Bacillus Bio-Organic Fertilizer Altered Soil Microorganisms and Improved Yield and Quality of Radish (Raphanus sativus L.)
Abstract
1. Introduction
2. Results
2.1. Soil Chemical Properties and Enzyme Activities
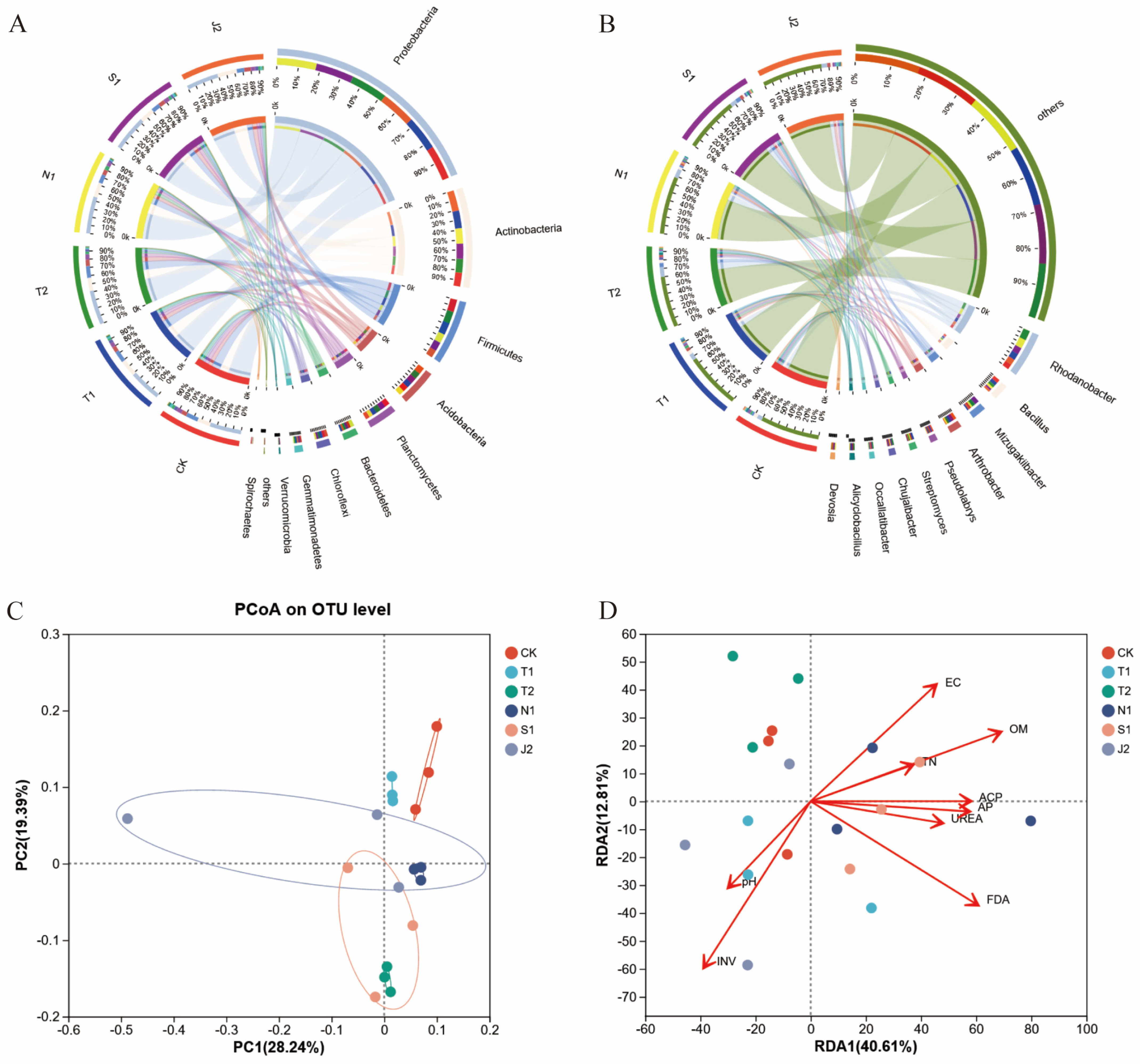
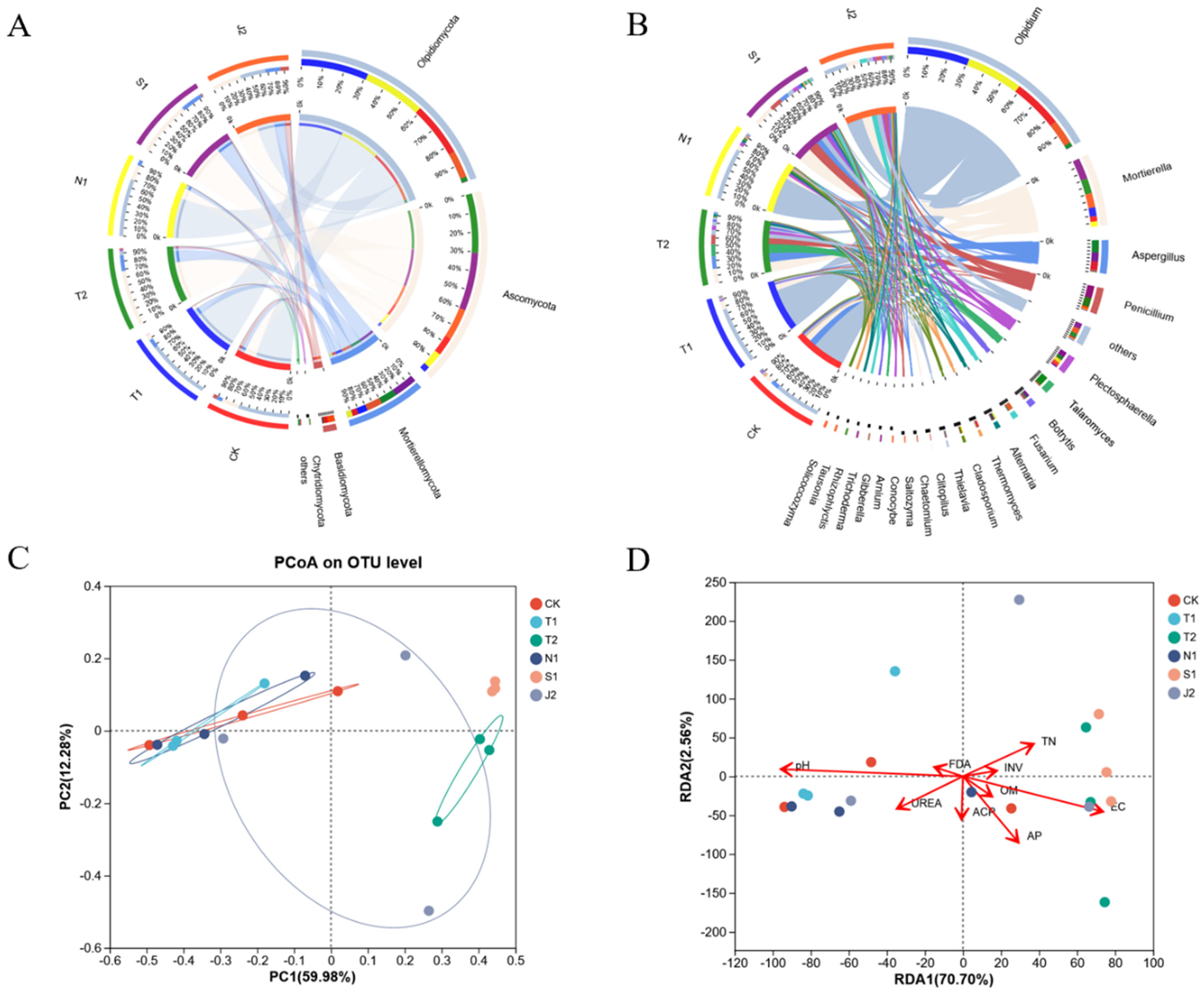
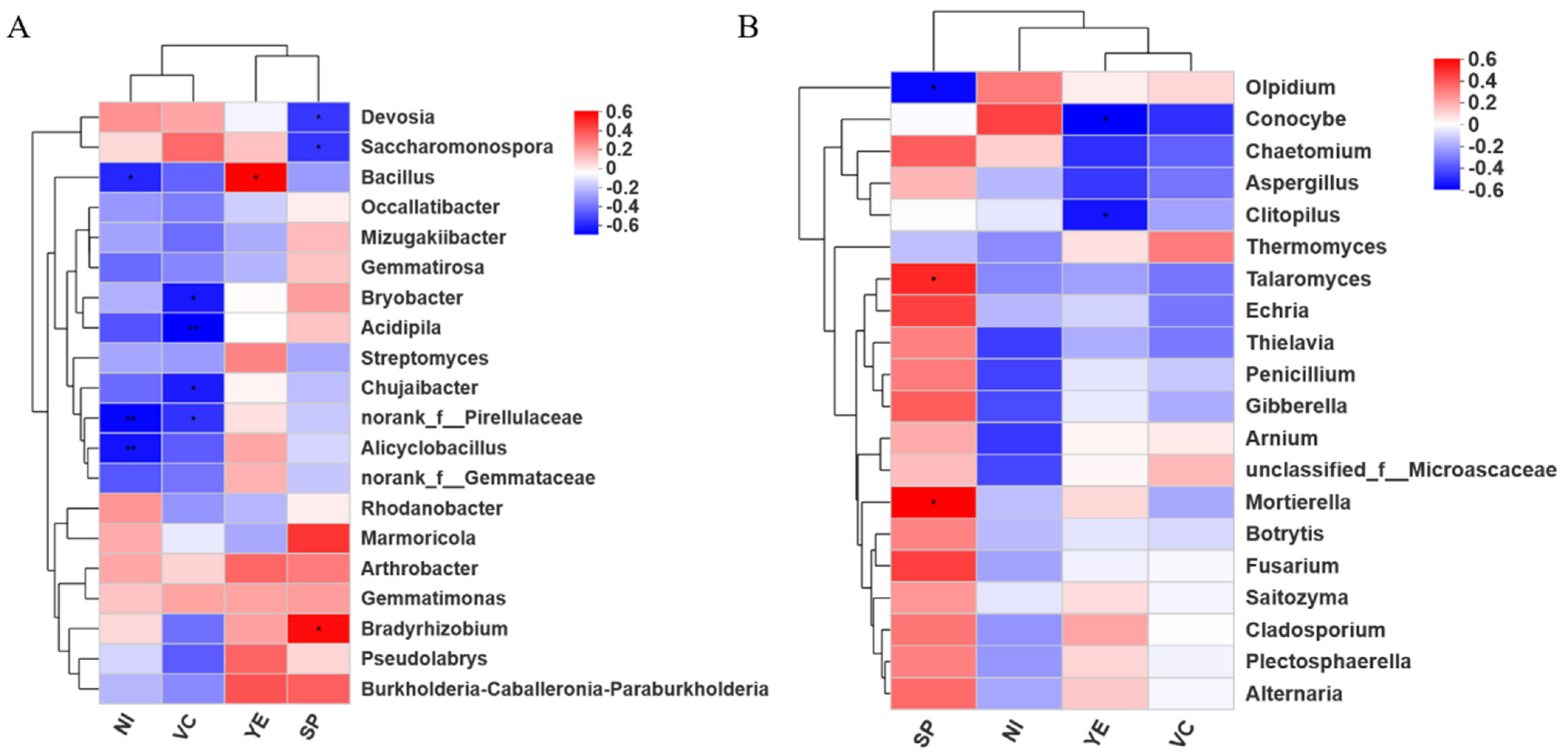
2.2. Soil Microbiomes
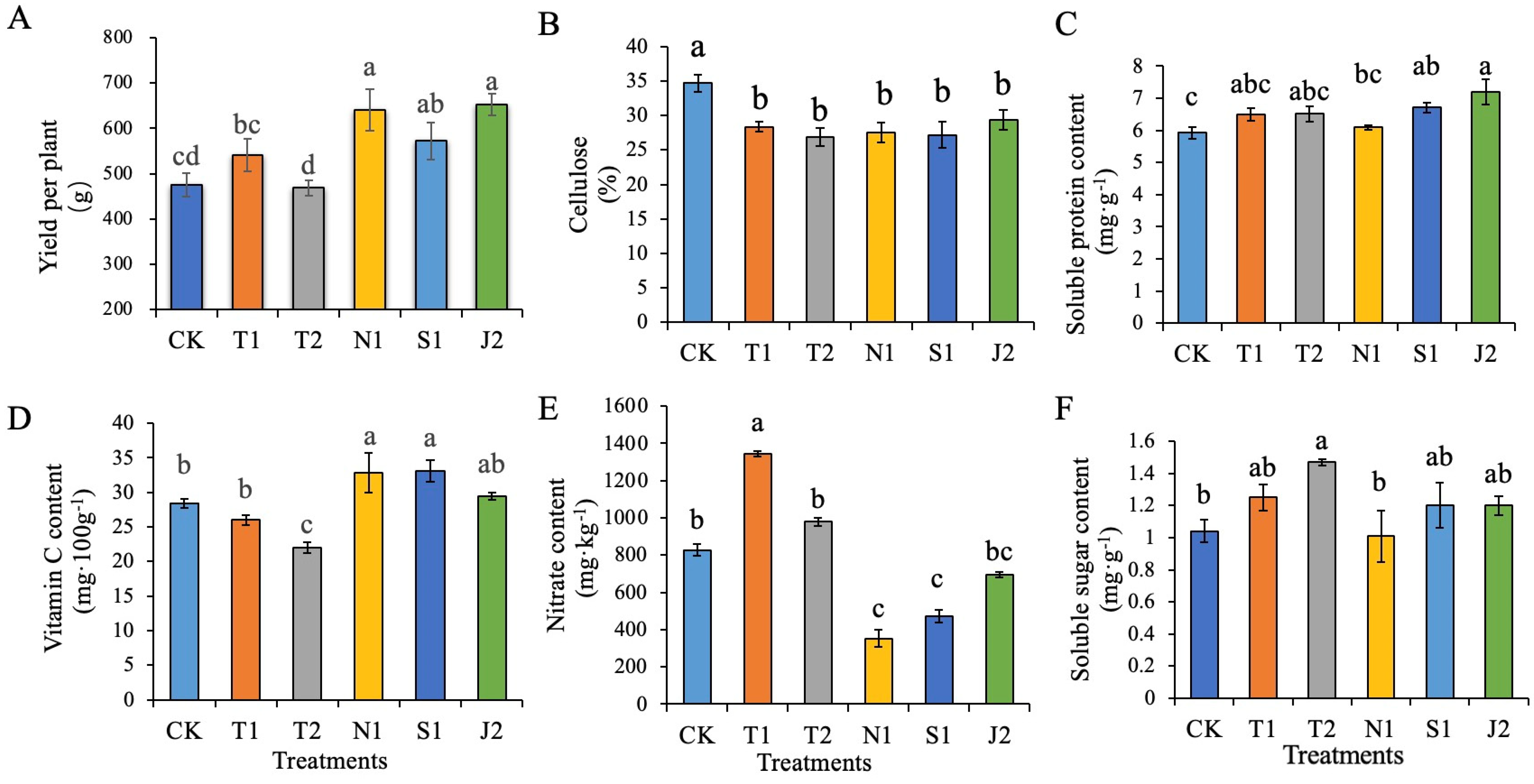
2.3. Photosynthetic Characteristics, Growth, Yield and Quality
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Design
4.3. Determination Indexes and Methods
4.3.1. Determination of Rhizosphere Soil Characteristics
4.3.2. DNA Extraction and PCR Amplification
4.3.3. Growth, Yield and Quality
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Wang, X.; Ma, L.; Chadwick, D.; Chen, X. Combined applications of organic and synthetic nitrogen fertilizers for improving crop yield and reducing reactive nitrogen losses from China’s vegetable systems: A meta-analysis. Environ. Pollut. 2021, 269, 116143. [Google Scholar] [CrossRef]
- Yang, F.; Tian, J.; Fang, H.; Gao, Y.; Xu, M.; Lou, Y.; Zhou, B.; Kobyakov, Y. Functional soil organic matter fractions, microbial community, and enzyme activities in a mollisol under 35 years manure and mineral fertilization. J. Soil Sci. Plant Nutr. 2019, 19, 430–439. [Google Scholar] [CrossRef]
- Zhu, J.; Peng, H.; Ji, X.; Li, C.; Li, S. Effects of reduced inorganic fertilization and rice straw recovery on soil enzyme activities and bacterial community in double-rice paddy soils. Eur. J. Soil Biol. 2019, 94, 103116. [Google Scholar] [CrossRef]
- Miao, Y.; Stewart, B.A.; Zhang, F. Long-term experiments for sustainable nutrient management in China. Rev. Agron. Sustain. Dev. 2011, 31, 397–414. [Google Scholar] [CrossRef]
- Flores-Félix, J.D.; Menéndez, E.; Rivera, P.L.; Marcos, M.R.; Hidalogo, M.P.; Mateos, P.; Martínez-Molina, E.; Velázquez, M.d.l.E.; García-Fraile, P.; Rivas, R. Use of rhizobium leguminosarum as a potential biofertilizer for lactuca sativa and daucus carota crops. J. Plant Nutr. Soil Sci. 2013, 176, 876–882. [Google Scholar] [CrossRef]
- Mahanta, R.; Bhattacharyya, R.; Gopinath, K.A.; Tuti, M.D.; Jeevanandan, K.; Chandrashekara, C.; Arunkumar, R.; Mina, B.L.; Pandey, B.M.; Mishra, P.K.; et al. Influence of farm yard manure application and mineral fertilization on yield sustainability, carbon sequestration potential and soil property of gardenpea–french bean. cropping system in the Indian Himalayas. Sci. Hortic. 2013, 164, 414–427. [Google Scholar] [CrossRef]
- Zhao, Z.; He, J.; Quan, Z.; Wu, C.; Sheng, R.; Zhang, L.; Geisen, S. Fertilization changes soil microbiome functioning, especially phagotrophic protists. Soil Biol. Biochem. 2020, 148, 107863. [Google Scholar] [CrossRef]
- Kang, A.; Zhang, N.; Xun, W.; Dong, X.; Xiao, M.; Liu, Z.; Xu, Z.; Feng, H.; Zou, J.; Shen, Q.; et al. Nitrogen fertilization modulates beneficial rhizosphere interactions through signaling effect of nitric oxide. Plant Physiol. 2021, 188, 1129–1140. [Google Scholar] [CrossRef]
- Gao, H.; Xi, Y.; Wu, X.; Pei, X.; Liang, G.; Bai, J.; Song, X.; Zhang, M.; Liu, X.; Han, Z.; et al. Partial substitution of manure reduces nitrous oxide emission with maintained yield in a winter wheat crop. J. Environ. Manag. 2023, 326, 116794. [Google Scholar] [CrossRef]
- Li, R.; Tao, R.; Ling, N.; Chu, G. Chemical, organic and bio-fertilizer management practices effect on soil physicochemical property and antagonistic bacteria abundance of a cotton field: Implications for soil biological quality. Soil Tillage Res. 2017, 167, 30–38. [Google Scholar] [CrossRef]
- Shen, W.; Lin, X.; Gao, N.; Zhang, H.; Yin, R.; Shi, W.; Duan, Z. Land use intensification affects soil microbial populations, functional diversity and related suppressiveness of cucumber Fusarium wilt in China’s Yangtze River Delta. Plant Soil 2008, 306, 117–127. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, X.; Bao, E.; Li, J.; Zou, Z.; Cao, K. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020, 10, 177. [Google Scholar] [CrossRef]
- Jin, N.; Jin, L.; Wang, Y.; Li, J.; Liu, F.; Liu, Z.; Luo, S.; Wu, Y.; Lyu, J.; Yu, J. Reduced chemical fertilizer combined with bio-organic fertilizer affects the soil microbial community and yield and quality of lettuce. Front. Microbiol. 2022, 13, 4. [Google Scholar] [CrossRef]
- Silvosa, S.M.; Macapeges, A.R.; Abad, R.G.; Bayogan, E.R. Growth and quality of screenhouse-grown radish in various compost amendments. J. Crop Improv. 2021, 35, 582–603. [Google Scholar] [CrossRef]
- Agnihotri, K.; Murari, D.; Pavitra, K. Effect of different organic manures on growth and yield parameters of radish (Raphanus Sativus L.) cv. Japanese white. SAARC J. Agric. 2021, 21, 17–22. [Google Scholar] [CrossRef]
- Cai, C.; Wei, P.; Ran, R.; Shen, Q. Colonization of Trichoderma harzianum strain SQR-T037 on tomato roots and its relationship to plant growth, nutrient availability and soil microflora. Plant Soil 2015, 388, 337–350. [Google Scholar] [CrossRef]
- Feng, N.; Liang, Q.; Feng, Y.; Xiang, L.; Zhao, H.; Li, Y.; Li, H.; Cai, Q.; Mo, C.; Wong, M. Improving yield and quality of vegetable grown in PAEs-contaminated soils by using novel bioorganic fertilizer. Sci. Total Environ. 2020, 739, 139883. [Google Scholar] [CrossRef]
- Robson, A. Soil Acidity and Plant Growth; Academic Press: Cambridge, MA, USA, 1989. [Google Scholar]
- Xiang, J.; Gu, J.; Wang, G.; Roland, B.; Yao, L.; Fang, Y.; Zhang, H. Soil pH controls the structure and diversity of bacterial communities along elevational gradients on Huangshan, China. Eur. J. Soil Biol. 2024, 120, 103586. [Google Scholar] [CrossRef]
- López, A.; Fenoll, J.; Hellín, P.; Flores, P. Physical characteristics and mineral composition of two pepper cultivars under organic, conventional and soilless cultivation. Sci. Hortic. 2013, 150, 259–266. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, F.; Zhou, R.; Wu, Y.; Hou, X.; Li, J.; Lin, W.; Wu, Z. Effects of reduced nitrogen with bio-organic fertilizer on soil properties, yield and quality of non-heading Chinese cabbage. Agronomy 2021, 11, 2196. [Google Scholar] [CrossRef]
- Pankhurst, C. Defining and assessing soil health and sustainable productivity. Biol. Fertil. Soils 1995, 19, 269–279. [Google Scholar]
- Zhong, S.; Zeng, H.; Jin, Z. Soil microbiological and biochemical properties as affected by different long-term banana-based rotations in the tropics. Pedosphere 2015, 25, 868–877. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Chen, Y.; Wu, L.; Huang, G.; Lv, J. Soil nitrogen cycling gene abundances in response to organic amendments: A meta-analysis. Sci. Total Environ. 2024, 921, 171048. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Soil fluorescein diacetate hydrolase activity in natural and degraded soil—A Review. Environ. Ecol. 2011, 29, 1699–1705. [Google Scholar]
- Yang, P.; Jian, L.; Sohail, H.; Yu, J.; Xie, J.; Li, J. Partial substitution of mineral fertilizer with biofertilizer enhances cauliflower nutritional quality, yield, and soil characteristics. Crop Sci. 2020, 60, 934–994. [Google Scholar] [CrossRef]
- Gou, J.; Suo, S.; Shao, K.; Zhao, Q.; Yao, D.; Li, H.; Zhang, J.; Rensing, C. Biofertilizers with beneficial rhizobacteria improved plant growth and yield in chili (Capsicum annuum L. ). World J. Microbiol. Biotechnol. 2020, 36, 86. [Google Scholar] [CrossRef]
- Wang, N.; Nan, H.; Feng, K. Effects of reduced chemical fertilizer with organic fertilizer application on soil microbial biomass, enzyme activity and cotton yield. Chin. J. Appl. Ecol. 2020, 31, 173–181. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, W.; Burger, M.; Yang, L.; Gong, P.; Wu, Z. Changes in soil carbon and enzyme activity as a result of different long-term fertilization regimes in a greenhouse field. PLoS ONE 2015, 10, e0118371. [Google Scholar] [CrossRef]
- Shen, Z.; Ruan, Y.; Chao, X.; Zhang, J.; Li, R.; Shen, Q. Rhizosphere microbial community manipulated by 2 years of consecutive biofertilizer application associated with banana fusarium wilt disease suppression. Biol. Fertil. Soil 2015, 51, 553–562. [Google Scholar] [CrossRef]
- Xiong, W.; Guo, S.; Jousset, A.; Zhao, Q.; Wu, H.; Li, R.; Kowalchuk, G.A.; Shen, Q. Bio-fertilizer application induces soil suppressiveness against fusarium wilt disease by reshaping the soil microbiome. Soil Biol. Biochem. 2017, 114, 238–247. [Google Scholar] [CrossRef]
- Fu, L.; Penton, R.; Ruan, Y.; Shen, Z.; Xue, C.; Li, R.; Shen, Q. Inducing the rhizosphere microbiome by biofertilizer application to suppress banana fusarium wilt disease. Soil Biol. Biochem. 2017, 104, 39–48. [Google Scholar] [CrossRef]
- Liao, J.; Ye, J.; Liang, Y.; Khalid, M.; Huang, D. Pakchoi antioxidant improvement and differential rhizobacterial community composition under organic fertilization. Sustainability 2019, 11, 2424. [Google Scholar] [CrossRef]
- Zhao, Z.; He, Z.; Geisen, S.; Han, L.; Wang, J.; Shen, J.; Wei, W.; Fang, Y.; Li, P.; Zhang, L. Protist communities are more sensitive to nitrogen fertilization than other microorganisms in diverse agricultural soils. Microbiome 2019, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Takaku, H.; Kodaira, S.; Kimoto, A.; Nashimoto, M.; Takagi, M. Microbial communities in the garbage composting with rice hull as an amendment revealed by culture-dependent and-independent approaches. J. Biosci. Bioeng. 2006, 101, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Ariesyady, H.; Ito, T.; Okabe, S. Functional bacterial and archaeal community structures of major trophic groups in a full-scale anaerobic sludge digester. Water Res. 2007, 41, 1554–1568. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral Vs. Organic amendments: Microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef]
- Bacon, C.W.; Palencia, E.R.; Hinton, D.M. Abiotic and biotic plant stress-tolerant and beneficial secondary metabolites produced by endophytic Bacillus species. In Plant Microbes Symbiosis: Applied Facets; Springer: New Delhi, India, 2015; pp. 163–177. [Google Scholar] [CrossRef]
- Gao, C.; EI-Sawah, A.M.; Ali, D.F.; Hamoud, Y.A.; Shaghaleh, H.; Sheteiwy, M. The integration of bio and organic fertilizers improve plant growth, grain yield, quality and metabolism of hybrid maize (Zea mays L.). Agron. J. 2020, 10, 319. [Google Scholar] [CrossRef]
- Liu, C.; Xia, R.; Tang, M.; Chen, X.; Zhong, B.; Liu, X.; Bian, R.; Yang, L.; Zheng, J.; Cheng, K.; et al. Improved ginseng production under continuous cropping through soil health reinforcement and rhizosphere microbial manipulation with biochar: A field study of Panax ginseng from Northeast China. Hortic. Res. 2022, 2022, 9. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, J.; Chen, D.; Yue, Y.; Wang, L.; Yang, X. A new strategy for sustainable agricultural development: Meta-analysis of the efficient interaction of plant growth-promoting rhizobacteria with nanoparticles. Plant Physiol. Biochem. 2025, 223, 109845. [Google Scholar] [CrossRef]
- Wang, J.; Xu, S.; Yang, R.; Zhao, W.; Zhu, D.; Zhang, X.; Huang, Z. Bacillus amyloliquefaciens fh-1 significantly affects cucumber seedlings and the rhizosphere bacterial community but not soil. Sci. Rep. 2021, 11, 12055. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, C.; Farmer, J.; Sun, J. Effects of bio-organic fertilizer on pepper growth and Fusarium wilt biocontrol. Sci. Hortic. 2015, 193, 114–120. [Google Scholar] [CrossRef]
- Yuan, J.; Ruan, Y.; Wang, B.; Zhang, J.; Waseem, R.; Huang, Q.; Shen, Q. Plant growth-promoting rhizobacteria strain Bacillus amyloliquefaciens NJN-6-enriched bio-organic fertilizer suppressed Fusarium wilt and promoted the growth of banana plants. J. Agric. Food Chem. 2013, 61, 3774–3780. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, Y.; Meng, L.; Li, H.; Fu, H. Simultaneous determination of total nitrogen and organic carbon in soil with an elemental analyzer. Chin. J. Anal. Lab. 2013, 32, 41–45. [Google Scholar]
- Raigón, M.; García, M.; Maquieira, A.; Puchades, R. Determination of available nitrogen (nitic and ammoniacal) in soils by flow-injection analysis. Chemistry 1992, 20, 483–487. [Google Scholar]
- Bao, S. Analysis Method of Soil and Agricultural Chemistry; China Agricultural Press: Beijing, China, 2000; pp. 25–108. [Google Scholar]
- Sun, X.; Zhu, L.; Wang, J.; Wang, J.; Su, B.; Liu, T.; Zhang, C.; Gao, C.; Shao, Y. Toxic effects of ionic liquid 1-octyl-3-methylimidazolium tetrafluoroborate on soil enzyme activity and soil microbial community diversity. Ecotoxicol. Environ. Saf. 2017, 135, 201208. [Google Scholar] [CrossRef]
- Taylor, J.; Wilson, B.; Mills, M.; Burns, R. Comparison of microbial numbers and enzymatic activities in surface soils and subsoils using various techniques. Soil Biol. Biochem. 2002, 34, 387–401. [Google Scholar] [CrossRef]
- Guan, Y. (Ed.) Methodology of soil enzyme measurement. In Methods of Soil Enzymology; China Agricultural Press: Beijing, China, 1986; pp. 274–314. [Google Scholar]
- Bates, S.; Berg, L.D.; Caporaso, J.; Walters, W.; Fierer, N. Examining the global distribution of dominant archaeal populations in soil. ISME J. 2011, 5, 908–917. [Google Scholar] [CrossRef]
- Rousk, J.; Baath, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Li, H. Principles and Techniques of Plant Physiology and Biochemistry Experiments; Higher Education Press: Beijing, China, 2000; Volume 7, pp. 134–136. [Google Scholar]
- Koudela, M.; Petkikova, K. Nutritional composition and yield of endive cultivars-Cichorium endivia L. Hortic. Sci. 2007, 34, 6–10. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996, 132, 361–373. [Google Scholar] [CrossRef] [PubMed]
| Treatments | pH | EC ms·m−1 | Organic Matter g·kg−1 | Available Phosphorus g·kg−1 | Total Nitrogen g·kg−1 | Ammonium Nitrogen g·kg−1 | Nitrate Nitrogen g·kg−1 |
|---|---|---|---|---|---|---|---|
| CK | 6.10 ± 0.02 c | 116.07 ± 18.08 c | 18.50 ± 0.71 b | 16.78 ± 2.6 ab | 1.84 ± 0.30 c | 1.00 ± 0.47 c | 20.30 ± 1.76 ab |
| TI | 5.87 ± 0.02 d | 165.07 ± 27.95 bc | 19.22 ± 1.78 b | 19.1 ± 1.24 ab | 2.26 ± 0.49 b | 0.39 ± 0.11 d | 18.30 ± 1.31 b |
| T2 | 6.05 ± 0.02 c | 238.00 ± 11.14 b | 23.25 ± 0.21 ab | 20.22 ± 3.4 ab | 2.35 ± 0.24 b | 1.83 ± 1.27 b | 23.70 ± 2.11 a |
| N1 | 6.42 ± 0.03 a | 225.33 ± 5.55 b | 28.09 ± 0.66 a | 22.64 ± 0.74 a | 2.65 ± 0.28 a | 2.51 ± 0.80 a | 23.50 ± 0.8 a |
| S1 | 6.29 ± 0.05 b | 310.00 ± 6.08 a | 24.48 ± 3.78 ab | 25.69 ± 0.93 a | 2.35 ± 0.17 b | 0.94 ± 1.27 c | 18.65 ± 1.48 b |
| J2 | 6.10 ± 0.02 c | 105.53 ± 9.90 c | 17.97 ± 0.85 b | 13.56 ± 1.15 b | 2.41 ± 1.06 b | 2.9 ± 1.02 a | 20.36 ± 0.25 ab |
| Treatments | Mineral Fertilizer | Bio-Organic Fertilizer | ||||
|---|---|---|---|---|---|---|
| N kg·ha−1 | P2O5 kg·ha−1 | K2O kg·ha−1 | NO. 1 kg·ha−1 | Seek kg·ha−1 | Jiajiapei kg·ha−1 | |
| No fertilizer (CK) | — | — | — | — | — | — |
| Conventional fertilizer (T1) | 168 | 150 | 195 | — | — | — |
| 80% N (T2) | 134.4 | 150 | 195 | — | — | — |
| 80% N + 20% NO. 1 (N1) | 89.4 | 75 | 184.5 | 1500 | — | — |
| 80% N + 20% Seek (S2) | 89.4 | 75 | 184.5 | — | 1500 | — |
| 80% N + 20% Jiajiapei (J2) | 120.8 | 128.4 | 184.2 | — | — | 1080 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Wu, Z.; Wang, Y.; Zhou, R.; Liu, L.; Wang, Y.; Zhao, J.; Jiang, F. Bacillus Bio-Organic Fertilizer Altered Soil Microorganisms and Improved Yield and Quality of Radish (Raphanus sativus L.). Plants 2025, 14, 1389. https://doi.org/10.3390/plants14091389
Qi Y, Wu Z, Wang Y, Zhou R, Liu L, Wang Y, Zhao J, Jiang F. Bacillus Bio-Organic Fertilizer Altered Soil Microorganisms and Improved Yield and Quality of Radish (Raphanus sativus L.). Plants. 2025; 14(9):1389. https://doi.org/10.3390/plants14091389
Chicago/Turabian StyleQi, Yingbin, Zhen Wu, Yachen Wang, Rong Zhou, Liwang Liu, Yan Wang, Jiying Zhao, and Fangling Jiang. 2025. "Bacillus Bio-Organic Fertilizer Altered Soil Microorganisms and Improved Yield and Quality of Radish (Raphanus sativus L.)" Plants 14, no. 9: 1389. https://doi.org/10.3390/plants14091389
APA StyleQi, Y., Wu, Z., Wang, Y., Zhou, R., Liu, L., Wang, Y., Zhao, J., & Jiang, F. (2025). Bacillus Bio-Organic Fertilizer Altered Soil Microorganisms and Improved Yield and Quality of Radish (Raphanus sativus L.). Plants, 14(9), 1389. https://doi.org/10.3390/plants14091389









