Non-Target Metabolomics Reveals Changes in Metabolite Profiles in Distant Hybrid Incompatibility Between Paeonia sect. Moutan and P. lactiflora
Abstract
1. Introduction
2. Results and Discussion
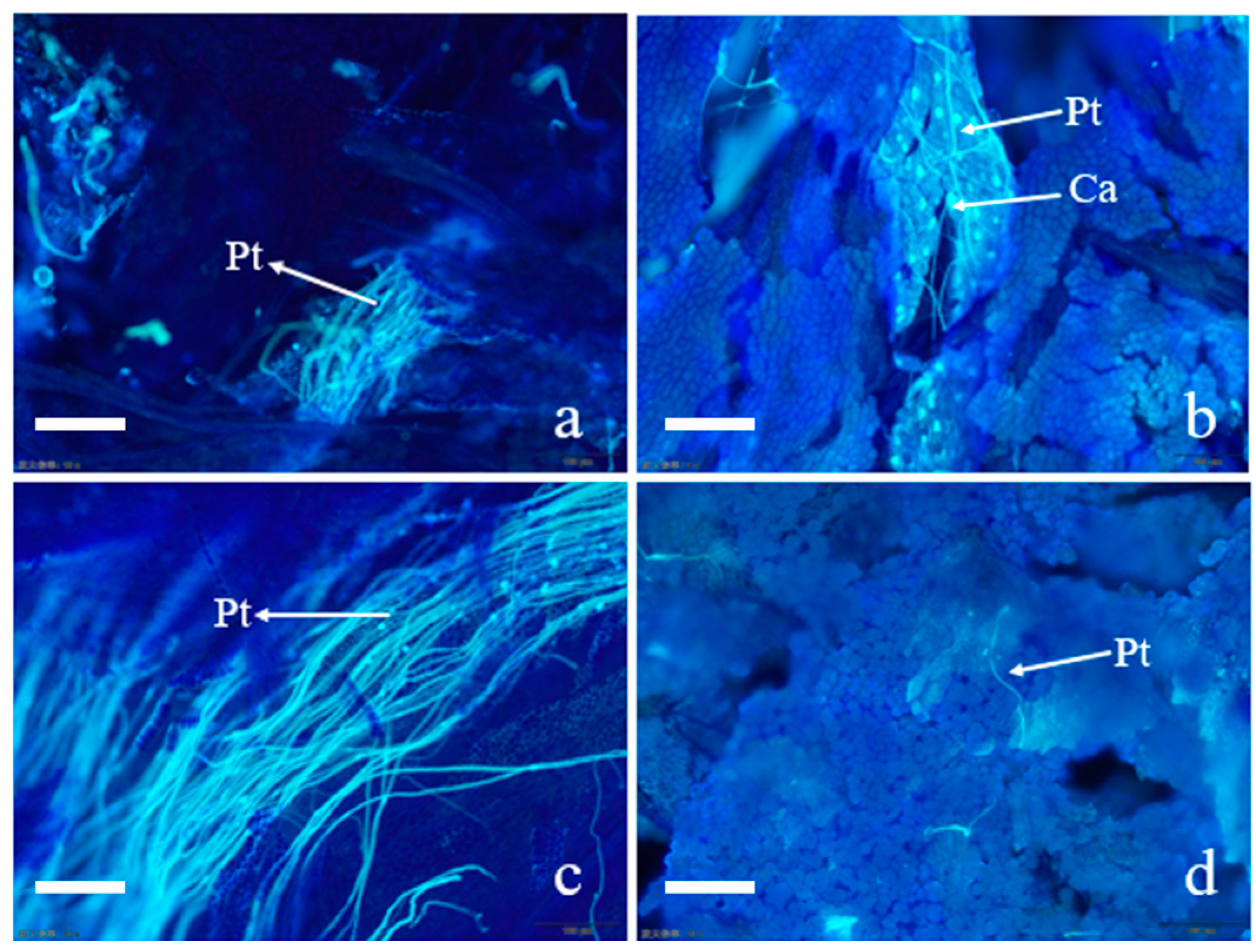
2.1. Effect of Cross-Pollination on Pollen Tube Growth
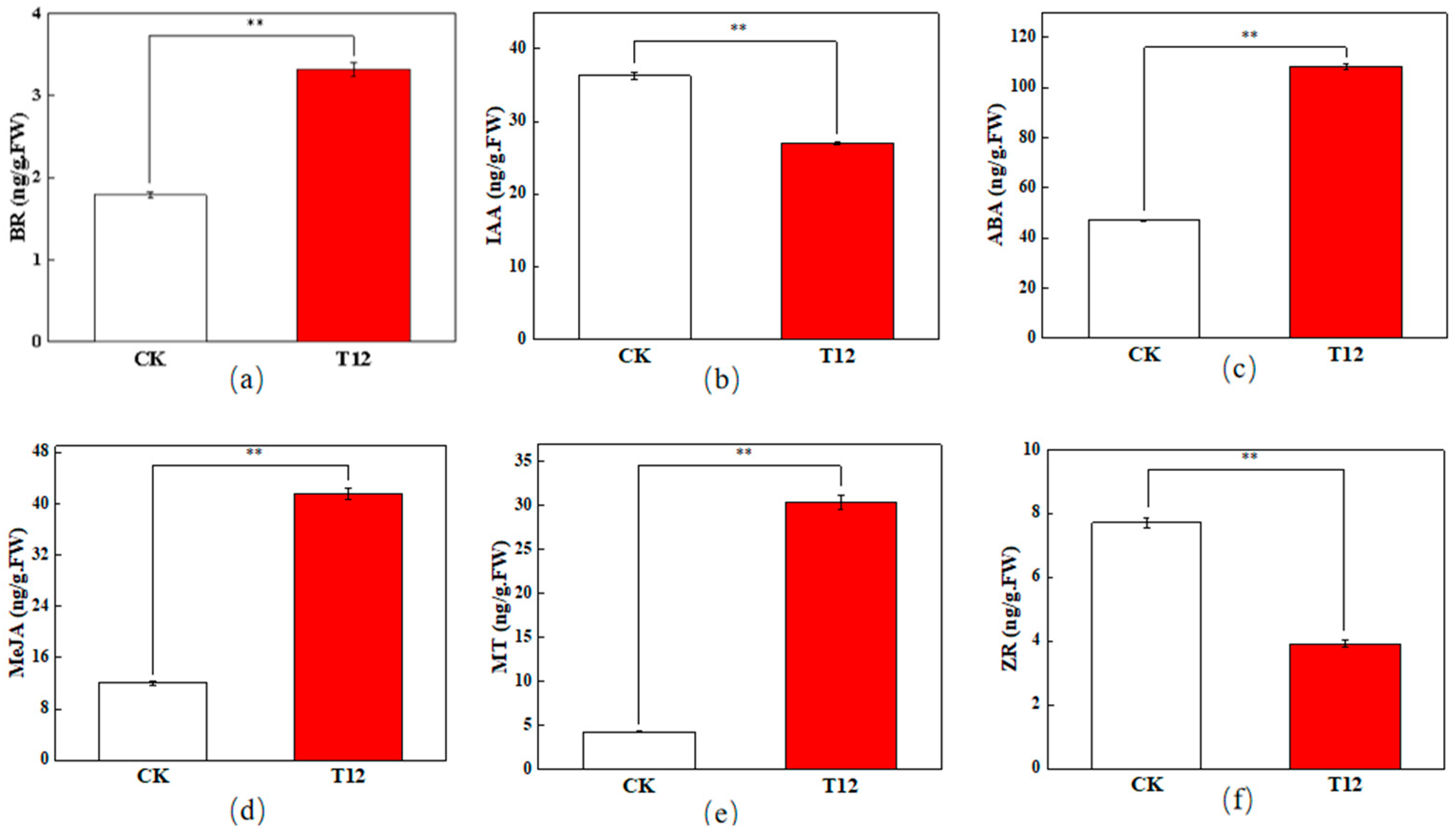
2.2. Changes in ZR, IAA, ABA, BR, MeJA, and MT Contents
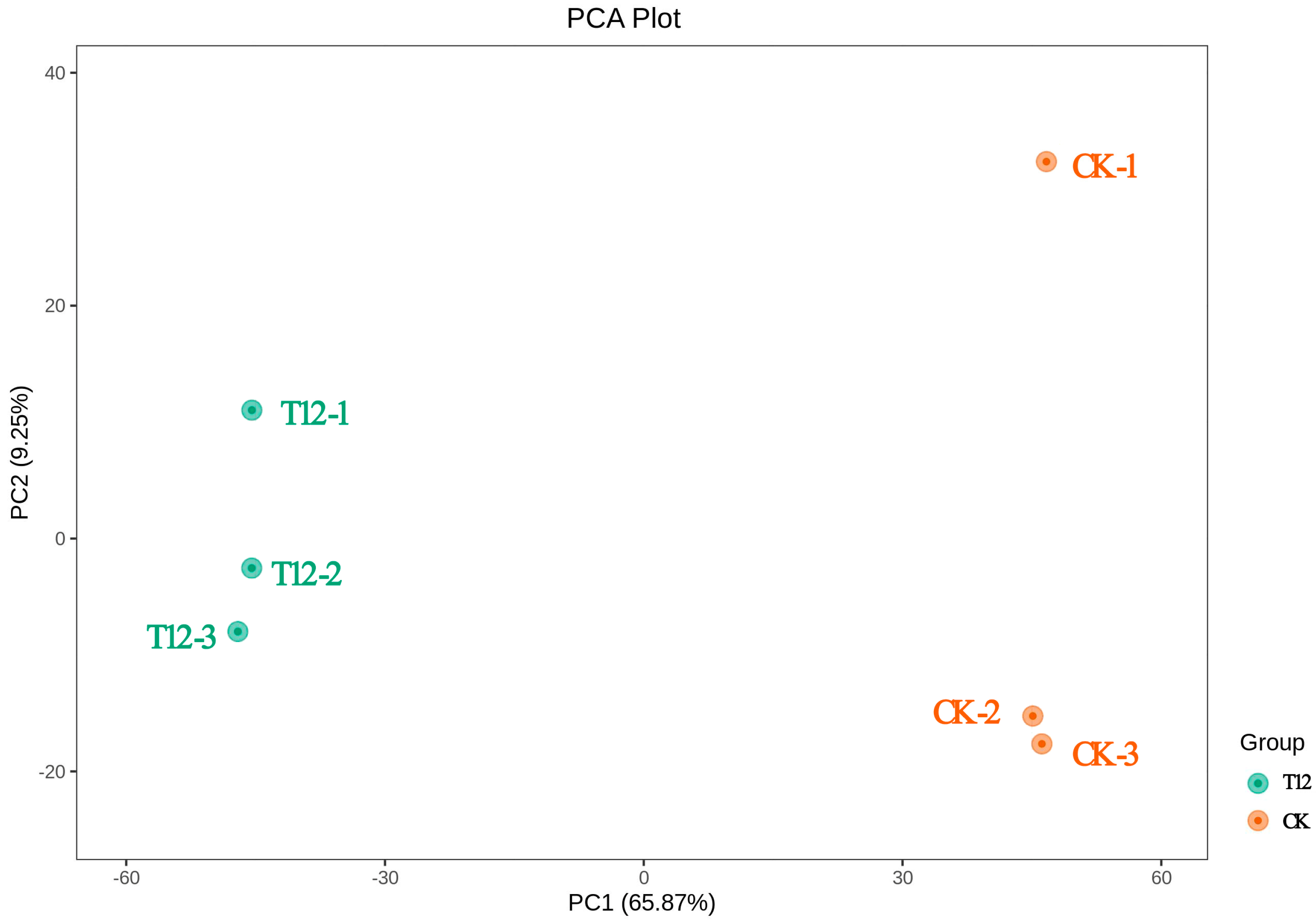
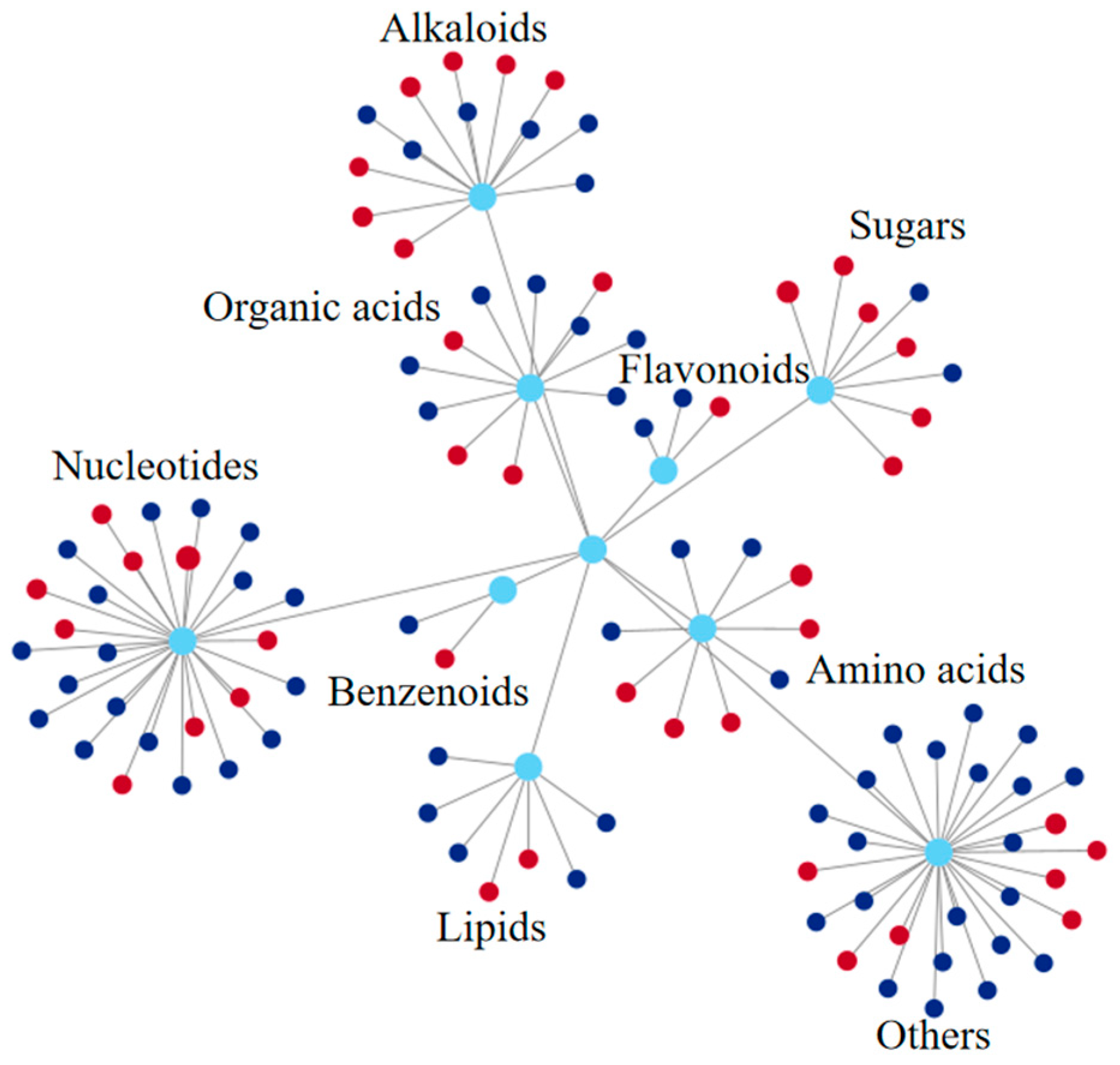
2.3. Metabolome Analysis
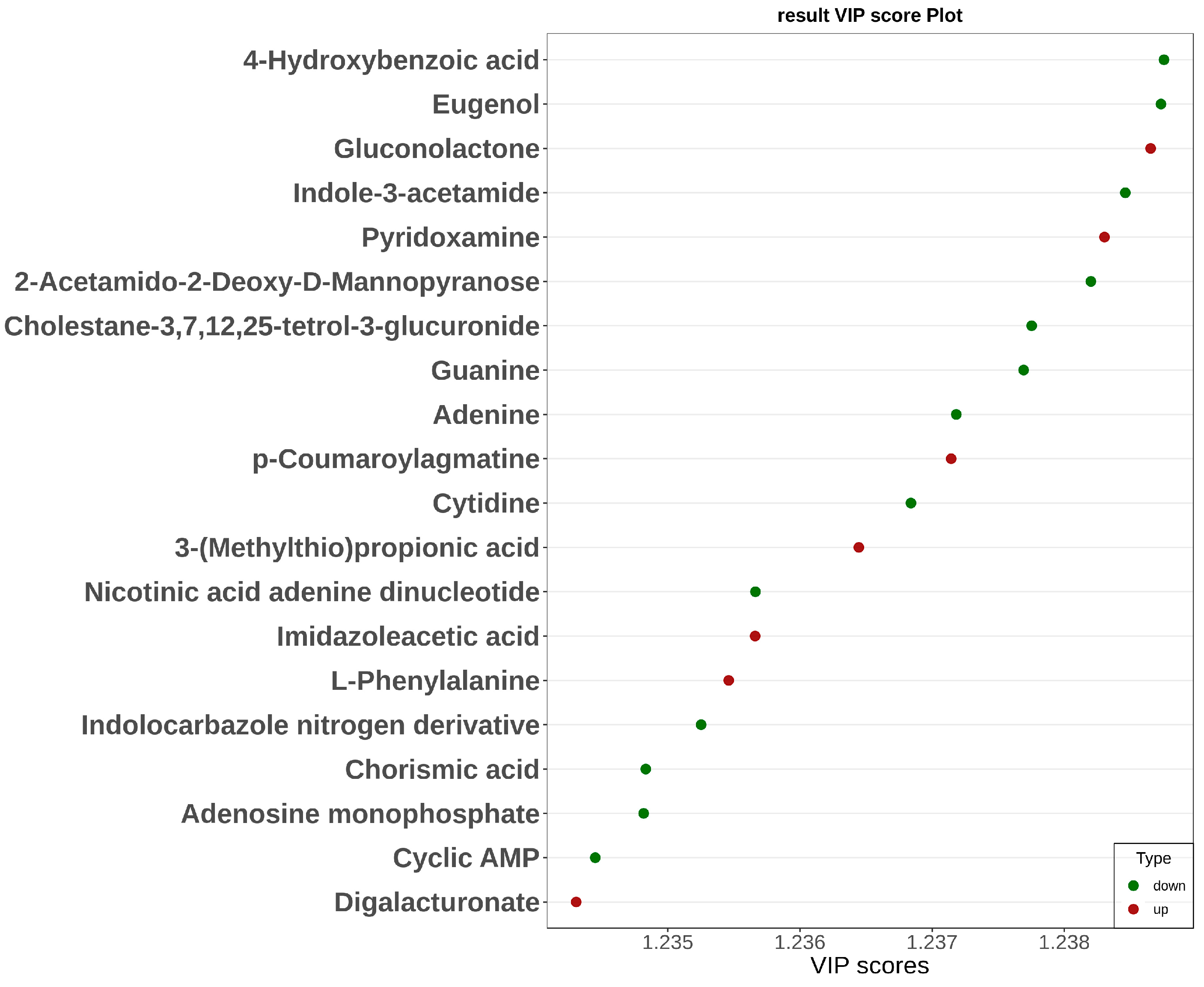
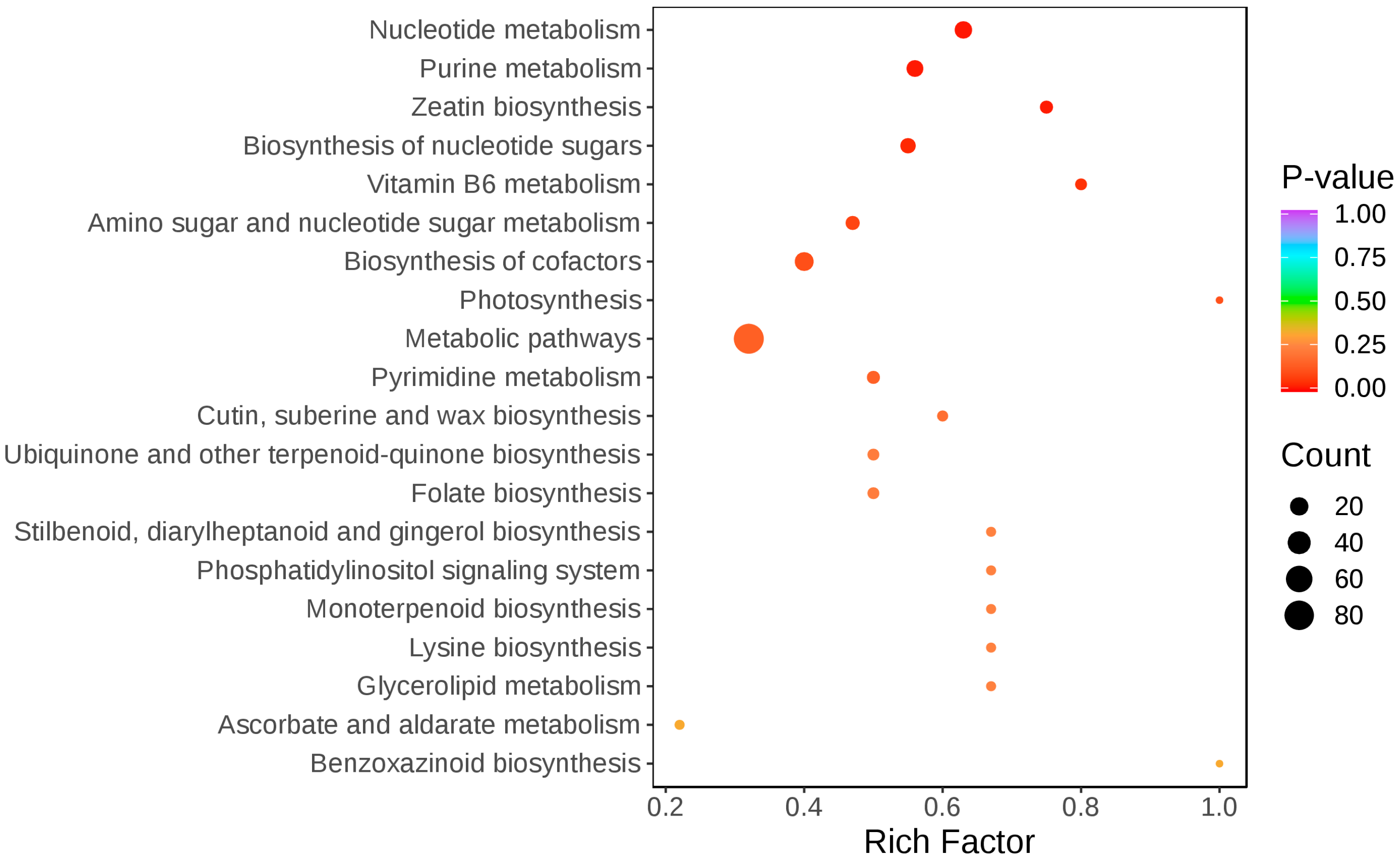
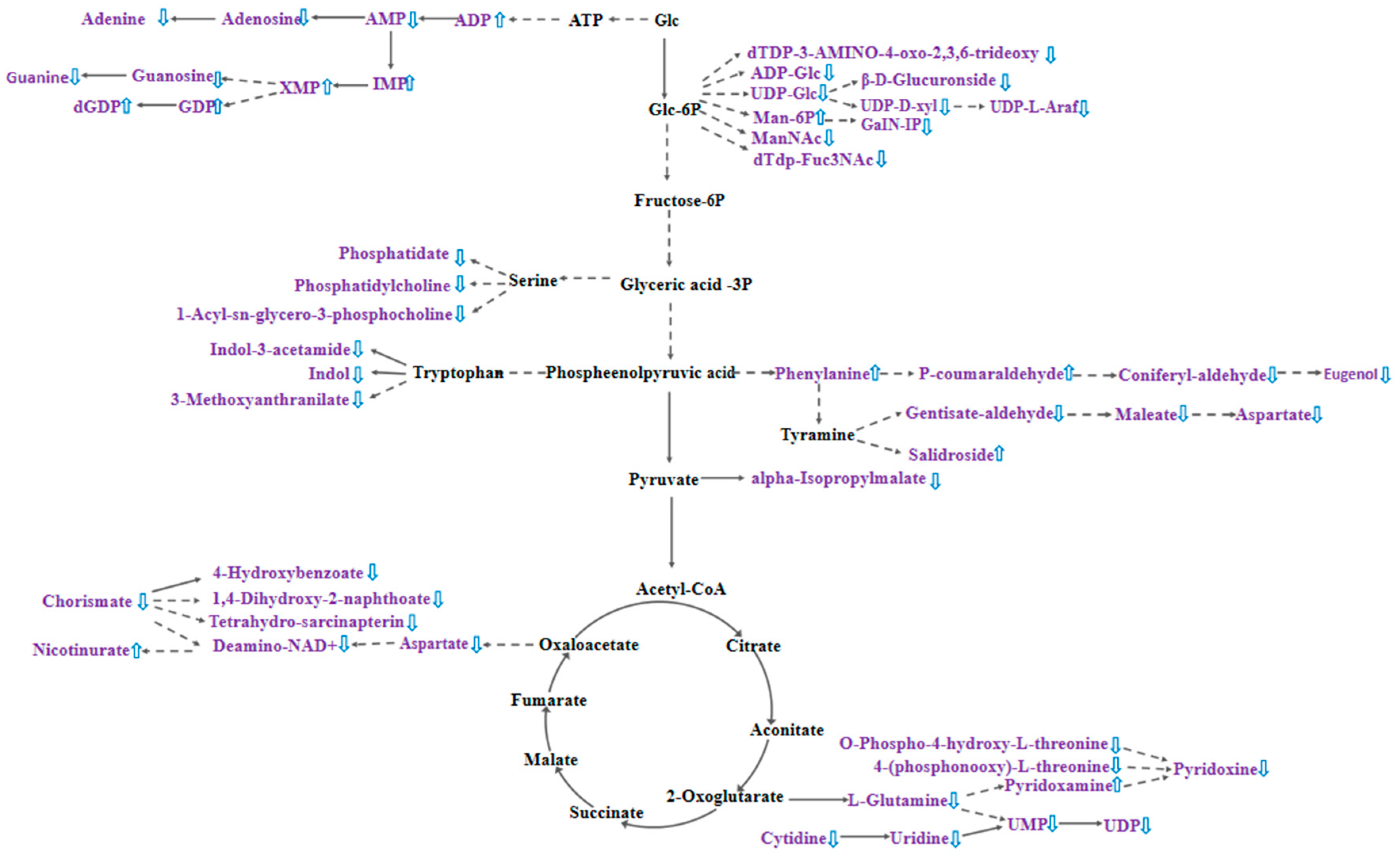
2.3.1. Changes in Metabolic Pathways
2.3.2. Changes in Sugars
2.3.3. Changes in Amino Acids
2.3.4. Changes in Nucleotides
2.3.5. Changes in Lipids
2.3.6. Changes in Flavonoids
2.3.7. Changes in Alkaloids
2.3.8. Changes in Organic Acids
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Pollen Tube Fluorescence Observation
3.2.2. Determination of Endogenous Hormone Content
3.2.3. Metabolite Identification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, S.-L.; Xu, C.; Liu, J.; Yu, Y.; Wu, P.; Cheng, T.; Hong, D.-Y. Out of the Pan-Himalaya: Evolutionary history of the Paeoniaceae revealed by phylogenomics. J. Syst. Evol. 2021, 59, 1170–1182. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, M.; Li, S.; Chen, Q.; Teixeira da Silva, J.A.; Wang, A.; Yu, X.; Wang, L. Germplasm resources and genetic breeding of Paeonia: A systematic review. Hortic. Res. 2020, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, J.; Fang, H.; Li, Z.; Li, M. Advances and challenges in medicinal plant breeding. Plant Sci. 2020, 298, 110573. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Zhao, Y.; Qiao, M.; Huang, Z.; Wei, L.; Zhou, Q.; Lu, W.; Sun, G.; Huang, Z.; Gao, H. Molecular dissection of the parental contribution in Paeonia Itoh hybrids. Plant Physiol. 2024, 196, 1953–1964. [Google Scholar] [CrossRef]
- He, D.; Guo, H.; He, S.; Zhang, M.; Chang, Y.; Wang, Z.; Liu, Y. Transcriptome analysis reveals the role of phytohormones in the distant hybridization of Peony embryo abortion. Horticulturae 2023, 9, 694. [Google Scholar] [CrossRef]
- Zhong, Y.; Du, M.-J.; Ji, R.-Z.; Rong, Q.; Guan, Z.-H.; Cheng, F.-Y. Cytogenetic analysis reveals a mechanism of compatibility in distant hybridization between tree peony and herbaceous peony. Euphytica 2024, 220, 66. [Google Scholar] [CrossRef]
- Wang, L.; Filatov, D.A. Mechanisms of prezygotic post-pollination reproductive barriers in plants. Front. Plant Sci. 2023, 14, 1230278. [Google Scholar] [CrossRef]
- Gong, H.; Chang, Y.; Xu, J.; Yu, X.; Gong, W. Unilateral cross-incompatibility between Camellia oleifera and C. yuhsienensis provides new insights for hybridization in Camellia spp. Front. Plant Sci. 2023, 14, 1182745. [Google Scholar] [CrossRef]
- Hao, Z.; Li, Y.; Yang, Y.; Song, J.; Meng, J.; Guan, W. Studies on distant hybridization compatibility between the Azalea (Rhododendron × hybridum hort.) and the Rhododendron decorum Franch. Native to China. Horticulturae 2024, 10, 1089. [Google Scholar] [CrossRef]
- Subasinghe Arachchige, E.C.W.; Evans, L.J.; Samnegård, U.; Rader, R. Morphological characteristics of pollen from triploid watermelon and its fate on stigmas in a hybrid crop production system. Sci. Rep. 2022, 12, 3222. [Google Scholar] [CrossRef]
- Kuligowska, K.; Lütken, H.; Christensen, B.; Skovgaard, I.; Linde, M.; Winkelmann, T.; Müller, R. Evaluation of reproductive barriers contributes to the development of novel interspecific hybrids in the Kalanchoë genus. BMC Plant Biol. 2015, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ferriol, M.; Simó, U.; Mansanet, C.J.; Torres, A.; Picó, B.; Monforte, A.J.; Romero, C. Pre-and post-zygotic barriers contribute to reproductive isolation and correlate with genetic distance in Cucumis. Plants 2023, 12, 926. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.Y.; Duan, Q.; Li, C.; Liu, M.-C.J.; Wu, H.-M. Pollen–pistil interactions: It takes two to tangle but a molecular cast of many to deliver. Curr. Opin. Plant Biol. 2022, 69, 102279. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, T.; Franklin-Tong, N. Male–female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol. Plant 2013, 6, 1018–1036. [Google Scholar] [CrossRef]
- Salam, U.; Ullah, S.; Tang, Z.H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant metabolomics: An overview of the role of primary and secondary metabolites against different environmental stress factors. Life 2023, 13, 706. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Huang, W.; Song, X.; Niu, J. Transcriptomics and metabolomics reveal purine and phenylpropanoid metabolism response to drought stress in Dendrobium sinense, an endemic orchid species in Hainan Island. Front. Genet. 2021, 12, 692702. [Google Scholar] [CrossRef]
- Shi, J.; Stahl, M.; de Vos, R.C.H.; Tielbörger, K.; Verhoeven, K.J.F.; Macel, M. Metabolomic profiling reveals shifts in defenses of an invasive plant. Biol. Invasions 2023, 25, 3293–3306. [Google Scholar] [CrossRef]
- Luo, K.; Li, T.; Wang, C.; Zhao, X.; Pan, J.; Zhu, S.; Li, Y.; Chen, W.; Yao, J.; Jiang, Y.; et al. Integrative transcriptomic and metabolomic analysis revealed the molecular mechanisms of fiber length difference between the micropylar end and chalazal end of ovule in interspecific hybrid cotton (Gossypium hirsutum × Gossypium barbadense). Ind. Crops Prod. 2024, 216, 118687. [Google Scholar] [CrossRef]
- Patel, M.K.; Pandey, S.; Kumar, M.; Haque, M.I.; Pal, S.; Yadav, N.S. Plants metabolome study: Emerging tools and techniques. Plants 2021, 10, 2409. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, L.; Zeng, B. Comparative physiological, transcriptomic, and metabolomic analyses of Acacia mangium provide new insights into Its molecular mechanism of self-incompatibility. Forests 2023, 14, 2034. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Zou, H.; Chen, J.; Wang, Z.; Luo, Z.; Yao, Z.; Fang, B.; Huang, L. Exploration of molecular mechanism of intraspecific cross-incompatibility in sweetpotato by transcriptome and metabolome analysis. Plant Mol. Biol. 2022, 109, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Gong, W.; Xu, J.; Gong, H.; Song, Q.; Xiao, S.; Yuan, D. Integration of semi-in vivo assays and multi-omics data reveals the effect of galloylated catechins on self-pollen tube inhibition in Camellia oleifera. Hortic. Res. 2023, 10, uhac248. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chen, S.; Chen, H.; Dai, L.; Qi, X.; Ahmad, M.Z.; Gao, K.; Qiu, S.; Jin, Y.; Deng, Y. Metabolomics reveals a key role of salicylic acid in embryo abortion underlying interspecific hybridization between Hydrangea macrophylla and H. arborescens. Plant Cell Rep. 2024, 43, 248. [Google Scholar] [CrossRef]
- Yu, J.L. Research on the Incompatibility Mechanism and Overcoming Techniques in Distant Hybridization Between Tree Peony (Paeonia sect. Moutan) and Herbaceous Peony (Paeonia lactiflora). Master’s Thesis, Henan Institute of Science and Technology, Xinxiang, China, 2024. [Google Scholar]
- Prasad, R. Cytokinin and its key role to enrich the plant nutrients and growth under adverse conditions-an update. Front. Genet. 2022, 13, 883924. [Google Scholar] [CrossRef]
- Breygina, M.; Voronkov, A.; Galin, I.; Akhiyarova, G.; Polevova, S.; Klimenko, E.; Ivanov, I.; Kudoyarova, G. Dynamics of endogenous levels and subcellular localization of ABA and cytokinins during pollen germination in spruce and tobacco. Protoplasma 2023, 260, 237–248. [Google Scholar] [CrossRef]
- Zhao, T. Microstructural Observation and Transcriptome Analysis of Pistil Abortion in ‘Li Guang’ Apricot. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2020. [Google Scholar] [CrossRef]
- Gomes, G.L.B.; Scortecci, K.C. Auxin and its role in plant development: Structure, signalling, regulation and response mechanisms. Plant Biol. 2021, 23, 894–904. [Google Scholar] [CrossRef]
- Skubacz, A.; Daszkowska-Golec, A.; Szarejko, I. The role and regulation of ABI5 (ABA-Insensitive 5) in plant development, abiotic stress responses and phytohormone crosstalk. Front. Plant Sci. 2016, 7, 1884. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) role in plant development and coping with different stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Vogler, F.; Schmalzl, C.; Englhart, M.; Bircheneder, M.; Sprunck, S. Brassinosteroids promote Arabidopsis pollen germination and growth. Plant Reprod. 2014, 27, 153–167. [Google Scholar] [CrossRef]
- Chang, Y.; Guo, X.; Xu, H.; Wu, Q.; Xie, A.; Zhao, Z.; Tian, R.; Gong, W.; Yuan, D. Methyl Jasmonate (MeJA) promotes the self-pollen tube growth of Camellia oleifera by regulating lignin biosynthesis. Int. J. Mol. Sci. 2024, 25, 10720. [Google Scholar] [CrossRef]
- Çetinbaş-Genç, A.; Vardar, F. Effect of methyl jasmonate on in-vitro pollen germination and tube elongation of Pinus nigra. Protoplasma 2020, 257, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, C.; Xu, L.; Niu, H.; Liu, Q.; Huang, Y.; Lv, G.; Yang, H.; Li, M. Melatonin and indole-3-acetic acid synergistically regulate plant growth and stress resistance. Cells 2022, 11, 3250. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.-Y.; Wang, K.-X.; Yan, M.-Y.; Kanwar, M.K.; Li, D.-Y.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P.; Zhou, J. Melatonin alleviates high temperature-induced pollen abortion in Solanum lycopersicum. Molecules 2018, 23, 386. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Cao, Y.; Loka, D.A.; Harris-Shultz, K.R.; Reiter, R.J.; Ali, S.; Liu, Y.; Zhou, Z. Exogenous melatonin improves cotton (Gossypium hirsutum L.) pollen fertility under drought by regulating carbohydrate metabolism in male tissues. Plant Physiol. Biochem. 2020, 151, 579–588. [Google Scholar] [CrossRef]
- Geserick, C.; Tenhaken, R. UDP-sugar pyrophosphorylase is essential for arabinose and xylose recycling, and is required during vegetative and reproductive growth in Arabidopsis. Plant J. 2013, 74, 239–247. [Google Scholar] [CrossRef]
- Kiyono, H.; Katano, K.; Suzuki, N. Links between regulatory systems of ROS and carbohydrates in reproductive development. Plants 2021, 10, 1652. [Google Scholar] [CrossRef]
- Selinski, J.; Scheibe, R. Pollen tube growth: Where does the energy come from? Plant Signal. Behav. 2014, 9, e977200. [Google Scholar] [CrossRef]
- Hirsche, J.; García Fernández, J.M.; Stabentheiner, E.; Großkinsky, D.K.; Roitsch, T. Differential effects of carbohydrates on Arabidopsis pollen germination. Plant Cell Physiol. 2017, 58, 691–701. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.C.; Li, Y.; Chen, L.-Q. Hexose transporter SWEET5 confers galactose sensitivity to Arabidopsis pollen germination via a galactokinase. Plant Physiol. 2022, 189, 388–401. [Google Scholar] [CrossRef]
- Martis, S.; Droux, M.; Deboudard, F.; Nasser, W.; Meyer, S.; Reverchon, S. Separation and quantification of 2-keto-3-deoxy-gluconate (KDG) a major metabolite in pectin and alginate degradation pathways. Anal. Biochem. 2021, 619, 114061. [Google Scholar] [CrossRef]
- Dewangan, B.P.; Gupta, A.; Sah, R.K.; Das, S.; Kumar, S.; Bhattacharjee, S.; Pawar, P.A.-M. Xylobiose treatment triggers a defense-related response and alters cell wall composition. Plant Mol. Biol. 2023, 113, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Linskens, H.F.; Tupý, J. The amino acids pool in the style of self-incompatible strains of Petunia after self-and cross-pollination. Der Züchter 1966, 36, 151–158. [Google Scholar] [CrossRef]
- Wang, J.; Kambhampati, S.; Allen, D.K.; Chen, L.-Q. Comparative metabolic analysis reveals a metabolic switch in mature, hydrated, and germinated pollen in Arabidopsis thaliana. Front. Plant Sci. 2022, 13, 836665. [Google Scholar] [CrossRef] [PubMed]
- Paupière, M.J.; Müller, F.; Li, H.; Rieu, I.; Tikunov, Y.M.; Visser, R.G.F.; Bovy, A.G. Untargeted metabolomic analysis of tomato pollen development and heat stress response. Plant Reprod. 2017, 30, 81–94. [Google Scholar] [CrossRef]
- Bellin, L.; Melzer, M.; Hilo, A.; Rieu, I.; Tikunov, Y.M.; Visser, R.G.F.; Bovy, A.G. Nucleotide limitation results in impaired photosynthesis, reduced growth and seed yield together with massively altered gene expression. Plant Cell Physiol. 2023, 64, 1494–1510. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Li, R.; Peng, G.; Chen, W.; Rautengarten, C.; Liu, M.; Zhu, L.; Xiao, Y.; Song, F.; et al. BOTRYOID POLLEN 1 regulates ROS-triggered PCD and pollen wall development by controlling UDP-sugar homeostasis in rice. Plant Cell 2023, 35, 3522–3543. [Google Scholar] [CrossRef]
- Zhang, W.; Qin, W.; Li, H.; Wu, A.-M. Biosynthesis and transport of nucleotide sugars for plant hemicellulose. Front. Plant Sci. 2021, 12, 723128. [Google Scholar] [CrossRef]
- Schmid, F.; Fliegert, R.; Westphal, T.; Bauche, A.; Guse, A.H. Nicotinic acid adenine dinucleotide phosphate (NAADP) degradation by alkaline phosphatase. J. Biol. Chem. 2012, 287, 32525–32534. [Google Scholar] [CrossRef]
- Blanco, E.; Fortunato, S.; Viggiano, L.; de Pinto, M.C. Cyclic AMP: A polyhedral signalling molecule in plants. Int. J. Mol. Sci. 2020, 21, 4862. [Google Scholar] [CrossRef]
- Wu, J.; Qu, H.; Jin, C.; Shang, Z.; Wu, J.; Xu, G.; Gao, Y.; Zhang, S. cAMP activates hyperpolarization-activated Ca2+ channels in the pollen of Pyrus pyrifolia. Plant Cell Rep. 2011, 30, 1193–1200. [Google Scholar] [CrossRef]
- Wolters-Arts, M.; Van Der Weerd, L.; Van Aelst, A.C.; Van Der Weerd, J.; Van As, H.; Mariani, C. Water-conducting properties of lipids during pollen hydration. Plant Cell Environ. 2002, 25, 513–519. [Google Scholar] [CrossRef]
- Wang, J.; Shan, Q.; Yi, T.; Ma, Y.; Zhou, X.; Pan, L.; Miao, W.; Zou, X.; Xiong, C.; Liu, F. Fine mapping and candidate gene analysis of CaFCD1 affecting cuticle biosynthesis in Capsicum annuum L. Theor. Appl. Genet. 2023, 136, 46. [Google Scholar] [CrossRef] [PubMed]
- Rotsch, A.H.; Kopka, J.; Feussner, I.; Ischebeck, T. Central metabolite and sterol profiling divides tobacco male gametophyte development and pollen tube growth into eight metabolic phases. Plant J. 2017, 92, 129–146. [Google Scholar] [CrossRef]
- Daryanavard, H.; Postiglione, A.E.; Mühlemann, J.K.; Muday, G.K. Flavonols modulate plant development, signaling, and stress responses. Curr. Opin. Plant Biol. 2023, 72, 102350. [Google Scholar] [CrossRef]
- Wu, H.-M.; Xie, D.-J.; Jia, P.-F.; Tang, Z.-S.; Shi, D.-Q.; Shui, G.-H.; Wang, G.-D.; Yang, W.-C. Homeostasis of flavonoids and triterpenoids most likely modulates starch metabolism for pollen tube penetration in rice. Plant Biotechnol. J. 2023, 21, 1757–1772. [Google Scholar] [CrossRef]
- Pérez-Alonso, M.M.; Ortiz-García, P.; Moya-Cuevas, J.; Lehmann, T.; Sánchez-Parra, B.; Björk, R.G.; Karim, S.; Amirjani, M.R.; Aronsson, H.; Wilkinson, M.D.; et al. Endogenous indole-3-acetamide levels contribute to the crosstalk between auxin and abscisic acid, and trigger plant stress responses in Arabidopsis. J. Exp. Bot. 2021, 72, 459–475. [Google Scholar] [CrossRef]
- Lu, C.; Tian, Y.; Hou, X.; Hou, X.; Jia, Z.; Li, M.; Hao, M.; Jiang, Y.; Wang, Q.; Pu, Q.; et al. Multiple forms of vitamin B6 regulate salt tolerance by balancing ROS and abscisic acid levels in maize root. Stress Biol. 2022, 2, 39. [Google Scholar] [CrossRef]
- Vanov, A.V.; Khomutov, A.R. Biogenic polyamines and related metabolites. Biomolecules 2021, 12, 14. [Google Scholar] [CrossRef]
- Çetinbaş-Genç, A. Putrescine modifies the pollen tube growth of tea (Camellia sinensis) by affecting actin organization and cell wall structure. Protoplasma 2020, 257, 89–101. [Google Scholar] [CrossRef]
- Yu, X.; Gu, C.; Guo, X.; Guo, R.; Zhu, L.; Qiu, X.; Chai, J.; Liu, F.; Feng, Z. Dynamic changes of microbiota and metabolite of traditional Hainan dregs vinegar during fermentation based on metagenomics and metabolomics. Food Chem. 2024, 444, 138641. [Google Scholar] [CrossRef]
- Wang, X.; Wang, K.; Yin, G.; Liu, X.; Liu, M.; Cao, N.; Duan, Y.; Gao, H.; Wang, W.; Ge, W.; et al. Pollen-expressed leucine-rich repeat extensins are essential for pollen germination and growth. Plant Physiol. 2018, 176, 1993–2006. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, W.; Yu, Y.; Mi, Z.; Zhang, Y.; Zhao, G.; Guo, Y.; Wang, Z.; Wang, E.; He, S. Non-Target Metabolomics Reveals Changes in Metabolite Profiles in Distant Hybrid Incompatibility Between Paeonia sect. Moutan and P. lactiflora. Plants 2025, 14, 1381. https://doi.org/10.3390/plants14091381
Jia W, Yu Y, Mi Z, Zhang Y, Zhao G, Guo Y, Wang Z, Wang E, He S. Non-Target Metabolomics Reveals Changes in Metabolite Profiles in Distant Hybrid Incompatibility Between Paeonia sect. Moutan and P. lactiflora. Plants. 2025; 14(9):1381. https://doi.org/10.3390/plants14091381
Chicago/Turabian StyleJia, Wenqing, Yingyue Yu, Zhaorong Mi, Yan Zhang, Guodong Zhao, Yingzi Guo, Zheng Wang, Erqiang Wang, and Songlin He. 2025. "Non-Target Metabolomics Reveals Changes in Metabolite Profiles in Distant Hybrid Incompatibility Between Paeonia sect. Moutan and P. lactiflora" Plants 14, no. 9: 1381. https://doi.org/10.3390/plants14091381
APA StyleJia, W., Yu, Y., Mi, Z., Zhang, Y., Zhao, G., Guo, Y., Wang, Z., Wang, E., & He, S. (2025). Non-Target Metabolomics Reveals Changes in Metabolite Profiles in Distant Hybrid Incompatibility Between Paeonia sect. Moutan and P. lactiflora. Plants, 14(9), 1381. https://doi.org/10.3390/plants14091381






