Integrated Omic Analyses Reveal Module Networks Regulating Growth and Bioactive Component Synthesis of Sophora tonkinensis via Calcium Modulation
Abstract
1. Introduction
2. Results
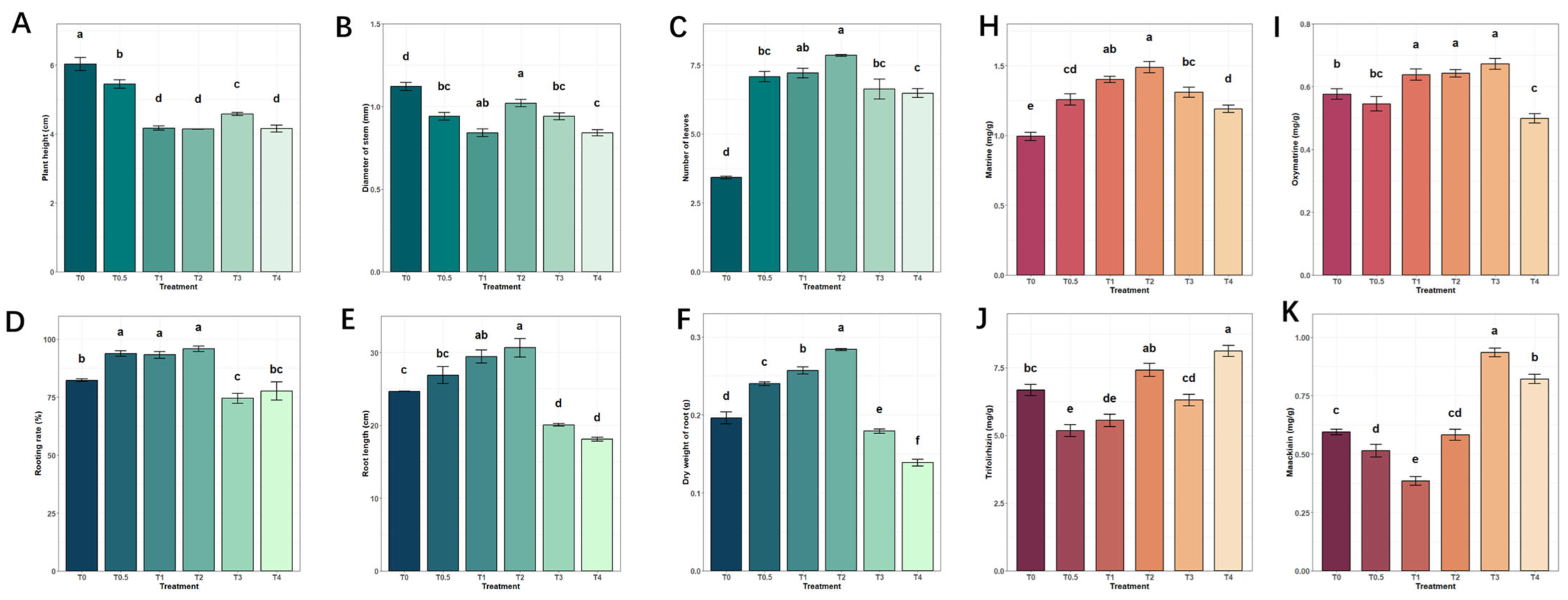
2.1. The Impact of Calcium on the Growth and Development of Tissue-Cultured Seedlings
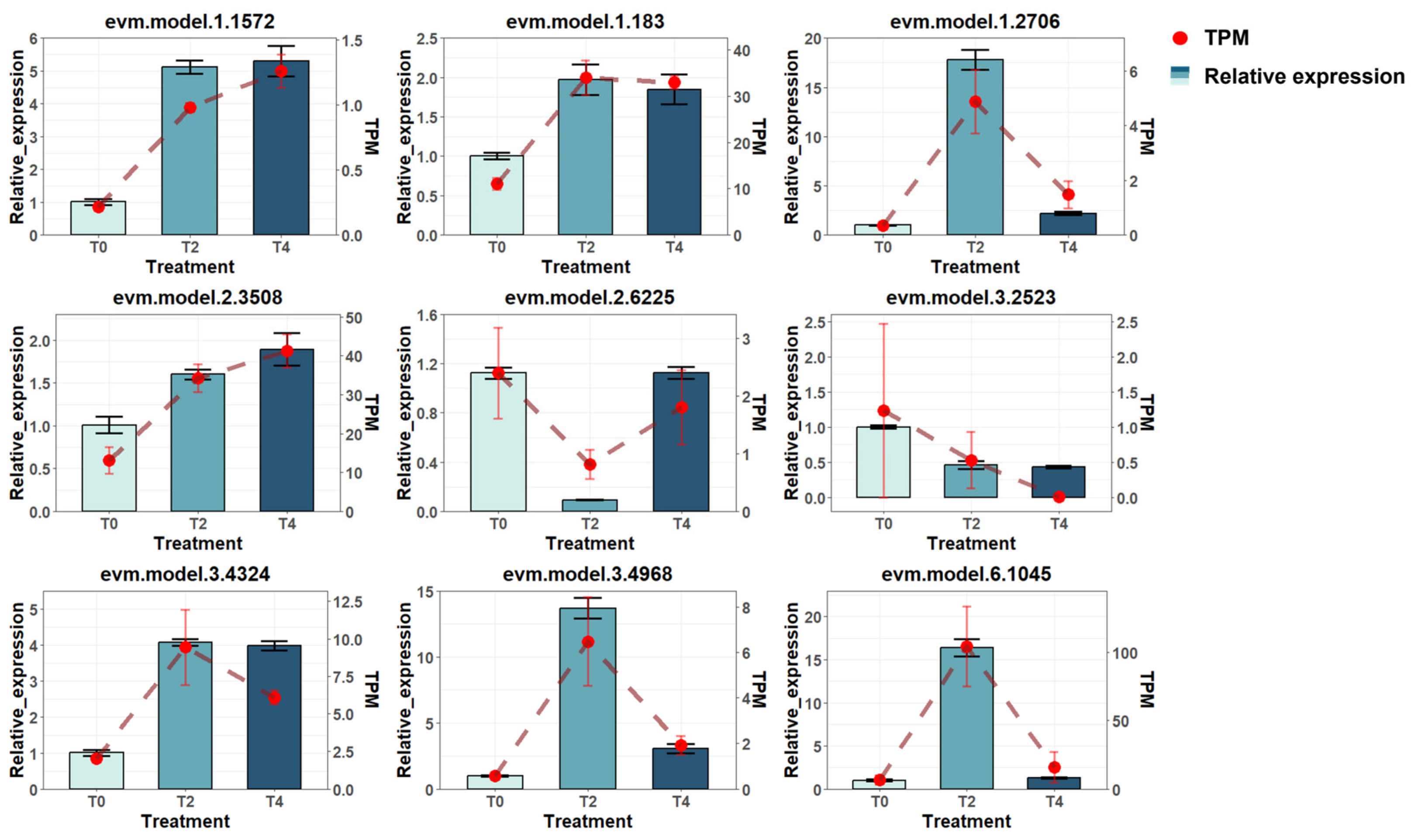
2.2. Initial Examination of Transcriptome Data
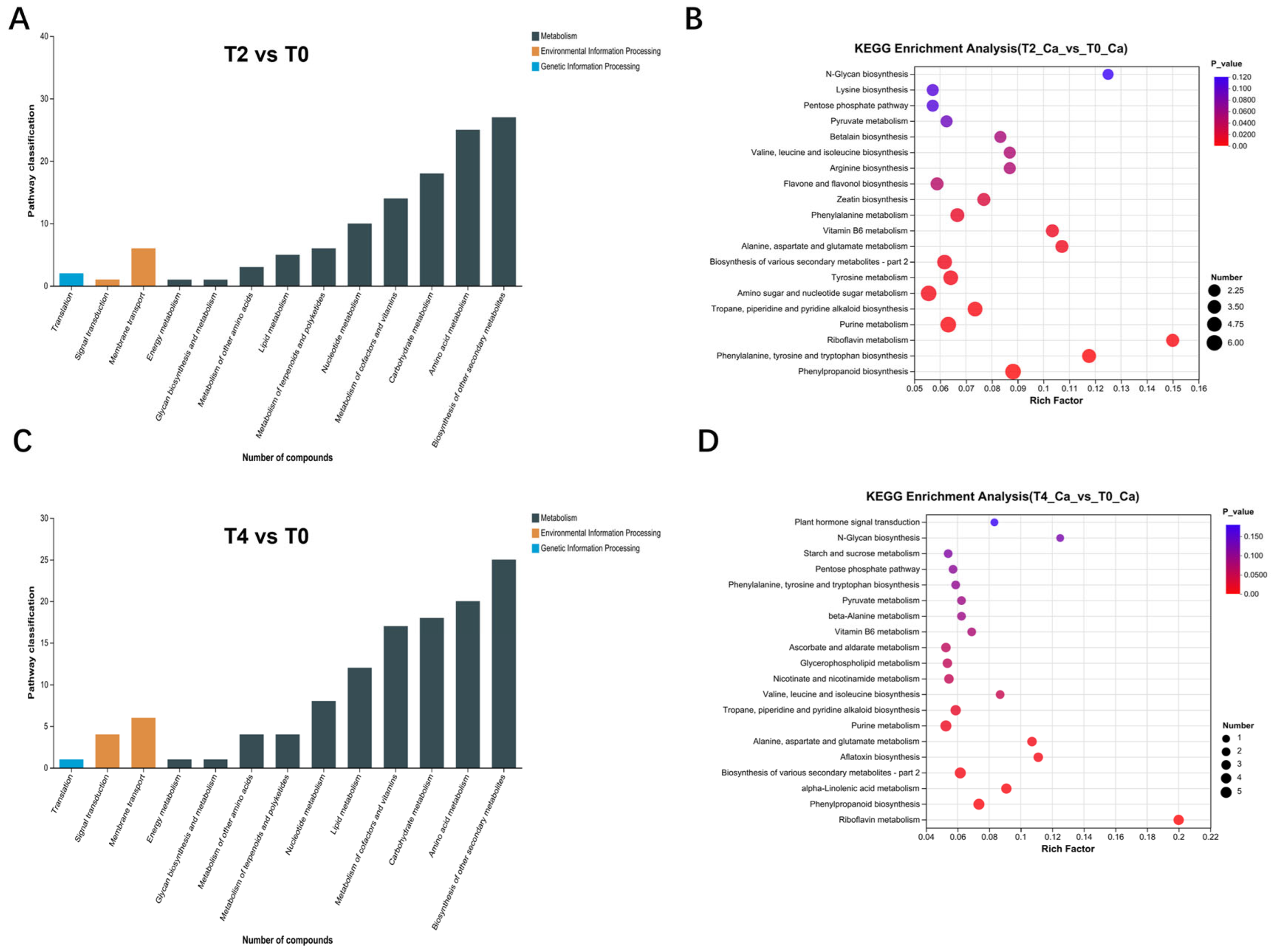
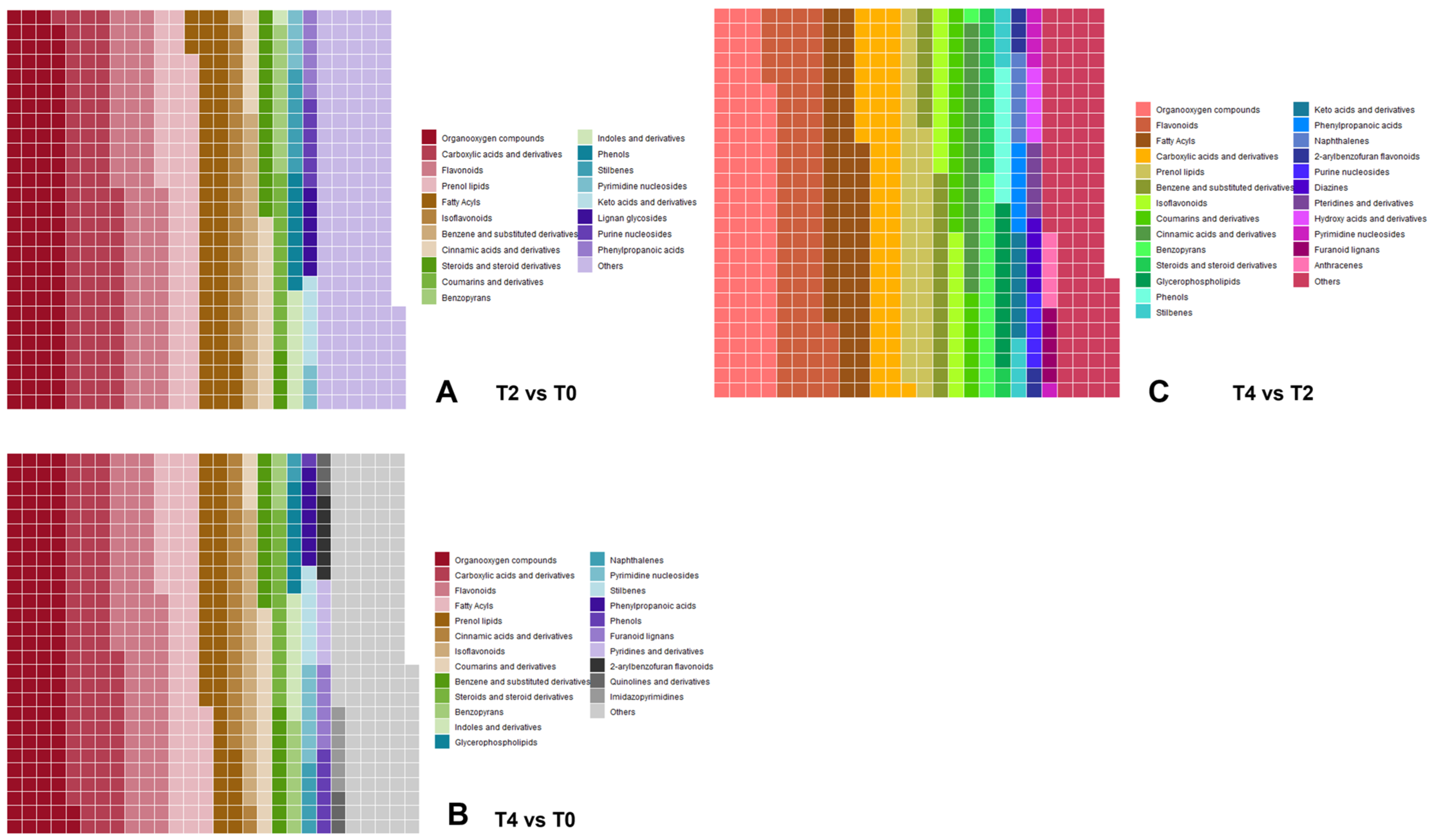
2.3. Initial Examination of Metabolome Data
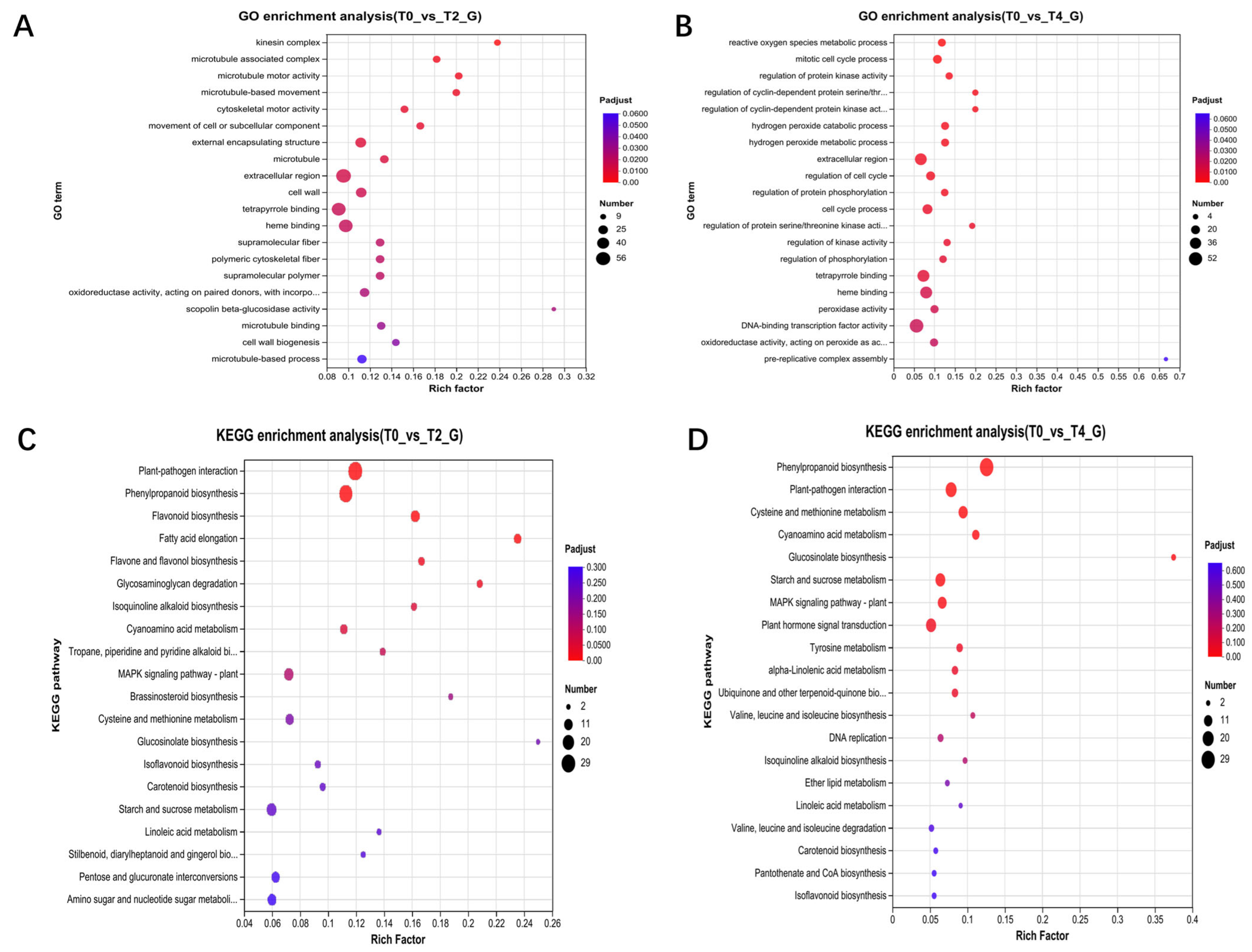
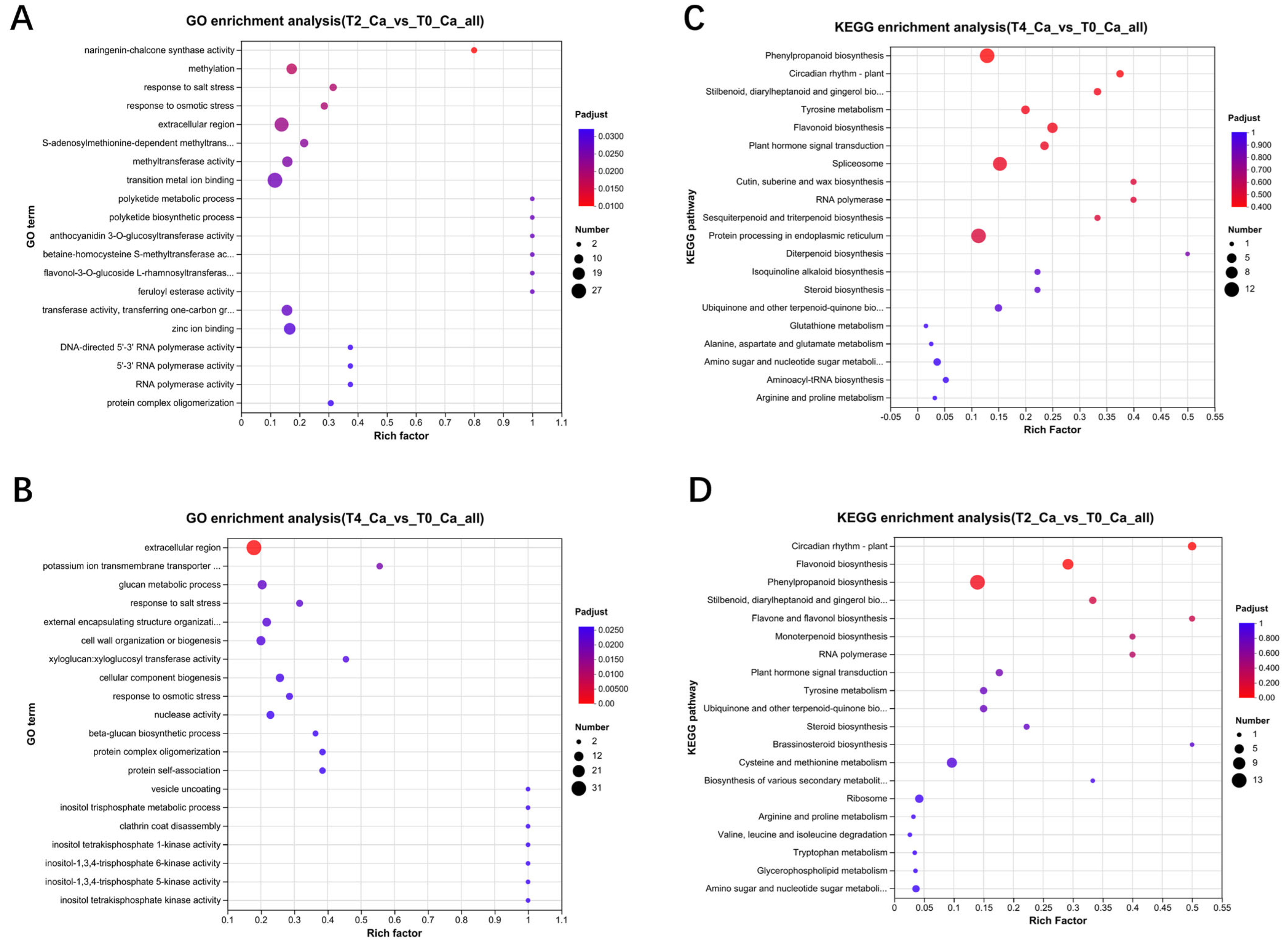
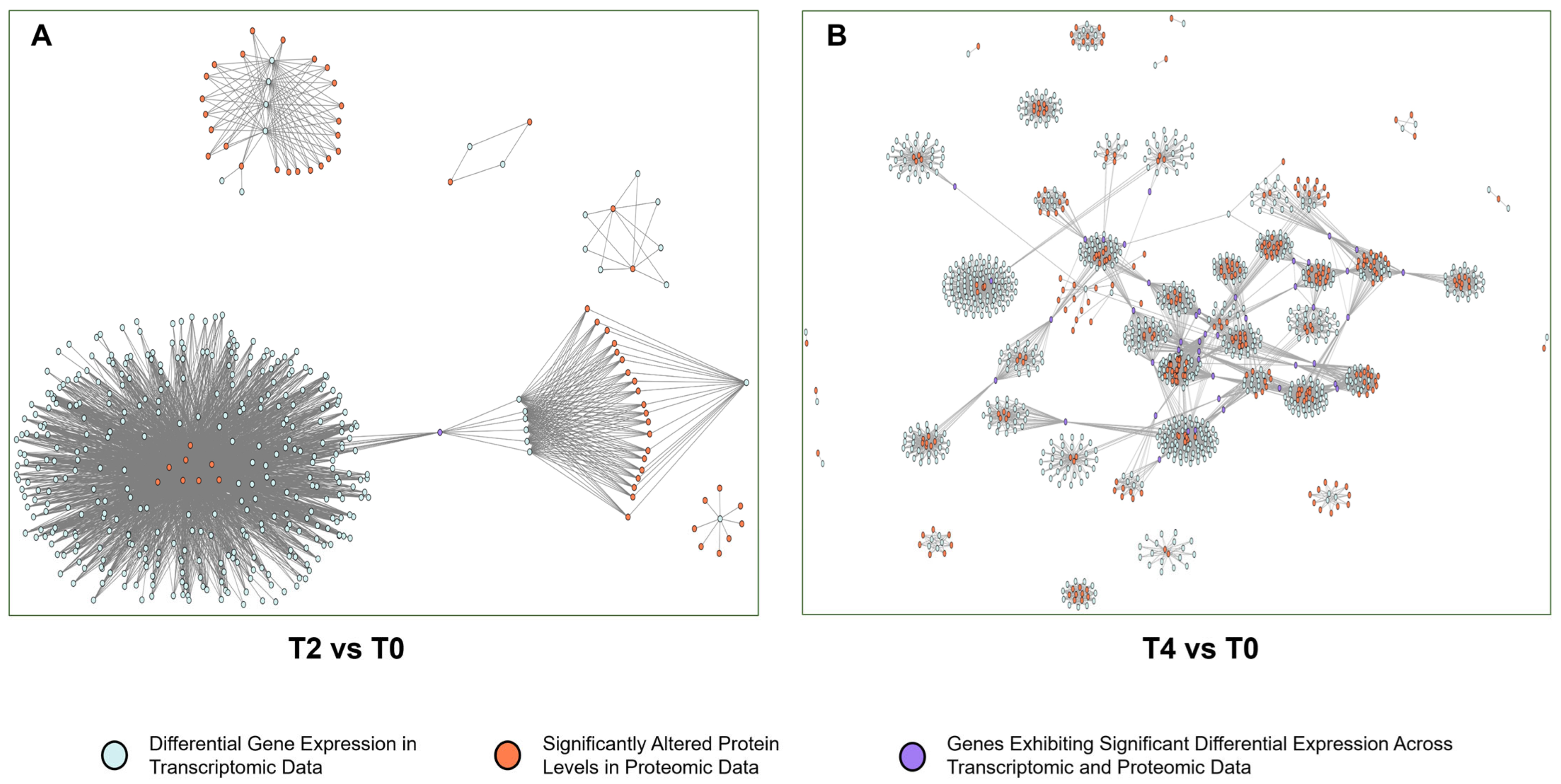
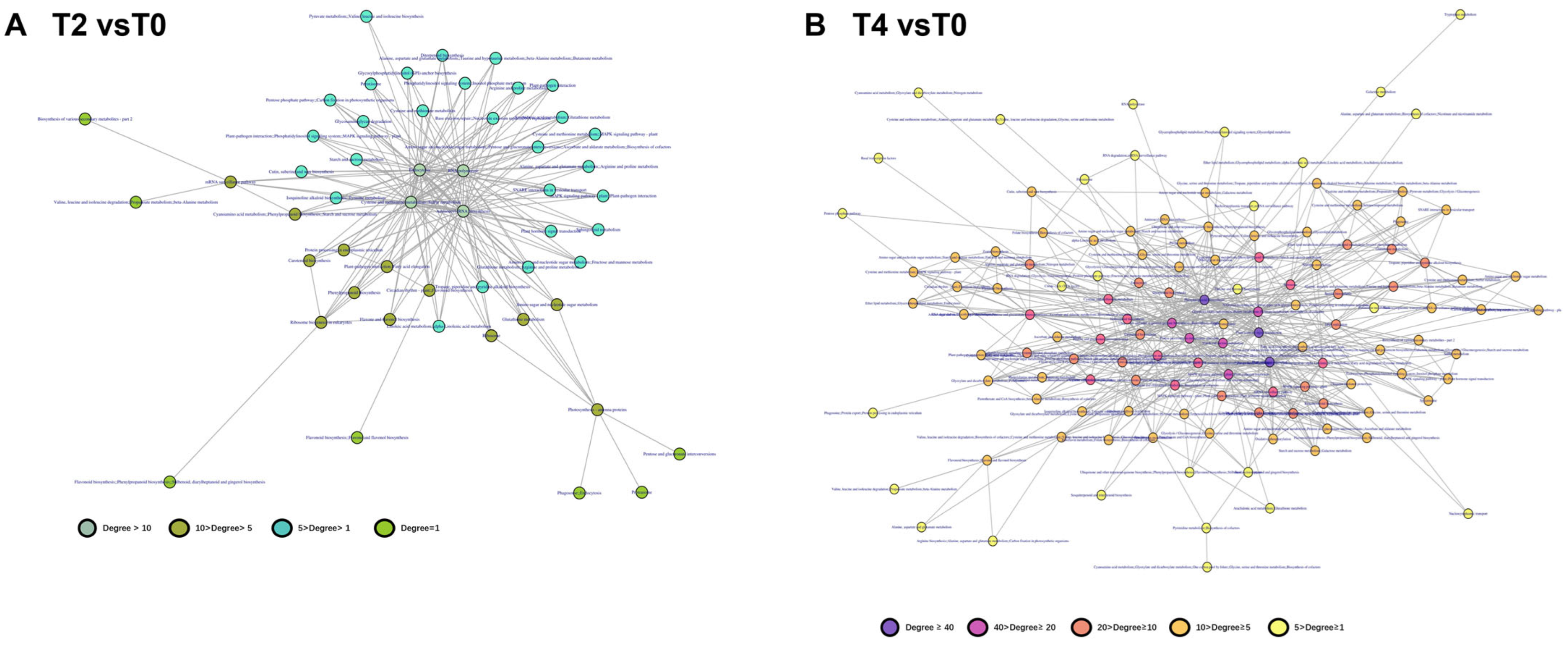
2.4. Multi-Omics Data Analyses
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Multi-Omics and Statistical Analyses
4.3. Determination of Growth Indicators
4.4. Determination of Pharmacological Substances
4.4.1. Determination of Matrine and Oxymatrine
4.4.2. Determination of Maackiain and Trifolirhizin
4.5. Collection of Omics Data
4.5.1. Transcriptome Data Collection
4.5.2. Protome Data Collection
4.5.3. Metabolomics Data Collection
4.6. Real-Time PCR
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DEGs | Differentially Expressed Genes |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GSEA | Gene Set Enrichment Analysis |
| ROS | Reactive Oxygen Species |
| HMDB | Human Metabolome Database |
| HPLC | High Performance Liquid Chromatography |
| IAA | 3-Indoleacetic acid |
| KT | Kinetin 6-Furfurylaminopurine |
| MAPK | Mitogen-activated Protein Kinase |
| TPM | Transcripts Per Million |
References
- Liang, Y.; Li, L.X.; Cai, J.Y.; Deng, C.H.; Qin, S.S.; Li, M.J.; Wei, K.H.; Zhang, Z.Y. Effect of exogenous Ca2+ on growth and accumulation of major components in tissue culture seedlings of Sophora tonkinensis gagnep. Pharmacogn. Mag. 2020, 16, 386–392. [Google Scholar]
- He, C.-M.; Cheng, Z.-H.; Chen, D.-F. Qualitative and quantitative analysis of flavonoids in Sophora tonkinensis by LC/MS and HPLC. Chin. J. Nat. Med. 2013, 11, 690–698. [Google Scholar] [CrossRef]
- Luan, S.; Wang, C. Calcium signaling mechanisms across kingdoms. Annu. Rev. Cell Dev. Biol. 2021, 37, 311–340. [Google Scholar] [CrossRef]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef]
- Pirayesh, N.; Giridhar, M.; Khedher, A.B.; Vothknecht, U.C.; Chigri, F. Organellar calcium signaling in plants: An update. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021, 1868, 118948. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Schiøtt, M.; Romanowsky, S.M.; Bækgaard, L.; Jakobsen, M.K.; Palmgren, M.G.; Harper, J.F. A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc. Natl. Acad. Sci. USA 2004, 101, 9502–9507. [Google Scholar] [CrossRef]
- Yuan, P.; Jauregui, E.; Du, L.; Tanaka, K.; Poovaiah, B.W. Calcium signatures and signaling events orchestrate plant–microbe interactions. Curr. Opin. Plant Biol. 2017, 38, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Zeng, W.; Bernard, S.; Liao, J.; Venkateshwaran, M.; Ane, J.-M.; Jiang, Y. Ca2+-regulated Ca2+ channels with an RCK gating ring control plant symbiotic associations. Nat. Commun. 2019, 10, 3703. [Google Scholar] [CrossRef]
- Toyota, M.; Spencer, D.; Sawai-Toyota, S.; Jiaqi, W.; Zhang, T.; Koo, A.J.; Howe, G.A.; Gilroy, S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 2018, 361, 1112–1115. [Google Scholar] [CrossRef]
- Farmer, E.E.; Gao, Y.Q.; Lenzoni, G.; Wolfender, J.L.; Wu, Q. Wound-and mechanostimulated electrical signals control hormone responses. New Phytol. 2020, 227, 1037–1050. [Google Scholar] [CrossRef]
- Tang, R.-J.; Luan, S. Regulation of calcium and magnesium homeostasis in plants: From transporters to signaling network. Curr. Opin. Plant Biol. 2017, 39, 97–105. [Google Scholar] [CrossRef]
- Wilkins, K.A.; Matthus, E.; Swarbreck, S.M.; Davies, J.M. Calcium-Mediated Abiotic Stress Signaling in Roots. Front. Plant Sci. 2016, 7, 1296. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, Y.; Huang, L.; Cui, X.; Liu, Y. From single-to multi-omics: Future research trends in medicinal plants. Brief. Bioinform. 2023, 24, bbac485. [Google Scholar] [CrossRef]
- He, S.; Yang, L.; Ye, S.; Lin, Y.; Li, X.; Wang, Y.; Chen, G.; Liu, G.; Zhao, M.; Zhao, X. MPOD: Applications of integrated multi-omics database for medicinal plants. Plant Biotechnol. J. 2022, 20, 797. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Meng, Y.; Wang, P.; Tang, Z.; Wang, H.; Xie, T. Bioinformatics-assisted, integrated omics studies on medicinal plants. Brief. Bioinform. 2020, 21, 1857–1874. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Y.; Adejobi, O.I.; Wang, Y.; Liu, A. Research Advances in Multi-Omics on the Traditional Chinese Herb Dendrobium officinale. Front. Plant Sci. 2022, 12, 808228. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Chen, C.; Gao, X.; Tan, C.; Bai, H.; Ning, K. Multi-omics profiling reveals comprehensive microbe–plant–metabolite regulation patterns for medicinal plant Glycyrrhiza uralensis Fisch. Plant Biotechnol. J. 2022, 20, 1874–1887. [Google Scholar] [CrossRef]
- Zhou, P.; Yin, M.; Dai, S.; Bao, K.; Song, C.; Liu, C.; Wu, Q. Multi-omics analysis of the bioactive constituents biosynthesis of glandular trichome in Perilla frutescens. BMC Plant Biol. 2021, 21, 277. [Google Scholar] [CrossRef]
- Li, C.; Wood, J.C.; Vu, A.H.; Hamilton, J.P.; Rodriguez Lopez, C.E.; Payne, R.M.E.; Serna Guerrero, D.A.; Yamamoto, K.; Vaillancourt, B.; Caputi, L. Single-cell multi-omics enabled discovery of alkaloid biosynthetic pathway genes in the medical plant Catharanthus roseus. bioRxiv 2022. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef]
- Sathyanarayanan, P.V.; Poovaiah, B.W. Decoding Ca2+ Signals in Plants. Crit. Rev. Plant Sci. 2004, 23, 1–11. [Google Scholar] [CrossRef]
- Zrimec, J.; Correa, S.; Zagorščak, M.; Petek, M.; Bleker, C.; Stare, K.; Schuy, C.; Sonnewald, S.; Gruden, K.; Nikoloski, Z. Evaluating plant growth–defense trade-offs by modeling the interaction between primary and secondary metabolism. Proc. Natl. Acad. Sci. USA 2025, 122, e2502160122. [Google Scholar] [CrossRef]
- Babalar, M.; Mumivand, H.; Hadian, J.; Tabatabaei, S.M.F. Effects of nitrogen and calcium carbonate on growth, rosmarinic acid content and yield of Satureja hortensis L. J. Agric. Sci. 2010, 2, 92. [Google Scholar] [CrossRef]
- Ullrich, S.F.; Rothauer, A.; Hagels, H.; Kayser, O. Influence of light, temperature, and macronutrients on growth and scopolamine biosynthesis in Duboisia species. Planta Medica 2017, 83, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, C.N.; Chatziartemiou, A.; Tsiknia, M.; Karyda, A.G.; Ehaliotis, C.; Gasparatos, D. Calcium- and Magnesium-Enriched Organic Fertilizer and Plant Growth-Promoting Rhizobacteria Affect Soil Nutrient Availability, Plant Nutrient Uptake, and Secondary Metabolite Production in Aloe vera (Aloe barbadensis Miller) Grown under Field Conditions. Agronomy 2023, 13, 482. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Jeyasri, R.; Muthuramalingam, P.; Karthick, K.; Shin, H.; Choi, S.H.; Ramesh, M. Methyl jasmonate and salicylic acid as powerful elicitors for enhancing the production of secondary metabolites in medicinal plants: An updated review. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 153, 447–458. [Google Scholar] [CrossRef]
- Shah, S.H.; Islam, S.; Mohammad, F. Sulphur as a dynamic mineral element for plants: A review. J. Soil Sci. Plant Nutr. 2022, 22, 2118–2143. [Google Scholar] [CrossRef]
- Aslam, S.; Gul, N.; Mir, M.A.; Asgher, M.; Al-Sulami, N.; Abulfaraj, A.A.; Qari, S. Role of Jasmonates, Calcium, and Glutathione in Plants to Combat Abiotic Stresses Through Precise Signaling Cascade. Front. Plant Sci. 2021, 12, 668029. [Google Scholar] [CrossRef]
- Gomez, L.D.; Noctor, G.; Knight, M.R.; Foyer, C.H. Regulation of calcium signalling and gene expression by glutathione. J. Exp. Bot. 2004, 55, 1851–1859. [Google Scholar] [CrossRef]
- Wang, Q.; Cang, X.; Yan, H.; Zhang, Z.; Li, W.; He, J.; Zhang, M.; Lou, L.; Wang, R.; Chang, M. Activating plant immunity: The hidden dance of intracellular Ca2+ stores. New Phytol. 2024, 242, 2430–2439. [Google Scholar] [CrossRef]
- Boudsocq, M.; Willmann, M.R.; McCormack, M.; Lee, H.; Shan, L.; He, P.; Bush, J.; Cheng, S.-H.; Sheen, J. Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 2010, 464, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.-W.; Chen, X.; Mei, Y. Function and regulation of phospholipid signalling in plants. Biochem. J. 2009, 421, 145–156. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, G.; Shi, C.; Liu, L.; Guo, Q.; Han, C.; Zhang, D.; Zhang, L.; Liu, B.; Gao, H. Majorbio Cloud: A one-stop, comprehensive bioinformatic platform for multiomics analyses. iMeta 2022, 1, e12. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. InterJournal Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef]
- Zhang, J. GseaVis: An Implement R Package to Visualize GSEA Results. 2022. Available online: https://github.com/junjunlab/GseaVis (accessed on 18 December 2024).
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.-F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
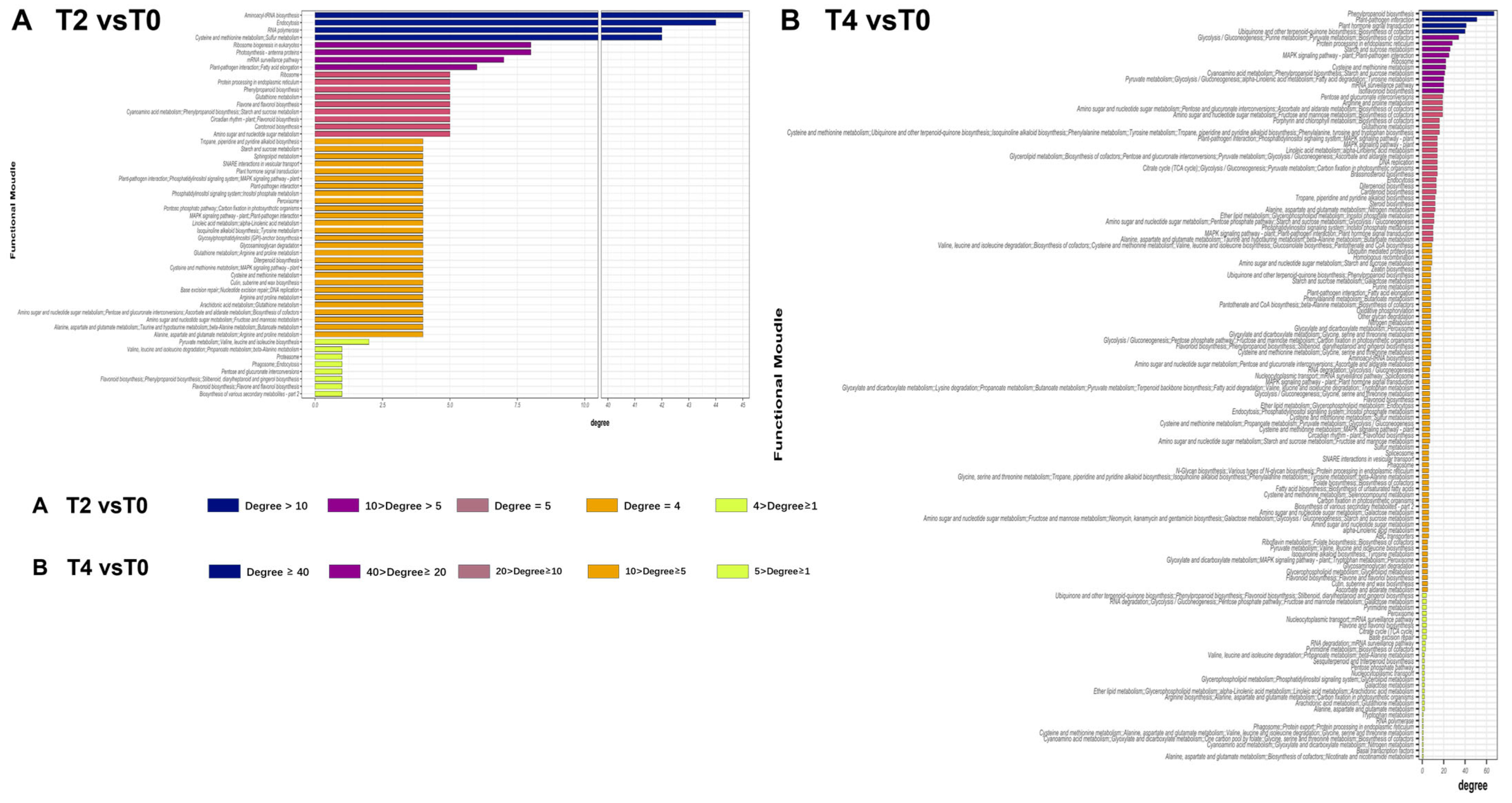
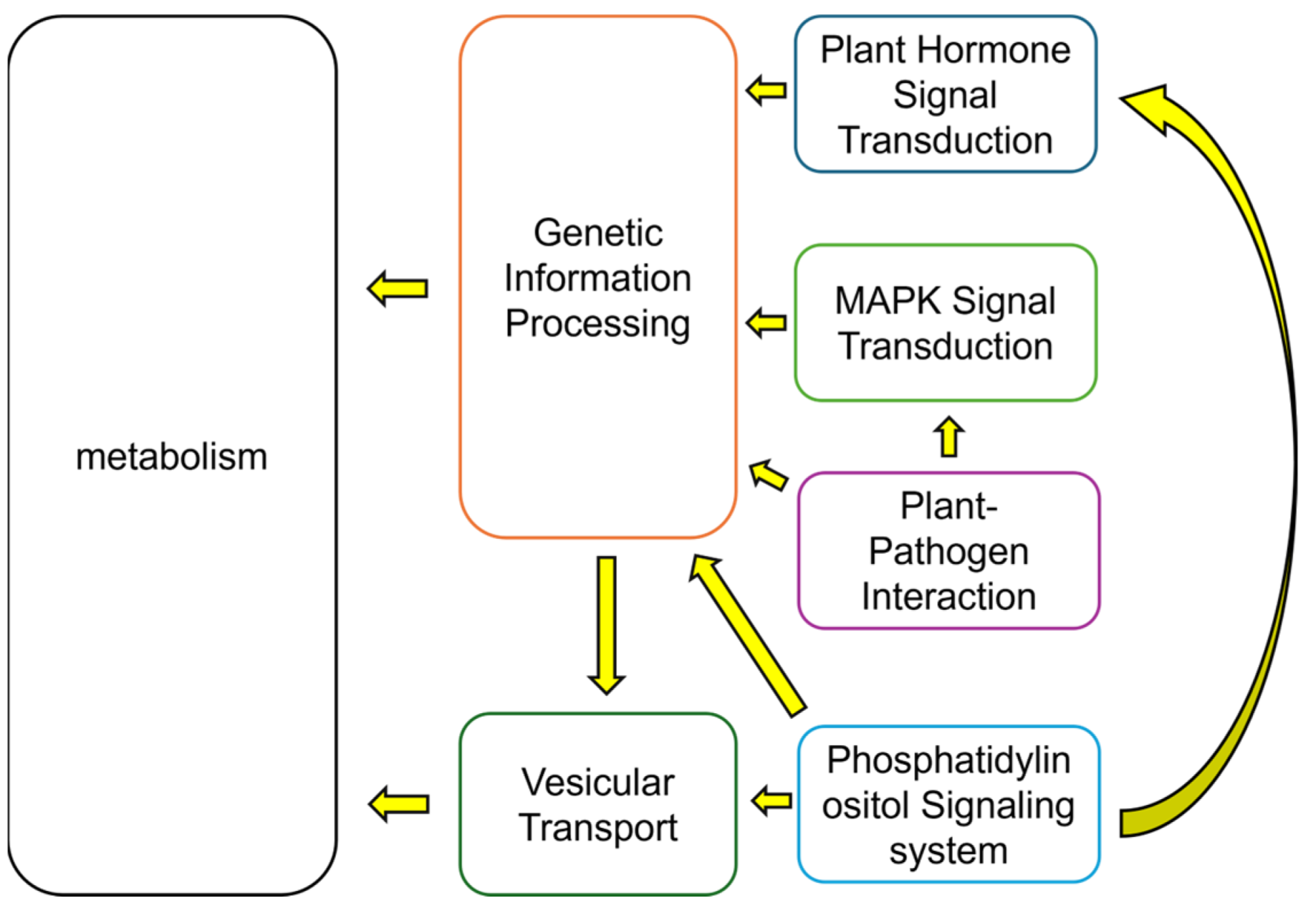
| Treatments | Calcium concentration |
|---|---|
| T0 | Ca2+ 0 mmol·L−1 |
| T0.5 | Ca2+ 0.748 mmol·L−1 |
| T1 | Ca2+ 1.495 mmol·L−1 |
| T2 | Ca2+ 2.99 mmol·L−1 |
| T3 | Ca2+ 4.485 mmol·L−1 |
| T4 | Ca2+ 5.98 mmol·L−1 |
| KEGG Pathway ID | KEGG Pathway |
|---|---|
| Map00196 | Photosynthesis—antenna proteins |
| Map04626 | Plant-pathogen interaction |
| Map00040 | Petose and glucuronate interconversions |
| Map00941 | Flavonoid biosynthesis |
| Map00531 | Glycosaminoglycan degradation |
| Map03010 | Ribosome |
| Map03050 | Proteasome |
| Map00280 | Valine, leucine and isoleucine degradation |
| Map04016 | MAPK signaling pathway—plant |
| Map00940 | Phenylpropanoid biosynthesis |
| Map00480 | Glutathione metabolism |
| Map00710 | Carbon fixation in photosynthetic organisms |
| Map00061 | Fatty acid biosynthesis |
| Map00030 | Pentose phosphate pathway |
| Map00944 | Flavone and flavonol biosynthesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Qiao, Z.; Fan, Z.-T.; Chen, L.-Y.; Li, L.-X.; Wei, F.; Qin, S.-S.; Wang, J.; Qin, B.; Liang, Y. Integrated Omic Analyses Reveal Module Networks Regulating Growth and Bioactive Component Synthesis of Sophora tonkinensis via Calcium Modulation. Plants 2026, 15, 133. https://doi.org/10.3390/plants15010133
Qiao Z, Fan Z-T, Chen L-Y, Li L-X, Wei F, Qin S-S, Wang J, Qin B, Liang Y. Integrated Omic Analyses Reveal Module Networks Regulating Growth and Bioactive Component Synthesis of Sophora tonkinensis via Calcium Modulation. Plants. 2026; 15(1):133. https://doi.org/10.3390/plants15010133
Chicago/Turabian StyleQiao, Zhu, Zhan-Tao Fan, Ling-Yun Chen, Ling-Xuan Li, Fan Wei, Shuang-Shuang Qin, Jing Wang, Ben Qin, and Ying Liang. 2026. "Integrated Omic Analyses Reveal Module Networks Regulating Growth and Bioactive Component Synthesis of Sophora tonkinensis via Calcium Modulation" Plants 15, no. 1: 133. https://doi.org/10.3390/plants15010133
APA StyleQiao, Z., Fan, Z.-T., Chen, L.-Y., Li, L.-X., Wei, F., Qin, S.-S., Wang, J., Qin, B., & Liang, Y. (2026). Integrated Omic Analyses Reveal Module Networks Regulating Growth and Bioactive Component Synthesis of Sophora tonkinensis via Calcium Modulation. Plants, 15(1), 133. https://doi.org/10.3390/plants15010133






