Dynamics of Short-Term Metabolic Profiling in Radish Sprouts (Raphanus sativus L.) in Response to Nitrogen Deficiency
Abstract
1. Introduction
2. Results and Discussion
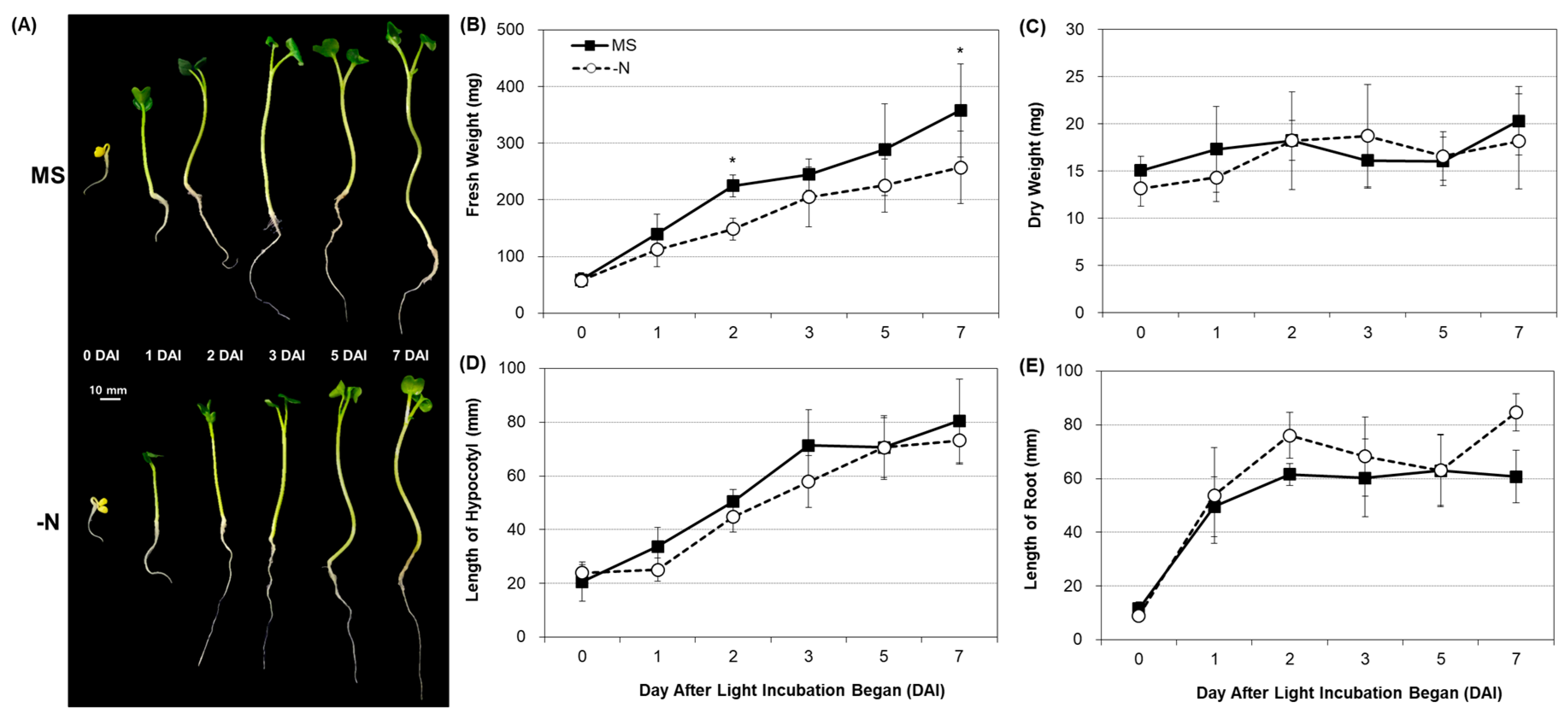
2.1. Phenotypic Distinction under Nitrogen Deficiency
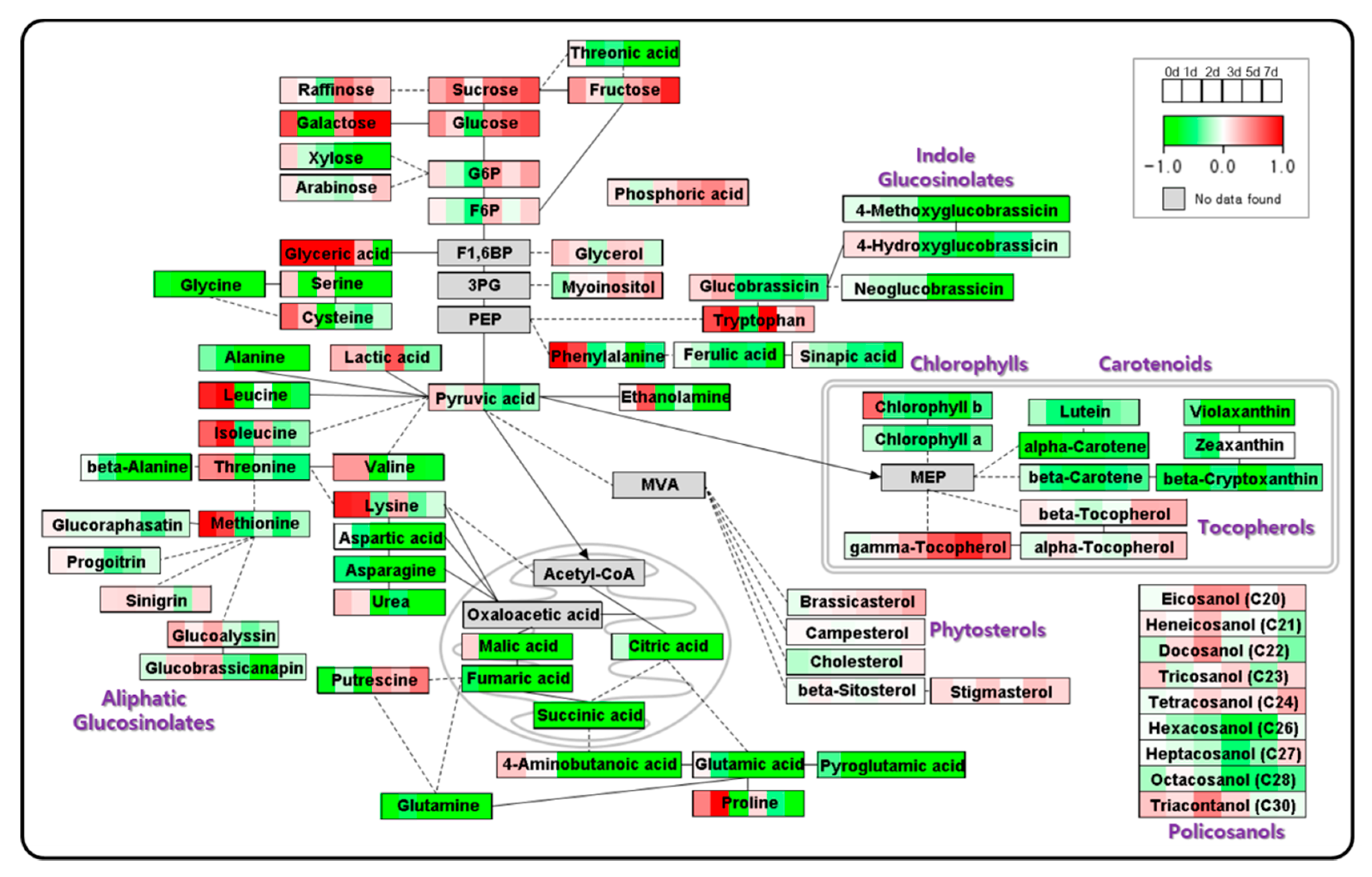
2.2. Metabolite Profiling of Radish Sprouts
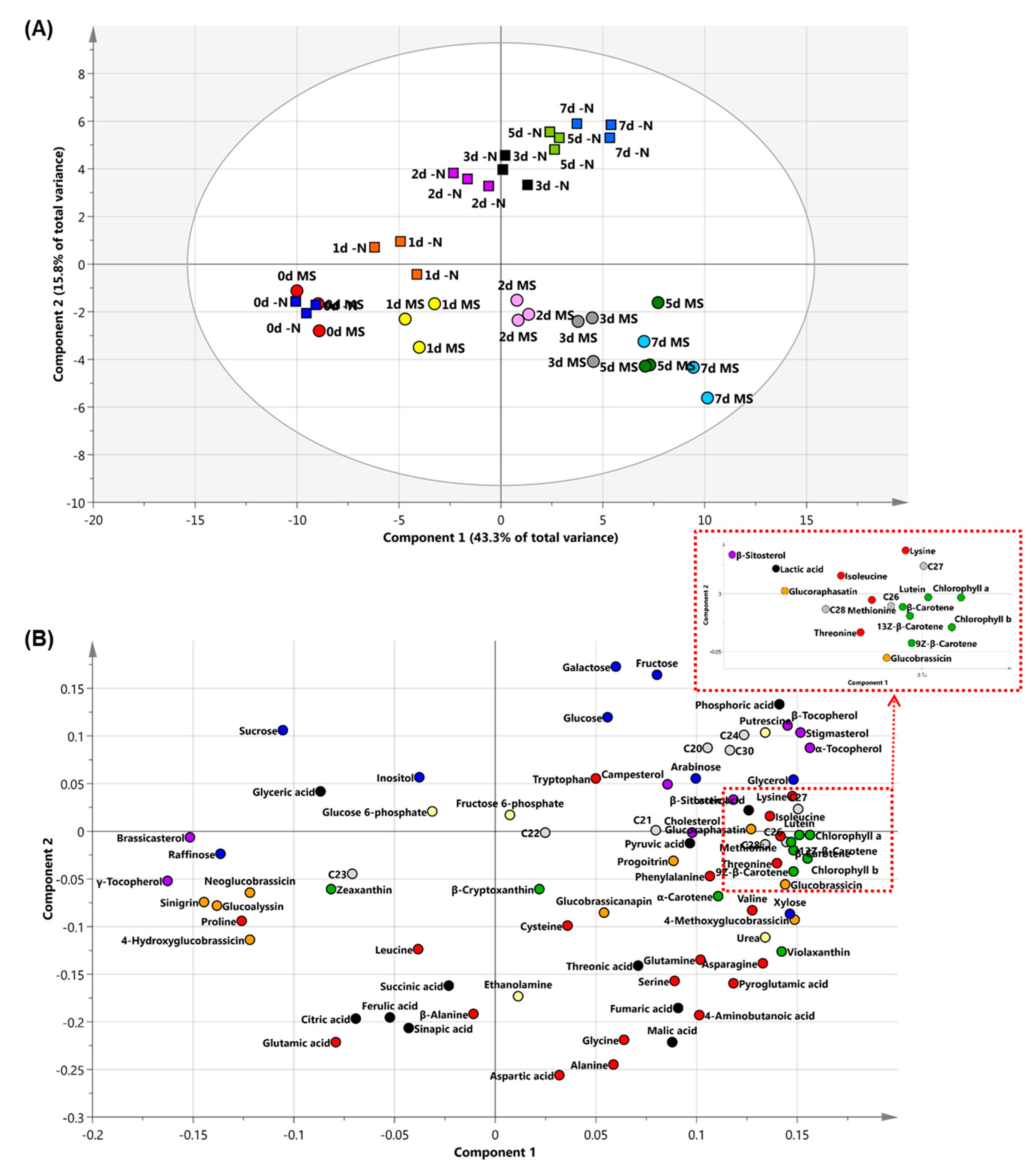
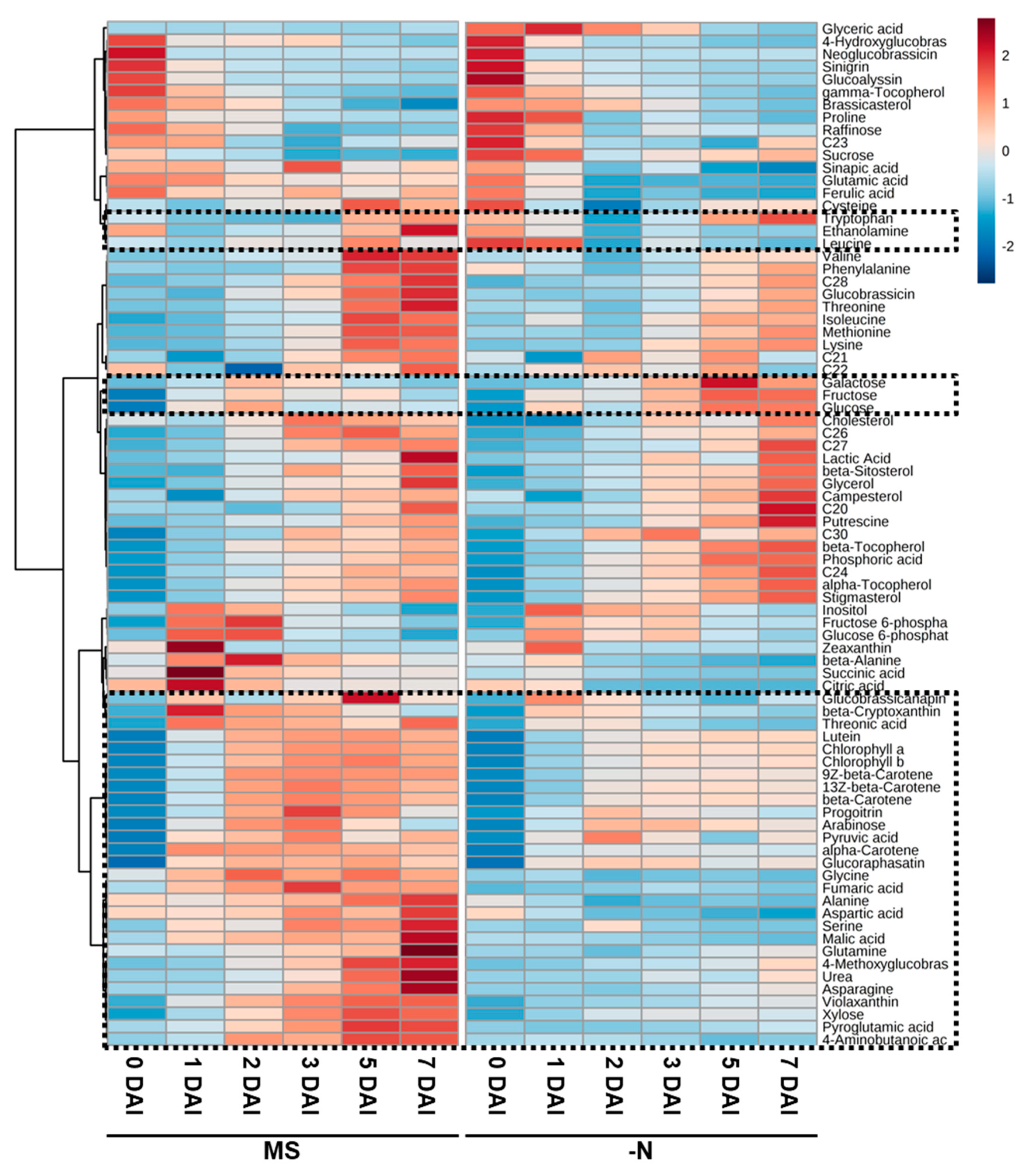
2.3. PCA and Heat Map
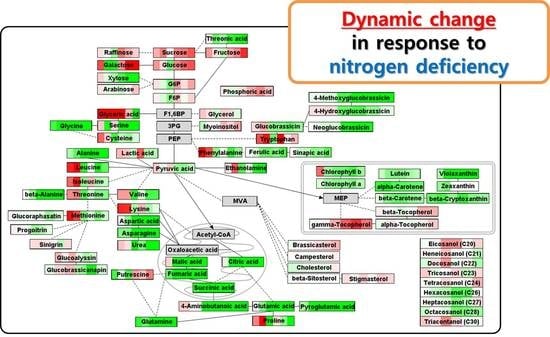
2.4. Metabolic Shifts during the Development of Radish Sprouts under Nitrogen Deficient Condition
3. Materials and Methods
3.1. Plant Materials and Culture Conditions
3.2. Extraction of Hydrophilic Metabolites and GC-TOFMS Analysis
3.3. Extraction of Policosanols, Tocopherols, and Phytosterols and GC-qMS Analysis
3.4. Extraction of Carotenoids and HPLC Analysis
3.5. Extraction of Chlorophylls and Analysis
3.6. Extraction of Desulfoglucosinolates and HPLC Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kim, Y.-N.; Giraud, D.W.; Driskell, J.A. Tocopherol and carotenoid contents of selected Korean fruits and vegetables. J. Food Compos. Anal. 2007, 20, 458–465. [Google Scholar] [CrossRef]
- Jing, P.; Zhao, S.-J.; Ruan, S.-Y.; Xie, Z.-H.; Dong, Y.; Yu, L. Anthocyanin and glucosinolate occurrences in the roots of Chinese red radish (Raphanus sativus L.), and their stability to heat and pH. Food Chem. 2012, 133, 1569–1576. [Google Scholar] [CrossRef]
- Park, C.H.; Baskar, T.B.; Park, S.-Y.; Kim, S.-J.; Arasu, M.V.; Al-Dhabi, N.A.; Kim, J.K.; Park, S.U. Metabolic profiling and antioxidant assay of metabolites from three radish cultivars (Raphanus sativus). Molecules 2016, 21, 157. [Google Scholar] [CrossRef] [PubMed]
- Takaya, Y.; Kondo, Y.; Furukawa, T.; Niwa, M. Antioxidant constituents of radish sprout (kaiware-daikon), Raphanus sativus L. J. Agric. Food Chem. 2003, 51, 8061–8066. [Google Scholar] [CrossRef] [PubMed]
- Barillari, J.; Cervellati, R.; Costa, S.; Guerra, M.C.; Speroni, E.; Utan, A.; Iori, R. Antioxidant and choleretic properties of Raphanus sativus L. sprout (Kaiware Daikon) extract. J. Agric. Food Chem. 2006, 54, 9773–9778. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhu, Y.; Luo, Y. Effects of sulfur fertilization on the accumulation of health promoting phytochemicals in radish sprouts. J. Agric. Food Chem. 2013, 61, 7552–7559. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Wu, Q.; Cui, J. Increased sucrose in the hypocotyls of radish sprouts contributes to nitrogen deficiency-induced anthocyanin accumulation. Front. Plant Sci. 2016, 7, 1976. [Google Scholar] [CrossRef]
- Li, R.; Zhu, Y. The primary active components, antioxidant properties, and differential metabolite profiles of radish sprouts (Raphanus sativus L.) upon domestic storage: Analysis of nutritional quality. J. Sci. Food Agric. 2018, 98, 5853–5860. [Google Scholar] [CrossRef]
- Strzetelski, P.; Smoleń, S.; Rożek, S.; Sady, W. The effect of diverse iodine fertilization on nitrate accumulation and content of selected compounds in radish plants (Raphanus sativus L.). ACTA Sci. Pol. Hort. Cult. 2010, 9, 65–67. [Google Scholar]
- Lee, C.B.; Koh, S.C.; Moon, B.Y.; Park, I.H.; Park, P.B.; Chun, H.S. Plant Physiology; Life Science Publishing Co.: Seoul, Korea, 2011; pp. 41–43. [Google Scholar]
- Kopsel, D.A.; Barickman, T.C.; Sams, C.E.; McElroy, J.S. Influence of nitrogen and sulfur on biomass production and carotenoid and glucosinolate concentrations in watercress (Nasturtium officinale R. Br.). J. Agric. Food Chem. 2007, 55, 10628–10634. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Lu, Y.; Xie, W.; Zhu, T.; Lian, X. Transcriptome response to nitrogen starvation in rice. J. Biosci. 2012, 37, 731–747. [Google Scholar] [CrossRef]
- Bi, Y.-M.; Wang, R.-L.; Zhu, T.; Rothstein, S.J. Global transcription profiling reveals differential responses to chronic nitrogen stress and putative nitrogen regulatory components in Arabidopsis. BMC Genomics. 2007, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bian, Y.; Cheng, K.; Zou, H.; Sun, S.S.-M.; He, J.-X. A comprehensive differential proteomic study of nitrate deprivation in Arabidopsis reveals complex regulatory networks of plant nitrogen responses. J. Proteome Res. 2012, 11, 2301–2315. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Yun, H.; Back, S.; Fernie, A.R.; Kim, Y.X.; Lee, Y.; Lee, S.; Lee, D.; Kim, J.K. Changes in mineral nutrient concentrations and C-N metabolism in cabbage shoots and roots following macronutrient deficiency. J. Plant Nutr. Soil Sci. 2018, 181, 777–786. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.-Y.; Lim, S.-H.; Yeo, Y.; Cho, H.S.; Ha, S.-H. Comparative metabolic profiling of pigmented rice (Oryza sativa L.) cultivars reveals primary metabolites are correlation with secondary metabolites. J. Cereal Sci. 2013, 57, 14–20. [Google Scholar] [CrossRef]
- Baek, S.A.; Jung, Y.H.; Lim, S.H.; Park, S.U.; Kim, J.K. Metabolic profiling in Chinese cabbage (Brassica rapa L. subsp. pekinensis) cultivars reveals that glucosinolate content is correlated with carotenoid content. J. Agric. Food Chem. 2016, 64, 4426–4434. [Google Scholar] [CrossRef]
- Park, Y.J.; Baek, S.A.; Choi, Y.; Kim, J.K.; Park, S.U. Metabolic profiling of nine Mentha species and prediction of their antioxidant properties using chemometrics. Molecules 2019, 24, 258. [Google Scholar] [CrossRef]
- Paul, M.J.; Stitt, M. Effects of nitrogen and phosphorus deficiencies on levels of carbohydrates, respiratory enzymes and metabolites in seedlings of tobacco and their response to exogenous sucrose. Plant Cell Environ. 1993, 16, 1047–1057. [Google Scholar] [CrossRef]
- Sung, J.; Lee, S.; Lee, Y.; Ha, S.; Song, B.; Kim, T.; Waters, B.M.; Krishnan, H.B. Metabolomic profiling from leaves and roots of tomato (Solanum lycopersicum L.) plants grown under nitrogen, phosphorus or potassium-deficient condition. Plant Sci. 2015, 241, 55–64. [Google Scholar] [CrossRef]
- Kusano, M.; Fukushima, A.; Redestig, H.; Saito, K. Metabolomic approaches toward understanding nitrogen metabolism in plants. J. Exp. Bot. 2011, 62, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Schonhof, I.; Krumbein, A.; Li, L.; Stützel, H.; Schreiner, M. Glucosinolate concentration in turnip (Brassica rapa ssp. rapifera L.) roots as affected by nitrogen and sulfur supply. J. Agric. Food Chem. 2007, 55, 8452–8457. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liang, G.; Li, Y.; Wang, F.; Yu, D. Two young microRNAs originating from target duplication mediate nitrogen starvation adaptation via regulation of glucosinolate synthesis in Arabidopsis thaliana. Plant Physiol. 2014, 164, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; He, H.; Yu, A. Identification of nitrogen starvation-responsive microRNAs in Arabidopsis thaliana. PLoS ONE 2012, 7, e48951. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.Y.; Yano, M.; Goodenowe, D.B.; Kanaya, S.; Kimura, T.; Awazuhara, M.; Arita, M.; Fujiwara, T.; Saito, K. Integration of transcriptomics and metabolomics for understanding of global responses to nutritional stresses in Arabidopsis thaliana. PNAS 2004, 101, 10205–10210. [Google Scholar] [CrossRef]
- Zhang, H.; Vasanthan, T.; Wettasinghe, M. Enrichment of tocopherols and phytosterols in canola oil during seed germination. J. Agric. Food Chem. 2007, 55, 355–359. [Google Scholar] [CrossRef]
- Seo, M.-S.; Kim, J.S. Understanding of MYB transcription factors involved in glucosinolate biosynthesis in Brassicaceae. Molecules 2017, 22, 1549. [Google Scholar] [CrossRef]
- Kutmon, M.; van Iersel, M.P.; Bohler, A.; Kelder, T.; Nunes, N.; Pico, A.R.; Evelo, C.T. PathVisio 3: An extendable pathway analysis toolbox. PLoS Comput. Biol. 2015, 11, e1004085. [Google Scholar] [CrossRef]
- Urbanczyk-Wochniak, E.; Fernie, A.R. Metabolic profiling reveals altered nitrogen nutrient regimes have diverse effects on the metabolism of hydroponically-grown tomato (Solanum lycopersicum) plants. J. Exp. Bot. 2005, 56, 309–321. [Google Scholar] [CrossRef]
- Obata, T.; Fernie, A.R. The use of metabolomics to dissect plant responses to abiotic stresses. Cell. Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef]
- Sønderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates-gene discovery and beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef]
- Schonhof, I.; Blankenburg, D.; Siegfried Müller, S.; Krumbein, A. Sulfur and nitrogen supply influence growth, product appearance, and glucosinolate concentration of broccoli. J. Plant Nutr. Soil Sci. 2007, 170, 65–72. [Google Scholar] [CrossRef]
- Miflin, B.J.; Habash, D.Z. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J. Exp. Bot. 2002, 53, 979–987. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Reisdorf-Cren, M.; Pageau, K.; Lelandais, M.; Grandjean, O.; Kronenberger, J.; Valadier, M.-H.; Feraud, M.; Jouglet, T.; Suzuki, A. Glutamine synthetase-glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink-source nitrogen cycle in tobacco. Plant Physiol. 2006, 140, 444–456. [Google Scholar] [CrossRef]
- Sánchez, E.; Garcia, P.C.; López-Lefebre, L.R.; Rivero, R.M.; Ruiz, J.M.; Romero, L. Proline metabolism in response to nitrogen deficiency in French Bean plants (Phaseolus vulgaris L. cv Strike). Plant Growth Regul. 2002, 36, 261–265. [Google Scholar] [CrossRef]
- Krapp, A.; Berthomé, R.; Orsel, M.; Mercey-Boutet, S.; Yu, A.; Castaings, L.; Elftieh, S.; Major, H.; Renou, J.P.; Daniel-Vedele, F. Arabidopsis roots and shoots show distinct temporal adaptation patterns toward nitrogen starvation. Plant Physiol. 2011, 157, 1255–1282. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef]
- Debolt, S.; Melino, V.; Ford, C.M. Ascorbate as a biosynthetic Precursor in plants. Ann. Bot. 2007, 99, 3–8. [Google Scholar] [CrossRef]
- Stefanelli, D.; Goodwin, I.; Jones, R. Minimal nitrogen and water use in horticulture: Effects on quality and content of selected nutrients. Food Res. Int. 2010, 43, 1833–1843. [Google Scholar] [CrossRef]
- Ferrer, A.; Altabella, T.; Arró, M.; Boronat, A. Emerging roles for conjugated sterols in plants. Prog. Lipid Res. 2017, 67, 27–37. [Google Scholar] [CrossRef]
- Üstün, S.; Hafrén, A.; Hofius, D. Autophagy as a mediator of life and death in plants. Curr. Opin. Plant Biol. 2017, 40, 122–130. [Google Scholar] [CrossRef]
- Avin-Wittenberg, T. Autophagy and its role in plant abiotic stress management. Plant Cell Environ. 2019, 42, 1045–1053. [Google Scholar] [CrossRef]
- Hanaoka, H.; Noda, T.; Shirano, Y.; Kato, T.; Hiroaki, H.; Shibata, D.; Tabata, S.; Ohsumi, Y. Leaf senescence and starvation-induced chlorosis are accelerated by the disruption of an Arabidopsis autophagy gene. Plant Physiol. 2002, 129, 1181–1193. [Google Scholar] [CrossRef]
- Marshall, R.S.; Li, F.; Gemperline, D.C.; Book, A.J.; Vierstra, R.D. Autophagic degradation of the 26S proteasome is mediated by the dual ATG8/Ubiquitin receptor RPN10 in Arabidopsis. Mol. Cell 2015, 58, 1053–1066. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.J.; Wang, K.X.; Xia, S.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Zhou, J. BZR1 mediates brassinosteroid-induced autophagy and nitrogen starvation in tomato. Am. Soc. Plant Biol. 2019, 179, 671–685. [Google Scholar] [CrossRef]
- Mei, H.-S.; Thimann, K.V. The relation between nitrogen deficiency and leaf senescence. Physiol. Plant. 1984, 62, 157–161. [Google Scholar] [CrossRef]
- Koeslin-Findeklee, F.; Rizi, V.S.; Becker, M.A.; Parra-Londono, S.; Arif, M.; Balazadeh, S.; Mueller-Roeber, B.; Kunze, R.; Horst, W.J. Transcriptomic analysis of nitrogen starvation- and cultivar-specific leaf senescence in winter oilseed rape (Brassica napus L.). Plant Sci. 2015, 233, 174–185. [Google Scholar] [CrossRef]
- Huang, Z.A.; Jiang, D.A.; Yang, Y.; Sun, J.W.; Jin, S.H. Effects of nitrogen deficiency on gas exchange, chlorophyll fluorescence, and antioxidant enzymes in leaves of rice plants. Photosynthetica 2004, 42, 357–364. [Google Scholar] [CrossRef]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Redd, V.R. Nitrogen deficiency effects on plant growth, leaf photosynthesis, and hyperspectral reflectance properties of sorghum. Eur. J. Agron. 2005, 22, 391–403. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, A.; Li, H.; Tang, Z.; Chen, X. Growth and physiological response to nitrogen deficiency and re-supply in leaf-vegetable sweetpotato (Ipomoea batatas Lam). HortScience 2015, 50, 754–758. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, S.R.; Lee, J.Y.; Park, S.Y.; Song, S.Y.; Na, J.H.; Kim, S.W.; Kim, S.J.; Nou, I.S.; Lee, Y.H.; et al. Metabolic differentiation of diamondback moth (Plutella xylostella (L.)) resistance in Cabbage (Brassica oleracea L. ssp. capitata). J. Agric. Food Chem. 2013, 61, 11222–11230. [Google Scholar] [CrossRef]
- Kim, T.J.; Lee, K.B.; Baek, S.-A.; Choi, J.; Ha, S.-H.; Lim, S.-H.; Park, S.Y.; Yeo, Y.; Park, S.U.; Kim, J.K. Determination of lipophilic metabolites for species discrimination and quality assessment of nine leafy vegetables. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 909–918. [Google Scholar] [CrossRef]
- Park, S.-Y.; Choi, S.R.; Lim, S.-H.; Yeo, Y.; Kweon, S.J.; Bae, Y.-S.; Kim, K.W.; Im, K.-H.; Ahn, S.K.; Park, S.U.; et al. Identification and quantification of carotenoids in paprika fruits and cabbage, kale, and lettuce leaves. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 355–358. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- European Community. Oil seeds-Determination of glucosinolates by high-performance liquid chromatography. Off. J. Eur. Communities. 1990, L170, 27–34. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.-A.; Im, K.-H.; Park, S.U.; Oh, S.-D.; Choi, J.; Kim, J.K. Dynamics of Short-Term Metabolic Profiling in Radish Sprouts (Raphanus sativus L.) in Response to Nitrogen Deficiency. Plants 2019, 8, 361. https://doi.org/10.3390/plants8100361
Baek S-A, Im K-H, Park SU, Oh S-D, Choi J, Kim JK. Dynamics of Short-Term Metabolic Profiling in Radish Sprouts (Raphanus sativus L.) in Response to Nitrogen Deficiency. Plants. 2019; 8(10):361. https://doi.org/10.3390/plants8100361
Chicago/Turabian StyleBaek, Seung-A, Kyung-Hoan Im, Sang Un Park, Sung-Dug Oh, Jaehyuk Choi, and Jae Kwang Kim. 2019. "Dynamics of Short-Term Metabolic Profiling in Radish Sprouts (Raphanus sativus L.) in Response to Nitrogen Deficiency" Plants 8, no. 10: 361. https://doi.org/10.3390/plants8100361
APA StyleBaek, S.-A., Im, K.-H., Park, S. U., Oh, S.-D., Choi, J., & Kim, J. K. (2019). Dynamics of Short-Term Metabolic Profiling in Radish Sprouts (Raphanus sativus L.) in Response to Nitrogen Deficiency. Plants, 8(10), 361. https://doi.org/10.3390/plants8100361