, J.; Peñuelas, J. Floral volatile organic compounds: Between attraction and deterrence of visitors under global change. Perspect. Plant Ecol. Evol. Syst. 2013, 15, 56–67. [Google Scholar] [CrossRef]
- Farré-Armengol, G.; Filella, I.; Llusià, J.; Peñuelas, J. β-Ocimene, a Key Floral and Foliar Volatile Involved in Multiple Interactions between Plants and Other Organisms. Molecules 2017, 22, 1148. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, C.; Zheng, R.; Cai, X.; Luo, J.; Zou, J.; Wang, C. Emission and Accumulation of Monoterpene and the Key Terpene Synthase (TPS) Associated with Monoterpene Biosynthesis in Osmanthus fragrans Lour. Front. Plant Sci. 2016, 6, 2739. [Google Scholar] [CrossRef]
- Chamorro, E.R.; Ballerini, G.; Sequeira, A.F.; Velasco, G.A.; Zalazar, M.F. Chemical composition of essential oil from Tagetes minuta L. leaves and flowers. J. Argent. Chem. Soc. 2008, 96, 80–86. [Google Scholar]
- Junker, R.R.; Tholl, D. Volatile Organic Compound Mediated Interactions at the Plant-Microbe Interface. J. Chem. Ecol. 2013, 39, 810–825. [Google Scholar] [CrossRef]
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008. [Google Scholar] [CrossRef]
- Gao, Y.; Jin, Y.-J.; Li, H.-D.; Chen, H.-J. Volatile Organic Compounds and Their Roles in Bacteriostasis in Five Conifer Species. J. Integr. Plant Boil. 2005, 47, 499–507. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Goepfert, M.; Liebl, P.; Herth, N.; Ciarlo, G.; Buentzel, J.; Huebner, J. Aroma oil therapy in palliative care: A pilot study with physiological parameters in conscious as well as unconscious patients. J. Cancer Res. Clin. Oncol. 2017, 143, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Haviland-Jones, J.; Simon, J.E.; Tepper, B.J. Influence of aroma intensity and nasal pungency on the ‘mood signature’ of common aroma compounds in a mixed ethnic population. Food Qual. Prefer. 2018, 65, 164–174. [Google Scholar] [CrossRef]
- Haehner, A.; Maass, H.; Croy, I.; Hummel, T. Influence of room fragrance on attention, anxiety and mood. Flavour Fragr. J. 2017, 32, 24–28. [Google Scholar] [CrossRef]
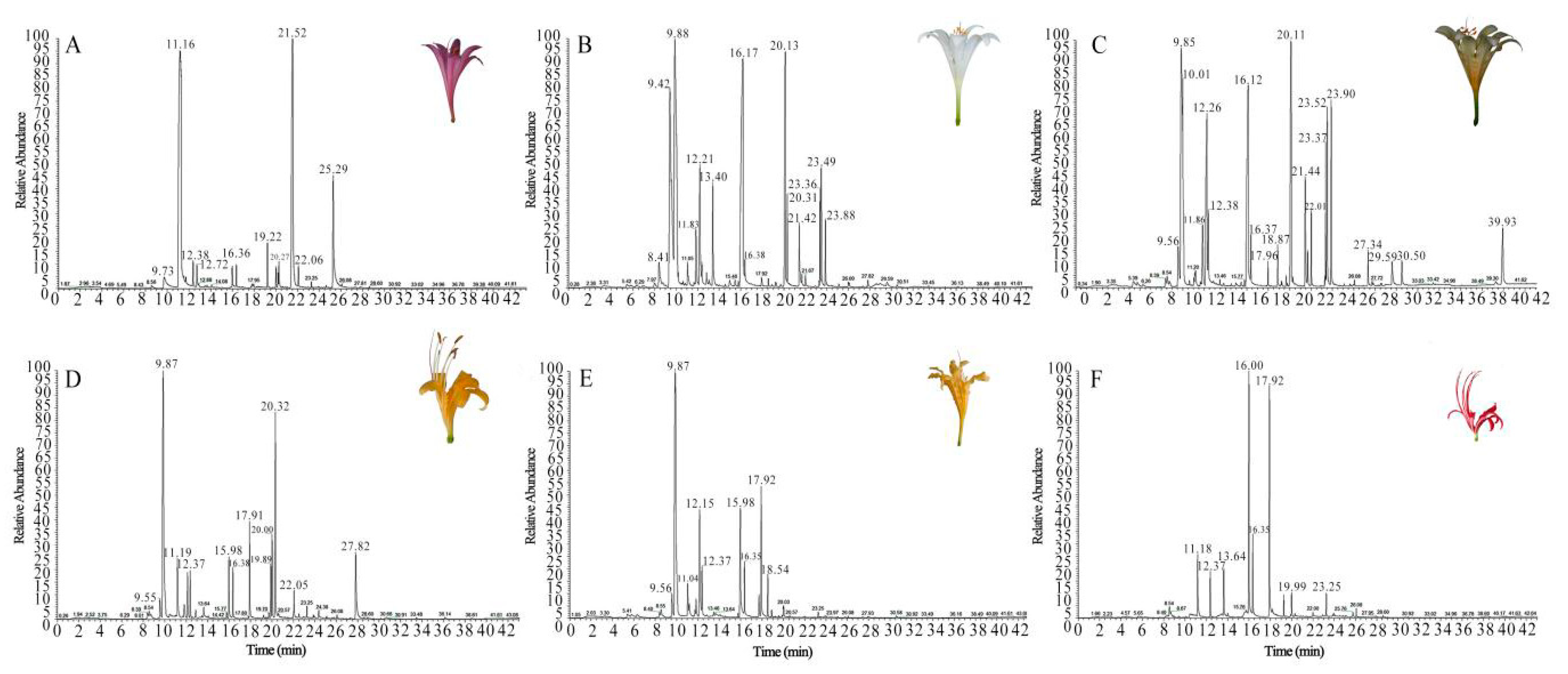
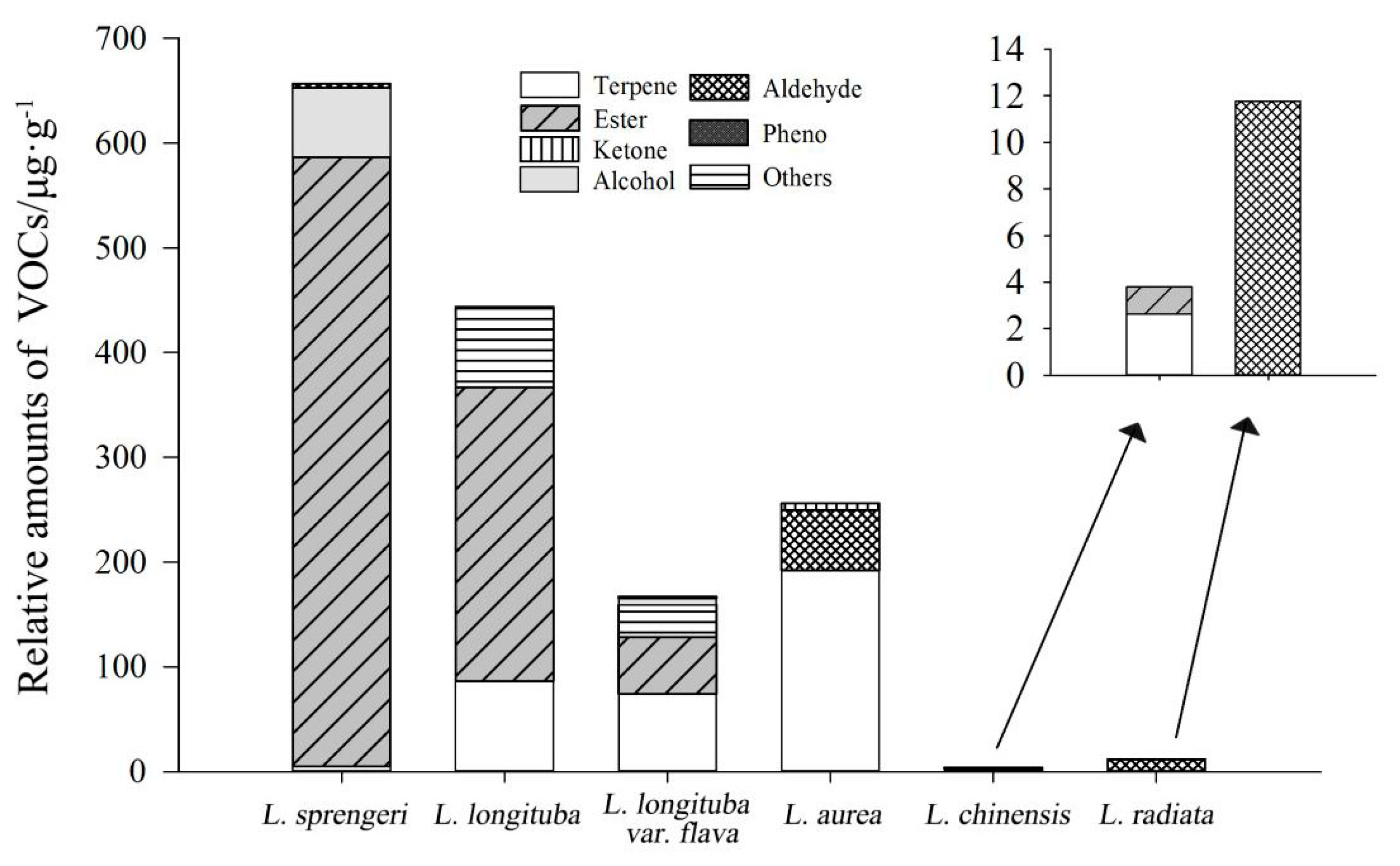

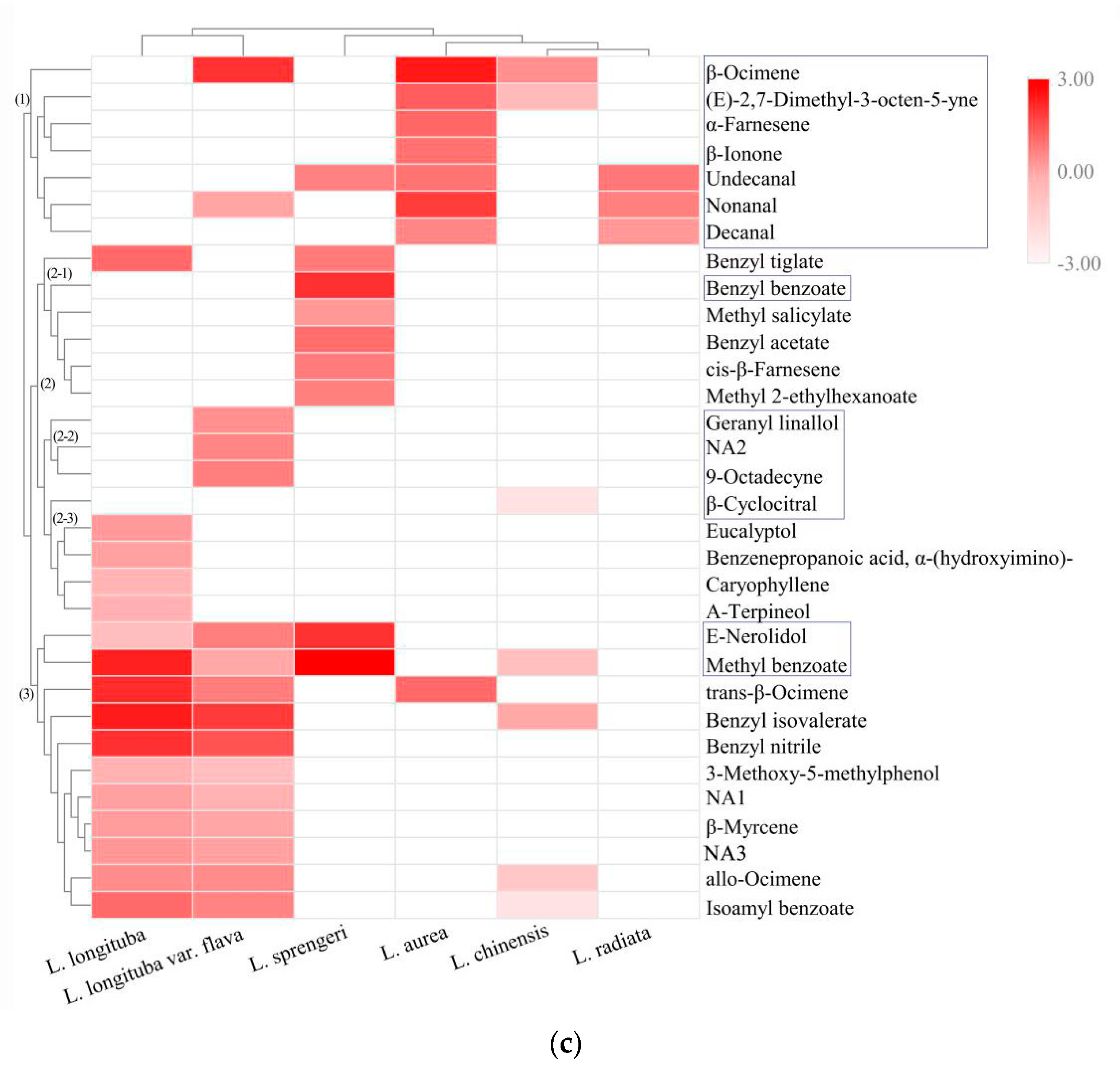
| NO. | Compounds | VIP* | P Value | Aroma Characteristics |
|---|---|---|---|---|
| 1 | β-ocimene | 1.32 | 0.000 | citrus, floral, woody |
| 2 | β-cyclocitral | 1.31 | 0.006 | saffron, rose, tobacco, fruity |
| 3 | NA2 | 1.31 | 0.000 | - |
| 4 | 9-octadecyne | 1.3 | 0.000 | - |
| 5 | undecanal | 1.28 | 0.001 | soapy, floral, citrus |
| 6 | (E)-2,7-dimethyl-3-octen-5-yne | 1.27 | 0.000 | - |
| 7 | β-ionone | 1.15 | 0.000 | woody, berry, floral, fruity |
| 8 | geranyl linallol | 1.15 | 0.000 | rose |
| 9 | methyl benzoate | 1.12 | 0.001 | wintergreen, cananga |
| 10 | α-farnesene | 1.12 | 0.000 | citrus, lavender, neroli |
| 11 | nonanal | 1.06 | 0.000 | rose, orris, citrus |
| 12 | benzyl benzoate | 1.04 | 0.000 | balsam, fruity |
| 13 | decanal | 1.01 | 0.001 | citrus, floral |
| 14 | E-nerolidol | 1 | 0.000 | floral, citrus, woody |
| Parameters | L. sprengeri | L. longituba | L. longituba var. flava | L. aurea | L. chinensis | L. radiate |
|---|---|---|---|---|---|---|
| Flower |  |  |  |  |  |  |
| Color | Purple | White | Light yellow | Yellow | Yellow | Red |
| Height (cm) | 13.56 ± 0.63c | 16.02 ± 0.87d | 16.24 ± 0.86de | 10.88 ± 1.07b | 17.78 ± 1.14e | 5.55 ± 0.60a |
| Breadth (cm) | 10.29 ± 0.76b | 13.59 ± 0.81cd | 14.77 ± 0.96d | 10.31 ± 0.54b | 13.01 ± 0.54c | 8.48 ± 0.59a |
| Aroma level | Scented | Scented | Scented | Scented | Non-scented | Non-scented |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Yue, Y.; Shi, M.; Chen, M.; Yang, X.; Wang, L. Exploration of Floral Volatile Organic Compounds in Six Typical Lycoris taxa by GC-MS. Plants 2019, 8, 422. https://doi.org/10.3390/plants8100422
Shi T, Yue Y, Shi M, Chen M, Yang X, Wang L. Exploration of Floral Volatile Organic Compounds in Six Typical Lycoris taxa by GC-MS. Plants. 2019; 8(10):422. https://doi.org/10.3390/plants8100422
Chicago/Turabian StyleShi, Tingting, Yuanzheng Yue, Man Shi, Min Chen, Xiulian Yang, and Lianggui Wang. 2019. "Exploration of Floral Volatile Organic Compounds in Six Typical Lycoris taxa by GC-MS" Plants 8, no. 10: 422. https://doi.org/10.3390/plants8100422
APA StyleShi, T., Yue, Y., Shi, M., Chen, M., Yang, X., & Wang, L. (2019). Exploration of Floral Volatile Organic Compounds in Six Typical Lycoris taxa by GC-MS. Plants, 8(10), 422. https://doi.org/10.3390/plants8100422




