Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits
Abstract
1. Introduction
2. Flavonoids and Phenolic Acids Composition of Oregano Species
3. Physiological Functions of Flavonoids and Phenolic Acids from Oregano Species
4. Health Benefits of Flavonoids and Phenolic Compounds from Oregano Species
- A planar ring system in the flavonoid molecule is needed for anti-inflammatory activity
- Hydroxyl groups in B ring and at C5 and C7 of A ring are necessary for anti-inflammatory activity
- The flavones and flavonoles having a hydroxyl group at 4′ position of B ring show higher anti-inflammatory activity
- The methylation of the hydroxyl groups at 3, 5 or 4′ positions improves activity
- The methylation of the 3-hydroxyl group reduces cytotoxicity
- Flavones exhibited higher activities than isoflavones, flavonoles and flavanones
- The 5, 6, 7-trihydroxyflavone core structure improves activity
- Aglycones are more bioactive than glycosides
4.1. Antioxidant Properties of FL and PA from Oregano
4.2. Anti-Inflammatory Properties of FL and PA from Oregano
4.3. Anti-Cancer Properties of FL and PA from Oregano
5. Enhancement of Flavonoid and Phenolic Acids Content in Oregano Species
5.1. Nutrient Manipulation
5.2. Light Quality
5.3. Plant Elicitors
5.3.1. Chitosan
5.3.2. Plant Growth Regulators
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| FL | Flavonoids |
| PA | Phenolic acids |
| BHT | Butylated hydroxytoluene |
| HAT | Hydrogen atom transfer |
| ET | Electron transfer |
| ORAC | Oxygen radical absorbance assay |
| DPPH | 1,1′-diphenyl-2-picrylhydrazyl radical |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| FRAP | Ferric reducing-antioxidant power assay |
| CUPRAC | Cupric ion reducing antioxidant capacity |
| TAC | Total antioxidant capacity |
| TPC | Total phenolic content |
| DW | Dried weight |
| DWE | Dried weight extract |
| NO | Nitric oxide |
| ROS | Reactive oxygen species |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-10 | Interleukin 10 |
| TNF-α | Tumor necrosis factor alpha |
| COX-2 | Cyclooxygenase-2 |
| LPS | Lipopolysaccharide |
| iNOS | Nitric oxide synthase |
| COXs | Cyclooxygenases |
References
- Calpouzos, L. Botanical aspects of oregano. Econ. Bot. 1954, 8, 222–223. [Google Scholar] [CrossRef]
- Franz, C.; Novak, J. Sources of essential oils. In Handbook of Essential Oils; CRC Press: Boca Raton, FL, USA, 2009; pp. 39–81. [Google Scholar]
- Pascual, M.E.; Slowing, K.; Carretero, E.; Sánchez Mata, D.; Villar, A. Lippia: Traditional uses, chemistry and pharmacology: A review. J. Ethnopharmacol. 2001, 76, 201–214. [Google Scholar] [CrossRef]
- Leyva-López, N.; Nair, V.; Bang, W.Y.; Cisneros-Zevallos, L.; Heredia, J.B. Protective role of terpenes and polyphenols from three species of oregano (Lippia graveolens, Lippia palmeri and Hedeoma patens) on the suppression of lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. J. Ethnopharmacol. 2016, 187, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Kroon, P.A.; Quideau, S.; Treutter, D. Plant phenolics—Secondary metabolites with diverse functions. In Recent Advances in Polyphenol Research; Daayf, F., Lattanzio, V., Eds.; Blackwell Publishing Ltw: Oxford, UK, 2008; Volume 1. [Google Scholar]
- Vermerris, W.; Nicholson, R. The role of phenols in plant defense. In Phenolic Compound Biochemistry; Springer: Dordrecht, The Netherlands, 2006; pp. 211–234. [Google Scholar]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural products (secondary metabolites). In Biochemistry & Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; American Society of Plants: Rockville, MD, USA, 2015; pp. 1250–1318. [Google Scholar]
- Leyva-López, N.; Gutiérrez-Grijalva, E.; Vazquez-Olivo, G.; Heredia, J. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Z.; Mukhopadhyay, S.; Robbins, R.J.; Harnly, J.M. Identification and quantification of flavonoids of Mexican oregano (Lippia graveolens) by LC-DAD-ESI/MS analysis. J. Food Compos. Anal. 2007, 20, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Moreira, E.; Grosso, C.; Andrade, P.B.; Valentão, P.; Romano, A. Phenolic profile, antioxidant activity and enzyme inhibitory activities of extracts from aromatic plants used in mediterranean diet. J. Food Sci. Technol. 2017, 54, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, A.M.; Roberts, H.T. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Vermerris, W.; Nicholson, R. Families of phenolic compounds and means of classification. In Phenolic Compound Biochemistry; Springer: Dordrecht, The Netherlands, 2008; pp. 1–34. [Google Scholar]
- Gutiérrez-Grijalva, E.P.; Ambriz-Pére, D.L.; Leyva-López, N.; Castillo-López, R.I.; Heredia, J.B. Review: Dietary phenolic compounds, health benefits and bioaccessibility. Arch. Latinoam. Nutr. 2016, 66, 87–100. [Google Scholar]
- Jiang, N.; Doseff, A.; Grotewold, E. Flavones: From biosynthesis to health benefits. Plants 2016, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.-K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Lacueva, C.; Medina-Remon, A.; Llorach, R.; Urpi-Sarda, M.; Khan, N.; Chiva-Blanch, G.; Zamora-Ros, R.; Rotches-Ribalta, M.; Lamuela-Raventos, R.M. Phenolic compounds: Chemistry and occurrence in fruits and vegetables. In Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value and Stability; Wiley-Blackwell: Ames, IA, USA, 2010; pp. 53–56. [Google Scholar]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-explorer 3.0: A major update of the phenol-explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef] [PubMed]
- Timóteo, P.; Karioti, A.; Leitão, S.G.; Vincieri, F.F.; Bilia, A.R. A validated HPLC method for the analysis of herbal teas from three chemotypes of brazilian Lippia alba. Food Chem. 2015, 175, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.G.; Lima, M.A.S.; Silveira, E.R. Total NMR assignments of new [C7-o-C7″]-biflavones from leaves of the limonene–carvone chemotype of Lippia alba (Mill) N. E. Brown. Magn. Reson. Chem. 2005, 43, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Pan, M.-H.; Wu, Q.-L.; Park, C.-H.; Juliani, H.R.; Ho, C.-T.; Simon, J.E. LC-MS method for the simultaneous quantitation of the anti-inflammatory constituents in oregano (Origanum species). J. Agric. Food Chem. 2010, 58, 7119–7125. [Google Scholar] [CrossRef] [PubMed]
- Baâtour, O.; Kaddour, R.; Tarchoun, I.; Nasri, N.; Mahmoudi, H.; Zaghdoudi, M.; Ghaith, H.; Marzouk, B.; Nasri-Ayachi, M.B.; Lachaâl, M. Modification of fatty acid, essential oil and phenolic contents of salt-treated sweet marjoram (Origanum majorana L.) according to developmental stage. J. Food Sci. 2012, 77, C1047–C1054. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, E.E.; Martínez, J.R.; Cala, M.P.; Durán, D.C.; Caballero, D. Chromatographic and mass spectrometric characterization of essential oils and extracts from Lippia (Verbenaceae) aromatic plants. J. Sep. Sci. 2013, 36, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Kogiannou, D.A.A.; Kalogeropoulos, N.; Kefalas, P.; Polissiou, M.G.; Kaliora, A.C. Herbal infusions; their phenolic profile, antioxidant and anti-inflammatory effects in HT29 and PC3 cells. Food Chem. Toxicol. 2013, 61, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Rinaldi Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Hennebelle, T.; Sahpaz, S.; Gressier, B.; Joseph, H.; Bailleul, F. Antioxidant and neurosedative properties of polyphenols and iridoids from Lippia alba. Phytother. Res. 2008, 22, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Quirantes-Piné, R.; Funes, L.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. High-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight and ion-trap tandem mass spectrometry to identify phenolic compounds from a lemon verbena extract. J. Chromatogr. A 2009, 1216, 5391–5397. [Google Scholar] [CrossRef] [PubMed]
- Bower, A.M.; Hernandez, L.M.R.; Berhow, M.A.; de Mejia, E.G. Bioactive compounds from culinary herbs inhibit a molecular target for type 2 diabetes management, dipeptidyl peptidase iv. J. Agric. Food Chem. 2014, 62, 6147–6158. [Google Scholar] [CrossRef] [PubMed]
- Berdowska, I.; Zieliński, B.; Fecka, I.; Kulbacka, J.; Saczko, J.; Gamian, A. Cytotoxic impact of phenolics from Lamiaceae species on human breast cancer cells. Food Chem. 2013, 141, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Koldaş, S.; Demirtas, I.; Ozen, T.; Demirci, M.A.; Behçet, L. Phytochemical screening, anticancer and antioxidant activities of Origanum vulgare L. ssp. viride (Boiss.) Hayek, a plant of traditional usage. J. Sci. Food Agric. 2015, 95, 786–798. [Google Scholar] [PubMed]
- Proestos, C.; Komaitis, M. Analysis of naturally occurring phenolic compounds in aromatic plants by RP-HPLC coupled to diode array detector (DAD) and GC-MS after silylation. Foods 2013, 2, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Kaliora, A.C.; Kogiannou, D.A.A.; Kefalas, P.; Papassideri, I.S.; Kalogeropoulos, N. Phenolic profiles and antioxidant and anticarcinogenic activities of greek herbal infusions; balancing delight and chemoprevention? Food Chem. 2014, 142, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Pizzale, L.; Bortolomeazzi, R.; Vichi, S.; Überegger, E.; Conte, L.S. Antioxidant activity of sage (Salvia officinalis and S fruticosa) and oregano (Origanum onites and O indercedens) extracts related to their phenolic compound content. J. Sci. Food Agric. 2002, 82, 1645–1651. [Google Scholar] [CrossRef]
- Engel, R.; Szabó, K.; Abrankó, L.; Rendes, K.; Füzy, A.; Takács, T. Effect of arbuscular mycorrhizal fungi on the growth and polyphenol profile of marjoram, lemon balm and marigold. J. Agric. Food Chem. 2016, 64, 3733–3742. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Zgórka, G.; Głowniak, K. Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. J. Pharm. Biomed. Anal. 2001, 26, 79–87. [Google Scholar] [CrossRef]
- Hossain, M.B.; Camphuis, G.; Aguiló-Aguayo, I.; Gangopadhyay, N.; Rai, D.K. Antioxidant activity guided separation of major polyphenols of marjoram (Origanum majorana L.) using flash chromatography and their identification by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Sep. Sci. 2014, 37, 3205–3213. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.; Carle, R.; Kammerer, D.R. Effects of blanching on polyphenol stability of innovative paste-like parsley (Petroselinum crispum (Mill.) Nym Ex A. W. Hill) and marjoram (Origanum majorana L.) products. Food Chem. 2013, 138, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Grevsen, K.; Frette, X.C.; Christensen, L.P. Content and composition of volatile terpenes, flavonoids and phenolic acids in greek oregano (Origanum vulgare L. ssp. hirtum) at different development stages during cultivation in cool temperate climate. Eur. J. Hortic. Sci. 2009, 74, 193–203. [Google Scholar]
- Tuttolomondo, T.; La Bella, S.; Licata, M.; Virga, G.; Leto, C.; Saija, A.; Trombetta, D.; Tomaino, A.; Speciale, A.; Napoli, E.M.; et al. Biomolecular characterization of wild sicilian oregano: Phytochemical screening of essential oils and extracts and evaluation of their antioxidant activities. Chem. Biodivers. 2013, 10, 411–433. [Google Scholar] [CrossRef] [PubMed]
- Vujicic, M.; Nikolic, I.; Kontogianni, V.G.; Saksida, T.; Charisiadis, P.; Orescanin-Dusic, Z.; Blagojevic, D.; Stosic-Grujicic, S.; Tzakos, A.G.; Stojanovic, I. Methanolic extract of Origanum vulgare ameliorates type 1 diabetes through antioxidant, anti-inflammatory and anti-apoptotic activity. Br. J. Nutr. 2015, 113, 770–782. [Google Scholar] [CrossRef] [PubMed]
- González, M.D.; Lanzelotti, P.L.; Luis, C.M. Chemical fingerprinting by HPLC-DAD to differentiate certain subspecies of Origanum vulgare L. Food Anal. Methods 2017, 10, 1460–1468. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Abramovič, H. Chapter 93—Antioxidant properties of hydroxycinnamic acid derivatives: A focus on biochemistry, physicochemical parameters, reactive species and biomolecular interactions a2—Preedy, victor r. In Coffee in Health and Disease Prevention; Academic Press: San Diego, CA, USA, 2015; pp. 843–852. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, M.L.F.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar]
- Samanta, A.; Das, G.; Das, S.K. Roles of flavonoids in plants. Carbon 2011, 100, 6. [Google Scholar]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Balaguer, F.; Goel, A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- García-Pérez, E.; Noratto, G.D.; García-Lara, S.; Gutiérrez-Uribe, J.A.; Mertens-Talcott, S.U. Micropropagation effect on the anti-carcinogenic activitiy of polyphenolics from mexican oregano (Poliomintha glabrescens Gray) in human colon cancer cells HT-29. Plant Foods Hum. Nutr. 2013, 68, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as α-glucosidases inhibitors: A review on structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Ni, X.; Kai, G.; Chen, X. A review on structure–activity relationship of dietary polyphenols inhibiting α-amylase. Crit. Rev. Food Sci. Nutr. 2013, 53, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. Anticancer properties of the flavone wogonin. Toxicology 2013, 314, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Ganai, S.A. Plant-derived flavone apigenin: The small-molecule with promising activity against therapeutically resistant prostate cancer. Biomed. Pharmacother. 2017, 85, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents wogonin, baicalein and baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Menezes, R.; Rodriguez-Mateos, A.; Kaltsatou, A.; González-Sarrías, A.; Greyling, A.; Giannaki, C.; Andres-Lacueva, C.; Milenkovic, D.; Gibney, R.E.; Dumont, J.; et al. Impact of flavonols on cardiometabolic biomarkers: A meta-analysis of randomized controlled human trials to explore the role of inter-individual variability. Nutrients 2017, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.; Barreiro, M.; Ferreira, I. Hydroxycinnamic acids and their derivatives: Cosmeceutical significance, challenges and future perspectives, a review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC technical report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 2. Hydrogen atom transfer (HAT)-based, mixed-mode (electron transfer (ET)/HAT) and lipid peroxidation assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Joseph, G.S.; Negi, P.S.; Varadaraj, M.C. Chemical composition and nutraceutical potential of indian borage (Plectranthus amboinicus) stem extract. J. Chem. 2013, 2013, 320329. [Google Scholar] [CrossRef]
- Singh, S.; Singh, D.R.; Banu, S.; Salim, K.M. Determination of bioactives and antioxidant activity in Eryngium foetidum L.: A traditional culinary and medicinal herb. Proc. Nat. Acad. Sci. India Sect. B Biol. Sci. 2013, 83, 453–460. [Google Scholar] [CrossRef]
- Martínez-Rocha, A.; Puga, R.; Hernández-Sandoval, L.; Loarca-Piña, G.; Mendoza, S. Antioxidant and antimutagenic activities of Mexican oregano (Lippia graveolens Kunth). Plant Foods Hum. Nutr. 2008, 63, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lagouri, V.; Alexandri, G. Antioxidant properties of greek O. dictamnus and R. officinalis methanol and aqueous extracts—HPLC determination of phenolic acids. Int. J. Food Prop. 2013, 16, 549–562. [Google Scholar] [CrossRef]
- Béjaoui, A.; Boulila, A.; Sanaa, A.; Boussaid, M.; Fernandez, X. Antioxidant activity and α-amylase inhibitory effect of polyphenolic-rich extract from Origanum glandulosum desf. J. Food Biochem. 2017, 41, e12271-n/a. [Google Scholar] [CrossRef]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Çelik, S.E.; Tufan, A.N.; Bekdeşer, B.; Özyürek, M.; Güçlü, K.; Apak, R. Identification and determination of phenolics in Lamiaceae species by UPLC-DAD-ESI-MS/MS. J. Chromatogr. Sci. 2017, 55, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Mahmoud, E.A. Egyptian herbal tea infusions’ antioxidants and their antiproliferative and cytotoxic activities against cancer cells. Nat. Prod. Res. 2015, 29, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Regueiro, J.; Alvarenga, J.F.R.; Martinez-Huelamo, M.; Leal, L.N.; Lamuela-Raventos, R.M. Characterization of the phenolic and antioxidant profiles of selected culinary herbs and spices: Caraway, turmeric, dill, marjoram and nutmeg. Food Sc. Technol. (Campinas) 2015, 35, 189–195. [Google Scholar] [CrossRef]
- Yan, F.; Azizi, A.; Janke, S.; Schwarz, M.; Zeller, S.; Honermeier, B. Antioxidant capacity variation in the oregano (Origanum vulgare L.) collection of the German National Genebank. Ind. Crop. Prod. 2016, 92, 19–25. [Google Scholar] [CrossRef]
- Hossain, M.B.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N.P. Optimisation of accelerated solvent extraction of antioxidant compounds from rosemary (Rosmarinus officinalis L.), marjoram (Origanum majorana L.) and oregano (Origanum vulgare L.) using response surface methodology. Food Chem. 2011, 126, 339–346. [Google Scholar] [CrossRef]
- Balkan, B.; Balkan, S.; Aydogdu, H.; Guler, N.; Ersoy, H.; Askin, B. Evaluation of antioxidant activities and antifungal activity of different plants species against pink mold rot-causing Trichothecium roseum. Arab. J. Sci. Eng. 2017, 42, 2279–2289. [Google Scholar] [CrossRef]
- Saija, A.; Speciale, A.; Trombetta, D.; Leto, C.; Tuttolomondo, T.; La Bella, S.; Licata, M.; Virga, G.; Bonsangue, G.; Gennaro, M.C.; et al. Phytochemical, ecological and antioxidant evaluation of wild sicilian thyme: Thymbra capitata (L.) cav. Chem. Biodivers. 2016, 13, 1641–1655. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins Basic Pathology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Hansson, G.K. Inflammation, atherosclerosis and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Cancer and inflammation: An old intuition with rapidly evolving new concepts. Annu. Rev. Immunol. 2012, 30, 677–706. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Yu, H.-M.; Shie, J.-J.; Cheng, T.-J.R.; Wu, C.-Y.; Fang, J.-M.; Wong, C.-H. Chemical constituents of Plectranthus amboinicus and the synthetic analogs possessing anti-inflammatory activity. Bioorgan. Med. Chem. 2014, 22, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, A.; Medjakovic, S. Anti-inflammatory properties of culinary herbs and spices that ameliorate the effects of metabolic syndrome. Maturitas 2012, 71, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Mekhora, C.; Muangnoi, C.; Chingsuwanrote, P.; Dawilai, S.; Svasti, S.; Chasri, K.; Tuntipopipat, S. Eryngium foetidum suppresses inflammatory mediators produced by macrophages. Asian Pac. J. Cancer Prev. 2012, 13, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutierrez-Grijalva, E.; Ambriz-Perez, D.; Heredia, J. Flavonoids as cytokine modulators: A possible therapy for inflammation-related diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef] [PubMed]
- Pecorino, L. Molecular Biology of Cancer—Mechanisms, Targets and Therapeutics, 3rd ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- National Cancer Institute. Antioxidants and Cancer Prevention. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/antioxidants-fact-sheet (accessed on 3 October 2017).
- Shukla, S.; Gupta, S. Apigenin and cancer chemoprevention. In Bioactive Foods in Promoting Health: Fruits and Vegetables; Watson, R.R., Preedy, V.R., Eds.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2010; pp. 663–689. [Google Scholar]
- El Babili, F.; Bouajila, J.; Souchard, J.P.; Bertrand, C.; Bellvert, F.; Fouraste, I.; Moulis, C.; Valentin, A. Oregano: Chemical analysis and evaluation of its antimalarial, antioxidant and cytotoxic activities. J. Food Sci. 2011, 76, C512–C518. [Google Scholar] [CrossRef] [PubMed]
- Calderón, Á.I.; Vázquez, Y.; Solís, P.N.; Caballero-George, C.; Zacchino, S.; Gimenez, A.; Pinzón, R.; Cáceres, A.; Tamayo, G.; Correa, M.; et al. Screening of Latin American plants for cytotoxic activity. Pharm. Biol. 2006, 44, 130–140. [Google Scholar] [CrossRef]
- Chaouki, W.; Leger, D.Y.; Eljastimi, J.; Beneytout, J.L.; Hmamouchi, M. Antiproliferative effect of extracts from Aristolochia baetica and Origanum compactum on human breast cancer cell line mcf-7. Pharm. Biol. 2010, 48, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Erenler, R.; Sen, O.; Aksit, H.; Demirtas, I.; Yaglioglu, A.S.; Elmastas, M.; Telci, I. Isolation and identification of chemical constituents from Origanum majorana and investigation of antiproliferative and antioxidant activities. J. Sci. Food Agric. 2016, 96, 822–836. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Cristaldi, B.; Menichini, F.; Conforti, F. Inhibitory effects of wild dietary plants on lipid peroxidation and on the proliferation of human cancer cells. Food Chem. Toxicol. 2015, 86, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Savini, I.; Arnone, R.; Catani, M.V.; Avigliano, L. Origanum vulgare induces apoptosis in human colon cancer Caco-2 cells. Nut. Cancer 2009, 61, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Nile, A.S.; Keum, Y.S. Total phenolics, antioxidant, antitumor and enzyme inhibitory activity of indian medicinal and aromatic plants extracted with different extraction methods. 3 Biotech 2017, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Chinou, I.; Liolios, C.; Moreau, D.; Roussakis, C. Cytotoxic activity of Origanum dictamnus. Fitoterapia 2007, 78, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Al-Kalaldeh, J.Z.; Abu-Dahab, R.; Afifi, F.U. Volatile oil composition and antiproliferative activity of Laurus nobilis, Origanum syriacum, Origanum vulgare and Salvia triloba against human breast adenocarcinoma cells. Nutr. Res. 2010, 30, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Berrington, D.; Lall, N. Anticancer activity of certain herbs and spices on the cervical epithelial carcinoma (HeLa) cell line. Evid.-Based Complement. Altern. Med. 2012, 2012, 564927. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Cardinali, A.; Ruta, C.; Fortunato, I.M.; Lattanzio, V.M.T.; Linsalata, V.; Cicco, N. Relationship of secondary metabolism to growth in oregano (Origanum vulgare L.) shoot cultures under nutritional stress. Environ. Exp. Bot. 2009, 65, 54–62. [Google Scholar] [CrossRef]
- Bernstein, N.; Chaimovitch, D.; Dudai, N. Effect of irrigation with secondary treated effluent on essential oil, antioxidant activity and phenolic compounds in oregano and rosemary. Agron. J. 2009, 101, 1–10. [Google Scholar] [CrossRef]
- Kwon, Y.-I.; Apostolidis, E.; Kim, Y.-C.; Shetty, K. Over-expression of proline-linked antioxidant pathway and modulation of phenolic metabolites in long life span clonal line of Origanum vulgare in response to ultraviolet radiation. J. Food Biochem. 2009, 33, 649–673. [Google Scholar] [CrossRef]
- Andarwulan, N.; Shetty, K. Influence of acetyl salicylic acid in combination with fish protein hydrolysates on hyperhydricity reduction and phenolic synthesis in oregano (Origanum vulgare) tissue cultures. J. Food Biochem. 1999, 23, 619–635. [Google Scholar] [CrossRef]
- Yin, H.; Fretté, X.C.; Christensen, L.P.; Grevsen, K. Chitosan oligosaccharides promote the content of polyphenols in Greek oregano (Origanum vulgare ssp. Hirtum). J. Agric. Food Chem. 2012, 60, 136–143. [Google Scholar] [CrossRef] [PubMed]
- García-Mier, L.; Guevara-González, R.; Mondragón-Olguín, V.; del Rocío Verduzco-Cuellar, B.; Torres-Pacheco, I. Agriculture and bioactives: Achieving both crop yield and phytochemicals. Int. J. Mol. Sci. 2013, 14, 4203–4222. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Pardossi, A.; Remorini, D.; Guidi, L. Antioxidant and photosynthetic response of a purple-leaved and a green-leaved cultivar of sweet basil (Ocimum basilicum) to boron excess. Environ. Exp. Bot. 2013, 85, 64–75. [Google Scholar] [CrossRef]
- Yépez-Hernández, F.-J.; Ferrera-Cerrato, R.; Alarcón, A.; Delgadillo-Martínez, J.; Mendoza-López, M.R.; García-Barradas, Ó. Fertilización nitrogenada en el crecimiento, contenido de compuestos fenólicos y actividad antioxidante de albahaca. Rev. Fitotec. Mex. 2016, 39, 33–40. [Google Scholar]
- Nguyen, P.M.; Niemeyer, E.D. Effects of nitrogen fertilization on the phenolic composition and antioxidant properties of basil (Ocimum basilicum L.). J. Agric. Food Chem. 2008, 56, 8685–8691. [Google Scholar] [CrossRef] [PubMed]
- Shiga, T.; Shoji, K.; Shimada, H.; Hashida, S.N.; Goto, F.; Yoshihara, T. Effect of light quality on rosmarinic acid content and antioxidant activity of sweet basil, Ocimum basilicum L. Plant Biotechnol. 2009, 26, 255–259. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ashkani, S.; Baghdadi, A.; Pazoki, A.; Jaafar, H.; Rahmat, A. Improvement in flavonoids and phenolic acids production and pharmaceutical quality of sweet basil (Ocimum basilicum L.) by ultraviolet-b irradiation. Molecules 2016, 21, 1203. [Google Scholar] [CrossRef] [PubMed]
- Jaganath, I.B.; Crozier, A. Flavonoid biosynthesis. In Plant Metabolism and Biotechnology; Ashihara, H., Crozier, A., Komamine, A., Eds.; John Wiley & Sons, Ltd.: West Sussex, UK, 2011; pp. 293–320. [Google Scholar]
- Malerba, M.; Cerana, R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Chen, F.; Wang, X.; Rajapakse, N.C. Effect of chitosan on the biological properties of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2005, 53, 3696–3701. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Pirbalouti, A.; Malekpoor, F.; Salimi, A.; Golparvar, A. Exogenous application of chitosan on biochemical and physiological characteristics, phenolic content and antioxidant activity of two species of basil (Ocimum ciliatum and Ocimum basilicum) under reduced irrigation. Sci. Hortic. 2017, 217, 114–122. [Google Scholar] [CrossRef]
- Koca, N.; Karaman, Ş. The effects of plant growth regulators and L-phenylalanine on phenolic compounds of sweet basil. Food Chem. 2015, 166, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Złotek, U.; Szymanowska, U.; Karaś, M.; Świeca, M. Antioxidative and anti-inflammatory potential of phenolics from purple basil (Ocimum basilicum L.) leaves induced by jasmonic, arachidonic and β-aminobutyric acid elicitation. Int. J. Food Sci. Technol. 2016, 51, 163–170. [Google Scholar] [CrossRef]
- Malekpoor, F.; Salimi, A.; Pirbalouti, A.G. Effect of jasmonic acid on total phenolic content and antioxidant activity of extract from the green and purple landraces of sweet basil. Acta Pol. Pharm. 2016, 73, 1229–1234. [Google Scholar]
- Kim, H.-J.; Chen, F.; Wang, X.; Rajapakse, N.C. Effect of methyl jasmonate on secondary metabolites of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2006, 54, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Pichyangkura, R.; Chadchawan, S. Biostimulant activity of chitosan in horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Trivellini, A.; Lucchesini, M.; Maggini, R.; Mosadegh, H.; Villamarin, T.S.S.; Vernieri, P.; Mensuali-Sodi, A.; Pardossi, A. Lamiaceae phenols as multifaceted compounds: Bioactivity, industrial prospects and role of “positive-stress”. Ind. Crop. Prod. 2016, 83, 241–254. [Google Scholar] [CrossRef]
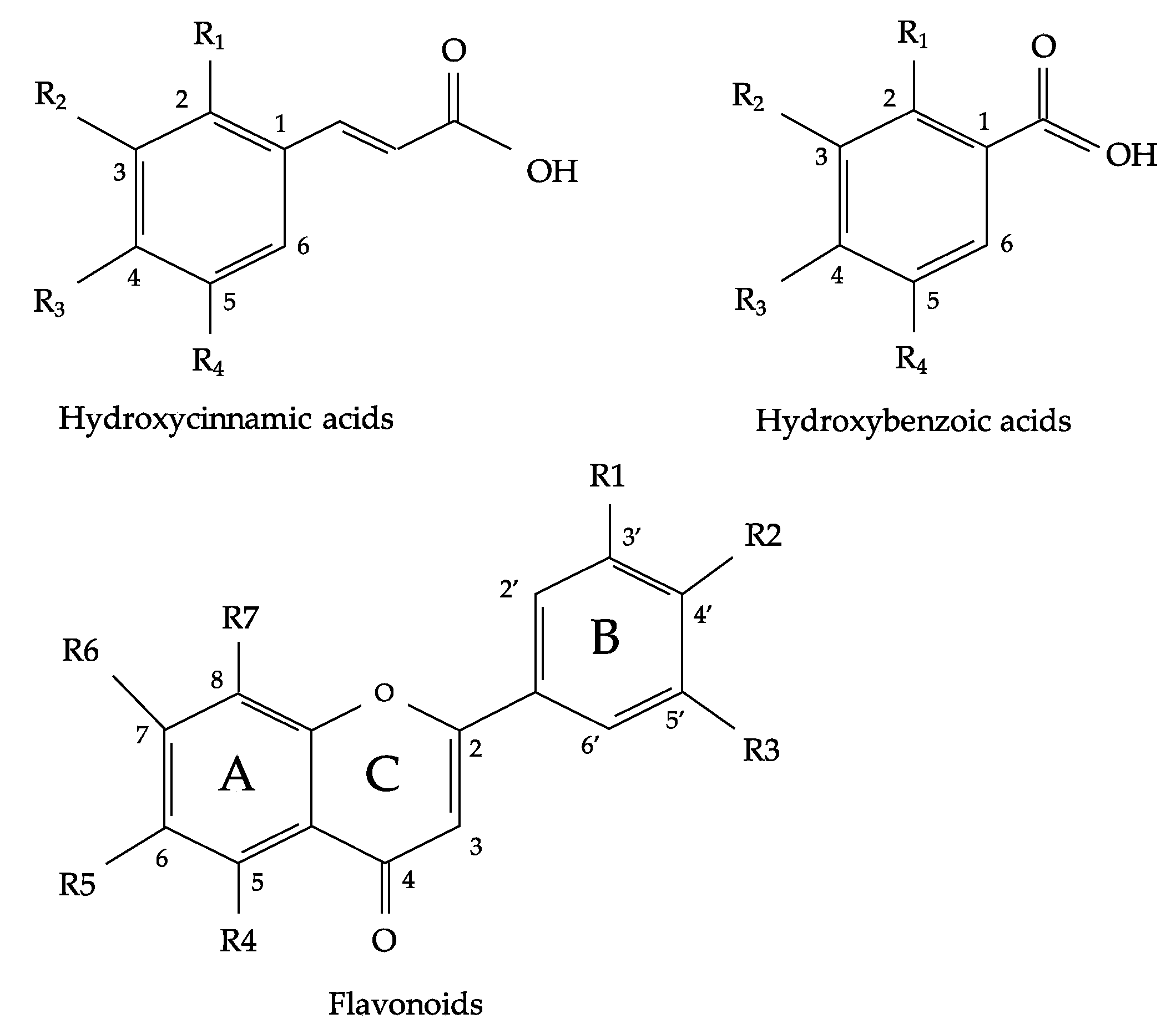
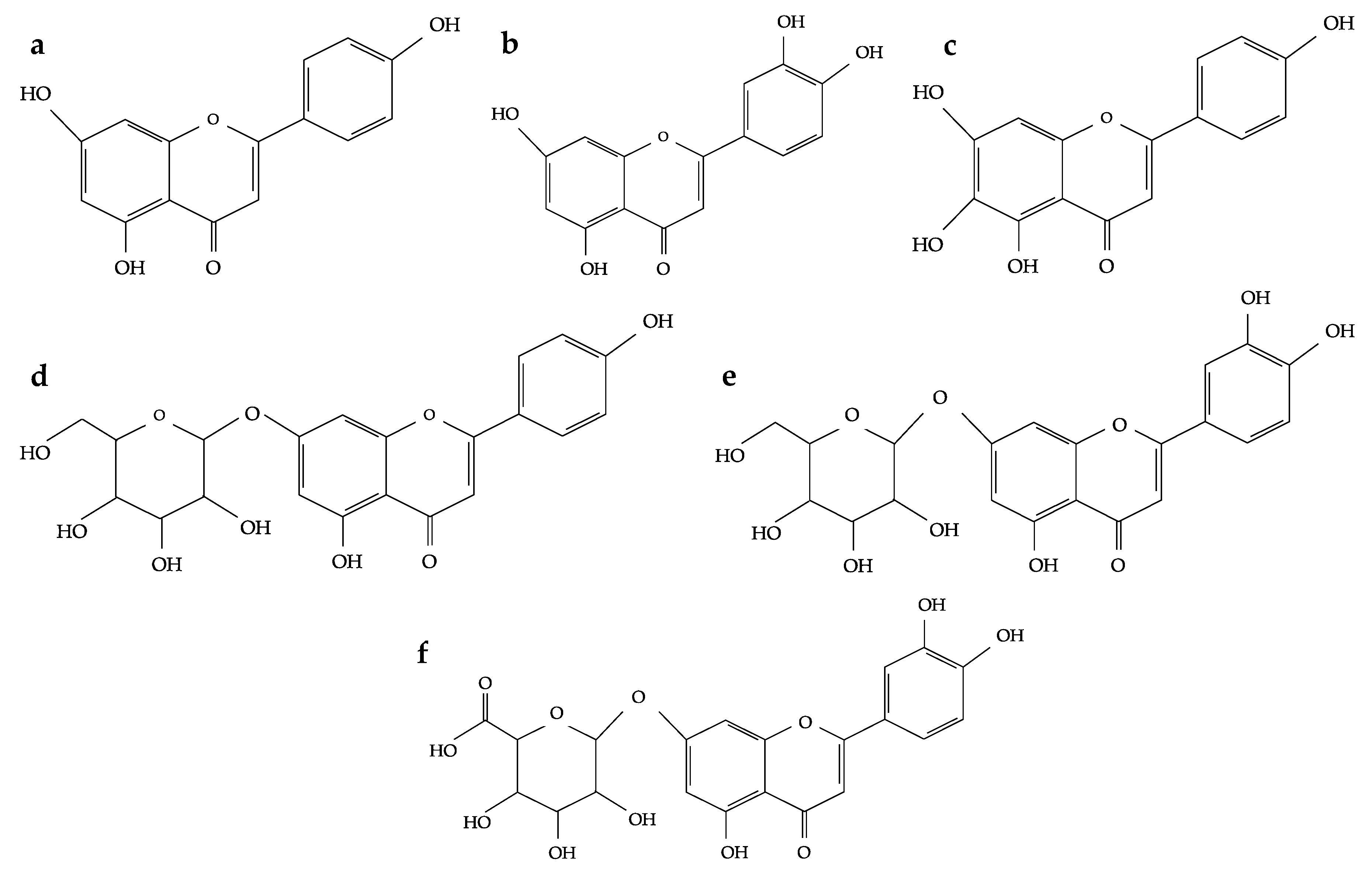
| Oregano Species | Origin | Extraction Solvent | Flavonoids and Phenolic Acids Constituents | Reference |
|---|---|---|---|---|
| Hedeoma patens | Mexico | Methanol/acetone/water (50:40:10) | 3-O-caffeoylquinic acid, luteolin-7-O-glucuronide, scutellarein-7-O-hexoside, salvianolic acid A | [4] |
| Lippia alba (Mill.) N. E. Brown | Brazil | Methanol 62.5% | Apigenin-7-O-diglucuronide, chrysoeriol-7-O-diglucuronide, luteolin-7-O-glucuronide, tricin-7-O-diglucuronide, tricin-7-O-glucuronide | [25] |
| Lippia alba (Mill.) N. E. Brown | France | Methanol/hydromethanol | Luteolin-7-diglucuronide, verbascoside, chlorogenic acid | [28] |
| Lippia alba (Mill.) N.E. Brown | Brazil | Chloroform | 5,5′′-dihydroxy-6,4′,6′′,3′′′,4′′′-pentamethoxy-[C7–O–C7′′]-biflavone, 4′,4,5,5′′-tetrahydroxy-6,6′′,3′′′-trimethoxy-[C7–O–C7′′]-biflavone | [22] |
| Lippia citriodora | Germany | Water | Verbasoside, luteolin-7-diglucuronide, apigenin-7-diglucuronide | [29] |
| Lippia graveolens | Mexico | Methanol/acetone/water (50:40:10) | Quercetin O-hexoside, scutellarein 7-O-hexoside, phloridzin, trihydroxy-methoxyflavone derivative, 6-O-methylscutellarein | [4] |
| Lippia graveolens | NR 1 | Methanol 70% | 6-Hydroxyluteolin 7-O-hexoside, pentaflavanone-B hexoside-1, pentaflavanone-B hexoside-2, scutellarein 7-O-hexoside, luteolin 7-O-glucoside, taxifolin, 6-hydroxyluteolin 7-O-rhamnoside, 6-hydroxyluteolin, 3-hydroxyphloretin 6′-O-hexoside, apigenin 7-O-glucoside, phloridzin, scutellarein, eriodictyol, luteolin, quercetin, naringenin, 6-methylscutellarein, 6,7-dimethylscutellarein, sakuranetin, pinocembrin, galangin, methylgalangin | [9] |
| Lippia graveolens | USA | Methanol 100% | Eriodictyol, naringenin, hispidulin, cirsimaritin | [30] |
| Lippia micromera | Colombia | Methanol/hydromethanol | Naringenin, apigenin | [28] |
| Lippia origanoides | Colombia | Methanol 62.5% | Quercetin, naringenina, luteolin, pinocembrin | [25] |
| Lippia palmeri | Mexico | Methanol/acetone/water (50:40:10) | Quercetin-O-hexoside, luteolin 7-O-glucuronide-3′-O-glucoside, scutellarein 7-O-hexoside, trihydroxy-methoxyflavone derivative | [4] |
| Majorana hortensis Moench. | Poland | Water | Caffeic acid, lithospermic acid, rosmarinic acid | [31] |
| O. acutidens | Turkey | Water | Gallic acid, caffeic acid, 4-hydroxybenzaldehyde, p-coumaric acid, rosmarinic acid | [32] |
| O. dictamus | Greece | Methanol 62.5%/BHT | Vanillic acid, protocatechuic acid, syringic acid, gallic acid, cinnamic acid, o-coumaric acid, p-coumaric acid, caffeic acid, chlorogenic acid, rosmarinic acid, chrysin, epicatechin, naringenin, catechin, genistein, quercetin | [33] |
| O. dictamus | Greece | Water | Chlorogenic acid, rutin, luteolin-7-O-glucoside, apigenin-7-O-glucoisde, rosmarinic acid, luteolin | [34] |
| O. indercedens | Greece | Methanol | Caffeic acid, rosmarinic acid | [35] |
| O. majoram L. | Germany | Methanol 60%/Formic acid 1% | Apigenin-6,8-di-C-glucoside, luteolin-7′-O-glucuronide, rosmarinic acid, apigenin-glucuronide, lithospermic acid A isomer a, salvianolic acid B, apigenin | [36] |
| O. majoram L. | Tunisia | Methanol | Dihydroxybenzoic acid hexose, syringic acid, vanillic acid, dihydroxybenzoic acid, p-coumaric acid, chlorogenic acid, salvianolic acid I, caffeoyl-arbutin, rosmarinic acid, gallocatechin isomer 1, gallocatechin isomer 2, 3-O-methyl-catechin, luteolin-6,8-C-dihexose, apigenin-6,8-di-C-hexoside, isoorientin, orientin, isovitexin, kaempferol-O-sumbubioside, kaempferol-O-glucuronide, luteolin-O-glycoside, diosmin, apigenin-O-glucuronide, acacetin rutinoside, luteolin, apigenin, taxifolin, taxifolin methyl ether isomer 1, taxifolin methyl ether isomer 2, dihydrokaempferide, hesperidin, eriodictyol, sakuranetin, O-methy-quercetin, dimethyl myriceitn, quercetin dimethyl ether, jaceidin isomer 1, jaceidin isomer 2 | [37] |
| Greece | Water | Vanillic acid, protocatechuic acid, syringic acid, gallic acid, cinnamic acid, o-coumaric acid, p-coumaric acid, ferulic acid, caffeic acid, sinapic acid, rosmarinic acid, chrysin, epicatechin, naringenin, catechin, kaempferol, quercetin | [34] | |
| Poland | Methanol | Protocatechuic acid, p-hydroxybenzoic acid, gentisic acid, chlorogenic acid, syringic acid | [38] | |
| Turkey | Methanol 80% | Caffeic acid glucoside, epigallocatechin, arbutin, luteolin ruitinoside, luteolin glucuronide, rosmarinic acid, dihydroquercetin, dihydroluteolin, apigenin, quercetin, quercetin arabinoside, luteolin-7-O-glucoside gallocatechin derivative | [39] | |
| Germany | Methanol 50% | Luteolin-6,8-di-C-glucoside, apigenin-6,8-di-C-glucoside, luteolin-glucuronide, rosmarinic acid, apigenin-glucuronide, lithospermic acid, apigenin | [40] | |
| O. majoram L. | USA | Methanol 80% | Eriodictyol 6,8-di-C-glucoside, eriodictyol 7-O-glucoside, apigenin 6,8-di-C-glucoside, luteolin 7,7′-di-O-glucuronide, luteolin 7-O-glucuronide-3′-O-glucoside, apigenin 7-O-diglucuronide, luteolin 7-O-glucuronide, luteolin 7-O-glucuronide, luteolin 7-O-glucoside, apigenin 7-O-glucoside, apigenin 7-O-glucuronide, rosmarinic acid | [41] |
| Turkey | Water | Gallic acid, caffeic acid, p-coumaric acid, rosmarinic acid, chicoric acid, apigenin-7-glucoside, quercetin, kaempferol | [32] | |
| Italy | Cascade extraction with ethyl acetate and ethanol | Eriodictyol 7-O-rutinoside, aromadendrin, eriodictyol, naringenin, luteolin, sorbifolin, cirsiliol, apigenin, cirsilineol, cirsimaritin, xanthomicrol, caffeic acid, rosmarinic acid | [42] | |
| Greece | Cascade extraction with hexane and ethyl acetate | Salvianolic acid H, salvianolic acid B, rosmarinic acid, salvianolic acid C, eriodictyol, naringenin | [43] | |
| Finland | Methanol 40% | Calleryanin 3,4-dihydroxybenzoate, gastrodin 3,4-dihydroxybenzoate, calleryanin 3-hydroxy,4-methoxybenzoate | [44] | |
| Portugal | Methanol 80% | Gallic acid, 3,4-dihydroxybenzoic acid, (+)-catechin, caffeic acid, (−)-epicatechin, rosmarinic acid | [10] | |
| Oregano 2 | Turkey | Methanol 80% | Gallic acid, syringic acid, vanillic acid, protocatechuic acid, chlorogenic acid, p-coumaric acid, quercetin-3-O-hexoside, luteolin-7-O-glucoside, ferulic acid, phloridzin, dicaffeoylquinic acid, apigenin-7-O-rutinoside, rutin, apigenin-7-O-glucoside, rosmarinic acid, luteolin-3-O-glucuronide, luteolin-7-O-rutinoside, quercetin, apigenin, 4′-methoxyapigenin, 6,7-dimethoxyscutellarein, luteolin | [45] |
| Poliomintha longiflora | NR 1 | Phosphate buffer | Vanillic acid, caffeic acid, luteolin, rosmarinic acid, hispidulin | [46] |
| Oregano Species | Extract | Compounds | Plant Part | Antioxidant Assay | Reference |
|---|---|---|---|---|---|
| Coleus aromaticus | Methanol | Rosmarinic, caffeic, p-coumaric and gallic acids; quercetin and rutin | Stem | DPPH, superoxide, TAC | [69] |
| Eryngium foetidum | Aqueous, methanol | Gallic, protocatechuic, syringic, p-coumaric, ferulic and sinapic acids | Leaves | TPC, DPPH | [70] |
| Lippia alba | Aqueous | Apigenin-7-O-diglucuronide, chrysoeriol-7-O-diglucuronide, tricin-7-O-diglucuronide, luteolin-7-O-glucuronide | Leaves | TPC, DPPH | [21] |
| Lippia graveolens | Methanol | Rosmarinic acid, naringenin | Aerial parts | TPC, DPPH | [71] |
| Methanol | Eriodictyol, naringenin, hispidulin, cirsimaritin | Leaves, commercial herbs | TPC, ORAC | [30] | |
| Origanum dictamnus | Sequentially with hexane, acetone and methanol; Aqueous | Methanolic: gallic, caffeic, ferulic and rosmarinic acids. Aqueous: gallic, caffeic, protocatechuic and rosmarinic acids | Leaves | TPC, DPPH, FRAP | [72] |
| Aqueous | Rosmarinic, caffeic and vanillic acids, epicatechin, catechin, genistein | Leaves and flowers | TPC, DPPH, FRAP | [34] | |
| Origanum glandulosum | Methanol, previously deffated with n-hexane | Caffeic acid, luteolin glucoside | Not specified | DPPH, FRAP | [73] |
| Origanum majorana | Methanol microwave-assisted | Rosmarinic and caffeic acid, apigenin, rutin | Aerial parts | TPC, DPPH, CUPRAC | [75] |
| Aqueous, methanol | Rosmarinic and caffeic acids | Leaves | TPC, DPPH, β-carotene bleaching | [76] | |
| Methanol | Rosmarinic acid, eriodictyol, naringenin, hispidulin, cirsimaritin | Leaves, commercial herbs | TPC, ORAC | [30] | |
| Methanol | Rosmarinic acid, epigallocatechin, quercetin, apigenin | Not specified | DPPH, FRAP | [39] | |
| Ethanol | Chlorogenic, ferulic, p-coumaric, p-hydroxybenzoic, protocatechuic, rosmarinic and syringic acids, quercetin | Not specified | TPC, DPPH, ABTS | [77] | |
| Origanum microphyllum | Aqueous | p-Hydroxybenzoic, protocatechuic, syringic and caffeic acids, naringenin | Leaves and flowers | TPC, FRAP | [26] |
| Origanum vulgare | Methanol | Rosmarinic acid | Leaves | TPC, ORAC | [78] |
| Methanol | Rosmarinic acid and (−)-epicatechin | Leaves | DPPH; ABTS, FRAP | [10] | |
| Water, methanol, ethyl acetate, hexane | Rosmarinic, caffeic, chicoric and p-coumaric acids | Leaves | TPC, DPPH, TAC, RP, superoxide | [32] | |
| Methanol | Rosmarinic acid, eriodictyol, naringenin | Leaves | TPC, ORAC | [30] | |
| Origanum vulgare | Methanol | Rosmarinic and caffeic acids, luteolin-7-O-glucoside, apigenin-7-O-glucoside | Not specified | TPC, FRAP | [79] |
| Aqueous | Eriodictyol, apigenin, caffeic acid, kaempferol | Not specified | TPC, DPPH | [80] | |
| Thymbra capitata | Ethyl acetate, ethanol | Ethyl acetate: taxifolin di-O-glucoside, thymusin. Ethanol: taxifolin di-O-glucoside, rosmarinic acid | Aerial parts | TPC, superoxide | [81] |
| Oregano Species | Compounds | Effect | Reference |
|---|---|---|---|
| Eryngium foetidum | Kaempferol, chlorogenic acid, caffeic acid | Reduction of NO and ROS production and inhibition of the protein levels and gene expression of IL-6, TNF-α, iNOS and COX-2 in LPS-stimulated RAW 264.7 murine macrophages | [88] |
| Hedeoma patens | Neochlorogenic acid, Luteolin-7-O-glucuronide, scutellarein-7-O-hexoside, salvianolic acid A | Reduction of the levels of NO and ROS produced in murine macrophage cells | [4] |
| Lippia graveolens | Quercetin-O-hexoside, scutellarein-7-O-hexoside, phloridzin, trihydroxy-methoxyflavone derivative, 6-O-methylscutellarein | Inhibition of the NO and ROS production in LPS-stimulated murine macrophage cells | [4] |
| Lippia palmeri | Quercetin-O-hexoside, luteolin-7-O-glucuronide-3′-O-glucoside, scutellarein-7-O-hexoside, trihydroxy-methoxyflavone derivative, 6-O-methylscutellarein | Decrease of the levels of NO and ROS produced in murine macrophage cells | [4] |
| Origanum vulgare | Caffeic acid | Diminution of IL-8 secretion in HT-29 and PC3 cells | [26] |
| Oregano Species | Anti-Cancer Activity | Effect | Reference |
|---|---|---|---|
| Origanum dictmnus | Cytotoxic | Activity against human bronchial epidermoid carcinoma (NSCLC-N6) and murine leukemia (P388) cells line. | [101] |
| Origanum compactum | Cytotoxic | Activity against human breast cancer cells (MCF-7) | [94] |
| Antiproliferative | Inhibit human breast cancer (MCF-7) cell proliferation. | [96] | |
| Origanum syriacum | Antiproliferative | Reduction in the proliferation of human breast cancer cells (MFC-7). | [102] |
| Origanum vulgare | Antiproliferative | Reduction in the proliferation of human breast cancer (MFC-7), colorectal cancer (LoVo), cervical epithelial carcinoma (HeLa) cells and selective antiproliferative activity on hepatic cancer cells (HepG2). | [32,98,102] |
| Cytotoxic | Showed signs of cells death on cervical epithelial carcinoma (HeLa) cell line and cytotoxicity for breast cancer and colon adenocarcinoma (Caco-2) cells. | [98,99,100,103] | |
| Lippia cardiostegia Benth | Cytotoxic | Activity against breast (MCF-7), lung (H-460) and central nervous system (SF-268) human cancer cell lines. | [95] |
| Origanum marjorana | Antiproliferative | Inhibit Rattus norvegicus brain glioma (C6) and cervical epithelial carcinoma (HeLa) cell proliferation. | [97] |
| Cytotoxic | Activity against fibrosarcoma (HT-1080) cell line. | [98] | |
| Origanum acutidens | Antiproliferative | Inhibit cervical epithelial carcinoma (HeLa) cell proliferation. | [32] |
| Poliomintha glabrescens Gray | Cytotoxic | Activity against human colon cancer cells (HT-29). | [57] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants 2018, 7, 2. https://doi.org/10.3390/plants7010002
Gutiérrez-Grijalva EP, Picos-Salas MA, Leyva-López N, Criollo-Mendoza MS, Vazquez-Olivo G, Heredia JB. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants. 2018; 7(1):2. https://doi.org/10.3390/plants7010002
Chicago/Turabian StyleGutiérrez-Grijalva, Erick P., Manuel A. Picos-Salas, Nayely Leyva-López, Marilyn S. Criollo-Mendoza, Gabriela Vazquez-Olivo, and J. Basilio Heredia. 2018. "Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits" Plants 7, no. 1: 2. https://doi.org/10.3390/plants7010002
APA StyleGutiérrez-Grijalva, E. P., Picos-Salas, M. A., Leyva-López, N., Criollo-Mendoza, M. S., Vazquez-Olivo, G., & Heredia, J. B. (2018). Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants, 7(1), 2. https://doi.org/10.3390/plants7010002








