Medicinal Plants Against Dental Caries: Research and Application of Their Antibacterial Properties
Abstract
1. Introduction
2. Search and Inclusion Methods
3. History and Evolution of Medicinal Plant Use in Dentistry
3.1. A Look at the History of Phytotherapy in Dentistry
3.2. Development of Plant-Based Treatments
4. Medicinal Plants and Extracts Used in Caries Treatment
5. Scientific Evidence: Clinical and Experimental Studies
6. Effectiveness and Safety of Plant-Based Therapies
6.1. Analysis of the Efficacy of Natural Therapies
6.2. Discussion and Comparison with Traditional Approaches
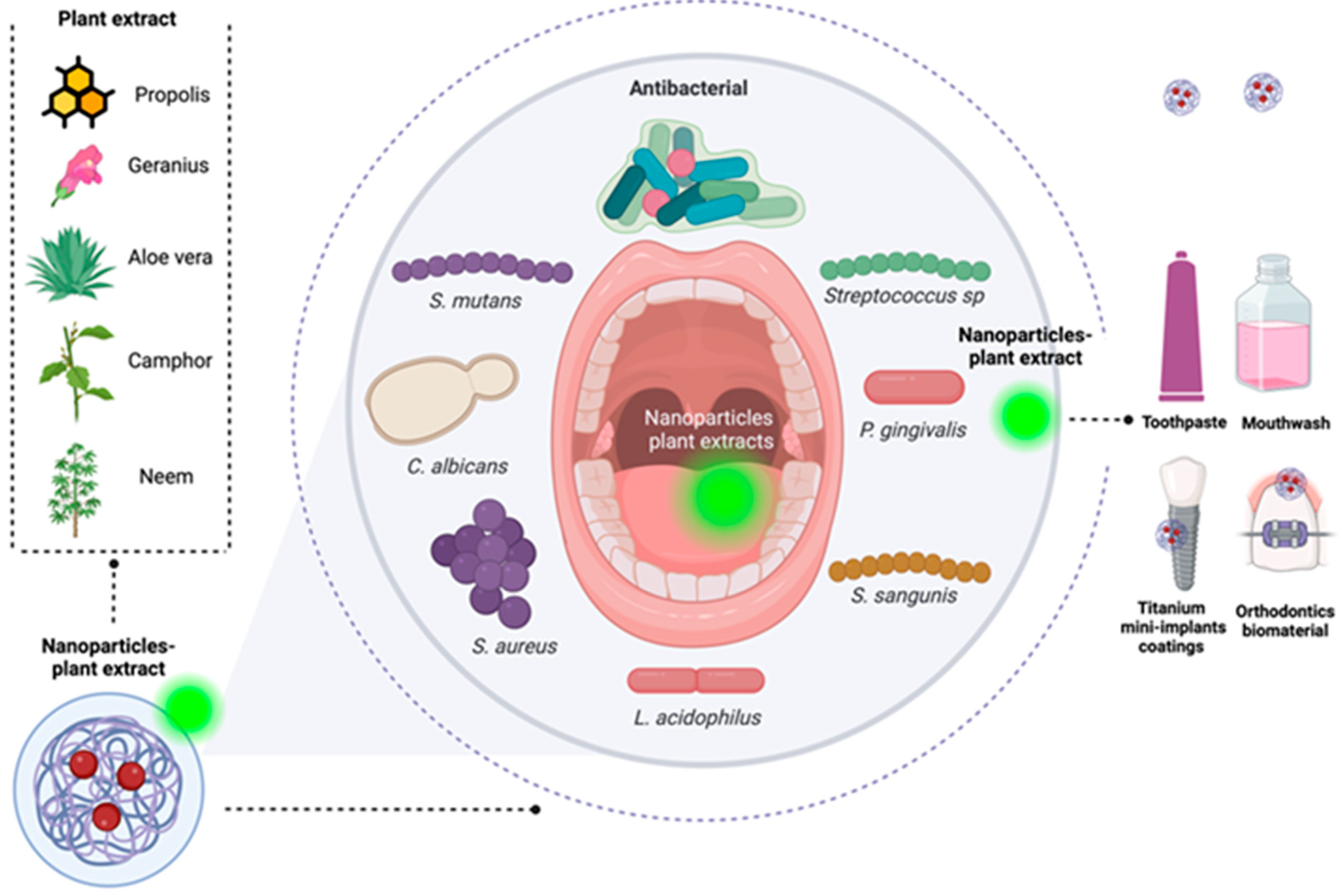
7. Plant-Based Nanoparticles and Coatings with Antibacterial Activity for Caries Treatment
7.1. Nanoparticles
7.2. Coatings
8. Future Perspectives and Challenges
9. Conclusions and Future Perspectives
10. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Homayouni Rad, A.; Pourjafar, H.; Mirzakhani, E. A Comprehensive Review of the Application of Probiotics and Postbiotics in Oral Health. Front. Cell. Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Daliri, E.B.-M.; Kim, N.; Kim, J.-R.; Yoo, D.; Oh, D.-H. Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The Virulence of Streptococcus mutans and the Ability to Form Biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Twetman, S.; Fisher, J.; Marsh, P.D. Understanding Dental Caries as a Non-Communicable Disease. Br. Dent. J. 2021, 231, 749–753. [Google Scholar] [CrossRef]
- Grabek-Lejko, D.; Hyrchel, T. The Antibacterial Properties of Polish Honey against Streptococcus mutans—A Causative Agent of Dental Caries. Antibiotics 2023, 12, 1640. [Google Scholar] [CrossRef]
- Mosaddad, S.A.; Tahmasebi, E.; Yazdanian, A.; Rezvani, M.B.; Seifalian, A.; Yazdanian, M.; Tebyanian, H. Oral Microbial Biofilms: An Update. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2005–2019. [Google Scholar] [CrossRef]
- Wolfoviz-Zilberman, A.; Kraitman, R.; Hazan, R.; Friedman, M.; Houri-Haddad, Y.; Beyth, N. Phage Targeting Streptococcus mutans In Vitro and In Vivo as a Caries-Preventive Modality. Antibiotics 2021, 10, 1015. [Google Scholar] [CrossRef]
- Ben-Zaken, H.; Kraitman, R.; Coppenhagen-Glazer, S.; Khalifa, L.; Alkalay-Oren, S.; Gelman, D.; Ben-Gal, G.; Beyth, N.; Hazan, R. Isolation and Characterization of Streptococcus mutans Phage as a Possible Treatment Agent for Caries. Viruses 2021, 13, 825. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhang, S.; Li, J.; Li, X.; Ying, Y.; Yuan, J.; Chen, K.; Deng, S.; Wang, Q. Association of Polymicrobial Interactions with Dental Caries Development and Prevention. Front. Microbiol. 2023, 14, 1162380. [Google Scholar] [CrossRef]
- Takenaka, S.; Ohsumi, T.; Noiri, Y. Evidence-Based Strategy for Dental Biofilms: Current Evidence of Mouthwashes on Dental Biofilm and Gingivitis. Jpn. Dent. Sci. Rev. 2019, 55, 33–40. [Google Scholar] [CrossRef]
- Chatzopoulos, G.S.; Karakostas, P.; Kavakloglou, S.; Assimopoulou, A.; Barmpalexis, P.; Tsalikis, L. Clinical Effectiveness of Herbal Oral Care Products in Periodontitis Patients: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 10061. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Kang, M.K. Biocompatibility and Antimicrobial Activity of Reynoutria elliptica Extract for Dental Application. Plants 2020, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Isola, G. Current Evidence of Natural Agents in Oral and Periodontal Health. Nutrients 2020, 12, 585. [Google Scholar] [CrossRef]
- Heliawati, L.; Lestari, S.; Hasanah, U.; Ajiati, D.; Kurnia, D. Phytochemical Profile of Antibacterial Agents from Red Betel Leaf (Piper crocatum Ruiz and Pav) against Bacteria in Dental Caries. Molecules 2022, 27, 2861. [Google Scholar] [CrossRef]
- Milia, E.P.; Sardellitti, L.; Eick, S. Antimicrobial Efficiency of Pistacia lentiscus L. Derivates against Oral Biofilm-Associated Diseases—A Narrative Review. Microorganisms 2023, 11, 1378. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Prakash, S.; Radha; Kumari, N.; Pundir, A.; Punia, S.; Saurabh, V.; Choudhary, P.; Changan, S.; Dhumal, S.; et al. Beneficial Role of Antioxidant Secondary Metabolites from Medicinal Plants in Maintaining Oral Health. Antioxidants 2021, 10, 1061. [Google Scholar] [CrossRef]
- Oluwasina, O.O.; Ezenwosu, I.V.; Ogidi, C.O.; Oyetayo, V.O. Antimicrobial Potential of Toothpaste Formulated from Extracts of Syzygium aromaticum, Dennettia tripetala and Jatropha curcas Latex against Some Oral Pathogenic Microorganisms. AMB Express 2019, 9, 20. [Google Scholar] [CrossRef]
- Yang, S.Y.; Choi, Y.R.; Lee, M.J.; Kang, M.K. Antimicrobial Effects against Oral Pathogens and Cytotoxicity of Glycyrrhiza uralensis Extract. Plants 2020, 9, 838. [Google Scholar] [CrossRef]
- Milutinovici, R.A.; Chioran, D.; Buzatu, R.; Macasoi, I.; Razvan, S.; Chioibas, R.; Corlan, I.V.; Tanase, A.; Horia, C.; Popovici, R.A.; et al. Vegetal Compounds as Sources of Prophylactic and Therapeutic Agents in Dentistry. Plants 2021, 10, 2148. [Google Scholar] [CrossRef]
- Delimont, N.M.; Carlson, B.N. Prevention of Dental Caries by Grape Seed Extract Supplementation: A Systematic Review. Nutr. Health 2020, 26, 43–52. [Google Scholar] [CrossRef]
- Singla, R.; Jaitak, V. Shatavari (Asparagus racemosus Wild): A Review on Its Cultivation, Morphology, Phytochemistry and Pharmacological Importance. Int. J. Pharm. Sci. Res. 2014, 5, 742. [Google Scholar] [CrossRef]
- Mishra, P.; Marwah, N.; Agarwal, N.; Chaturvedi, Y.; Suohu, T. Comparison of Punica granatum, Terminalia chebula, and Vitis vinifera Seed Extracts Used as Mouthrinse on Salivary Streptococcus mutans Levels in Children. J. Contemp. Dent. Pract. 2019, 20, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Coelho dos Santos, D.; da Silva Barboza, A.; Ribeiro, J.S.; Rodrigues Junior, S.A.; Campos, Â.D.; Lund, R.G. Bixa orellana L. (Achiote, Annatto) as an Antimicrobial Agent: A Scoping Review of Its Efficiency and Technological Prospecting. J. Ethnopharmacol. 2022, 287, 114961. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, R.; Sharma, M.; Kumar, M.; Barbhai, M.D.; Lorenzo, J.M.; Sharma, S.; Samota, M.K.; Atanassova, M.; Caruso, G.; et al. Carica papaya L. Leaves: Deciphering Its Antioxidant Bioactives, Biological Activities, Innovative Products, and Safety Aspects. Oxid. Med. Cell. Longev. 2022, 2022, 2451733. [Google Scholar] [CrossRef]
- Moghadam, E.T.; Yazdanian, M.; Tahmasebi, E.; Tebyanian, H.; Ranjbar, R.; Yazdanian, A.; Seifalian, A.; Tafazoli, A. Current Herbal Medicine as an Alternative Treatment in Dentistry: In Vitro, In Vivo and Clinical Studies. Eur. J. Pharmacol. 2020, 889, 173665. [Google Scholar] [CrossRef]
- Shaalan, O.; El-Rashidy, A. Antibacterial Effect of Miswak Herbal Toothpaste Compared to Fluoride Toothpaste in High Caries Risk Patients: Randomized Clinical Trial. J. Clin. Exp. Dent. 2023, 15, e526–e534. [Google Scholar] [CrossRef]
- Sedigh-Rahimabadi, M.; Fani, M.; Rostami-chijan, M.; Zarshenas, M.M.; Shams, M. A Traditional Mouthwash (Punica granatum var pleniflora) for Controlling Gingivitis of Diabetic Patients: A Double-Blind Randomized Controlled Clinical Trial. J. Evid.-Based Complement. Altern. Med. 2017, 22, 59–67. [Google Scholar] [CrossRef]
- Eltay, E.G.; Gismalla, B.G.; Mukhtar, M.M.; Awadelkarim, M.O.A. Punica Granatum Peel Extract as Adjunct Irrigation to Nonsurgical Treatment of Chronic Gingivitis: Punica granatum Peel Extract as Adjunct Oral Irrigation. Complement. Ther. Clin. Pract. 2021, 43, 101383. [Google Scholar] [CrossRef]
- Amanpour, S.; Javar, M.A.; Sarhadinejad, Z.; Doustmohammadi, M.; Moghadari, M.; Sarhadynejad, Z. A Systematic Review of Medicinal Plants and Herbal Products’ Effectiveness in Oral Health and Dental Cure with Health Promotion Approach. J. Educ. Health Promot. 2023, 12, 306. [Google Scholar] [CrossRef]
- Flemming, J.; Meyer-Probst, C.T.; Speer, K.; Kölling-Speer, I.; Hannig, C.; Hannig, M. Preventive Applications of Polyphenols in Dentistry—A Review. Int. J. Mol. Sci. 2021, 22, 4892. [Google Scholar] [CrossRef]
- Valadas, L.A.R.; Gurgel, M.F.; Mororó, J.M.; da Cruz Fonseca, S.G.; Fonteles, C.S.R.; de Carvalho, C.B.M.; Fechine, F.V.; Rodrigues Neto, E.M.; de França Fonteles, M.M.; Chagas, F.O.; et al. Dose-Response Evaluation of a Copaiba-Containing Varnish against Streptococcus mutans In Vivo. Saudi Pharm. J. 2019, 27, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Elgamily, H.; Safy, R.; Makharita, R. Influence of Medicinal Plant Extracts on the Growth of Oral Pathogens Strep Tococcus mutans and Lactobacillus acidophilus: An In-Vitro Study. Open Access Maced. J. Med. Sci. 2019, 7, 2328–2334. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, Ê.S.; Ayres, V.F.S.; Oliveira, M.R.; Corrêa, G.M.; Takeara, R.; Guimarães, A.C.; Santiago, M.B.; Oliveira, T.A.S.; Martins, C.H.G.; Crotti, A.E.M.; et al. Anticariogenic Activity of Three Essential Oils from Brazilian Piperaceae. Pharmaceuticals 2022, 15, 972. [Google Scholar] [CrossRef]
- Pourmoslemi, S.; Larki-Harchegani, A.; Daneshyar, S.; Dastan, D.; Nili-Ahmadabadi, A.; Jazaeri, M. Antibacterial and Anti-Glucosyltransferase Activity of Verbascum speciosum against Cariogenic Streptococci. J. Pharmacopunct. 2023, 26, 139–146. [Google Scholar] [CrossRef]
- AlFawaz, Y.F. Antibacterial Efficacy of NanoCare, Fullerene (C60) Activated by UV Light, and Moringa oleifera against S. mutans and Bond Integrity of Composite Resin to Caries Affected Dentin. Photodiagnosis Photodyn. Ther. 2024, 45, 103926. [Google Scholar] [CrossRef]
- Mandava, K.; Batchu, U.R.; Kakulavaram, S.; Repally, S.; Chennuri, I.; Bedarakota, S.; Sunkara, N. Design and Study of Anticaries Effect of Different Medicinal Plants against S. mutans glucosyltransferase. BMC Complement. Altern. Med. 2019, 19, 197. [Google Scholar] [CrossRef]
- Khoramian Tusi, S.; Jafari, A.; Marashi, S.M.A.; Faramarzi Niknam, S.; Farid, M.; Ansari, M. The Effect of Antimicrobial Activity of Teucrium polium on Oral Streptococcus mutans: A Randomized Cross-over Clinical Trial Study. BMC Oral. Health 2020, 20, 130. [Google Scholar] [CrossRef]
- Ancuceanu, R.; Anghel, A.I.; Ionescu, C.; Hovaneț, M.V.; Cojocaru-Toma, M.; Dinu, M. Clinical Trials with Herbal Products for the Prevention of Dental Caries and Their Quality: A Scoping Study. Biomolecules 2019, 9, 884. [Google Scholar] [CrossRef]
- Shekar, C.; Nagarajappa, R.; Singh, R.; Thakur, R. Antimicrobial Efficacy of Acacia Nilotica, Murraya koenigii L. Sprengel, Eucalyptus Hybrid, and Psidium guajava on Primary Plaque Colonizers: An In Vitro Comparison between Hot and Cold Extraction Process. J. Indian. Soc. Periodontol. 2015, 19, 174–181. [Google Scholar] [CrossRef]
- Martíınez, C.C.; Gómez, M.D.; Oh, M.S. Use of Traditional Herbal Medicine as an Alternative in Dental Treatment in Mexican Dentistry: A Review. Pharm. Biol. 2017, 55, 1992–1998. [Google Scholar] [CrossRef]
- Muddathir, A.M.; Mohieldin, E.A.M.; Mitsunaga, T. In Vitro Activities of Acacia nilotica (L.) Delile Bark Fractions against Oral Bacteria, Glucosyltransferase and as Antioxidant. BMC Complement. Med. Ther. 2020, 20, 360. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.; Jeyakumar, E.; Gupta, A. Antibacterial Activity of Acacia arabica (Bark) Extract against Selected Multi Drug Resistant Pathogenic Bacteria. Int. J. Curr. Microbiol. Appl. Sci. 2015, 1, 213–222. [Google Scholar]
- Magryś, A.; Olender, A.; Tchórzewska, D. Antibacterial Properties of Allium sativum L. against the Most Emerging Multidrug-Resistant Bacteria and Its Synergy with Antibiotics. Arch. Microbiol. 2021, 203, 2257–2268. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.S.; Shaukat, M.S.; Qureshi, A.A.; Abdur, R. Comparative Effectiveness of Chewing Stick and Toothbrush: A Randomized Clinical Trial. N. Am. J. Med. Sci. 2014, 6, 333–337. [Google Scholar] [CrossRef]
- Pachava, S.; Chandu, V.C.; Yaddanapalli, S.C.; Dasari, A.B.; Assaf, H.M. Comparing Caries Experience between Azadirachta indica Chewing Stick Users and Toothbrush Users among 35–44-Year-Old Rural Population of Southern India. J. Int. Soc. Prev. Community Dent. 2019, 9, 417–422. [Google Scholar] [CrossRef]
- Kazemipoor, M.; Fadaei Tehrani, P.; Zandi, H.; Golvardi Yazdi, R. Chemical Composition and Antibacterial Activity of Berberis vulgaris (Barberry) against Bacteria Associated with Caries. Clin. Exp. Dent. Res. 2021, 7, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Anita, P.; Balan, I.N.; Ethiraj, S.; Madan Kumar, P.; Sivasamy, S. In Vitro Antibacterial Activity of Camellia sinensis Extract against Cariogenic Microorganisms. J. Basic. Clin. Pharm. 2015, 6, 35. [Google Scholar] [CrossRef]
- Hattarki, S.A.; Bogar, C.; Bhat, K.G. Green Tea Catechins Showed Antibacterial Activity on Streptococcus mutans—An In Vitro Study. Indian J. Dent. Res. 2021, 32, 226–229. [Google Scholar] [CrossRef]
- Shetty, S.B.; Mahin-Syed-Ismail, P.; Varghese, S.; Thomas-George, B.; Kandathil-Thajuraj, P.; Baby, D.; Haleem, S.; Sreedhar, S.; Devang-Divakar, D. Antimicrobial Effects of Citrus sinensis Peel Extracts against Dental Caries Bacteria: An In Vitro Study. J. Clin. Exp. Dent. 2016, 8, e71–e77. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Medical Importance of Cichorium intybus—A Review. IOSR J. Pharm. 2016, 6, 41–56. [Google Scholar]
- Baqueta, M.R.; Diniz, P.H.G.D.; Pereira, L.L.; Almeida, F.L.C.; Valderrama, P.; Pallone, J.A.L. An Overview on the Brazilian Coffea canephora Scenario and the Current Chemometrics-Based Spectroscopic Research. Food Res. Int. 2024, 194, 114866. [Google Scholar] [CrossRef] [PubMed]
- Abrão, F.; Alves, J.A.; Andrade, G.; de Oliveira, P.F.; Ambrósio, S.R.; Veneziani, R.C.S.; Tavares, D.C.; Bastos, J.K.; Martins, C.H.G. Antibacterial Effect of Copaifera duckei Dwyer Oleoresin and Its Main Diterpenes against Oral Pathogens and Their Cytotoxic Effect. Front. Microbiol. 2018, 9, 201. [Google Scholar] [CrossRef]
- Freires, I.A.; Denny, C.; Benso, B.; De Alencar, S.M.; Rosalen, P.L. Antibacterial Activity of Essential Oils and Their Isolated Constituents against Cariogenic Bacteria: A Systematic Review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef]
- Bist, M.; Baboo Prasad, S. Embelia Ribes: A Valuable Medicinal Plant. J. Chem. Pharm. Res. 2016, 8, 1229–1233. [Google Scholar]
- Balhaddad, A.A.; AlSheikh, R.N. Effect of Eucalyptus Oil on Streptococcus mutans and Enterococcus faecalis Growth. BDJ Open 2023, 9, 26. [Google Scholar] [CrossRef]
- Landeo-Villanueva, G.E.; Salazar-Salvatierra, M.E.; Ruiz-Quiroz, J.R.; Zuta-Arriola, N.; Jarama-Soto, B.; Herrera-Calderon, O.; Pari-Olarte, J.B.; Loyola-Gonzales, E. Inhibitory Activity of Essential Oils of Mentha spicata and Eucalyptus globulus on Biofilms of Streptococcus mutans in an In Vitro Model. Antibiotics 2023, 12, 369. [Google Scholar] [CrossRef]
- Mirpour, M.; Gholizadeh Siahmazgi, Z.; Sharifi Kiasaraie, M. Antibacterial Activity of Clove, Gall Nut Methanolic and Ethanolic Extracts on Streptococcus mutans PTCC 1683 and Streptococcus salivarius PTCC 1448. J. Oral. Biol. Craniofac Res. 2015, 5, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Kurz, H.; Karygianni, L.; Argyropoulou, A.; Hellwig, E.; Skaltsounis, A.L.; Wittmer, A.; Vach, K.; Al-Ahmad, A. Antimicrobial Effects of Inula viscosa Extract on the In Situ Initial Oral Biofilm. Nutrients 2021, 13, 4029. [Google Scholar] [CrossRef]
- Verma, G.; Sharma, V. A Scientific Update on Juglans regia Linn. Asian J. Pharm. Res. Dev. 2020, 8, 166–175. [Google Scholar] [CrossRef]
- Carrol, D.H.; Chassagne, F.; Dettweiler, M.; Quave, C.L. Antibacterial Activity of Plant Species Used for Oral Health against Porphyromonas gingivalis. PLoS ONE 2020, 15, e0239316. [Google Scholar] [CrossRef]
- De Assis, E.L.; Silveira, F.D.; Da Ponte, A.V.A.; Regis, R.R. A Systematic Review of the Potential Effects of Lippia sidoides on Dental Plaque and Periodontal Diseases. Planta Med. 2022, 88, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Shah, G.; Singh, D.; Baghel, U.S. Isolation and Identification of Compounds from the Leaf Extract of Melaleuca alternifolia. Pharmacogn. J. 2017, 9, s52–s55. [Google Scholar] [CrossRef]
- Besra, M.; Kumar, V. Antimicrobial Activity of Essential Oils and Herbal Extracts against Etiological Agent of Dental Caries. J. Essent. Oil Bear. Plants 2016, 19, 1807–1815. [Google Scholar] [CrossRef]
- Mekhemar, M.; Hassan, Y.; Dörfer, C. Nigella sativa and Thymoquinone: A Natural Blessing for Periodontal Therapy. Antioxidants 2020, 9, 1260. [Google Scholar] [CrossRef]
- Herdiyati, Y.; Astrid, Y.; Shadrina, A.A.N.; Wiani, I.; Satari, M.H.; Kurnia, D. Potential Fatty Acid as Antibacterial Agent against Oral Bacteria of Streptococcus mutans and Streptococcus sanguinis from Basil (Ocimum americanum): In Vitro and In Silico Studies. Curr. Drug Discov. Technol. 2020, 18, 532–541. [Google Scholar] [CrossRef]
- Pai, K.R.; Pallavi, L.K.; Bhat, S.S.; Hegde, S.K. Evaluation of Antimicrobial Activity of Aqueous Extract of “Ocimum Sanctum-Queen of Herb” on Dental Caries Microorganisms: An In Vitro Study. Int. J. Clin. Pediatr. Dent. 2022, 15, S176–S179. [Google Scholar] [CrossRef]
- Shetty, S.; Shetty, R.M.; Rahman, B.; Vannala, V.; Desai, V.; Shetty, S.R. Efficacy of Psidium guajava and Allium sativum Extracts as Antimicrobial Agents against Periodontal Pathogens. J. Pharm. Bioallied Sci. 2020, 12, S589–S594. [Google Scholar] [CrossRef]
- Mohammadi-Sichani, M.; Karbasizadeh, V.; Dokhaharani, S.C. Evaluation of Biofilm Removal Activity of Quercus infectoria Galls against Streptococcus mutans. Dent. Res. J. 2016, 13, 46–51. [Google Scholar] [CrossRef]
- De Oliveira, J.R.; Camargo, S.E.A.; De Oliveira, L.D. Rosmarinus officinalis L. (Rosemary) as Therapeutic and Prophylactic Agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef]
- Günther, M.; Karygianni, L.; Argyropoulou, A.; Anderson, A.C.; Hellwig, E.; Skaltsounis, A.L.; Wittmer, A.; Vach, K.; Al-Ahmad, A. The Antimicrobial Effect of Rosmarinus officinalis Extracts on Oral Initial Adhesion Ex Vivo. Clin. Oral. Investig. 2022, 26, 4369–4380. [Google Scholar] [CrossRef]
- Al-Dabbagh, S.A.; Qasim, H.J.; Al-Derzi, N.A. Efficacy of Miswak Toothpaste and Mouthwash on Cariogenic Bacteria. Saudi Med. J. 2016, 37, 1009–1014. [Google Scholar] [CrossRef]
- Khan, M.; Alkhathlan, H.Z.; Khan, S.T. Antibiotic and Antibiofilm Activities of Salvadora persica L. Essential Oils against Streptococcus mutans: A Detailed Comparative Study with Chlorhexidine Digluconate. Pathogens 2020, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Nordin, A.; Bin Saim, A.; Ramli, R.; Abdul Hamid, A.; Mohd Nasri, N.W.; Idrus, R.B.H. Miswak and Oral Health: An Evidence-Based Review. Saudi J. Biol. Sci. 2020, 27, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Phumat, P.; Khongkhunthian, S.; Wanachantararak, P.; Okonogi, S. Effects of Piper Betle Fractionated Extracts on Inhibition of Streptococcus mutans and Streptococcus intermedius. Drug Discov. Ther. 2018, 12, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Rathinamoorthy, R.; Thilagavathi, G. Terminalia chebula-Review on Pharmacological and Biochemical Studies. Int. J. PharmTech Res. 2014, 6, 97–116. [Google Scholar]
- Herman, A.; Młynarczyk, A. Essential Oils and Plant Extracts with Activity against Oral Microorganisms: Prevention and Treatment of Oral Diseases. Isr. J. Plant Sci. 2015, 62, 250–257. [Google Scholar] [CrossRef]
- Woźniewicz, M.; Nowaczyk, P.M.; Kurhańska-Flisykowska, A.; Wyganowska-Świątkowska, M.; Lasik-Kurdyś, M.; Walkowiak, J.; Bajerska, J. Consumption of Cranberry Functional Beverage Reduces Gingival Index and Plaque Index in Patients with Gingivitis. Nutr. Res. 2018, 58, 36–45. [Google Scholar] [CrossRef]
- Zare Javid, A.; Hormoznejad, R.; Yousefimanesh, H.A.; Zakerkish, M.; Haghighi-zadeh, M.H.; Dehghan, P.; Ravanbakhsh, M. The Impact of Resveratrol Supplementation on Blood Glucose, Insulin, Insulin Resistance, Triglyceride, and Periodontal Markers in Type 2 Diabetic Patients with Chronic Periodontitis. Phytother. Res. 2017, 31, 108–114. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral Diseases: A Global Public Health Challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Singhal, R.; Agarwal, V.; Rastogi, P.; Khanna, R.; Tripathi, S. Efficacy of Acacia arabica Gum as an Adjunct to Scaling and Root Planing in the Treatment of Chronic Periodontitis: A Randomized Controlled Clinical Trial. Saudi Dent. J. 2018, 30, 53–62. [Google Scholar] [CrossRef]
- Sasi, M.; Kumar, S.; Kumar, M.; Thapa, S.; Prajapati, U.; Tak, Y.; Changan, S.; Saurabh, V.; Kumari, S.; Kumar, A.; et al. Garlic (Allium sativum L.) Bioactives and Its Role in Alleviating Oral Pathologies. Antioxidants 2021, 10, 1847. [Google Scholar] [CrossRef]
- Bin, C.; Al-Dhabi, N.A.; Esmail, G.A.; Arokiyaraj, S.; Arasu, M.V. Potential Effect of Allium sativum Bulb for the Treatment of Biofilm Forming Clinical Pathogens Recovered from Periodontal and Dental Caries. Saudi J. Biol. Sci. 2020, 27, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, T.; Krishnan, V.; Rajendran, R.; Madhusudhanan, N. Azadirachta indica: A Herbal Panacea in Dentistry—An Update. Pharmacogn. Rev. 2015, 9, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Pandit, N.; Changela, R.; Bali, D.; Tikoo, P.; Gugnani, S. Porphyromonas gingivalis: Its Virulence and Vaccine. J. Int. Clin. Dent. Res. Organ. 2015, 7, 51. [Google Scholar] [CrossRef]
- Passos, V.F.; de Melo, M.A.S.; Lima, J.P.M.; Marçal, F.F.; Costa, C.A.G.D.A.; Rodrigues, L.K.A.; Santiago, S.L. Active Compounds and Derivatives of Camellia sinensis Responding to Erosive Attacks on Dentin. Braz. Oral. Res. 2018, 32, e40. [Google Scholar] [CrossRef] [PubMed]
- Birsa, M.L.; Sarbu, L.G. Health Benefits of Key Constituents in Cichorium intybus L. Nutrients 2023, 15, 1322. [Google Scholar] [CrossRef]
- Tsou, S.H.; Hu, S.W.; Yang, J.J.; Yan, M.; Lin, Y.Y. Potential Oral Health Care Agent from Coffee against Virulence Factor of Periodontitis. Nutrients 2019, 11, 2235. [Google Scholar] [CrossRef]
- Yadav, M.; Kaushik, M.; Roshni, R.; Reddy, P.; Mehra, N.; Jain, V.; Rana, R. Effect of Green Coffee Bean Extract on Streptococcus mutans Count: A Randomised Control Trial. J. Clin. Diagn. Res. 2017, 11, ZC68–ZC71. [Google Scholar] [CrossRef]
- Karygianni, L.; Cecere, M.; Skaltsounis, A.L.; Argyropoulou, A.; Hellwig, E.; Aligiannis, N.; Wittmer, A.; Al-Ahmad, A. High-Level Antimicrobial Efficacy of Representative Mediterranean Natural Plant Extracts against Oral Microorganisms. Biomed. Res. Int. 2014, 2014, 839019. [Google Scholar] [CrossRef]
- Casarin, M.; Pazinatto, J.; Santos, R.C.V.; Zanatta, F.B. Melaleuca alternifolia and Its Application against Dental Plaque and Periodontal Diseases: A Systematic Review. Phytother. Res. 2018, 32, 230–242. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Naiktari, R.S.; Gaonkar, P.; Gurav, A.N.; Khiste, S.V. A Randomized Clinical Trial to Evaluate and Compare the Efficacy of Triphala Mouthwash with 0.2% Chlorhexidine in Hospitalized Patients with Periodontal Diseases. J. Periodontal Implant. Sci. 2014, 44, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Nowaczyk, P.M.; Bajerska, J.; Lasik-Kurdyś, M.; Radziejewska-Kubzdela, E.; Szwengiel, A.; Woźniewicz, M. The Effect of Cranberry Juice and a Cranberry Functional Beverage on the Growth and Metabolic Activity of Selected Oral Bacteria. BMC Oral. Health 2021, 21, 660. [Google Scholar] [CrossRef]
- Pellerin, G.; Bazinet, L.; Grenier, D. Deacidification of Cranberry Juice Reduces Its Antibacterial Properties against Oral Streptococci but Preserves Barrier Function and Attenuates the Inflammatory Response of Oral Epithelial Cells. Foods 2021, 10, 1634. [Google Scholar] [CrossRef]
- Mazur, M.; Ndokaj, A.; Jedlinski, M.; Ardan, R.; Bietolini, S.; Ottolenghi, L. Impact of Green Tea (Camellia Sinensis) on Periodontitis and Caries. Systematic Review and Meta-Analysis. Jpn. Dent. Sci. Rev. 2021, 57, 1–11. [Google Scholar] [CrossRef]
- Taweechaisupapong, S.; Pinsuwan, W.; Suwannarong, W.; Kukhetpitakwong, R.; Luengpailin, S. Effects of Streblus asper leaf extract on the biofilm formation of subgingival pathogens. S. Afr. J. Bot. 2014, 94, 1–5. [Google Scholar] [CrossRef]
- Joshi, A.; Sharma, A.; Bachheti, R.K.; Pandey, D.P. A Comparative Study of the Chemical Composition of the Essential Oil from Eucalyptus globulus Growing in Dehradun (India) and around the World. Orient. J. Chem. 2016, 32, 331–340. [Google Scholar] [CrossRef]
- Choi, H.A.; Cheong, D.E.; Lim, H.D.; Kim, W.H.; Ham, M.H.; Oh, M.H.; Wu, Y.; Shin, H.J.; Kim, G.J. Antimicrobial and Anti-Biofilm Activities of the Methanol Extracts of Medicinal Plants against Dental Pathogens Streptococcus mutans and Candida albicans. J. Microbiol. Biotechnol. 2017, 27, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.P. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Clinical Laboratory Standards Institute: Malvern, PA, USA, 2018; ISBN 1562388363. [Google Scholar]
- Alexander, B.D. Performance Standards for Antifungal Susceptibility Testing of Yeasts; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2017; ISBN 1562388282. [Google Scholar]
- Zini, A.; Mann, J.; Mazor, S.; Vered, Y. Beneficial Effect of Aged Garlic Extract on Periodontitis: A Randomized Controlled Doubleeblind Clinical Study. J. Clin. Biochem. Nutr. 2020, 67, 297–301. [Google Scholar] [CrossRef]
- Elheeny, A.A.H. Allium Sativum Extract as an Irrigant in Pulpectomy of Primary Molars: A 12-Month Short-Term Evaluation. Clin. Exp. Dent. Res. 2019, 5, 420–426. [Google Scholar] [CrossRef]
- Yikici, C.; Özcan, S. Remineralization Activities of Toothpastes with and without Aloe vera with Different Ratios of Fluoride on Demineralized Enamel. Niger. J. Clin. Pract. 2022, 25, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Penmetsa, G.; Pitta, S. Efficacy of Ocimum sanctum, Aloe vera and Chlorhexidine Mouthwash on Gingivitis: A Randomized Controlled Comparative Clinical Study. AYU (Int. Q. J. Res. Ayurveda) 2019, 40, 23. [Google Scholar] [CrossRef] [PubMed]
- Valones, M.A.A.; Silva, I.C.G.; Gueiros, L.A.M.; Leão, J.C.; Caldas, A.F.; Carvalho, A.A.T. Clinical Assessment of Rosemary-based Toothpaste (Rosmarinus officinalis Linn.): A Randomized Controlled Double-Blind Study. Braz. Dent. J. 2019, 30, 146–151. [Google Scholar] [CrossRef]
- Flores-Villa, E.; Sáenz-Galindo, A.; Castañeda-Facio, A.O.; Narro-Céspedes, R.I. Romero (Rosmarinus officinalis L.): Su Origen, Importancia y Generalidades de Sus Metabolitos Secundarios. TIP Rev. Espec. En. Cienc. Químico-Biológicas 2020, 23, e20200266. [Google Scholar] [CrossRef]
- Nadar, B.; Usha, G.; Lakshminarayan, N. Comparative Evaluation of Efficacy of 4% Tulsi Extract (Ocimum sanctum), Fluoridated and Placebo Dentifrices against Gingivitis and Plaque among 14–15 Years School Children in Davangere City, India-A Triple Blinded Randomized Clinical Trial. Contemp. Clin. Dent. 2020, 11, 67–75. [Google Scholar] [CrossRef]
- Annigeri, R.G.; Mangala, G.K.; Thimmasetty, J.; Sharma, N.; Kanjani, V.; Sravya, G. Evaluation of Tulasi Extract Mouthwash in the Management of Oral Candidiasis. J. Adv. Clin. Res. Insights 2018, 5, 30–34. [Google Scholar] [CrossRef]
- Halawany, H.S. A review on miswak (Salvadora persica) and its effect on various aspects of oral health. Saudi Dent. J. 2012, 24, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Sajadi, F.; Shokrizadeh, M.; Sharifi, M.; Aftabi, R. Evaluating the Effects of Camellia sinensis (Green Tea) and Teucrium polium Extracts on Salivary Streptococcus mutans Levels in Children. J. Dent. 2023, 24, 19–27. [Google Scholar] [CrossRef]
- Tafazoli, A.; Tafazoli Moghadam, E. Camellia sinensis Mouthwashes in Oral Care: A Systematic Review. J. Dent. 2020, 21, 249–262. [Google Scholar] [CrossRef]
- del López-Rodríguez, G.P. Evaluación in vitro del efecto antibacteriano de la Camellia sinensis (té verde) frente al Streptococcus mutans (ATCC 25175) y al Streptococcus sanguinis (ATCC) 10556). Bachelor’s Thesis, Universidad Peruana de Ciencias Aplicadas (UPC), Santiago de Surco, Peru, 2014. [Google Scholar]
- Lingström, P.; Zaura, E.; Hassan, H.; Buijs, M.J.; Hedelin, P.; Pratten, J.; Spratt, D.; Daglia, M.; Karbowiak, A.; Signoretto, C.; et al. The Anticaries Effect of a Food Extract (Shiitake) in a Short-Term Clinical Study. J. Biomed. Biotechnol. 2012, 2012, 217164. [Google Scholar] [CrossRef]
- Bezerra, M.S.; Zeferino, K.S.; Menezes, L.D.; Bezerra, A.S.; Lopes, L.Q.S.; Marquezan, F.K.; Marquezan, P.K. Antimicrobial and Antibiofilm Activities of Cichorium intybus: A Review. Res. Soc. Dev. 2022, 11, e10911225384. [Google Scholar] [CrossRef]
- Babaei, H.; Forouzandeh, F.; Maghsoumi-Norouzabad, L.; Yousefimanesh, H.A.; Ravanbakhsh, M.; Zare Javid, A. Effects of Chicory Leaf Extract on Serum Oxidative Stress Markers, Lipid Profile and Periodontal Status in Patients with Chronic Periodontitis. J. Am. Coll. Nutr. 2018, 37, 479–486. [Google Scholar] [CrossRef]
- Selvaraj, K.; Bharath, N.; Natarajan, R.; Dinesh, S.; Murugesan, S.; Selvaraj, S. Comparative Evaluation of Antimicrobial Efficacy of Toothpastes Containing Probiotic and Neem as Primary Ingredient on Salivary Streptococcus mutans in Melmaruvathur Population: An In Vivo Study. J. Pharm. Bioallied Sci. 2020, 12, S595–S600. [Google Scholar] [CrossRef] [PubMed]
- Taweechaisupapong, S.; Wongkham, S.; Chareonsuk, S.; Suparee, S.; Srilalai, P.; Chaiyarak, S. Selective activity of Streblus asper on Mutans streptococci. J Ethnopharmacol. 2020, 70, 73–79. [Google Scholar] [CrossRef]
- Suleiman, W.B. In Vitro Estimation of Superfluid Critical Extracts of Some Plants for Their Antimicrobial Potential, Phytochemistry, and GC-MS Analyses. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 29. [Google Scholar] [CrossRef]
- Cheng, L.; Li, J.; He, L.; Zhou, X. Natural Products and Caries Prevention. Caries Res. 2015, 49 (Suppl. S1), 38–45. [Google Scholar] [CrossRef]
- Furquim dos Santos Cardoso, V.; Amaral Roppa, R.H.; Antunes, C.; Silva Moraes, A.N.; Santi, L.; Konrath, E.L. Efficacy of Medicinal Plant Extracts as Dental and Periodontal Antibiofilm Agents: A Systematic Review of Randomized Clinical Trials. J. Ethnopharmacol. 2021, 281, 114541. [Google Scholar] [CrossRef]
- López-Villarreal, S.M.; Elizondo-Luévano, J.H.; Pérez-Hernández, R.A.; Sánchez-García, E.; Verde-Star, M.J.; Castro-Ríos, R.; Garza-Tapia, M.; Rodríguez-Luis, O.E.; Chávez-Montes, A. Preliminary Study of the Antimicrobial, Anticoagulant, Antioxidant, Cytotoxic, and Anti-Inflammatory Activity of Five Selected Plants with Therapeutic Application in Dentistry. Int. J. Environ. Res. Public Health 2022, 19, 7927. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.; Das, S.M. Antimicrobial Efficacy of Some Medicinal Plant Extract against Streptococcus mutans Causing Dental Caries. J. Med. Plants Stud. 2017, 5, 315–317. [Google Scholar]
- Chinsembu, K.C. Plants and Other Natural Products Used in the Management of Oral Infections and Improvement of Oral Health. Acta Trop. 2016, 154, 6–18. [Google Scholar] [CrossRef]
- Kermeoglu, F.; Aksoy, U.; Kalender, A.; Oztan, M.D.; Oguz, E.I.; Kıyan, M. Determination of the Minimum Inhibitory Concentrations of Alexidine and Chlorhexidine against Enterococcus faecalis and Candida albicans: An In Vitro Study. Cureus 2018, 10, e2221. [Google Scholar] [CrossRef] [PubMed]
- Kutsch, V.K. Dental Caries: An Updated Medical Model of Risk Assessment. J. Prosthet. Dent. 2014, 111, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.G.M.; Hashizume, L.N.; Maltz, M. The Effect of Different Formulations of Chlorhexidine in Reducing Levels of Mutans Streptococci in the Oral Cavity: A Systematic Review of the Literature. J. Dent. 2007, 35, 359–370. [Google Scholar] [CrossRef]
- Dong, L.; Tong, Z.; Linghu, D.; Lin, Y.; Tao, R.; Liu, J.; Tian, Y.; Ni, L. Effects of Sub-Minimum Inhibitory Concentrations of Antimicrobial Agents on Streptococcus mutans Biofilm Formation. Int. J. Antimicrob. Agents 2012, 39, 390–395. [Google Scholar] [CrossRef]
- Ahmed, O.A.K.; Sibuyi, N.R.S.; Fadaka, A.O.; Maboza, E.; Olivier, A.; Madiehe, A.M.; Meyer, M.; Geerts, G. Prospects of Using Gum Arabic Silver Nanoparticles in Toothpaste to Prevent Dental Caries. Pharmaceutics 2023, 15, 871. [Google Scholar] [CrossRef]
- Noronha, V.T.; Paula, A.J.; Durán, G.; Galembeck, A.; Cogo-Müller, K.; Franz-Montan, M.; Durán, N. Silver Nanoparticles in Dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.A.S.; Barbosa, D.B.; Berretta, A.A.; Do Amaral, J.G.; Gorup, L.F.; De Souza Neto, F.N.; Fernandes, R.A.; Fernandes, G.L.; Camargo, E.R.; Agostinho, A.M.; et al. Green Synthesis of Silver Nanoparticles Combined to Calcium Glycerophosphate: Antimicrobial and Antibiofilm Activities. Future Microbiol. 2018, 13, 345–357. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Emmanuel, R.; Palanisamy, S.; Chen, S.M.; Chelladurai, K.; Padmavathy, S.; Saravanan, M.; Prakash, P.; Ali, M.A.; Al-Hemaid, F.M.A. Antimicrobial Efficacy of Green Synthesized Drug Blended Silver Nanoparticles against Dental Caries and Periodontal Disease Causing Microorganisms. Mater. Sci. Eng. C 2015, 56, 374–379. [Google Scholar] [CrossRef]
- Chandana, C.S.; Gayathri, R.; Priya, V.V.; Geetha, R.V. Synthesis and Characterization of Silver Nano Particles from Plectranthus ambionicus Extract and Its Antimicrobial Activity against Enterococcus faecalis and Candida albicans. J. Pharm. Sci. Res. 2017, 9, 2423–2425. [Google Scholar]
- Yazdanian, M.; Rostamzadeh, P.; Rahbar, M.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Yazdanian, A. The Potential Application of Green-Synthesized Metal Nanoparticles in Dentistry: A Comprehensive Review. Bioinorg. Chem. Appl. 2022, 2022, 2311910. [Google Scholar] [CrossRef] [PubMed]
- Wassel, M.O.; Khattab, M.A. Antibacterial Activity against Streptococcus mutans and Inhibition of Bacterial Induced Enamel Demineralization of Propolis, Miswak, and Chitosan Nanoparticles Based Dental Varnishes. J. Adv. Res. 2017, 8, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Adapa, S. A Review on Silver Nanoparticles-The Powerful Nanoweapon against Oral Pathogens. Int. J. Oral Health Sci. 2020, 10, 6. [Google Scholar] [CrossRef]
- Acosta-Torres, L.S.; Mendieta, I.; Nuñez-Anita, R.E.; Cajero-Juárez, M.; Castaño, V.M. Cytocompatible Antifungal Acrylic Resin Containing Silver Nanoparticles for Dentures. Int. J. Nanomed. 2012, 7, 4777. [Google Scholar] [CrossRef]
- Hossain, N.; Mobarak, M.H.; Hossain, A.; Khan, F.; Mim, J.J.; Chowdhury, M.A. Advances of Plant and Biomass Extracted Zirconium Nanoparticles in Dental Implant Application. Heliyon 2023, 9, e15973. [Google Scholar] [CrossRef]
- Nikam, A.; Pagar, T.; Ghotekar, S.; Pagar, K.; Pansambal, S. A Review on Plant Extract Mediated Green Synthesis of Zirconia Nanoparticles and Their Miscellaneous Applications. J. Chem. Rev. 2019, 1, 154–163. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green Synthesis of Nanoparticles Using Plant Extracts: A Review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Sreenivasagan, S.; Subramanian, A.K.; Rajeshkumar, S. Assessment of Antimicrobial Activity and Cytotoxic Effect of Green Mediated Silver Nanoparticles and Its Coating onto Mini-Implants. Ann. Phytomedicine Int. J. 2020, 9, 207–212. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver Nanoparticles as Antimicrobial Agent: A Case Study on E. coli as a Model for Gram-Negative Bacteria. J. Colloid. Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Guevara Ruiz, L.M.; Bonilla Valladares, P.M.; Caicedo Breedy, M.F. Actividad Antimicrobiana de Adhesivo Ortodóntico Con Nanopartículas de Plata Sobre Streptococcus mutans. Odontologia 2020, 22, 33–44. [Google Scholar] [CrossRef]
- Alsamhary, K.E. Moringa oleifera Seed Based Green Synthesis of Copper Nanoparticles: Characterization, Environmental Remediation and Antimicrobial Activity. Saudi J. Biol. Sci. 2023, 30, 103820. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.C.; Rolim, W.R.; Viana, M.M.; Souza, T.R.; Gonçalves, F.; Tanaka, C.J.; Bueno-Silva, B.; Seabra, A.B. Biogenic Synthesis and Antimicrobial Activity of Silica-Coated Silver Nanoparticles for Esthetic Dental Applications. J. Dent. 2020, 96, 103327. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Jiménez, C.; Bolaños-Carmona, M.V.; Enrich-Essvein, T.; Rodríguez-Navarro, A.B.; González-López, S.; Yamauti, M.; Álvarez-Lloret, P. Green Synthesis of Chitosan- and Fluoride-Functionalized Silver Nanoparticles Using Camellia sinensis: Characterization and Dental Applications. Int. J. Biol. Macromol. 2024, 268, 131676. [Google Scholar] [CrossRef]
- Goel, M.; Sharma, A.; Sharma, B. Recent Advances in Biogenic Silver Nanoparticles for Their Biomedical Applications. Sustain. Chem. 2023, 4, 61–94. [Google Scholar] [CrossRef]
- Rajagopal, S.; Sugumaran, S. The Antibacterial Effectiveness of Citrullus Lanatus-Mediated Stannous Nanoparticles on Streptococcus mutans. Cureus 2023, 15, e45504. [Google Scholar] [CrossRef] [PubMed]
- Radmand, F.; Baseri, M.; Memar, M.Y.; Ebrahimi, A.; Hamishehkar, H.; Asnaashari, S.; Naseri, A.; Kouhsoltani, M. Anti-Biofilm and Anti-Glucosyltransferase Effects of Nano Liposomal Plant Extracts against Streptococcus mutans. Sci. Rep. 2024, 14, 27304. [Google Scholar] [CrossRef]
- Thangavelu, L.; Adil, A.H.; Arshad, S.; Devaraj, E.; Mallineni, S.K.; Sajja, R.; Chakradhar, A.; Karobari, M.I. Antimicrobial Properties of Silver Nitrate Nanoparticle and Its Application in Endodontics and Dentistry: A Review of Literature. J. Nanomater. 2021, 2021, 9132714. [Google Scholar] [CrossRef]
- Thomas, R.; Snigdha, S.; Bhavitha, K.B.; Babu, S.; Ajith, A.; Radhakrishnan, E.K. Biofabricated Silver Nanoparticles Incorporated Polymethyl Methacrylate as a Dental Adhesive Material with Antibacterial and Antibiofilm Activity against Streptococcus mutans. 3 Biotech 2018, 8, 404. [Google Scholar] [CrossRef]
- Manikandan, V.; Velmurugan, P.; Park, J.-H.; Chang, W.-S.; Park, Y.-J.; Jayanthi, P.; Cho, M.; Oh, B.-T. Green Synthesis of Silver Oxide Nanoparticles and Its Antibacterial Activity against Dental Pathogens. 3 Biotech 2017, 7, 72. [Google Scholar] [CrossRef]
- Pellegrini, P.; Sauerwein, R.; Finlayson, T.; McLeod, J.; Covell, D.A.; Maier, T.; Machida, C.A. Plaque Retention by Self-Ligating vs Elastomeric Orthodontic Brackets: Quantitative Comparison of Oral Bacteria and Detection with Adenosine Triphosphate-Driven Bioluminescence. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 426.e1–427. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Bharath, L.V. Mechanism of Plant-Mediated Synthesis of Silver Nanoparticles—A Review on Biomolecules Involved, Characterisation and Antibacterial Activity. Chem. Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Della Valle, C.; Visai, L.; Santin, M.; Cigada, A.; Candiani, G.; Pezzoli, D.; Arciola, C.R.; Imbriani, M.; Chiesa, R. A Novel Antibacterial Modification Treatment of Titanium Capable to Improve Osseointegration. Int. J. Artif. Organs 2012, 35, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Kirmanidou, Y.; Sidira, M.; Bakopoulou, A.; Tsouknidas, A.; Prymak, O.; Papi, R.; Choli-Papadopoulou, T.; Epple, M.; Michailidis, N.; Koidis, P.; et al. Assessment of Cytotoxicity and Antibacterial Effects of Silver Nanoparticle-Doped Titanium Alloy Surfaces. Dent. Mater. 2019, 35, e220–e233. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Jang, Y.-S.; Jang, J.-H.; Bae, T.-S.; Lee, S.-J.; Lee, M.-H. Enhanced Antibacterial Activity of Titanium by Surface Modification with Polydopamine and Silver for Dental Implant Application. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019847067. [Google Scholar] [CrossRef]
- Asahi, Y.; Naito, K.; Kanda, H.; Niwano, K.; Takegawa, D.; Yumoto, H.; Noiri, Y.; Hayashi, M. Clinical Investigation of the Inhibitory Effects of Tooth-Coating Materials on Initial Active Root Caries: A Pilot Randomized Controlled Trial. Medicina 2024, 60, 150. [Google Scholar] [CrossRef]
- Dong, T.; Wang, X.; Li, F.; Zhu, Y.; Fu, X. Study on the Wear Resistance of Ni-Co-ZrO2 Composite Coatings with Different ZrO2 Nanoparticle Concentrations Prepared Using Electrodeposition on the Micro-Surface of Spindle Hook Teeth. Metals 2023, 13, 1251. [Google Scholar] [CrossRef]
- Oyane, A.; Sakamaki, I.; Nakamura, M.; Koga, K.; Shitomi, K.; Tanaka, S.; Miyaji, H. Fluoridated Apatite Coating on Human Dentin via Laser-Assisted Pseudo-Biomineralization with the Aid of a Light-Absorbing Molecule. Int. J. Mol. Sci. 2022, 23, 15981. [Google Scholar] [CrossRef]
- Chaharmahali, R.; Fattah-alhosseini, A.; Nouri, M.; Babaei, K. Improving Surface Characteristics of PEO Coatings of Mg and Its Alloys with Zirconia Nanoparticles: A Review. Appl. Surf. Sci. Adv. 2021, 6, 100131. [Google Scholar] [CrossRef]
- Sanaullah, I.; Bashir, M.; Batool, T.; Riaz, S.; Ali, D.; Sabri, A.N.; Naseem, S. Tangerine Mediated Synthesis of Zirconia as Potential Protective Dental Coatings. Mater. Sci. Eng. C 2021, 120, 111653. [Google Scholar] [CrossRef]
- Ramesh, G.; Nagarajappa, R.; Madhusudan, A.; Sandesh, N.; Batra, M.; Sharma, A.; Patel, S. Estimation of Salivary and Tongue Coating PH on Chewing Household Herbal Leaves: A Randomized Controlled Trial. Anc. Sci. Life 2012, 32, 69. [Google Scholar] [CrossRef]
- Melero, H.; Fernández, J.; Dosta, S.; Guilemany, J.M. Caracterización de Nuevos Recubrimientos Biocompatibles de Hidroxiapatita-TiO2 Obtenidos Mediante Proyección Térmica de Alta Velocidad. Boletín Soc. Española Cerámica Vidr. 2011, 50, 59–64. [Google Scholar] [CrossRef]
- García, Ó.L. Desarrollo de Recubrimientos Antimicrobianos de Polihidroxialcanoatos Sobre Muestras de Tántalo. Optimización Del Recubrimiento En Muestras Lisas. Primeros Estudios En Muestras Porosas. Master’s Thesis, Universitat Politècnica de Catalunya-BarcelonaTech, Barcelona, Spain, 2015. [Google Scholar]
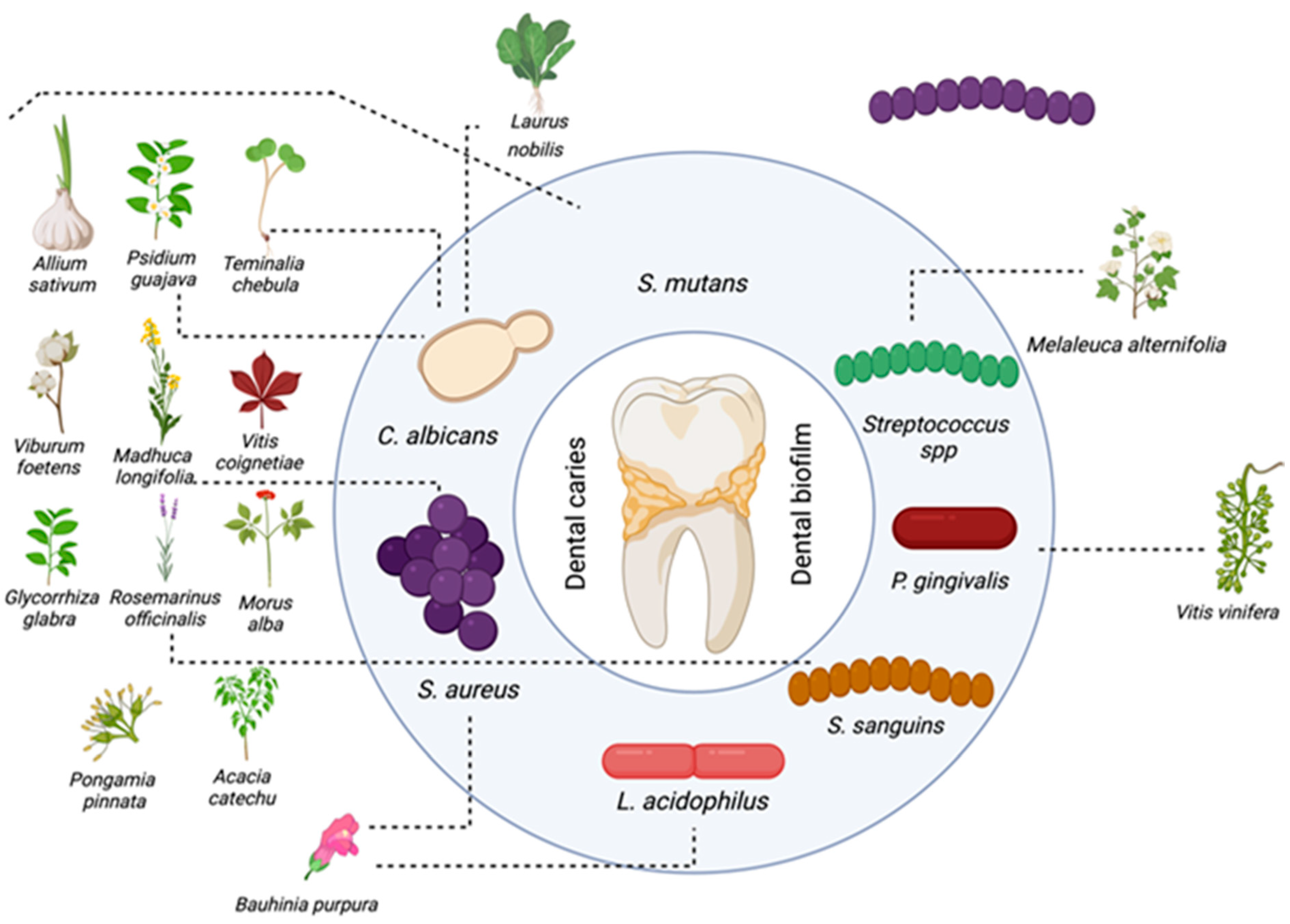
| Species | In Vitro | Reference |
|---|---|---|
| Allium sativum | Antibacterial effect on multi-drug-resistant species of Streptococcus mutans, antitumor and antiproliferative effect on human oral squamous cancer cells | [15] |
| Cotinus coggygria | Potential against dental calculus | [29] |
| Propolis | Antimicrobial against S. mutans and anticandidal activities | [15] |
| Cocos nucifera | Antimicrobial activity Antifungal activity | [15] |
| Inula viscosa | Antibacterial activity against cariogenic bacteria | [27] |
| Carica papaya | Removal of dental caries | [15] |
| Cistus incanus | Antibacterial effect against S. mutans, prevention of bacterial adhesion, inhibition of glucosyltransferase (GTF) | [27] |
| A variety of plant species | Anticariogenic activity and antibiofilm formation of a variety of polyphenols | [30] |
| Glycyrrhiza glabra | Antibacterial effects on both Gram-positive and -negative bacterial species. Antibacterial effects on the species Porphyromonas gingivalis. Antifungal effects on Candida albicans | [15] |
| Hypericum perforatum | Antibacterial effect against Aggregatibacter actinomycetemcomitans and P. gingivalis | [15] |
| Mentha piperita Thymus vulgaris | Antibacterial effects on S. mutans, C. albicans and A. actinomycetemcomitans Antimicrobial effects on S. mutans | [15] |
| Cinnamomum zeylanicum | Antimicrobial effects on S. mutans and Lactobacillus acidophilus | [31] |
| Aloe vera | Bactericidal effects against cariogenica and periodontopatogenic bacterias | [15] |
| Medicinal Plant Extracts of Cinnamon, Turmeric, Ginger, Clove and Black seed | Antimicrobial effects on S. mutans and L. acidophilus | [32] |
| Melaleuca alternifolia Syzygium aromaticum | Antimicrobial activity Antimicrobial effects on S. mutans and L. acidophilus | [15,31] |
| Peperomia pellucida (EOs) Piper marginatum (EOs) Piper callosum (EOs) | Antimicrobial effects against S. mutans, Streptococcus mitis, Streptococcus sanguinis, Streptococcus salivarius, S. sobrinus, Enterococcus faecalis, and Lactobacillus casei | [33] |
| Nigella sativa | Antimicrobial effects on S. mutans and L. acidophilus | [31] |
| Citrus aurantifolia | Antimicrobial effects on S. mutans | [15] |
| Camelia sinensis | Antimicrobial effects against S. mutans, P. gingivalis and Prevotella nigrescens | [15] |
| Vitis vinifera (Grape seed extract) | Antimicrobial effects against S. mutans | [15] |
| Curcuma longa | Antimicrobial effects on S. mutans | [15] |
| Zingiber officinale | Antimicrobial effects on S. mutans and L. acidophilus | [31] |
| Verbascum speciosum | Anticariogenic activity against S. mutans and Streptococcus sobrinus | [34] |
| Morinda Oleifera | Antimicrobial effects on S. mutans | [35] |
| Medicinal Plants | Formulation | Year |
|---|---|---|
| Camellia sinensis Terminalia chebula Glycyrrhiza uralensis | Mouthwash Toothpastes | [38] |
| Terminalia chebula Psidium guajava Azadirachta indica Pongamia pinnata Syzygium aromaticum Mentha piperita | Mouthwash | [34] |
| Syzygium aromaticum Dennettia tripetala Jatropha curcas | Toothpastes | [13] |
| Copaifera langsdorffii | Dental varnish | [28] |
| Teucrium polium | Mouthwash | [35] |
| Salvadora persica Asparagus racemosus Streblus asper Rosamarinus officinalis | Toothpastes | [36] |
| Piper crocatum | Mouthwash | [37] |
| Miswak | Toothpastes | [23] |
| Plant Specie | Family | Part Used | Microorganism | Reference |
|---|---|---|---|---|
| Acacia nilotica | Fabaceae | Leaves, stem, bark | S. mutans, Streptococcus sobrinus, Porphyromonas gingivalis | [41,42] |
| Allium sativum | Amaryllidaceae | Bulb of the plant | Enerecoccus faecalis, Stapylococcus aureus, Escherichia coli | [43] |
| Asparagus racemosus | Asparagaceae | Aerial parts | S. mutans, L. acidophilus | [18] |
| Azadirachta indica | Meliaceae | Bark, leaves, seeds | S. mutans, Sreptococcus salivarius, Streptococcus sanguis and S. mitis | [44,45] |
| Berberis vulgaris | Berberidaceae | Roots | S. mutans, S. sobrinus, S. sanguinis, S. salivaris, Lactobacillus rhamnosus | [46] |
| Camellia sinensis | Theaceae | Leaf buds, stems | S. mutans, L. acidophilus | [47,48] |
| Citrus sinensis | Rutaceae | Peel, leaves, wood of the tree | S. mutans, L. acidophilus | [49] |
| Cichorium intybus | Asteraceae | Aerial parts | S. aureus, E. coli, Salmonella typhi, Prevotella intermedia, S. mutans | [50] |
| Coffee canephora | Rubiaceae | Beans, stems leaves | P. gingivalis | [51] |
| Copaifera pubiflora | Fabaceae | Oleoresin, bark | P. gingivalis Aggregatibacter actinomycetemcomitans | [52] |
| Coriandrum sativum | Apiaceae | Seeds, leaves | S. mutans, C. albicans | [53] |
| Embelia ribes | Primulaceae | Stem, bark | Bacillus subtilis, S. mutans, S. sanguinis | [36,54] |
| Eucalyptus globulus | Myrtaceae | Leaves | S. mutans, E. faecalis | [55,56] |
| Eugenia caryophyllata | Myrtaceae | Gall | S. mutans, L. acidophilus | [31,57] |
| Inula viscosa | Asteraceae | Leaves, stem | P. gingivalis, S. sobrinus | [58] |
| Juglans regia | Juglandaceae | Bark | P. gingivalis S. salivarius, S. mutans, S. aureus, S. sanguis | [59,60] |
| Lippia sidoides | Verbenaceae | Leaves, aerial parts, flowers | S. mutans | [53,61] |
| Melaleuca alternifolia | Myrtaceae | Leaves | P. gingivalis, Porphyromonas endodontalis, A. actinomycetemcomitans | [62] |
| Nigella sativa | Ranunculaceae | Seeds | S. mutans P. intermedia, P. gingivalis, A. actinomycetemcomitans | [63,64] |
| Ocimum americanum | Lamiaceae | Leaves | S. mutans L. casei, S. sanguinis | [65] |
| Ocimum sanctum | Lamiaceae Labiatae | Leaves, stem, flower, root, seeds | S. mutans, S. sanguinis A. actinomycetemcomitans | [66] |
| Psidium guajava | Myrtaceae | Bark, leaf, stem, | P. gingivalis, P. intermedia, S. aureus, E. coli | [67] |
| Quercus infectoria | Fagaceae | Galls | S. mutans | [68] |
| Rosmarinus officinalis | Lamiaceae | Leaves, aerial parts | S. sanguinus S. mutans S. sobrinus | [69,70] |
| Salvadora persica | Salvadoraceae | Roots, branches | S. mutans, S. aureus and Streptococcus sp. isolates | [71,72,73] |
| Streblus asper | Moraceae | Leaves, steam bark, aerial parts | S. mutans, P. gingivalis A. actinomycetemcomitans | [74] |
| Terminalia chebula | Combretaceae | Fruit | A. actinomycetemcomitans, dental plaque bacteria, S. mutans | [75] |
| Thymus vulgaris | Lamiaceae | Leaves | S. mutans C. albicans | [76] |
| Vaccinium macrocarpon | Ericaceae | Fruit | S. mutans, S. sobrinus, P. gingivalis, Fusobacterium nucleatum, S. mutans, Actinomyces naeslundii | [77] |
| Vitis vinifera | Vitaceae | Seeds, leaves | P. gingivalis | [78] |
| Plant Species | Extracts/Key Compounds | Biological Activity | References |
|---|---|---|---|
| Acacia nilotica | Stem and bark extracts contain quercetin, luteolin, saponins, anthraquinones, amino acids, fatty acids, diverse carbohydrates, and polyphenols like chebulinic acid alongside tannin, gallic acid, catechin, epigallocatechin-7-gallate, catechin derivatives, ellagic acid, kaempferol and quercetin. | A. nilotica are used as antibacterial agents (S. sobrinus and P. gingivalis) targeting oral pathogens and are effective in managing dental plaque. Additionally, they can inhibit GTF and its antioxidant properties, with a focus on promoting oral hygiene, by decreasing bacteria counts and controlling dental plaque. It is used as a chewing stick, mouthwash, or gum. | [41,79,80] |
| Allium sativum | Tannins, flavonoids, and alkaloids (alliin, methiin, sodium acetate). | The substances exhibit antibacterial, antifungal, and antiviral characteristics, making them suitable for treating dental cavities. They are formulated into gels, gums, toothpaste, and pharmaceutical strips for oral care applications. | [81,82] |
| Asparagus racemosus | Borneol, myrtanol, pinocarveol, 2-ethylhexanol, perillaldehyde | Antibacterial properties against S.mutans and L. acidophilus | [18] |
| Azaridachta indica | Extracts from bark, leaves, and seeds display antifungal, anti-ulcer, and antinociceptive properties. Contains approximately 300 structurally distinct constituents, with the majority being limonoids. The composition includes hexadecanoic acid, oleic acid, 5,6-dihydro-2,4,6-triethyl-(4H)-1,3,5-dithiazine, methyl oleate, and eudesm-7(11)-en-4-ol, tannins, lignins, flavonoids | Extract from neem demonstrates having efficacy against bacteria such as S. mutans, S. salivarius, S. sanguis, and S. mitis. A blend of chewing sticks proves advantageous in eliminating organisms responsible for dental caries; chewing these sticks increases saliva secretion, cleansing, and antibacterial, antioxidant, and anti-inflammatory properties, and also it helps to reduce the oxidative stress that comes with periodontal disease. It has also shown antimicrobial properties by destructing bacterial cell membranes which reduces the surface of adhesion of specific bacteria and inhibits bacterial growth. Moreover, a mucoadhesive dental gel infused with A. indica exhibits significant benefits by reducing both the plaque index and salivary bacterial count, surpassing the effectiveness of CHX gluconate mouthwash. | [34,83] |
| Berberis vulgaris | Protoberberine, alkaloids, roots | Dental gel. Antibacterial properties, plaque formation index decreasedThe growth of microorganisms is inhibited by berberine. | [84] |
| Camellia sinensis | Epicatechin-3-gallate, epigallocatechin-3-gallate | An effective treatment and preventive measure against periodontal disease, possessing antioxidant, anti-inflammatory, antibacterial, antiviral, and antimutagenic properties. They can potentially be used in the preparation of toothpastes and mouthwashes. | [85] |
| Citrus sinensis | Orange peel extract | Antibacterial properties: numerous studies have revealed that it can treat periodontal disease | [49,60] |
| Cichorium intybus | The extract comprises primarily carvacrol, cinnamic aldehyde, thymol, camphor, linalool, carvone, and terpineol. Additionally, it contains inulin, caffeic acid derivatives like ferulic acid, caftaric acid, chicoric acid, and chlorogenic acid (3-CQA), as well as isochlorogenic acid (3,5-diCQA), dicaffeoyl tartaric acid, sugars, proteins, hydroxycoumarins, flavonoids, terpenoids, sesquiterpene lactones, alkaloids, steroids, oils, volatile compounds, vitamins, and polyynes. | Potential in preventing virulence-linked properties of oral pathogens such as P. intermedia and S. mutans | [86] |
| Coffea canephora | Phenolic acid, green coffee extract | Green coffee extract’s chlorogenic acid decreased the oral bacteria S. mutans in a clinical experiment. | [87,88] |
| Copaifera pubiflora | Oleoresin, hydroalcoholic extract | Antibacterial in endodontic infections and dental caries; antimicrobial properties against P. gingivalis and A. actinomycetemcomitans. | [52] |
| Coriandrum sativum | The leaves predominantly contain decanal, trans-2-decenal, and 2-decen-1-ol. Fatty acids (AKFAs) were extracted from the endophytic fungus Arthrographis kalrae, which was isolated from Coriandrum sativum leaves. The leaves also contain linalool, terpenoids, phenolic compounds, and fatty acids. The contents of Fraction Cs4 isolated from the leaves include 1-decanol, thymol, trans-caryophyllene, trans-2-dodecen-1-ol, spathulenol, globulol, and α-cadinol. | The essential oil of C. sativum exhibits antifungal activity, significantly reduces the viability of C. albicans cells, and demonstrates potential against S. mutans biofilms, combating dental caries. | [53] |
| Eucalyptus globulus | The essential oil extracted from leaves contains a total of 27 compounds. The key compounds present in the oil include β-pinene, eucalyptol (1,8-cineole), α-pinene, α-phellandrene, gamma-eudesmol, para-cymene, β-eudesmol, limonene, terpinen-4-ol, and piperitone. | Demonstrates significant antimicrobial efficacy against bacteria commonly present in the oral cavity. The EO notably hindered both the planktonic and biofilm growth of S. mutans and E. faecalis. In this way, EOs can be used to generate pharmaceutical products for oral health. | [55] |
| Eugenia caryophyllata | The primary constituents present in clove oil are phenylpropanoids, notably eugenol and β-caryophyllene, 2-methoxy-4-(2-propenyl)-, acetate. | Clove oil, renowned for its antiseptic, analgesic, and anesthetic effects, displays robust antimicrobial activity against streptococci, particularly S. mutans. Eugenol, a major component of clove oil, showcases potent antimicrobial properties against cariogenic bacteria, specifically targeting S. mutans. Moreover, the methanolic extract of clove demonstrates significant antimicrobial efficacy against both S. mutans and L. acidophilus, suggesting its potential application in minimally invasive and adhesive dentistry practices. Also, clove exhibits sensibility to pathogens and bacteria associated with tooth decay and periodontal diseases, offering antibacterial activity against P. gingivalis and P. intermedia, alongside antioxidant properties. | [53,57] |
| Inula viscosa | 2,5-Dihydroxy-isocostic acid, 2,3-Dihydroxycostic acid, hydroxy-allylic, dichloromethane-MeOH. | Inhibits microbial adhesion in the oral cavity. It has antibacterial activity against P. gingivalis, and S. sobrinus. | [58,89] |
| Juglans regia | The bark extract contains juglone, terpenoids, alkaloids, steroids, phenols flavonoids, saponins, polyphenols, acid, mono, di, tri acylglycerol, free fatty acids, oleic and linoleic acids, proteins, naphthaquinones, ascorbic acid, sitosterol, and tannins. | Extract from Juglans regia with juglone exhibits antibiofilm and growth inhibitory effects against oral pathogen P. gingivalis, enhancing oral hygiene. Bark of J. regia is side effect-free, beneficial against plaque and caries bacteria, contrasting with harmful mouthwashes and antibiotics. It also reduces P. gingivalis growth and has antibiofilm action against S. sobrinus, A. viscosus, and S. mutans, with antiplaque formation properties. | [59] |
| Lippia sidoides | Essential oil rich in thymol, carvacrol, flavonoids. | Potent antibacterial activity against cariogenic bacteria; effective against plaque and gingivitis. Extracts from L. sidoides significantly reduce extracellular polysaccharides and bacterial cells in S. mutans biofilm, without affecting biofilm thickness. L. sidoides essential oil shows strong antibacterial activity and clinical efficacy as a mouthwash, making it promising for combating plaque and gingivitis. It also manages supragingival biofilm, inhibits plaque formation, and possesses antigingivitis properties. | [51,53] |
| Melaleuca alternifolia | The essential oil extracted from leaves and branches contains a variety of compounds including terpinen-4-ol, 1,8-cineole, alpha-terpineol, gamma-terpinene, terpinene, terpinolene, cymene, limonene, pinene, sabinene, viridiflorol, and globulol. | Displayed inhibitory effects on bacterial growth linked with various dental conditions including dental caries, periodontitis, dental plaque, and gingivitis. The bacteria affected include P. gingivalis, P. endodontalis, and A. actinomycetemcomitans. | [90] |
| Nigella sativa | The active extract, especially in nanoemulsion form, is attributed to its key components, including carvacrol, longifolene, ρ-cymene, t-anethole, 4-terpineol, and thymoquinone. | The essential oil exhibits anticarcinogenic, antioxidant, and antimicrobial properties. It has also demonstrates sensitivity against intermedia, P. gingivalis, and A. actinomycetemcomitans. Its suggests that thymoquinone could be significant in both the treatment and prevention of periodontal diseases. | [64] |
| Ocium americanum | The essential oil of Ocium leaf contains medium-chain free fatty acids derivative of lauric acid; it is a type of fatty acid that interacts with MurA enzyme, which is necessary for the formation of the cell wall of cariogenic bacteria. | The essential oil extracted from the leaves of Ocimum americanum demonstrates grand antimicrobial activity against cariogenic bacteria, notably S. mutans, S. sanguinis and L. casei, in both planktonic and biofilm cultures, comparable efficacy to CHX solution in reducing bacterial counts. This plant also showed activity against periodontal microorganisms P. gingivalis, P. intermedia, and F. nucleatum. | [53,65] |
| Ocimum sanctum | The essential oil contains caryophyllene, β-caryophyllene, β-pinene, copaene, and eugenol. Additionally, O. sanctum essential oil comprises a diverse range of groups, including monoterpenes hydrocarbons (e.g., α-pinene, camphene), sesquiterpene hydrocarbons (e.g., caryophyllene, Copaene, α-caryophyllene, α-bourbonene, α-cubebene), oxygenated monoterpenes (e.g., caryophyllene oxide), and aromatic compounds (e.g., eugenol, borneol, methyl iso-eugenol). | The ethanol extract displays antibacterial effectiveness against S. mutans and has long been utilized for alleviating toothaches and pulpitis traditionally. These components contribute to the antimicrobial attributes of Tulsi, rendering it efficient against bacteria responsible for dental caries. The essential oil of O. sanctum exhibits antimicrobial and antifungal properties against oral pathogens associated with dental issues. It is utilized as an ingredient in mouthwash and toothpaste formulations by pharmaceutical companies for treating toothaches and pulpitis. Eugenol, extensively utilized in dentistry, is one of its key compounds. | [66] |
| Psidium guajava | Bark, leaf, stem. Flavonoids: guaijaverin, quercetin, tannins. | Paste and mouthwash. Anti-inflammatory, antioxidant, antimicrobial and wound-healer properties. Inhibits P. gingivalis and P. intermedia growth, antiplaque formation | [67] |
| Quercus infectoria | The extract is rich in tannins; it also contains gallic acid and ellagic acid. | The extract demonstrates antimicrobial properties, proving effective against the causative agents of both periodontitis and dental caries. The extract significantly reduced the formation of biofilm biomass by S. mutans, indicating its potential in combating dental caries. The EO showed activity against various oral pathogens, including F. nucleatum, S. mutans, S. salivarius and P. gingivalis. | [68] |
| Rosmarinus officinalis | Essential oil contains terpenoids, flavonoids, phenols, essential oils, borneol, camphor and verbenone. | R. officianalis essential oil has antioxidant, antibacterial (S. sanguinus, S. mutans, S. sobrinus), antifungal and antibiofilm properties; this aids in preventing plaque formation, and the reduction in biofilm formation suggests potential application in new anticaries treatment protocols. | [91] |
| Salvadora persica | The mixture comprises oxygenated monoterpenes, sesquiterpene hydrocarbons, and monoterpene with primary constituents including α-caryophellene, 9-epi-(E.)-caryophellene, 1,8-cineole (eucalyptol), and β-pinene. Additionally, it contains chrysin-8-c-β-D-glucopyranoside, ferulic acid, gallic acid, stigmasterol, butylated hydroxytoluene, and benzene (isothiocyanatomethyl). Other components include tannins, vitamin C, potassium, sodium chloride, silica, salvadorine, salvadourea, and saponins, along with fibrous branches. | The compounds exhibit plaque formation inhibition and have a traditional use as toothbrushes, recent studies highlighting their effectiveness in combating gingivitis and enhancing oral hygiene. They significantly contribute to the antibacterial activity observed in Salvadora persica extract against S. aureus and Streptococcus sp. isolates from patients with plaque-induced gingivitis. These compounds, present in chewing sticks and mouthwash, possess antibacterial, anti-inflammatory, and antioxidant properties. They also inhibit plaque formation and prevent periodontal disease by blocking the function of the glucosyltransferase enzyme. | [73] |
| Streblus asper | From the aerial bark, compounds such as n-triacontane, β-sitosterol, stigmasterol, tetraiacontan-3-one, oleanolic acid, and botulin are found. Additionally, flavonoids and lignans are derived from the heartwood. | Significantly reduced S. mutans colonies; effectiveness against P. gingivalis and A. actinomycetemcomitans colonies. These results revealed that extract is able to inhibit in vitro subgingival biofilm formation and reduce the numbers of P. gingivalis, A. actinomycetemcomitans and total bacteria. | [74] |
| Terminalia chebula | Fruit. Poluphenols, terpenes, anthocyanins, flavonoids, alkaloids, glycosides | Mouth rinses, toothpaste. Antibacterial properties against A. actinomycetemcomitans and S. mutans, anti-inflammatory and antioxidant properties.Prevents and treats dental caries and gingivitis. | [92] |
| Thymus vulgaris | Thyme essential oil major compounds were found as thymol, α-thymol, camphene, caryophyllene, humulene, α-terpineol and ρ-cymene. | T. vulgaris essential oil holds promise for inclusion in toothpaste formulations, EO exhibits antimicrobial activity against clinical isolates of pathogenic bacteria, including S. mutans, P. gingivalis, S. pyogenes, and C. albicans. | [76] |
| Vaccinium macrocarpon | Anthocyanins, flavonols, proanthocyanidins | Inhibits the colonization of bacterias such as P. gingivalis, F. nucleatum Prevents P. gingivalis from adhering to various proteins, which could lead to periodontal disorders. Antibacterial properties against S. mutans, S. sobrinus, S. oralis. | [93,94] |
| Vitis vinifera | Phenolic compounds | Anti-inflammatory, antioxidant, cytoprotective properties. Antibacterial, antifungal and antiviral activity against oral infections. Controls the bacterial-induced inflammatory response and oxidative stress imbalance in periodontal diseases. | [78] |
| Plant Specie | Compound(s) | Study Model | Administration | Effect | References |
|---|---|---|---|---|---|
| Allium sativum | Allicin, Alliin, Diallyl trisulfide, S-allyl cysteine, Allyl mercaptan, Ajoene. | Human clinical trial; 200 subjects (20–60 years old). Human clinical trial, 90 children (4–6 years old). | Oral consumption; 8 tablets (300 mg AGE powder/tablet) during 18 months. | Consumption of AGE tablets to be effective as a preventive measure of periodontitis. Antibacterial activity. Garlic extract can be used safely for irrigation of root canals of primary molars. | [101,102] |
| Aloe vera | Anthraquinones | Randomized clinical study; 72 extracted human molars. Comparative clinical trals: A. vera, O. sanctum, chlorhexidin mouthwashes. | Toothpaste containing 1000–1450 ppm fluoride and A. vera. Mouthwash with A. vera; daily use, for 30 days. | Improvement in the enamel density values after demineralization. Usage of A. vera- mouthwash showed a significant reduction in plaque, gingival and bleeding indices over 30 days. | [103,104] |
| Rosmarinus officinalis | Borneol, camphor, limonene, camphene, pinene, cineol, myrcene, verbenone and caryophyllene. | Clinical study/n = 110 subjects | Toothpaste of daily use. | Reduced risk of gingival bleeding, prevented the increase in plaque formation. | [105,106] |
| Ocimum sanctum | Caryophyllene, pinene, copaene Civsilineol, Civsimavatine, Isothymonin, Apigenin, Rosavinic acid, Eugenol and Linoleic acid. | Triple-blinded trial: 84 participants (14–15-year-old). Comparative clinical trals: O. sanctum, and A. vera CHX mouthwashes. | Toothpaste, daily use for 21 days. Mouthwash added with O. santum; daily use, 30 days. | Significant reduction in the plaque and gingival scores. Antigingivitis effect. Usage of O. sanctum mouthwash reduced plaque, gingival and bledding indices over 30 days. | [104,107,108] |
| Salvadora persica | Caryophellene, cineole (eucalyptol), caryophellene, pinene | Comparative study (clinical trial; 40 students (16–18 years old). Miswak toothpaste vs. Miswak mouthwash or ordinary toothpaste. Comparative study (clinical trial; 60 girls (18–22 years old). | Twice daily (morning and before sleeping) for 2 weeks. S. persica extracts /10 g/100 mL) Daily use, during 6 months. | Mouthwash group presents antigingivitis, anticariogenic, antiplaque, whitening properties; orthodontic chain preservation and promotion of gingival wound healing. | [44,71,109] |
| Camellia sinensis | Polyphenols, epigallocatechin gallate (EGCG). | In vivo study; 90 children (4–6 year-old). | Extracts in the form of a gel for 2 weeks and analysis after breakfast, without tootbrush/toothpaste use. | Extracts diminished salivary S. mutans levels and has antibacterial activity against predominant cariogenic bacteria. | [25,110,111,112] |
| Vitis vinifera | Pro-anthocyanidins catechin, epicatechin and procyanidins. | Comparative study of pomegranate and guava extracts; 40 children (8–10 years old). Comparative study: 80 children of 8–15 years of age. | Aqueous extracts GSE (100 mg/mL) added to mouthwash was used (7 days) | Prevention of dental caries. Reduction in the oral streptococci counts. Inhibition of S. mutans biofilms. Plaque reduction (antioxidant and phytochemical properties). | [15,16,17,18] |
| Punica granatum | Plyphenolic flavonoids (punicalagins and ellagic acid), tannins. | Comparative study of grape seed and guava extracts; 40 children (8–10 years old). Comparative study: 80 children of 8–15 years of age. | Aqueous extracts (100 mg/mL) added to mouthwash was used (7 days). Fruit extracts from P. granatum and T. chebula added to mouthwash. | Reduction in the oral streptococci counts. Plaque reduction (antioxidant and phytochemical properties). | [16,18] |
| Psidium guajava | Saponins, tannins, flavanoids and alkaloids. Guaijaverin. | Comparative study of pomegranate and grape seed extracts; 40 children (8–10 years old). | Aqueous extracts (100 mg/mL) added to mouthwash was used (7 days). | Activity vs. S. aureus, E.coli, C. albicans and S. mutans | [18] |
| Terminalia chebula | Chebulic acid, Chebulagic acid, Corilagin, and Gallic acid. | Comparative study of pomegranate and grape seed extracts; 80 children (8–15 years old). | Mouthwash used daily, for 15, 30 days. | Diminished count of S. mutans in saliva (microbiologic assay). | [16] |
| Lentinula edodes | Eritadenina, Lentinan, Emitanina, Quitina, Ergosterols | In vivo studies/ 65 healthy adults. | Rinsing with extract (<5.000 Da) of shiitake (L. edodes) for 14–15 weeks. | Metabolic activity of dental plaque was reduced. But no reduction in plaque scores and no inhibition of the production of organic acids in plaque. | [113] |
| Cichorium intybus | Phenylpropenoids, anthocyanins, flavonoids, polysaccharides (such as inulin), sesquiterpenoids, triterpenoids, proteins, steroids, lipids, caffeine, and organic acids. | Double-blind, controlled clinical trial (40 patients with periodontitis). | 1 g (capsules), twice daily, over 8 weeks. | It had potent antimicrobial activity against S. mutans; antioxidant and antiproliferative activity. Chicory leaf extract may be helpful in controlling periodontal status. | [114,115] |
| Azadirachta indica | Hexadecanoic acid, Hentriacontane, Phytol. | Comparative clinical study (60 patients) Neem-based toothpaste vs. probiotic-based toothpaste. | Neem-based toothpaste, used for 60 days. | The neem-based toothpaste showed antimicrobial activity in terms of reduction in the level of S. mutans. | [116] |
| Streblus asper | Amyrin acetate, sitosterol, streblosid, lupeol acetate, diol, Sioraside, amyrin, mansonin, Threo-streblusol B, streblusquinone, streblusol A, E, C y D, triacontane, tetraiacontan-3-one, oleanolic acid, botulin. | Randomized controlled trial: 76 subjects (14–18 years old). | Mouthwash used for 60 sec. then saliva sample collection (0, 2, 30, 60 and 120 min). | Bactericidal activity towards S. mutans. | [117] |
| Plant Extract | Pathogen | MIC Value (mg/mL) | Reference |
|---|---|---|---|
| Melaleuca alternifolia | Streptococcus spp. | 1.2–20 | [63] |
| Allium sativum | Streptococcus mutans | 0.5–32.0 | [63] |
| Psidium guajava | S. mutans | 0.076 | [63] |
| Glycorrhiza glabra | S mutans | <12.5 | [63] |
| Bauhinia purpura | S. mutans | 0.029 | [63] |
| Viburum foetens | S. mutans | 0.08 | [63] |
| Madhuca longifolia | S. mutans | 0.0183–0.0212 | [63] |
| Morus alba | S. mutans | 0.008 | [63] |
| Rosmarinus officinalis | S. mutans | 4.0 | [63] |
| Terminalia chebula | S. mutans | 0.076 | [63] |
| Vitis coignetiae | S. mutans | 7.50 | [63] |
| Camellia sinensis | S. mutans | 80.0 | [112] |
| Camellia sinensis | Streptococcus sanguinis | 80.0–250.0 | [112] |
| Bauhinia purpura | Lactobacillus acidophilus | 0.0202 | [63] |
| Laurus nobilis | Candida albicans | 0.25 | [63] |
| Psidium guajava | C. albicans | 0.152 | [63] |
| Vitis vinifera | Porphyromonas gingivalis | 0.48 | [123] |
| Pongamia pinnata | S. mutans | 0.20 | [122] |
| Acacia catechu | S. mutans | 0.20 | [122] |
| Aloe vera | S. mutans | 0.25–3.0 | [121] |
| Equisetum arvense | S. mutans | 0.25–3.0 | [121] |
| Mimosa tenuiflora | S. mutans | 0.25–3.0 | [121] |
| Lippia graveolens | S. mutans | 0.25–3.0 | [121] |
| Syzygium aromaticum | S. mutans | 0.25–3.0 | [121] |
| Aloe vera | Streptococcus sobrinus | 2.0–3.0 | [121] |
| Equisetum arvense | S. sobrinus | 2.0–3.0 | [121] |
| Melaleuca tenuiflora | S. sobrinus | 2.0–3.0 | [121] |
| Lippia graveolens | S. sobrinus | 2.0–3.0 | [121] |
| Syzygium aromaticum | S. sobrinus | 2.0–3.0 | [121] |
| Antimicrobial Agent | MIC (mg/mL) | MBC (mg/mL) | Reference |
|---|---|---|---|
| Sodium fluoride | 0.625 | 2.5 | [127] |
| Tea polyphenol | 1.25 | 2.5 | |
| Chlorhexidine | 0.0025 | 0.0025 | |
| Penicillin | 0.000047 | 0.000094 | |
| Metronidazole | 0.250 | 0.5 |
| NPs Based | Plant Encapsulated | Microorganism Effect | MIC | Reference |
|---|---|---|---|---|
| AgNPs | Punica granatum associated with CaGP | S. mutans and C. albicans | ND | [130] |
| AgNPs | Collagen | S. mutans | 125 ppm | [143] |
| CuNPs | Moringa oleifera | E. coli, Klebsiella pneumonia, E. faecalis, S. aureus and C. albicans | 0.3 mg/mL | [144] |
| SiNPs | Green tea powder | S. mutans | 600 μg/mL | [145] |
| AgNPs + chitosan + fluoride | Camellia sinensis | ND | ND | [146] |
| AgNPs | Ulva flexuosa, Neochloris oleoabundans and Fucus vesiculosus | B. subtilis, S. aureus, E. coli, and Pseudomonas aeruginosa | ND | [147] |
| SnCl2 | Citrullus lanatus | S. mutans | 0.531–1.176 μg/mL | [148] |
| Nanoliposomes | Aqueous plant extracts of licorice, ginger, pomegranate, and rose | S. mutans | 2.0–2048 µg/mL | [149] |
| Coating | Treatment | Microorganism | Reference |
|---|---|---|---|
| Tooth-Coating ZIF-C | GIC contains zinc mixture. Randomized controlled trial. | S. mutans | [158] |
| Coatings Ni-Co-ZrO2 | Electrodeposition on the micro-surface of spindle hook teeth | - | [159] |
| Coating of fluoridated apatite (FAp) | LAB. | S. mutans | [160] |
| Coating of magnesium (Mg) | Alloys with ZrNPs. | Actinomyces spp., P. gingivalis and C. albicans | [161] |
| Coating of synthesis of zirconia (ZrO2) | Tangerine-mediated synthesis of ZrO2 NPs as potential protective dental coatings. | Streptococcus, E. coli and Bacillus subtilis | [162] |
| Coatings of salvia | A randomized controlled trial. | S. mutans, P. intermedia and P. gingivalis. | [163] |
| Coatings of Hidroxiapatit-TiO2 | Dental implants. | S. mutans, S. aureus and C. albicans | [164] |
| Polyhydroxyalkanoates coatings | Dental implants. | S. aureus and E. coli | [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gloria-Garza, M.A.; Reyna-Martínez, G.R.; Jiménez-Salas, Z.; Campos-Góngora, E.; Kačániová, M.; Aguirre-Cavazos, D.E.; Bautista-Villarreal, M.; Leos-Rivas, C.; Elizondo-Luevano, J.H. Medicinal Plants Against Dental Caries: Research and Application of Their Antibacterial Properties. Plants 2025, 14, 1390. https://doi.org/10.3390/plants14091390
Gloria-Garza MA, Reyna-Martínez GR, Jiménez-Salas Z, Campos-Góngora E, Kačániová M, Aguirre-Cavazos DE, Bautista-Villarreal M, Leos-Rivas C, Elizondo-Luevano JH. Medicinal Plants Against Dental Caries: Research and Application of Their Antibacterial Properties. Plants. 2025; 14(9):1390. https://doi.org/10.3390/plants14091390
Chicago/Turabian StyleGloria-Garza, Marcela Alejandra, Gustavo Raúl Reyna-Martínez, Zacarías Jiménez-Salas, Eduardo Campos-Góngora, Miroslava Kačániová, Diana Elena Aguirre-Cavazos, Minerva Bautista-Villarreal, Catalina Leos-Rivas, and Joel Horacio Elizondo-Luevano. 2025. "Medicinal Plants Against Dental Caries: Research and Application of Their Antibacterial Properties" Plants 14, no. 9: 1390. https://doi.org/10.3390/plants14091390
APA StyleGloria-Garza, M. A., Reyna-Martínez, G. R., Jiménez-Salas, Z., Campos-Góngora, E., Kačániová, M., Aguirre-Cavazos, D. E., Bautista-Villarreal, M., Leos-Rivas, C., & Elizondo-Luevano, J. H. (2025). Medicinal Plants Against Dental Caries: Research and Application of Their Antibacterial Properties. Plants, 14(9), 1390. https://doi.org/10.3390/plants14091390









