Quantification of (1→4)-β-d-Galactans in Compression Wood Using an Immuno-Dot Assay
Abstract
:1. Introduction
2. Results
2.1. Assays Using Reference Lupin Seed (1→4)-β-Galactans
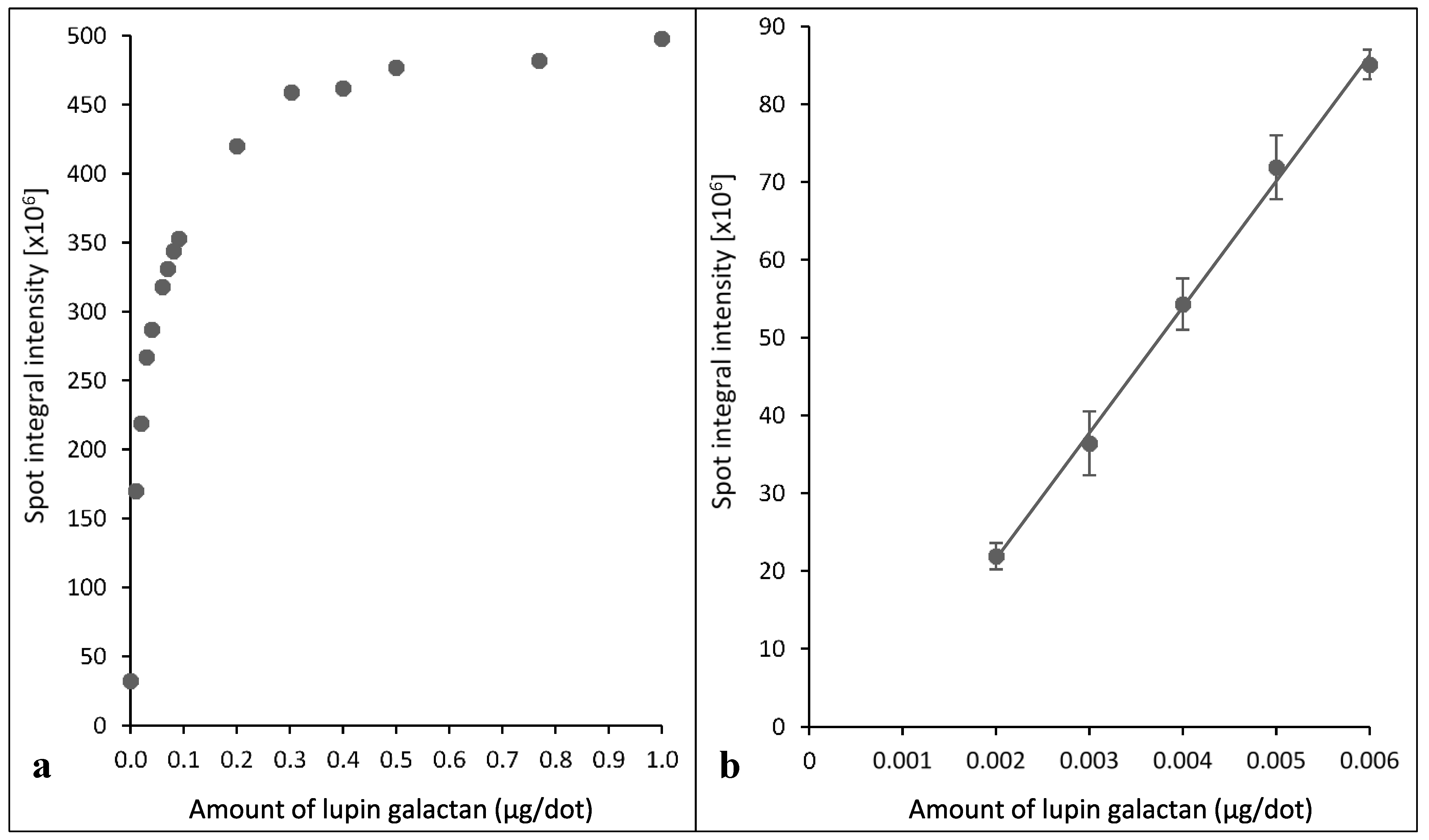
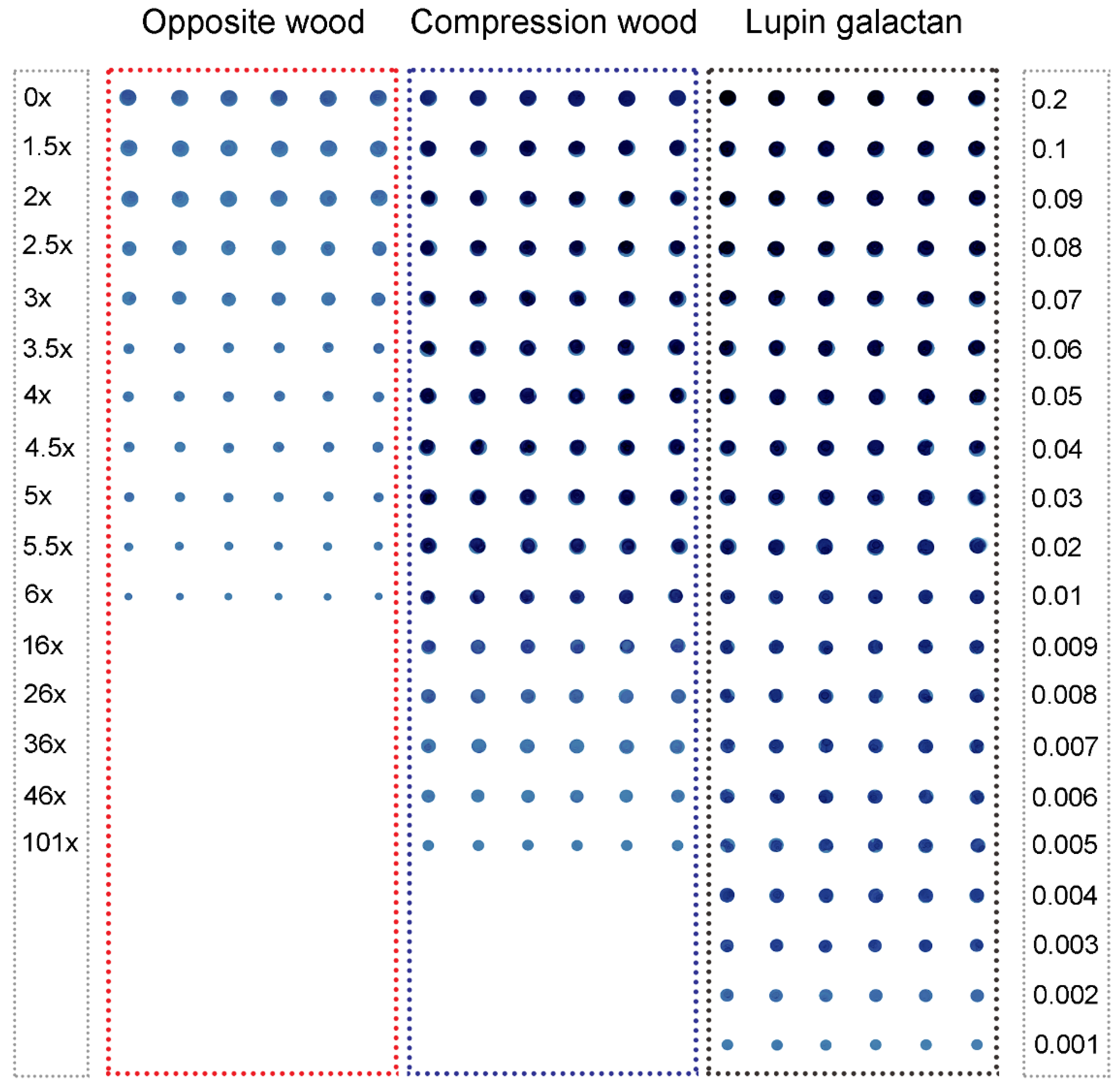
2.2. Assays Using Extracts of Radiata Pine Wood
| Tree | Amounts of (1→4)-β-Galactans Extracted Without Delignification | Amounts of (1→4)-β-Galactans Extracted after Delignification | ||||
|---|---|---|---|---|---|---|
| OW | CW | CW/OW | OW | CW | CW/OW | |
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |||
| Tree 1 | 0.03 ± 0.00 | 0.35 ± 0.03 | 11.7 | 0.38 ± 0.09 | 9.95 ± 1.39 | 26.2 |
| Tree 2 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.5 | 0.60 ± 0.11 | 8.34 ± 0.60 | 14.0 |
| Tree 3 | 0.01 ± 0.00 | 0.01 ± 0.00 | 1.0 | 0.42 ± 0.08 | 8.25 ± 0.02 | 19.5 |
| Tree 4 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.7 | 0.60 ± 0.03 | 6.63 ± 0.95 | 11.1 |
| Tree 5 | 0.01 ± 0.00 | 0.02 ± 0.00 | 2.0 | 0.39 ± 0.02 | 5.92 ± 0.76 | 15.3 |
| Tree 6 | 0.03 ± 0.00 | 0.04 ± 0.00 | 1.3 | 0.22 ± 0.04 | 4.27 ± 0.50 | 19.5 |
| Tree 7 | 0.03 ± 0.00 | 0.03 ± 0.00 | 1.0 | 0.74 ± 0.08 | 3.14 ± 0.10 | 4.2 |

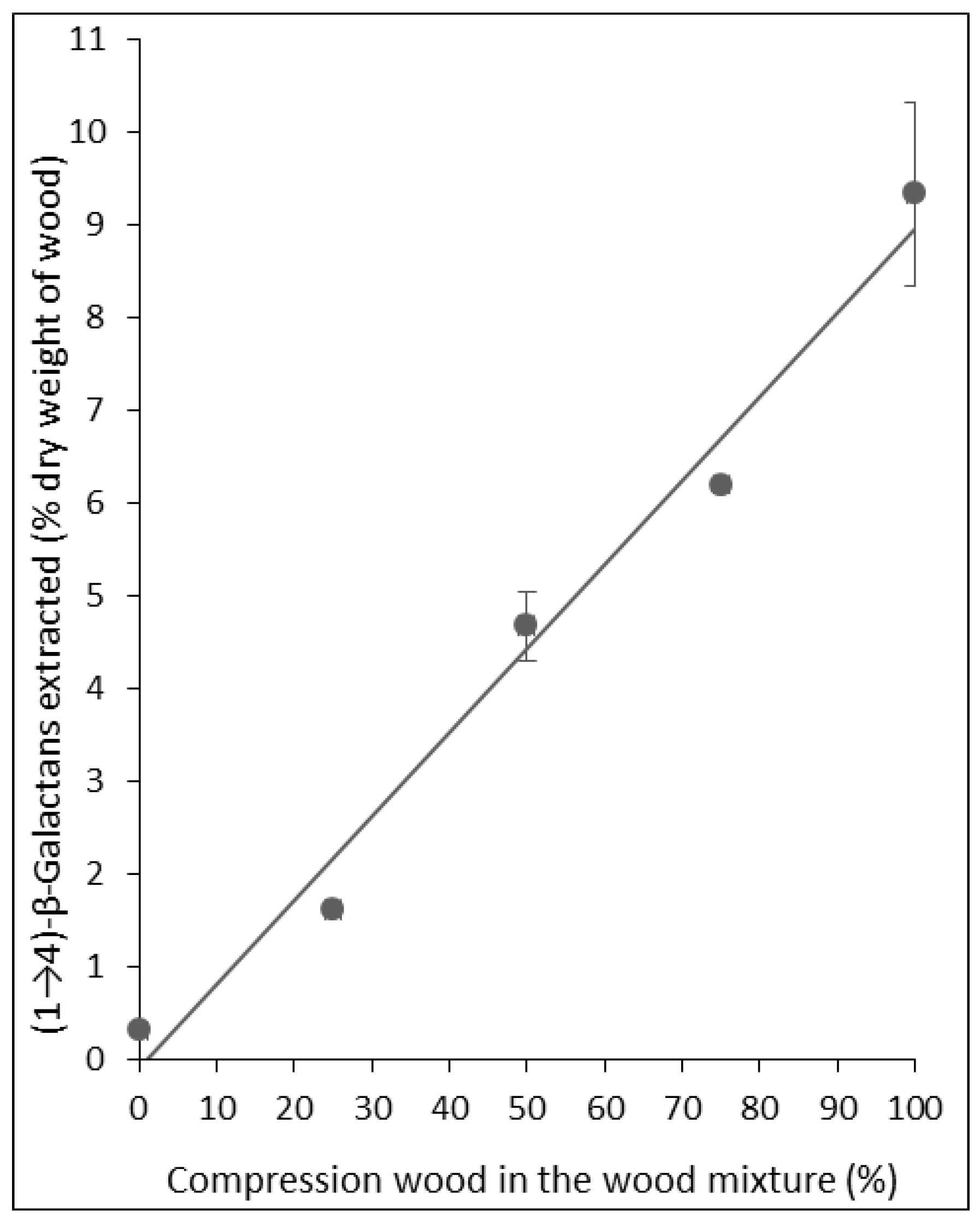
2.3. Monosaccharide Compositions of Radiata Pine Wood
| Monosaccharides | |||||||
|---|---|---|---|---|---|---|---|
| Arabinose | Xylose | Galactose | Glucose | Mannose | CW/OW Galactose | ||
| Tree | Wood Type | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |
| Tree 1 | OW | 10.2 ± 0.53 | 32.2 ± 0.12 | 8.4 ± 0.07 | 13.6 ± 0.26 | 35.6 ± 0.24 | 6.3 |
| CW | 4.7 ± 0.36 | 15.3 ± 0.29 | 52.7 ± 0.25 | 11.4 ± 0.11 | 16.0 ± 0.28 | ||
| Tree 2 | OW | 13.2 ± 0.23 | 35.8 ± 0.47 | 10.5 ± 0.20 | 9.4 ± 0.30 | 31.2 ± 0.18 | 5.5 |
| CW | 5.8 ± 0.06 | 13.1 ± 0.13 | 56.9 ± 0.49 | 9.8 ± 0.23 | 14.3 ± 0.25 | ||
| Tree 3 | OW | 12.0 ± 0.33 | 38.7 ± 0.11 | 7.7 ± 0.10 | 10.1 ± 0.37 | 31.8 ± 0.46 | 7.6 |
| CW | 5.3 ± 0.26 | 11.4 ± 0.17 | 58.5 ± 0.28 | 12.2 ± 0.27 | 12.6 ± 0.29 | ||
| Tree 4 | OW | 13.3 ± 0.35 | 31.4 ± 0.71 | 10.2 ± 0.17 | 12.4 ± 0.25 | 32.7 ± 1.03 | 4.9 |
| CW | 6.5 ± 0.24 | 15.9 ± 0.18 | 49.3 ± 0.12 | 10.4 ± 0.34 | 17.8 ± 0.78 | ||
| Tree 5 | OW | 10.2 ± 1.44 | 30.4 ± 0.61 | 10.5 ± 0.05 | 13.1 ± 0.31 | 35.8 ± 0.89 | 4.6 |
| CW | 7.6 ± 0.29 | 15.7 ± 0.08 | 47.7 ± 0.89 | 11.9 ± 0.16 | 17.3 ± 0.39 | ||
| Tree 6 | OW | 12.3 ± 0.21 | 29.5 ± 0.31 | 7.9 ± 0.08 | 12.5 ± 0.23 | 37.8 ± 0.19 | 6.8 |
| CW | 6.8 ± 0.02 | 11.9 ± 0.07 | 53.7 ± 0.41 | 11.2 ± 0.29 | 16.5 ± 0.11 | ||
| Tree 7 | OW | 10.2 ± 0.90 | 34.4 ± 0.35 | 8.9 ± 0.09 | 11.1 ± 0.23 | 35.4 ± 0.29 | 4.3 |
| CW | 7.4 ± 0.13 | 18.9 ± 0.39 | 38.1 ± 1.46 | 11.3 ± 0.29 | 24.4 ± 0.85 | ||
3. Discussion
4. Experimental
4.1. Wood Samples
4.2. Extraction of Non-Cellulosic Polysaccharides from the Wood Samples
4.3. Immuno-Dot Quantification of (1→4)-β-Galactans
4.4. Neutral Monosaccharide Compositions of the Wood Samples
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Westing, A.H. Formation and function of compression wood in gymnosperms. Bot. Rev. 1965, 31, 381–480. [Google Scholar] [CrossRef]
- Westing, A.H. Formation and function of compression wood in gymnosperms, II. Bot. Rev. 1968, 34, 51–78. [Google Scholar] [CrossRef]
- Robards, A.W. The effect of gravity on the formation of wood. Sci. Prog. (Oxf.) 1969, 57, 513–532. [Google Scholar]
- Scurfield, G. Reaction wood: Its structure and function. Science 1973, 179, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Timell, T.E. Compression Wood in Gymnosperms; Springer-Verlag: Berlin, Germany, 1986. [Google Scholar]
- Donaldson, L.A.; Singh, A.P. Formation and structure of compression wood. In Cellular Aspects of Wood Formation; Fromm, J., Ed.; Springer-Verlag: Berlin, Germany, 2013; pp. 225–256. [Google Scholar]
- Harwood, V.D. Studies on the cell wall polysaccharides of Pinus radiata 1. Isolation and structure of a xylan. Sven. Papp. 1972, 75, 207–212. [Google Scholar]
- Harwood, V.D. Studies on the cell wall polysaccharides of Pinus radiata II. Structure of a glucomannan. Sven. Papp. 1973, 76, 377–379. [Google Scholar]
- Whistler, R.L.; Chen, C.-C. Hemicelluloses. In Wood Structure and Composition; Lewin, M., Goldstein, I.S., Eds.; Marcel Dekker: New York, NY, USA, 1991; pp. 287–319. [Google Scholar]
- Harris, P.J.; Stone, B.A. Chemistry and molecular organization of plant cell walls. In Biomass Recalcitrance: Deconstructing the Plant Cell Wall for Bioenergy; Himmel, M.E., Ed.; Blackwell Publishing: Oxford, UK, 2008; pp. 61–93. [Google Scholar]
- Teleman, A. Hemicelluloses and pectin. In Pulp and Paper Chemistry and Technology. Vol. 1. Wood Chemistry and Biotechnology; Ek, M., Gellerstedt, G., Henriksson, G., Eds.; de Gruyter: Berlin, Germany, 2009; pp. 101–120. [Google Scholar]
- Brennan, M.; McLean, J.P.; Altaner, C.; Ralph, J.; Harris, P.J. Cellulose microfibril angles and cell-wall polymers in different wood types of Pinus radiata. Cellulose 2012, 19, 1385–1404. [Google Scholar] [CrossRef]
- Nanayakkara, B.; Manley-Harris, M.; Suckling, I.D.; Donaldson, L.D. Quantitative chemical indicators to assess the gradation of compression wood. Holzforschung 2009, 63, 431–439. [Google Scholar] [CrossRef]
- Jones, L.; Seymour, G.B.; Knox, J.P. Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1→4)-β-d-galactan. Plant Physiol. 1997, 113, 1405–1412. [Google Scholar] [PubMed]
- Willats, W.G.T.; Orfila, C.; Limberg, G.; Buchholt, H.C.; van Alebeek, G.-J.W.M.; Voragen, A.G.J.; Marcus, S.E.; Christenen, T.M.I.E.; Mikkelsen, J.D.; Murray, B.S.; et al. Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls. J. Biol. Chem. 2001, 276, 19404–19413. [Google Scholar] [CrossRef] [PubMed]
- Moller, I.; Sørensen, I.; Bernal, A.J.; Blaukopf, C.; Lee, K.J.D.; Øbro, J.; Pettolino, F.; Roberts, A.; Mikkelsen, J.D.; Knox, J.P.; et al. High-throughput mapping of cell-wall polymers within and between plants using novel microarrays. Plant J. 2007, 50, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Moller, I.E.; Pettolino, F.A.; Hart, C.; Lampugnani, E.R.; Willats, W.G.T.; Bacic, A. Glycan profiling of plant cell wall polymers using microarrays. JoVE 2012, 70, e4238. [Google Scholar] [PubMed]
- Jin, G. Analytical approaches to assessing poor wood quality in Pinus radiata. M.Sc. Thesis, The University of Auckland, Auckland, New Zealand, 2011. [Google Scholar]
- Wise, L.E. Quantitative isolation of hemicelluloses from coniferous woods. Preliminary communication. Ind. Eng. Chem. Anal. Ed. 1945, 17, 63–64. [Google Scholar] [CrossRef]
- Lindberg, B. Recent advances in methods of isolating and purifying hemicelluloses. Pure Appl. Chem. 1962, 5, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Wise, L.E.; Murphy, M.; D’Addieco, A.A. Chlorite holocellulose, its fractionation and bearing on summative wood analysis and on studies on hemicelluloses. Paper Trade J. 1946, 122, 35–43. [Google Scholar]
- Bouveng, H.O.; Meier, H. Studies on a galactan from Norwegian spruce compression wood (Picea abies Karst.). Acta Chem. Scand. 1959, 13, 1884–1889. [Google Scholar] [CrossRef]
- Ahlgren, P.A.; Goring, D.A.I. Removal of wood components during chlorite delignification of black spruce. Can. J. Chem. 1971, 49, 1272–1275. [Google Scholar] [CrossRef]
- Watanabe, T.; Ohnishi, J.; Yamasaki, Y.; Kaizu, S.; Koshijima, T. Binding-site analysis of the ether linkages between lignin and hemicelluloses in lignin-carbohydrate complexes by DDQ-oxidation. Agric. Biol. Chem. 1989, 53, 2233–2252. [Google Scholar] [CrossRef]
- Ralph, J.; Brunow, G.; Boerjan, W. Lignins. In Encyclopedia of Life Sciences; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 1–10. [Google Scholar]
- Altaner, C.M.; Tokareva, E.N.; Jarvis, M.C.; Harris, P.J. Distribution of (1→4)-β-galactans, arabinogalactan proteins, xylans and (1→3)-β-glucans in tracheid cell walls of softwoods. Tree Physiol. 2010, 30, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.A.; Knox, J.P. Localization of cell wall polysaccharides in normal and compression wood of radiata pine: Relationships with lignification and microfibril orientation. Plant Physiol. 2012, 158, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.J. Diversity in plant cell walls. In Plant Diversity and Evolution: Genotypic and Phenotypic Variation in Higher Plants; Henry, R.J., Ed.; CAB International Publishing: Wallingford, Oxon, UK, 2005; pp. 201–227. [Google Scholar]
- Kim, J.S.; Awano, T.; Yoshinaga, A.; Takabe, K. Immunolocalization of β-1-4-galactan and its relationship with lignin distribution in developing compression wood of Cryptomeria japonica. Planta 2010, 232, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.S.; Timell, T.E. Polysaccharides in compression wood of tamerack (Larix laricina) 4. Constitution of an acidic galactan. Sven. Papp. 1972, 75, 522–594. [Google Scholar]
- Willför, S.; Holmbom, B. Isolation and characterisation of water soluble polysaccharides from Norway spruce and Scots pine. Wood Sci. Technol. 2004, 38, 173–179. [Google Scholar] [CrossRef]
- Willför, S.; Sundberg, A.; Hemming, J.; Holmbom, B. Polysaccharides in some industrially important softwood species. Wood Sci. Technol. 2005, 39, 245–258. [Google Scholar] [CrossRef]
- Goellner, E.M.; Utermoehlen, J.; Kramer, R.; Classen, B. Structure of arabinogalactan from Larix laricina and its reactivity with antibodies directed against type-II-arabinogalactans. Carbohydr. Polym. 2011, 86, 1739–1744. [Google Scholar] [CrossRef]
- Saeman, J.F.; Moore, W.E.; Millett, M.A. Sugar units present. Hydrolysis and quantitative paper chromatography. In Methods in Carbohydrate Chemistry; Whistler, R.L., Ed.; Academic Press: New York, NY, USA, 1963; Volume 3, pp. 54–69. [Google Scholar]
- Albersheim, P.; Nevins, D.G.; English, P.D.; Karr, A. A method for analysis of sugars in plant cell-wall polysaccharides by gas-liquid chromatography. Carbohydr. Res. 1967, 5, 340–345. [Google Scholar] [CrossRef]
- Mankarios, A.T.; Jones, C.F.G.; Jarvis, M.C.; Thelfall, D.R.; Friend, J. Hydrolysis of plant polysaccharides and GLC analysis of their constituent neutral sugars. Phytochemistry 1979, 18, 419–422. [Google Scholar] [CrossRef]
- Blakeney, A.B.; Harris, P.J.; Henry, R.J.; Stone, B.A. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr. Res. 1983, 113, 291–299. [Google Scholar] [CrossRef]
- Smith, B.G.; Harris, P.J. Polysaccharide composition of unlignified cell walls of pineapple [Ananas comosus (L.) Merr.] fruit. Plant Physiol. 1995, 107, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Cantu, S.L.; Tornqvist, C.-E.; Falbel, T.G.; Bolivar, J.L.; Patterson, S.E.; Pauly, M.; Walton, J.D. A high-throughput platform for screening milligram quantities of plant biomass for lignocellulose digestibility. Bioenergy Res. 2010, 3, 93–102. [Google Scholar] [CrossRef]
- Apiolaza, L.A.; Butterfield, B.; Chauhan, S.S.; Walker, J.C.F. Characterization of mechanically perturbed young stems: Can it be used for wood quality screening? Ann. For. Sci. 2011, 68, 407–414. [Google Scholar] [CrossRef]
- Apiolaza, L.A.; Chauhan, S.S.; Walker, J.C.F. Genetic control of very early compression and opposite wood in Pinus radiata and its implications for selection. Tree Genet. Genomes 2011, 7, 563–571. [Google Scholar] [CrossRef]
- McLean, J.P.; Jin, G.; Brennan, M.; Nieuwoudt, M.K.; Harris, P.J. Using NIR and ATR-FTIR spectroscopy to rapidly detect compression wood in Pinus radiata. Can. J. For. Res. 2014, 44, 820–830. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavan, R.R.; Fahey, L.M.; Harris, P.J. Quantification of (1→4)-β-d-Galactans in Compression Wood Using an Immuno-Dot Assay. Plants 2015, 4, 29-43. https://doi.org/10.3390/plants4010029
Chavan RR, Fahey LM, Harris PJ. Quantification of (1→4)-β-d-Galactans in Compression Wood Using an Immuno-Dot Assay. Plants. 2015; 4(1):29-43. https://doi.org/10.3390/plants4010029
Chicago/Turabian StyleChavan, Ramesh R., Leona M. Fahey, and Philip J. Harris. 2015. "Quantification of (1→4)-β-d-Galactans in Compression Wood Using an Immuno-Dot Assay" Plants 4, no. 1: 29-43. https://doi.org/10.3390/plants4010029
APA StyleChavan, R. R., Fahey, L. M., & Harris, P. J. (2015). Quantification of (1→4)-β-d-Galactans in Compression Wood Using an Immuno-Dot Assay. Plants, 4(1), 29-43. https://doi.org/10.3390/plants4010029






