Differential Effects of Increasing Salinity on Germination and Seedling Growth of Native and Exotic Invasive Cordgrasses
Abstract
:1. Introduction
2. Results
2.1. Germination Responses to Salinity
2.2. Germination Responses after Salinity Exposure
2.3. Initial Seedling Growth Responses to Salinity
3. Discussion
4. Materials and Methods
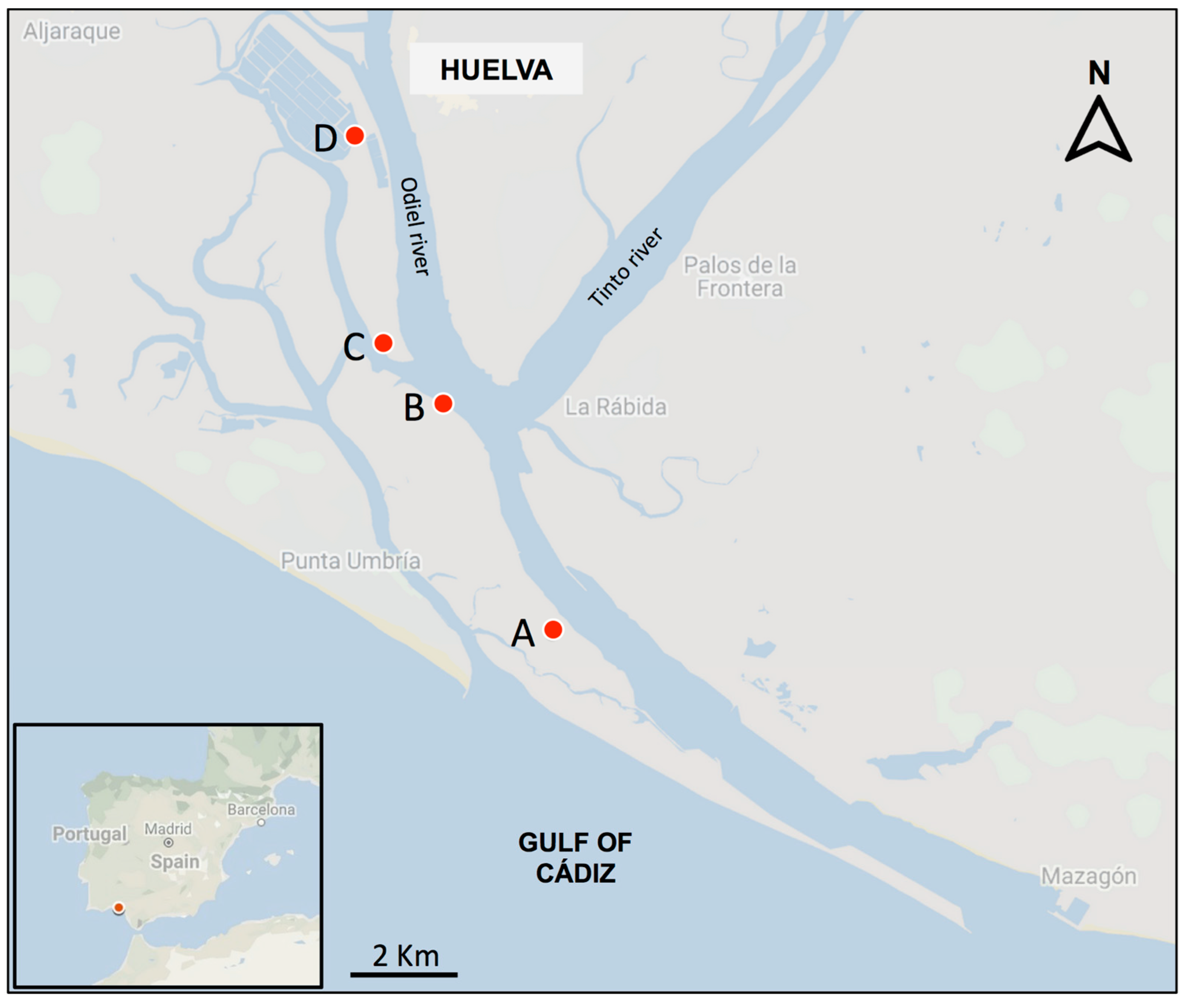
4.1. Study Area and Plant Material
4.2. Salinity Germination Experiment
4.3. Post-Salinity Exposure Recovery Experiment
4.4. Initial Seedling Growth
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ungar, I.A. Population ecology of halophyte seeds. Bot. Rev. 1987, 53, 301–334. [Google Scholar] [CrossRef]
- Ungar, I.A. Seed germination and seed-bank ecology of halophytes. In Seed Development and Germination; Kigel, J., Galili, G., Eds.; Marcel Dekker: New York, NY, USA, 1995; pp. 599–628. [Google Scholar]
- Rubio-Casal, A.E.; Castillo, J.M.; Luque, C.J.; Figueroa, M.E. Nucleation and facilitation in salt pans in Mediterranean salt marshes. J. Veg. Sci. 2001, 12, 761–770. [Google Scholar] [CrossRef]
- Ungar, I.A. Seed banks and seed population dynamics of halophytes. Wetl. Ecol. Manag. 2001, 9, 499–510. [Google Scholar] [CrossRef]
- Song, J.; Feng, G.; Tian, C.Y.; Zhang, F.S. Strategies for adaptation of Suaeda physophora, Haloxylon ammodendron and Haloxylon persicum to a saline environment during seed germination stage. Ann. Bot. 2005, 96, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Gul, B.; Ansari, R.; Flowers, T.J.; Khan, M.A. Germination strategies of halophyte seeds under salinity. Environ. Exp. Bot. 2013, 92, 4–18. [Google Scholar] [CrossRef]
- Woodell, S.R.J. Salinity and seed germination patterns in coastal plants. Vegetatio 1985, 61, 223–229. [Google Scholar] [CrossRef]
- Muñoz-Rodríguez, A.F.; Sanjosé, I.; Márquez-García, B.; Infante-Izquierdo, M.D.; Polo-Ávila, A.; Nieva, F.J.J.; Castillo, J.M. Germination syndromes in response to salinity of Chenopodiaceae halophytes along the intertidal gradient. Aquat. Bot. 2017, 139, 48–56. [Google Scholar] [CrossRef]
- Keiffer, C.H.; Ungar, I.A. The effect of extended exposure to hypersaline conditions on the germination of five inland halophyte species. Am. J. Bot. 1997, 84, 104–111. [Google Scholar] [CrossRef]
- Khan, M.A.; Ungar, I.A. Effects of thermoperiod on recovery of seed germination of halophytes from saline conditions. Am. J. Bot. 1997, 84, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Gulzar, S. Light, salinity, and temperature effects on the seed germination of perennial grasses. Am. J. Bot. 2003, 90, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Zedler, J.B.; Kercher, S. Causes and consequences of invasive plants in wetlands: Opportunities, opportunists, and outcomes. Crit. Rev. Plant Sci. 2004, 23, 431–452. [Google Scholar] [CrossRef]
- Weber, E.; D’Antonio, C.M. Germination and growth responses of hybridizing Carpobrotus species (Aizoaceae) from coastal California to soil salinity. Am. J. Bot. 1999, 86, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Dethier, M.N.; Hacker, S.D. Physical factors vs. biotic resistance in controlling the invasion of an estuarine marsh grass. Ecol. Appl. 2005, 15, 1273–1283. [Google Scholar] [CrossRef]
- Yuan, Z.; Shi, F. Ecological adaptation strategies in alien species: Effects of salinity, temperature and photoperiod on Spartina alterniflora Loisel. seed germination. Pol. J. Ecol. 2009, 57, 677–684. [Google Scholar]
- Stralberg, D.; Brennan, M.; Callaway, J.C.; Wood, J.K.; Schile, L.M.; Jongsomjit, D.; Kelly, M.; Parker, V.T.; Crooks, S. Evaluating tidal marsh sustainability in the face of sea-level rise: A hybrid modeling approach applied to San Francisco Bay. PLoS ONE 2011, 6, e27388. [Google Scholar] [CrossRef]
- Pennings, S.C.; Bertness, M.D. Using latitudinal variation to examine effects of climate on coastal salt marsh pattern and process. Curr. Top. Wetl. Biogeochem. 1999, 3, 100–111. [Google Scholar]
- Scavia, D.; Field, J.C.; Boesch, D.F.; Buddemeier, R.W.; Burkett, V.; Cayan, D.R.; Fogarty, M.; Harwell, M.A.; Howarth, R.W.; Mason, C.; et al. Climate change impacts on US coastal and marine ecosystems. Estuaries 2002, 25, 149–164. [Google Scholar] [CrossRef]
- Gilman, E.L.; Ellison, J.; Duke, N.C.; Field, C. Threats to mangroves from climate change and adaptation options: A review. Aquat. Bot. 2008, 89, 237–250. [Google Scholar] [CrossRef]
- Strong, D.R.; Ayres, D.R. Ecological and evolutionary misadventures of Spartina. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 389–410. [Google Scholar] [CrossRef]
- Nieva, F.J.J.; Díaz-Espejo, A.; Castellanos, E.M.; Figueroa, M.E. Field variability of invading populations of Spartina densiflora Brong. in different habitats of the Odiel Marshes (SW Spain). Estuar. Coast. Shelf Sci. 2001, 52, 515–527. [Google Scholar] [CrossRef]
- Marchant, C.J.; Goodman, P.J. Spartina maritima (Curtis) Fernald. J. Ecol. 1969, 57, 287–291. [Google Scholar] [CrossRef]
- Castellanos, E.M.; Figueroa, M.E.; Davy, A.J. Nucleation and facilitation in saltmarsh succession: Interactions between Spartina maritima and Arthrocnemum perenne. J. Ecol. 1994, 82, 239–248. [Google Scholar] [CrossRef]
- Castillo, J.M.; Ayres, D.R.; Leira-Doce, P.; Bailey, J.; Blum, M.; Strong, D.R.; Luque, T.; Figueroa, E. The production of hybrids with high ecological amplitude between exotic Spartina densiflora and native S. maritima in the Iberian Peninsula. Divers. Distrib. 2010, 16, 547–558. [Google Scholar] [CrossRef]
- Infante-Izquierdo, M.D.; Castillo, J.M.; Nieva, F.J.J.; Rotundu, I.D.; David, F.T.; Grewell, B.J.; Muñoz-Rodríguez, A.F. Fruit set, seed viability and germination of the European native Spartina maritima in Southwest Iberian Peninsula. Wetlands 2019. [Google Scholar] [CrossRef]
- Bortolus, A. The austral cordgrass Spartina densiflora Brong: Its taxonomy, biogeography and natural history. J. Biogeogr. 2006, 33, 158–168. [Google Scholar] [CrossRef]
- Castillo, J.M.; Rubio-Casal, A.E.; Redondo, S.; Álvarez-Lopez, A.A.; Luque, T.; Luque, C.; Nieva, F.J.J.; Castellanos, E.M.; Figueroa, M.E. Short-term responses to salinity of an invasive cordgrass. Biol. Invasions 2005, 7, 29–35. [Google Scholar] [CrossRef]
- Di Bella, C.E.; Striker, G.G.; Escaray, F.J.; Lattanzi, F.A.; Rodríguez, A.M.; Grimoldi, A.A. Saline tidal flooding effects on Spartina densiflora plants from different positions of the salt marsh. Diversities and similarities on growth, anatomical and physiological responses. Environ. Exp. Bot. 2014, 102, 27–36. [Google Scholar] [CrossRef]
- Nieva, F.J.J.; Castellanos, E.M.; Figueroa, M.E. Effects of light and salinity on seed germination in the marsh invader Spartina densiflora Brong., 1829 (Gramineae) from Gulf of Cádiz, Spain. Bol. R. Soc. Esp. Hist. Nat. 2001, 96, 117–124. [Google Scholar]
- Kittelson, P.M.; Boyd, M.J. Mechanisms of expansion for an introduced species of cordgrass, Spartina densiflora, in Humboldt Bay, California. Estuaries 1997, 20, 770–778. [Google Scholar] [CrossRef]
- Mateos-Naranjo, E.; Redondo-Gómez, S. Interpopulation differences in salinity tolerance of the invasive cordgrass Spartina densiflora: Implications for invasion process. Estuar. Coast. 2016, 39, 98–107. [Google Scholar] [CrossRef]
- Redondo, S.; Rubio-Casal, A.E.; Castillo, J.M.; Luque, C.J.; Álvarez, A.A.; Luque, T.; Figueroa, M.E. Influences of salinity and light on germination of three Sarcocornia taxa with contrasted habitats. Aquat. Bot. 2004, 78, 255–264. [Google Scholar] [CrossRef]
- Contreras-Cruzado, I.; Infante-Izquierdo, M.D.; Márquez-García, B.; Hermoso-López, V.; Polo, A.; Nieva, F.J.J.; Cartes-Barroso, J.B.; Castillo, J.M.; Muñoz-Rodríguez, A. Relationships between spatio-temporal changes in the sedimentary environment and halophytes zonation in salt marshes. Geoderma 2017, 305, 173–187. [Google Scholar] [CrossRef]
- Wijte, A.H.; Gallagher, J.L. Effect of oxygen availability and salinity on early life history stages of salt marsh plants. I. Different germination strategies of Spartina alterniflora and Phragmites australis (Poaceae). Am. J. Bot. 1996, 83, 1337–1342. [Google Scholar] [CrossRef]
- Li, R.; Shi, F.; Fukuda, K. Interactive effects of salt and alkali stresses on seed germination, germination recovery, and seedling growth of a halophyte Spartina alterniflora (Poaceae). S. Afr. J. Bot. 2010, 76, 380–387. [Google Scholar] [CrossRef]
- Cordazzo, C.V. Effects of salinity on seed germination, seedling growth and survival of Spartina ciliata Brong. Acta Bot. Bras. 1999, 13, 317–322. [Google Scholar] [CrossRef]
- Van Zandt, P.A.; Mopper, S. The effects of maternal salinity and seed environment on germination and growth in Iris hexagona. Evol. Ecol. Res. 2004, 6, 813–832. [Google Scholar]
- Zia, S.; Khan, M.A. Effect of light, salinity, and temperature on seed germination of Limonium stocksii. Can. J. Bot. 2004, 82, 151–157. [Google Scholar] [CrossRef]
- Muñoz-Rodríguez, A.F.; Rodríguez-Rubio, P.; Nieva, F.J.J.; Fernández-Illescas, F.; Sánchez-Gullón, E.; Soto, J.M.; Hermoso-López, V.; Márquez-García, B. The importance of bracteoles in ensuring Atriplex halimus germination under optimal conditions. Fresen. Environ. Bull. 2012, 21, 3521–3526. [Google Scholar]
- Pujol, J.A.; Calvo, J.F.; Ramírez-Diaz, L. Recovery of germination from different osmotic conditions by four halophytes from southeastern Spain. Ann. Bot. 2000, 85, 279–286. [Google Scholar] [CrossRef]
- Greipsson, S.; Davy, A.J. Germination of Leymus arenarius and its significance for land reclamation in Iceland. Ann. Bot. 1994, 73, 393–401. [Google Scholar] [CrossRef]
- Wulff, R.D. Environmental maternal effects on seed quality and germination. In Seed Development and Germination; Kigel, J., Galili, G., Eds.; Marcel Dekker: New York, NY, USA, 1995; pp. 491–505. [Google Scholar]
- Donohue, K.; Schmitt, J. Maternal environmental effects in plants: Adaptive plasticity? In Maternal Effects as Adaptations; Mousseau, T.A., Fox, C.W., Eds.; Oxford University Press: New York, NY, USA, 1998; pp. 137–158. [Google Scholar]
- Galloway, L.F. Maternal effects provide phenotypic adaptation to local environmental conditions. New Phytol. 2005, 166, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, S.; Defina, A.; Marani, M. Tidal regime, salinity and salt marsh plant zonation. Estuar. Coast. Shelf Sci. 2005, 62, 119–130. [Google Scholar] [CrossRef]
- Castillo, J.M.; Gallego-Tévar, B.; Figueroa, E.; Grewell, B.J.; Vallet, D.; Rousseau, H.; Keller, J.; Lima, O.; Dréano, S.; Salmon, A.; et al. Low genetic diversity contrasts with high phenotypic variability in heptaploid Spartina densiflora populations invading the Pacific coast of North America. Ecol. Evol. 2018, 8, 4992–5007. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Tévar, B.; Grewell, B.J.; Rousseau, H.; Keller, J.; Ainouche, A.; Lima, O.; Dréano, S.; Salmon, A.; Figueroa, E.; Aïnouche, M.; et al. Genetic structure of Spartina hybrids between native Spartina maritima and invasive Spartina densiflora in Southwest Europe. Perspect. Plant Ecol. Evol. Syst. 2019, 37, 26–38. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, C.; Zhang, L.; Zhu, Z.; Tian, K.; Gao, W. Seed dispersal capacity and post-dispersal fate of the invasive Spartina alterniflora in saltmarshes of the Yangtze Estuary. Estuar. Coast. Shelf Sci. 2016, 169, 158–163. [Google Scholar] [CrossRef]
- Ungar, I.A. Effects of the parental environment on the temperature requirements and salinity tolerance of Spergularia marina seeds. Bot. Gaz. 1988, 149, 432–436. [Google Scholar] [CrossRef]
- Wang, L.; Baskin, J.M.; Baskin, C.C.; Cornelissen, J.H.C.; Dong, M.; Huang, Z. Seed dimorphism, nutrients and salinity differentially affect seed traits of the desert halophyte Suaeda aralocaspica via multiple maternal effects. Bmc Plant Biol. 2012, 12, 170. [Google Scholar] [CrossRef]
- Fenner, M. The effects of the parent environment on seed germinability. Seed Sci. Res. 1991, 1, 75–84. [Google Scholar] [CrossRef]
- Gutterman, Y. Maternal effects on seeds during development. In Seeds: The Ecology of Regeneration in Plant Communities, 2nd ed.; Fenner, M., Ed.; CABI Publishing: Wallingford, UK, 2000; pp. 59–84. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds. Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998; p. 666. [Google Scholar]
- Luzuriaga, A.L.; Escudero, A.; Pérez-García, F. Environmental maternal effects on seed morphology and germination in Sinapis arvensis (Cruciferae). Weed Res. 2006, 46, 163–174. [Google Scholar] [CrossRef]
- Fernández-Illescas, F.; Nieva, F.J.J.; Silva, I.; Tormo, R.; Muñoz, A.F. Pollen production of Chenopodiaceae species at habitat and landscape scale in Mediterranean salt marshes: An ecological and phenological study. Rev. Palaeobot. Palynol. 2010, 161, 127–136. [Google Scholar] [CrossRef]
- Long, S.P.; Mason, C.F. Saltmarsh Ecology; Blackie: Glasgow, UK; London, UK, 1983; p. 160. [Google Scholar]
- Barba-Brioso, C.; Fernández-Caliani, J.C.; Miras, A.; Cornejo, J.; Galán, E. Multi-source water pollution in a highly anthropized wetland system associated with the estuary of Huelva (SW Spain). Mar. Pollut. Bull. 2010, 60, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D.B. The measurement of viability. In Viability of Seeds; Roberts, E.H., Ed.; Chapman and Hall: London, UK, 1972; pp. 172–208. [Google Scholar]
- Márquez-García, B.; Márquez, C.; Sanjosé, I.; Nieva, F.J.J.; Rodríguez-Rubio, P.; Muñoz-Rodríguez, A.F. The effects of heavy metals on germination and seedling characteristics in two halophyte species in Mediterranean marshes. Mar. Pollut. Bull. 2013, 70, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Grewell, B.J.; Baye, P.R.; Fiedler, P.L. Shifting mosaics: Vegetation of Suisun Marsh. In Suisun Marsh: Ecological History and Possible Futures; Moyle, P.B., Manfree, A.D., Fiedler, P.L., Eds.; University of California Press: Berkeley, CA, USA, 2014; pp. 65–102. [Google Scholar]
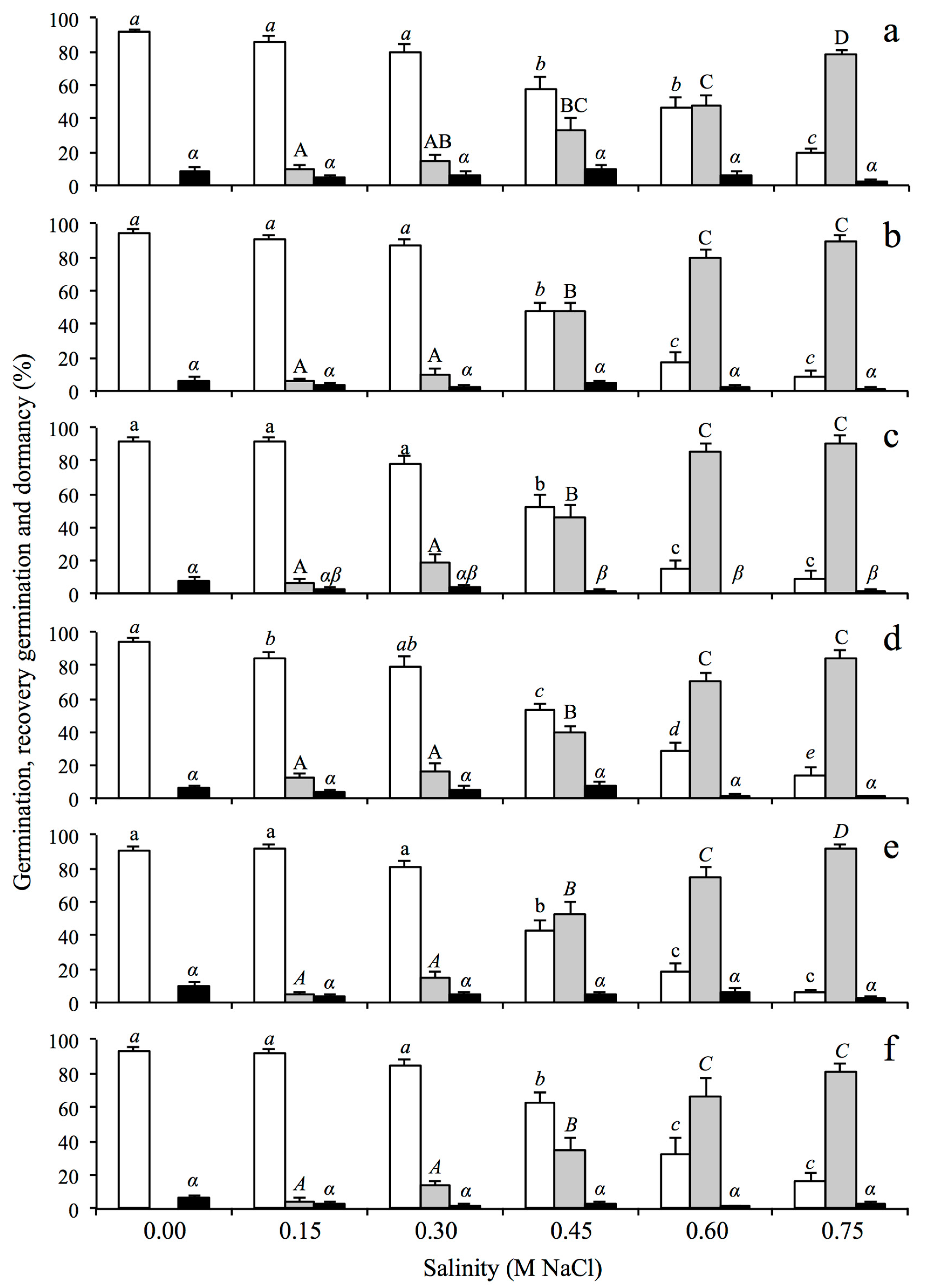
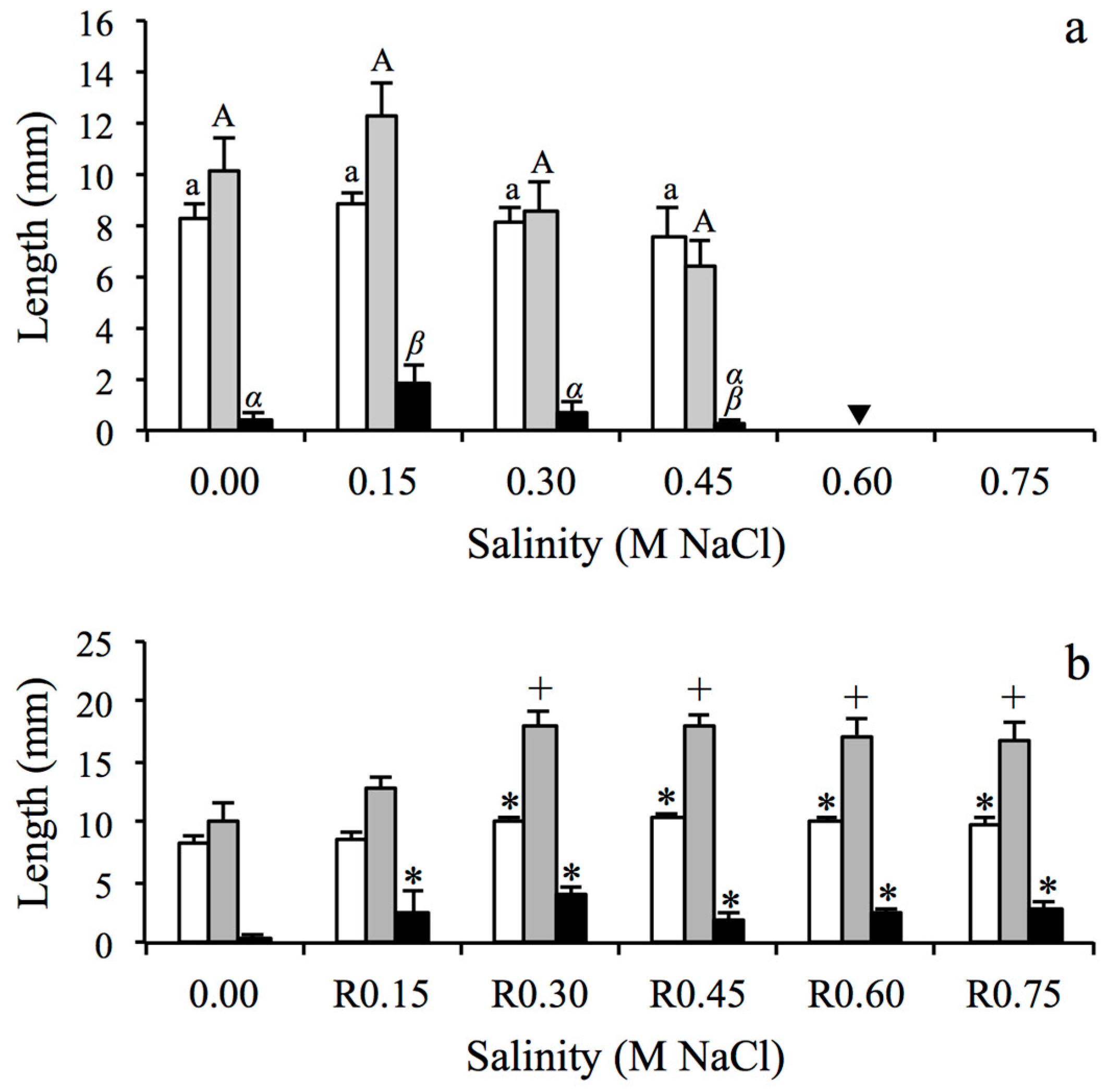

| Salinity (M NaCl) | G (%) | T50 G (days) | RG (%) | T50 R (days) | V (%) |
|---|---|---|---|---|---|
| 0.00 | 100 ± 0a | 23 ± 1a | - | - | 76 ± 6a |
| 0.15 | 91 ± 4a | 28 ± 2ab | 9 ± 4a | 3 ± 1a | 76 ± 8a |
| 0.30 | 51 ± 6b | 32 ± 4abc | 49 ± 6b | 6 ± 1a | 76 ± 7a |
| 0.45 | 19 ± 4c | 38 ± 6bc | 81 ± 4c | 11 ± 1b | 78 ± 1a |
| 0.60 | 12 ± 5cd | 43 ± 3c | 88 ± 5cd | 12 ± 1b | 71 ± 3a |
| 0.75 | 0 ± 0d | - | 100 ± 0d | 13 ± 0b | 78 ± 3a |
| one-way ANOVA (F) or Kruskal–Wallis (H) test | F = 119.17, df = 5, p < 0.0001 | F = 5.43, df = 4, p < 0.01 | F = 74.59, df = 4, p < 0.0001 | H4,19 = 14.64, p < 0.01 | F = 0.22, df = 5, p > 0.05 |
| Salinity (M NaCl) | V (%) | T50 G (days) | T50 R (Days) | V (%) | T50 G (days) | T50 R (days) |
|---|---|---|---|---|---|---|
| Low marsh | ‘Almendral’ | |||||
| 0.00 | 79 ± 3a | 19 ± 1a | - | 65 ± 6a | 22 ± 1a | - |
| 0.15 | 82 ± 3a | 22 ± 1ab | 24 ± 5a | 65 ± 6a | 25 ± 1ab | 20 ± 4a |
| 0.30 | 80 ± 3a | 25 ± 2b | 15 ± 3ab | 62 ± 7a | 27 ± 2abd | 12 ± 3ab |
| 0.45 | 79 ± 4a | 27 ± 2bc | 9 ± 1bc | 57 ± 8a | 32 ± 3bc | 8 ± 1bc |
| 0.60 | 73 ± 4a | 33 ± 3c | 6 ± 1cd | 55 ± 7a | 40 ± 4c | 5 ± 1c |
| 0.75 | 71 ± 3a | 36 ± 2c | 5 ± 1d | 52 ± 8a | 39 ± 4cd | 6 ± 0bc |
| one-way ANOVA (F) or Kruskal–Wallis (H) test | F = 1.77, df = 5 p > 0.05 | F = 14.16, df = 5 p < 0.0001 | H4,55 = 17.51 p < 0.005 | F = 0.61, df = 5 p > 0.05 | F = 9.38, df = 5 p < 0.0001 | H4,53 = 19.85 p < 0.001 |
| Middle marsh | ‘Bacuta’ | |||||
| 0.00 | 70 ± 4a | 26 ± 1a | - | 66 ± 5a | 23 ± 1a | - |
| 0.15 | 64 ± 2ab | 28 ± 2ab | 11 ± 2a | 63 ± 5a | 27 ± 2ab | 19 ± 6a |
| 0.30 | 56 ± 2bc | 31 ± 2ab | 9 ± 3a | 57 ± 6a | 28 ± 2b | 13 ± 3a |
| 0.45 | 45 ± 3cd | 37 ± 4bc | 12 ± 4a | 44 ± 6a | 31 ± 3b | 13 ± 4a |
| 0.60 | 41 ± 3d | 39 ± 8abc | 7 ± 1a | 47 ± 6a | 31 ± 5abc | 7 ± 1a |
| 0.75 | 39 ± 3d | 43 ± 5c | 7 ± 0a | 46 ± 5a | 43 ± 4c | 6 ± 1a |
| one-way ANOVA (F) or Kruskal–Wallis (H) test | F = 18.08, df = 5 p < 0.0001 | H5,62 = 13.71 p < 0.05 | H4,51 = 5.08 p > 0.05 | F = 2.91, df = 5 p < 0.05 | H5,63 = 20.42 p < 0.005 | H4,51 = 6.27 p = 0.180 |
| High marsh | ‘Calatilla’ | |||||
| 0.00 | 54 ± 4a | 25 ± 1a | - | 71 ± 3a | 24 ± 2a | - |
| 0.15 | 46 ± 3ab | 25 ± 1a | 31 ± 9a | 64 ± 3a | 24 ± 2a | 25 ± 8a |
| 0.30 | 37 ± 3bc | 28 ± 2ab | 13 ± 3ab | 54 ± 5ab | 29 ± 3ab | 11 ± 3a |
| 0.45 | 32 ± 3c | 36 ± 4abc | 7 ± 1b | 55 ± 5ab | 38 ± 5ab | 7 ± 1a |
| 0.60 | 30 ± 2c | 37 ± 5bc | 6 ± 1b | 43 ± 5b | 37 ± 5b | 6 ± 1a |
| 0.75 | 30 ± 3c | 42 ± 6c | 7 ± 1b | 42 ± 5b | 33 ± 1b | 6 ± 1a |
| one-way ANOVA (F) or Kruskal–Wallis (H) test | F = 10.14, df = 5 p < 0.0001 | H5,57 = 16.94 p < 0.005 | H4,49 = 14.91 p < 0.005 | F = 6.81, df = 5 p < 0.0001 | H5,62 = 12.99 p < 0.05 | H4,51 = 6.21 p > 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Infante-Izquierdo, M.D.; Castillo, J.M.; Grewell, B.J.; Nieva, F.J.J.; Muñoz-Rodríguez, A.F. Differential Effects of Increasing Salinity on Germination and Seedling Growth of Native and Exotic Invasive Cordgrasses. Plants 2019, 8, 372. https://doi.org/10.3390/plants8100372
Infante-Izquierdo MD, Castillo JM, Grewell BJ, Nieva FJJ, Muñoz-Rodríguez AF. Differential Effects of Increasing Salinity on Germination and Seedling Growth of Native and Exotic Invasive Cordgrasses. Plants. 2019; 8(10):372. https://doi.org/10.3390/plants8100372
Chicago/Turabian StyleInfante-Izquierdo, María Dolores, Jesús M. Castillo, Brenda J. Grewell, F. Javier J. Nieva, and Adolfo F. Muñoz-Rodríguez. 2019. "Differential Effects of Increasing Salinity on Germination and Seedling Growth of Native and Exotic Invasive Cordgrasses" Plants 8, no. 10: 372. https://doi.org/10.3390/plants8100372
APA StyleInfante-Izquierdo, M. D., Castillo, J. M., Grewell, B. J., Nieva, F. J. J., & Muñoz-Rodríguez, A. F. (2019). Differential Effects of Increasing Salinity on Germination and Seedling Growth of Native and Exotic Invasive Cordgrasses. Plants, 8(10), 372. https://doi.org/10.3390/plants8100372






