1. Introduction
In their natural environment, plants are at risks of infections by pests and pathogens. Plant resistance to such infections is attributed to multiple defenses that comprise constitutive/preformed and inducible chemical barriers. Inducible chemical barriers involve the synthesis of pathogenesis-related proteins (
PR) and the accumulation of phytoalexins [
1]. An example of a phytoalexin is tricin, which is a flavonoid that confers resistance against brown planthopper in rice [
2]. Some phytoalexins are highly species-specific; for example, in pea (
Pisum sativum L.), the synthesis and localization of pisatin are primarily associated with resistance against Fusarium wilt [
3].
“Esca complex” is a widespread and destructive grapevine trunk affliction that affects grape yield and quality [
4,
5]. The disease is generally associated with the development of diverse wood pathogens among which the ascomycetes
Phaeomoniella chlamydospora and
Phaeoacremonium minimun and the basidiomycete
Fomitiporia mediterranea are most commonly cited [
6,
7,
8,
9]. The hyphae of these fungi spread into the xylem vessel and the parenchyma cells of trunks, leading to necrosis [
10]. Sometimes, these hyphae invade other woody tissues including cordons, arms, spurs, and canes [
11,
12,
13,
14].
“Esca complex” exhibits a long latency time (several years) between wood colonization and visible foliar symptoms [
5] and has become increasingly frequent worldwide. A ten-year survey conducted in different vine-growing regions of France revealed that the simplification of the woody vine structure may have resulted in an increase in the incidence of the affliction in the country [
15]. Some basic and practical cultural measures for preventing “Esca complex” have been proposed. For example, it was determined that increasing the length of cordons [
15] and opting for a minimal pruning system instead of the standard spur-pruning [
7] may help minimize the consequences of wood necroses. The foliar application of fertilizer mixtures containing calcium, magnesium, and Fucales seaweed was found effective in reducing foliar symptoms and increasing the yield and quality of berries [
4,
16]. The correlation between the symptomatic expression of “Esca complex” and the host physiology was highlighted by some authors [
13,
14,
17]. This implies that characterizing the impact of the affliction on grapevine physiology could help in finding candidate biomarkers associated with disease resistance.
Several studies have indicated that phytoalexins and in particular phenolic compounds (phenolic acids, flavonoids, anthocyanins, proanthocyanidins, and stilbenes) play a role in limiting the development of “Esca complex”. A typical reaction to wood colonization by esca-associated fungi is the accumulation of a mixture of polysaccharides (tyloses and gummosis) and the formation of polyphenol-rich reaction zones that obstruct the xylem to compartmentalize the fungi [
18]. However, decreased levels of most phenolic compounds were observed in the xylem sap of vines with severe wood symptoms [
19], as well as a decreasing trend for the levels of amino acids involved in the biosynthesis of phenolic compounds [
20].
Rusjan et al. [
10] found that esca-associated fungi caused the accumulation of flavonoids and stilbenes in both asymptomatic and necrotic trunks of vines. In particular, there was a high degree of flavonoid polymerization and a high level of procyanidins in the necrotic wood. However, reduction in the levels of phenolic compounds in asymptomatic wood and no effect on the levels in symptomatic wood were reported for
Pa. chlamydospora-infected young vines [
12]. Further, no difference was observed in total analyzed phenolics in asymptomatic stems of healthy and infected vines in the study by Magnin-Robert et al. [
13]; however, a considerable accumulation of stilbenes—
trans-resveratrol and
trans-vitisin B—was observed in the affected vines.
It was also demonstrated that vines respond to “Esca complex” by accumulating stilbenes in naturally infected leaves [
21] and leaves infected ex vivo with
Pa. chlamydospora [
22]. These increases were accompanied with the up-regulation of phenylalanine ammonia-lyase (
PAL) and stilbene synthase (
StSy)—two genes involved in the biosynthesis of polyphenolic compounds—in green [
22] and dry leaves [
23]. The accumulation of phenolic acids and flavonoids in symptomatic and asymptomatic leaves of field-grown vines was also reported [
24]. However, in the study by Martín et al. [
25], it was demonstrated that the appearance of foliar symptoms led to a decrease in the levels of flavonoids, proanthocyanidins, and hydroxycinnamic acids in the leaves of
Vitis vinifera L. ‘Tempranillo’ grown under a dry and warm temperature. For the same cultivar grown under a hot and humid temperature, hydroxycinnamic acids levels increased in symptomatic leaves whereas flavonoids levels decreased. Interestingly, levels of
trans-resveratrol in asymptomatic leaves of affected vines were slightly higher than those in healthy leaves of non-affected vines in some vineyards in Italy [
26].
It is evident from the abovementioned findings that there are different responses of phenolic compounds to “Esca complex”. These different results can be attributed to the types and complexities of symptomatic and asymptomatic materials studied by different authors.
Internal wood symptoms in adult vines are characterized by two diverse shapes of necrotic areas and discolorations. One shape/discoloration is caused by
F. mediterranea and is called “white rot” or simply “esca” it is characterized by a clear/yellowish soft and spongy mass of wood usually in the center of the trunk or cordons, which can be observed alone or with dark-brown to black spots in the xylem vessels [
10,
11,
14,
19]. The second shape/discoloration refers to different types of brown wood necrosis of which “dark/brown wood streaking” (BWS) is most commonly reported; BWS consists of extended columnar strips of xylem necrosis with pink-brown to dark-brown areas or black spots around the annual growth section [
13,
19]. A third type of shape/discoloration (wood stripe), which is present in external vine wood, is also reported and the symptoms appear as a longitudinal and superficial yellowish-orange stripe and orange-brown discolorations of the young wood vessels located immediately below the bark [
27].
Two typical severity levels of leaf symptoms are observed in esca-affected grapevines. A chronic form, characterized by tiger-striped symptoms (GLS) (also named by some authors GLSD for “Grapevine Leaf Stripe Disease”), is initially characterized by chlorosis and then light-green irregular spots and/or scorching between the main veins and/or along the leaf margins. The chlorotic and drying areas gradually expand from the basal to the distal part of the leaves, and then they coalesce to become partial necrotic stripes. As the chlorotic tissues turn yellow-brown or red-brown, the leaves exhibit a tiger stripe pattern [
17,
19,
25,
26,
27,
28]. GLSD symptoms are also reported in the berries and consist of tiny dark-brown or purple speckling distributed irregularly over the entire surface or scattered at the far end (termed “black measles” by some authors) and sometimes of shriveling/withering of grape bunches [
18]. “Apoplexy” consists of partial or complete sudden wilting of the crown and is considered an acute form of the leaf symptomatic expression of “Esca complex” [
23,
27] or an acute form of GLSD by some authors [
14]. BWS and GLSD vines are associated with a large procession of inhabiting fungi, although
Pa. chlamydospora and
Pm. minimun are most commonly found [
18]. Although the percentage of necrotic areas within the wood from which pathogens can be isolated is often a key factor to determine the severity of ”Esca complex,” wood necrosis is not always related to the incidence of foliar symptoms [
15]. Given this observation, the term “esca proper” is used by some authors to indicate the coexistence of “white rot” and GLSD in the same vine [
13,
14]. “Esca complex” is most commonly noted in established vineyards. In newly planted vines, scattered brown-black spots of necrotic xylem (without decay), often with a dark viscous ooze and a moderate/diffuse chlorosis of the leaves are observed, and the disease is termed “Petri disease” [
6,
8,
12].
Studies have shown that many abiotic factors and cultural practices (alone or combined) may influence the development of “Esca complex” and the variability of its damage [
15,
20,
26]. For example, it has been observed that heavy rainfall followed by hot winds in mid-summer favors the onset of apoplexy [
18]. These observations were confirmed by other authors [
25], who then reported that the biosynthesis of phenolic compounds in esca-affected leaves depended on the climate under which the vines were grown.
The above literature review shows that potential defense mechanisms developed by grapevine to resist esca-associated fungi need to be explored further. Therefore, the experiment in this study was designed to produce complementary data that would help improve the understanding of defense events occurring during an “Esca” invasion. It was hypothesized that esca-associated fungi induce the production of defensive compounds in leaves as part of both a locally- and systemically-induced defense response; local induction is defined as the enhancement of defensive traits in the organ that is attacked, while systemic induction is the enhancement of defenses in distant and undamaged organs, conferring broad-spectrum resistance throughout the plant [
29,
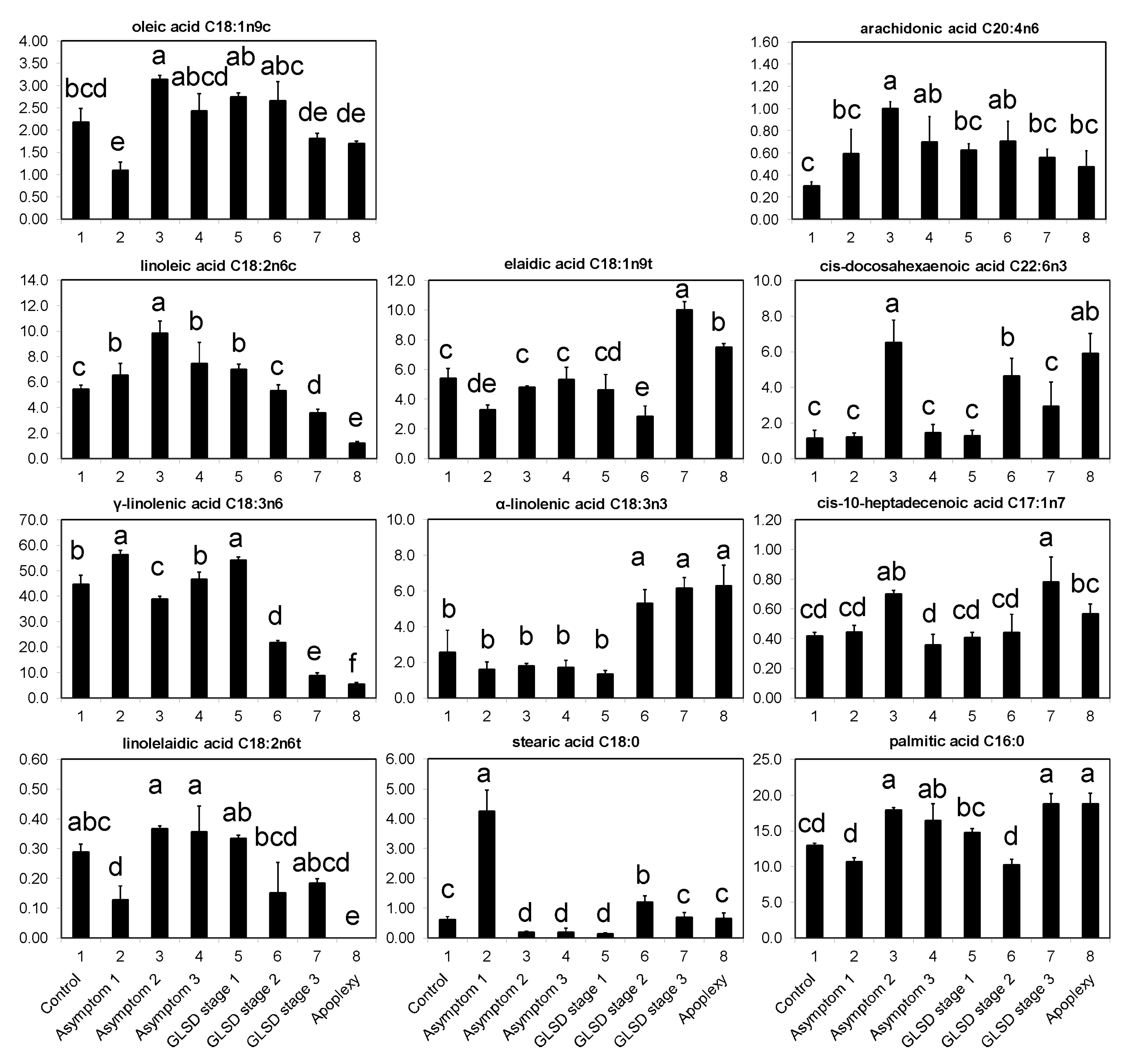
30]. To test this hypothesis, the accumulation of polyphenols in the leaves of vineyard-grown plants was monitored and levels of fatty acids were determined. Recent studies demonstrated that fatty acids play an important role in the modulation of signal transduction pathways in systemically acquired pathogen resistance. In several plants, the degree of resistance to pathogens was found to be directly correlated with the levels of C16:1 (palmitoleic acid), C18:1 (oleic and elaidic acids), C18:2 (linoleic and linolelaidic acids), C18:3 (α-linolenic and γ-linolenic acids), and C20:4 (achidonic acid) [
1]. For example, rhizobacteria-induced enhanced resistance to
Botrytis cinerea is associated with the accumulation of C18:2 and C18:3 in
Phaseolus vulgaris L. [
31], while reduction in C18:1 level induces defense responses against several pathogens by upregulating expressions of a variety of structurally diverse
R genes in
Arabidopsis [
32]. Therefore, the levels of phenolic compounds and fatty acids in asymptomatic and symptomatic leaves of grapevine affected by BWS, GLSD and apoplexy were investigated to identify infection stages at which plant resistance mechanisms were more efficiently activated.
4. Materials and Methods
4.1. Site Characterization: Cultivar, Location, and Weather
Experiments were performed on V. vinifera L. ‘Malvasia’ composed of 21–24-year-old plants in the vineyard of Quinta de Nossa Senhora de Loures (465 m, 41° 17.12’ 31’’ N, 7° 44.07’ 22’’ W) in Vila Real, Portugal. The vineyard has 1247 vines grafted on 196-17-Castel rootstock and trained to a bilateral cordon according to the royal-type trellis system, on an area of 0.27 ha. The vines were planted at a distance of 1.80 × 1.20 m in 22 longitudinal rows. The climatic conditions in the Quinta are characterized by an average annual air temperature of 14.35 °C (2.04/29.23 °C day/night) and 814 mm annual precipitation, with 75% relative humidity and a 16-h photoperiod (1350 μmol·m−2·s−1). The vineyard is located on Anthrosol (62% sand, 25% silt, 13% clay; pH 4.2) and the vines are managed without irrigation. Pruning, fertilization, and plant protection practices are undertaken annually according to local practices. The position of the vineyard in the Quinta allows all vines to be grown under the same soil and climatic conditions. The vineyard is naturally infected with esca-associated fungi, and since 2010, research at the experimental field focused on the prevention and cure of “Esca complex.”
4.2. Sampling Procedure
A characteristic trait of “Esca complex” is the unpredictable year-to-year discontinuity in foliar symptomatic expression [
5,
15,
19,
25]. Thus, to assess the incidence of the affliction, vines were inspected over four years through visual observations of leaf and berry symptoms and internal observations of wood symptoms by destructive means.
Several vines that did not show external symptoms since 2010 when work started at the experimental site were inspected during a four-year study period for the presence of discolorations associated with “Esca complex;” these vines were characterized as “apparently healthy” by several authors [
5,
11,
13,
14,
17,
23,
26,
28]. In this study, it was decided that an internal inspection of the wood was necessary before selecting “apparently healthy” vines. Therefore, wood cores were retrieved with a sterilized Pressler increment borer at 30 and 110 cm above the ground from the trunk of the vines, as described in [
19]. Based on the analysis of wood cores, the vines were categorized into two groups. The first group consisted of vines that did not exhibit symptoms either in the trunk or in the leaves; these vines were presumed healthy and considered as “controls,” as suggested in several papers [
7,
10,
19,
25]. Woods cores were subsequently subjected to fungal isolation and identification as described in [
6];
Pa. chlamydospora,
Pm. minimun, and
F. mediterranea were usually not identified in these wood cores. The second group consisted of vines with brown necrosis and dark streaking of the xylem vessels, or BWS vines. These vines did not exhibit visible leaf or berry symptoms during the four-year survey.
Phaeomoniella chlamydospora and
Pm. minimun were identified in these wood cores, along with some
Phaeoacremonium,
Botryospaeriaceae, and other species (data not shown). The wood deterioration characteristic of “white rot” was not observed. GLSD was the prevalent form of “Esca complex” in the vineyard. Some GLSD vines had both symptomatic and asymptomatic shoots (one cordon symptomatic and one cordon asymptomatic), and they were selected for the study; such vines were also studied by several authors [
4,
14,
17,
24,
28]. Other vines that showed GLSD leaf symptoms in a one or more inspection years and in some years only berry symptoms were also studied; however, this was a rare observation in the vineyard.
GLSD leaf symptoms at different degrees of severity were easily identifiable in the field. Leaf symptoms appeared between late June and early August, and although they usually increased in severity with plant growth, this increase was highly variable. In order to understand the biosynthesis of phenolic compounds by symptomatic expression, rather than selecting leaves with different degrees of symptom severity, vines with the majority of their leaves showing the same degree of symptom severity at harvest were targeted. In some vines, small chloroses characteristic of GLSD appeared, but did not evolve rapidly into spotting/scorching or tiger stripes. At the time of berry harvest, the surface of most leaves on these vines was still covered with discolorations, although some leaves started producing spotting/scorching or assuming the “tiger stripes” pattern (GLSD severity stage 1). At harvest, GLSD symptoms appeared in some vines as mainly chlorotic/spotting/scorching zones scattered over the leaf lamina (GLSD severity stage 2) or mainly tiger striped leaves (GLSD severity stage 3). An attempt was made to group vines exhibiting apoplectic symptoms; these symptoms appeared in a highly discontinuous manner in time (usually between early August and early September) and space in the vineyard. All selected vines were numbered and marked according to their place in the lines and rows.
4.3. Sample Collection
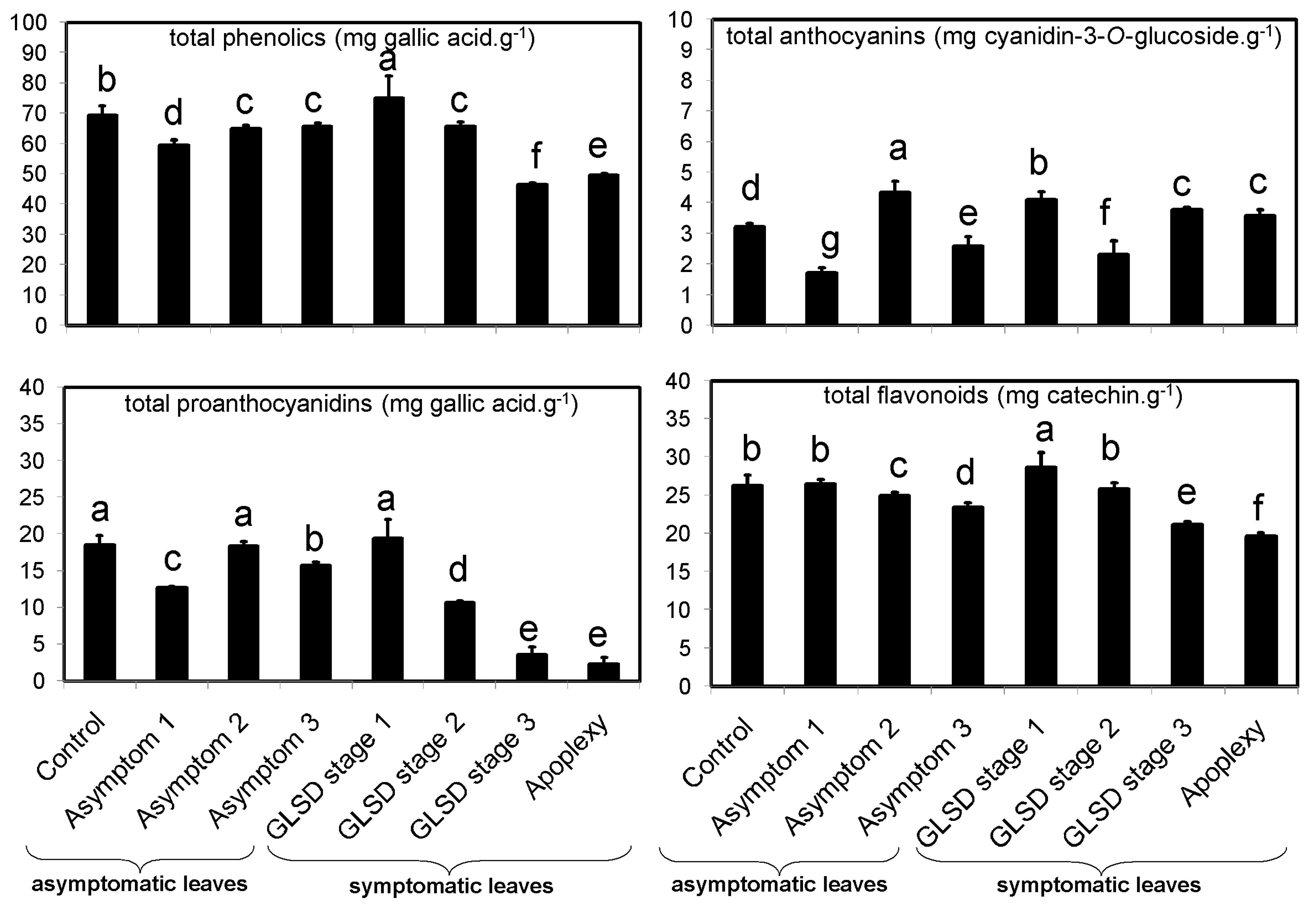
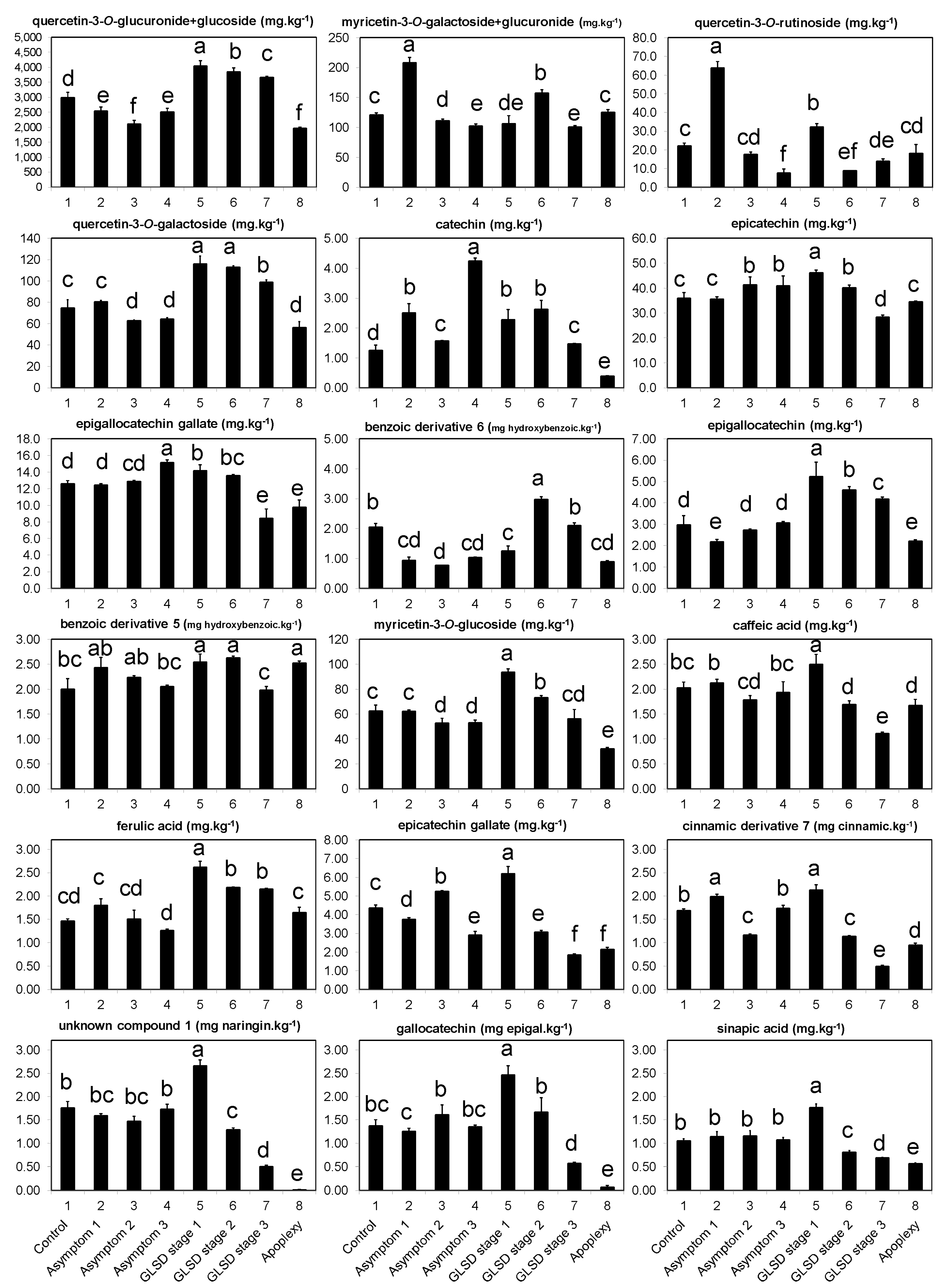
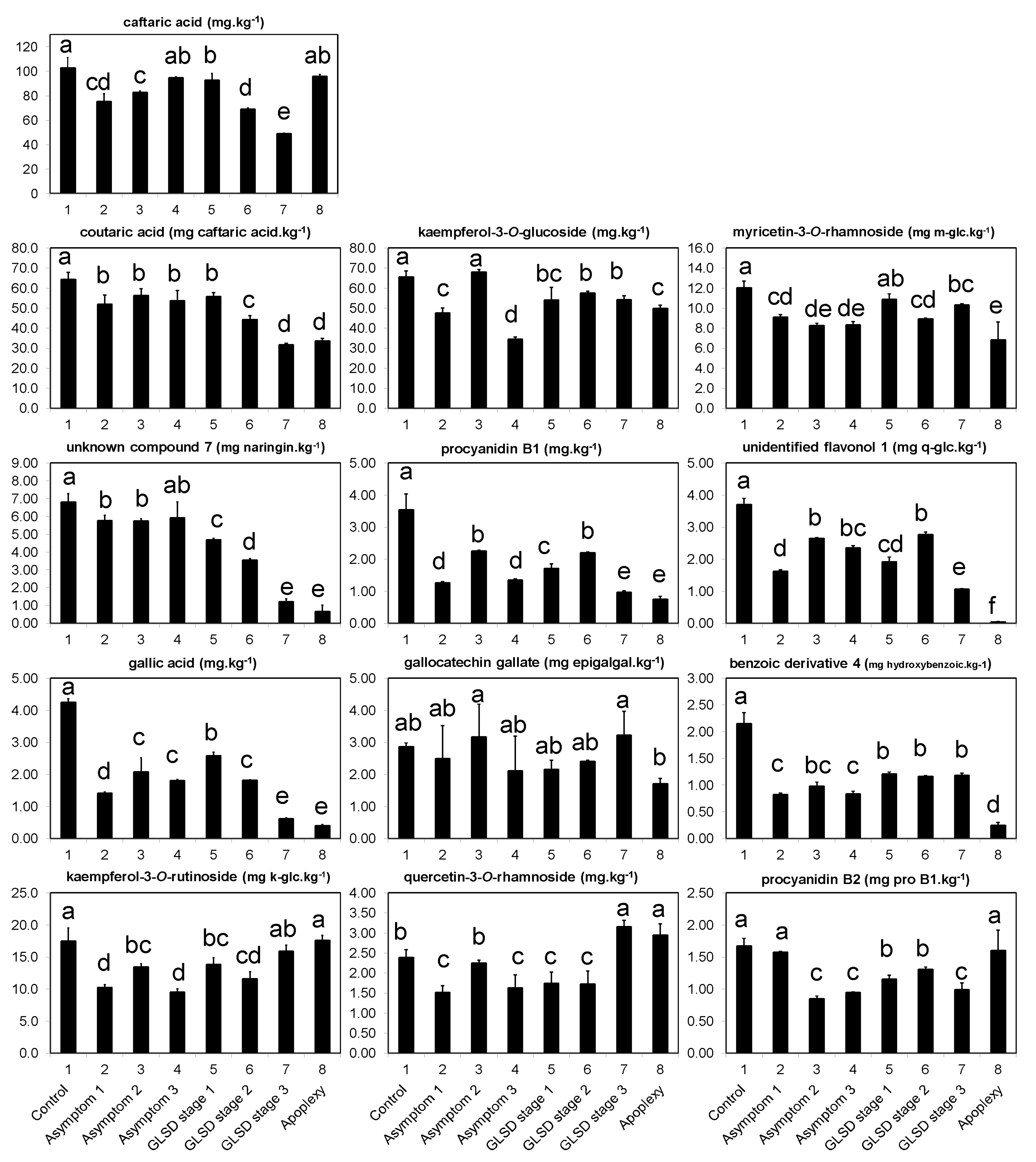
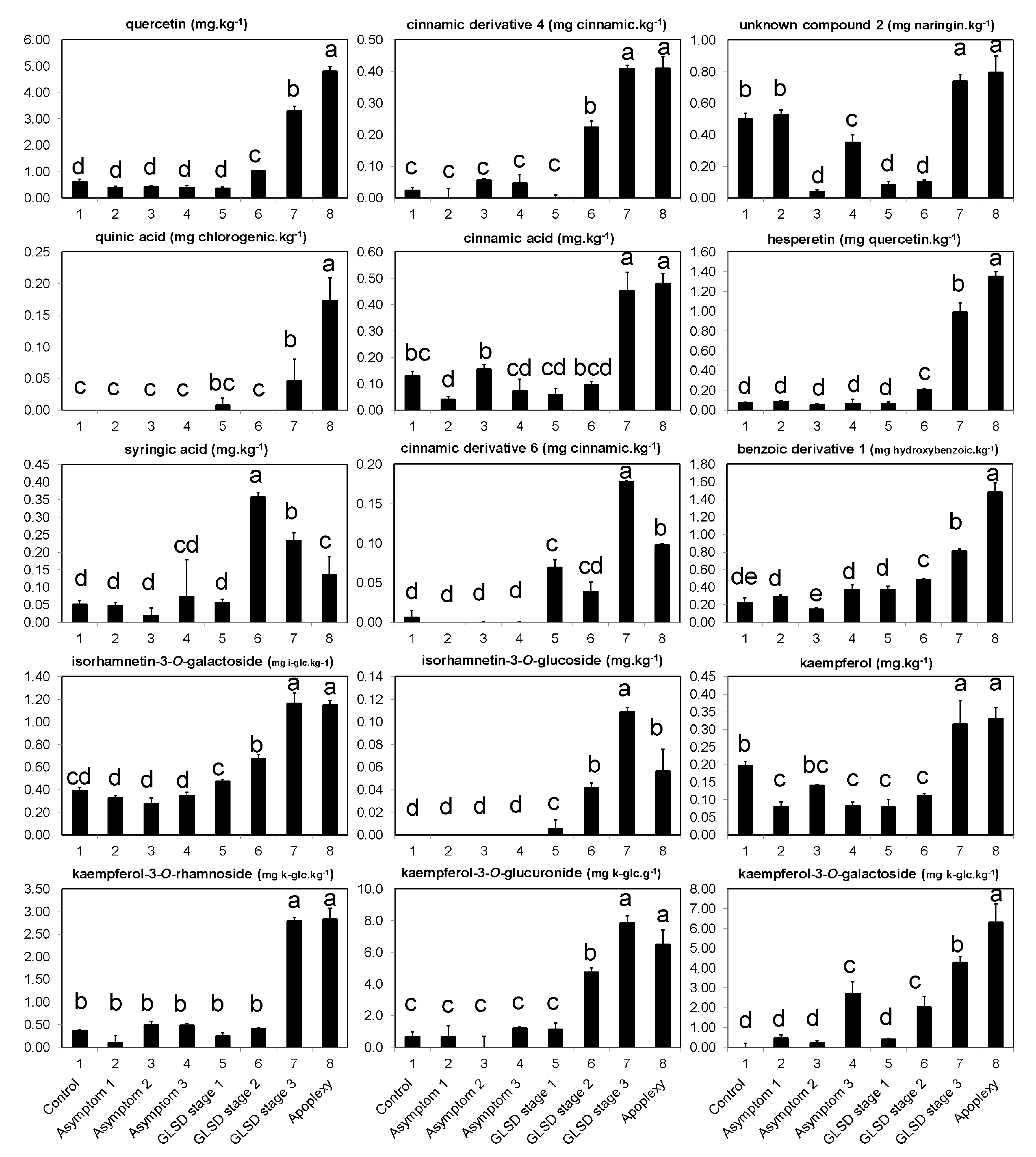
The occurrence of symptoms in the vineyard allowed the collection of different sets of leaves, which were divided into eight groups (
Figure 6): (1) Asymptomatic leaves from apparently healthy vines (control); (2) asymptomatic leaves from BWS vines (asymptomatic 1), to analyze the systemic effects of trunk-localized fungi attack; (3) asymptomatic leaves from asymptomatic cordons on GLSD vines (asymptomatic 2), to assess whether the biosynthesis of defensive compounds was similar in symptomatic and asymptomatic parts of the same vine; (4) asymptomatic leaves from GLSD vines with berry symptoms (asymptomatic 3), to analyze the systemic effects of berry-localized infection; (5) symptomatic leaves from vines with initial symptoms of GLSD i.e., chlorotic leaves (GLSD stage 1); (6) symptomatic leaves from vines with moderate symptoms of GLSD i.e., chlorotic/spotting/scorching leaves (GLSD stage 2); (7) symptomatic leaves from vines with advanced symptoms of GLSD i.e., tiger striped leaves (GLSD stage 3); (8) symptomatic leaves from apoplectic vines (apoplexy). In the field, apoplexy appeared quickly, affecting the entire vine with total wilt and immediate drying caused by the hot weather (an average of 32 °C day temperature during apoplexy expression); thus, apoplectic leaves were harvested and studied already dried as in [
23]. All symptomatic leaves were collected to study locally induced defenses.
All samples were collected mid-September, one day prior to berry harvesting. This ensured that the leaves were at the same stage of maturity. For each leaf-group, four vines were used for sampling and were considered as replicates. Six to twelve leaves of the same size from different parts of a vine were selected. Only two vines exhibited berry symptoms at harvest; hence, two sets of leaves were harvested from each vine to make four replicates, allowing for statistical comparisons. Leaves were immediately frozen in the field with liquid nitrogen to halt enzymatic activities and stored at −80 °C. Prior to use, the leaves were lyophilized, finely powdered with a hand blender, and sieved (0.2-mm mesh).
4.4. Determination of Total Amounts of Phenolic Compounds
Phenolic compounds were extracted using an optimized laboratory protocol. After defatting with 1 mL hexane for 16 h, 0.2 g samples were extracted using 1 mL 70% methanol added with 10 μL naringin as an internal standard, during ultrasonication in ice water for 20 min. The extract was centrifuged at 13,000×
g for 15 min (25 °C), and the extraction was repeated using the pellet. The combined supernatants were pre-purified on a Sep-Pak C18 cartridge (Waters, Milford, MA, USA) to remove chlorophylls, and then filtered through a Spartan 13/0.2 RC filter (Whatman, Dassel, Germany). The filtrate was used for the determination of total phenolic content (TPC) in mg gallic acid equivalent [GAE]·g
−1 using the Folin–Ciocalteu method as described in [
36]; total flavonoid content (TFC) in mg catechin equivalent [CAE]·g
−1 using aluminum chloride as described in [
36]; and total proanthocyanidin content (TPAC) in mg [GAE]·g
−1 using polyvinylpyrrolidone, as described in [
37]. The total anthocyanin content (TAC) was estimated using the pH differential assay [
38], and the results were expressed in mg cyanidin 3-
O-glucoside [CGE]·g
−1.
4.5. Chromatographic Separation and Identification of Phenolic Compounds
The quantitative analysis of individual phenolic compounds was carried out on a Gilson (Villers-le-bel, France) high-performance liquid chromatography (HPLC) instrument consisting of an autosampler, binary pump, column compartment, and a Finnigan photodiode array detector (DAD 81401; Thermo Electron, San Jose, CA, USA). Chromatography was performed on 10 μL samples of the phenolic filtrate injected into the HPLC onto a C18 column (5 μm, 250 × 4.5 mm i.d.) supplied from Sigma/Aldrich (Steinheim, Germany), and maintained at 25 °C. The solvent system consisted of 0.1% trifluoroacetic acid in water (mobile phase A) and 0.1% trifluoroacetic acid in acetonitrile (mobile phase B). Elution was performed at a constant flow rate of 1 mL.min−1 using a linear gradient program starting with 100% mobile phase A for 5 min, decreasing to 80% at 15 min, 50% at 30 min, 0% at 45 min, and then reverting to 100% at 55 until reaching 60 min.
The detection of compounds by DAD was conducted by scanning between 210–520 nm, with a resolution of 1.2 nm. Eluting peaks were monitored at 280, 320, 360, and 520 nm for hydroxybenzoic acids and other low molecular weight compounds, hydroxycinnamic acids and stilbenes, flavonoids, and anthocyanins, respectively, using the software Excalibur 2.0, which generated a three-dimensional dataset (absorbance, retention time, and wavelength). Eluting peaks at 450 nm were also monitored because two peaks were consistently observed with large areas at that wavelength. The peaks were selected using both the Gensis and the ICIS detection algorithms of Xcalibur. The threshold for quantification by peak areas was 5000 μAU·min−1, and compounds whose peak areas were below this value were considered “non-detected.”
For identification, 38 reference compounds previously reported in grapevine leaves [
10,
12,
13,
19,
22,
25,
26,
33], and representatives of the chemical classes under study were purchased (
Table S1); they were also separated by HPLC. Peaks were identified with “some certainty” to compounds by matching UV/vis spectra and retention times with those of the reference compounds. The remaining peaks were putatively identified by comparison with UV/vis bibliographic data. Some peaks could not match to any compounds or phenolic group and were labeled as “unknown.” Compounds were quantified by dividing their peak areas with that of the internal standard (naringin) and the results were converted to mg·kg
−1 after correction by the peak area of the reference, its response factor, and the amount of biomass extracted. For compounds identified putatively, quantification was carried out using reference compounds with similar chemical characteristics as shown in
Table S1.
4.6. Extraction, Separation, and Identification of Fatty Acids
The extraction of lipids was based on the method presented in [
39]. Leaf samples (5 mg) were added with 0.8 mL water and 2 mL methanol in a DSR-2800V rotary shaker (Digisystem Laboratory Instruments Inc, Taipei, Taiwan) at room temperature; after continuous shaking for 5 min, 1 mL chloroform was added, and it was followed by agitation for 5 min. The mixture was centrifuged for 5 min at 2000×
g (25 °C). The supernatant was collected and 2 mL chloroform/water (1/1,
v/
v) and five drops of 100 mM KCl were added. After vortexing, the mixture was centrifuged for 5 min at 2000×
g (25 °C). The lipid fraction in the bottom layer was collected and the chloroform phase was evaporated to dryness under nitrogen. The dried extract was then transesterified with 5 mL 14% boron trifluoride in methanol under nitrogen at 70 °C for 60 min. Transesterified lipids were extracted by adding 5 mL hexane, followed by 3 min of vortexing. The upper phase, constituting fatty acid methyl esters (FAME), was collected and 1 g Na
2SO
4 was added to remove water.
FAME were separated via capillary gas chromatography (CG) using Shimadzu GC-2010 Plus (Shimadzu, Kyoto, Japan) equipped with an autosampler and an automatic split/splitless injector. Exactly 1 µL of FAME extract was injected into the GC at an inlet temperature of 270 °C and a split ratio of 5:1; compounds were separated on a 30 m long, 0.25-μm-thick-film DB-225MS column with a 0.25 mm i.d. (Agilent, Wilmington, DE, USA). The flow rate of the carrier gas (helium) was maintained at a constant value of 1 mL·min−1 at an inlet pressure of 200 kPa. The column temperature was maintained at 200 °C for 10 min, and it was then increased to 220 °C at a rate of 5 °C·min−1.
The resolved compounds were detected using a flame ionization detector (FID-2010 Plus) set at 270 °C; the compounds were identified by comparing their retention times to those of a standard FAME mixture (FAME 37, Supelco, Bellefonte, PA, USA) run under the same conditions. Quantification was achieved by integrating the peaks with the Lab Solution 5.71 software, setting the minimum peak area/height at 2000 count. The amount of each FAME was expressed as a weight percentage of the total FAMEs represented in the chromatogram.
4.7. Statistical analyses
All data from four replications were subjected to an analysis of variance (ANOVA) using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). In the case of TPC, TPAC, TFC and TAC, each replicate was analyzed two times and the average values used in statistical analyses. The Tukey’s test was applied for assessing the mean differences, and a P value of ≤ 0.05 was considered as meaning statistical difference between the leaf groups.