Physiological Response of Olive Trees Under Xylella fastidiosa Infection and Thymol Therapy Monitored Through Advanced IoT Sensors
Abstract
1. Introduction
2. Results
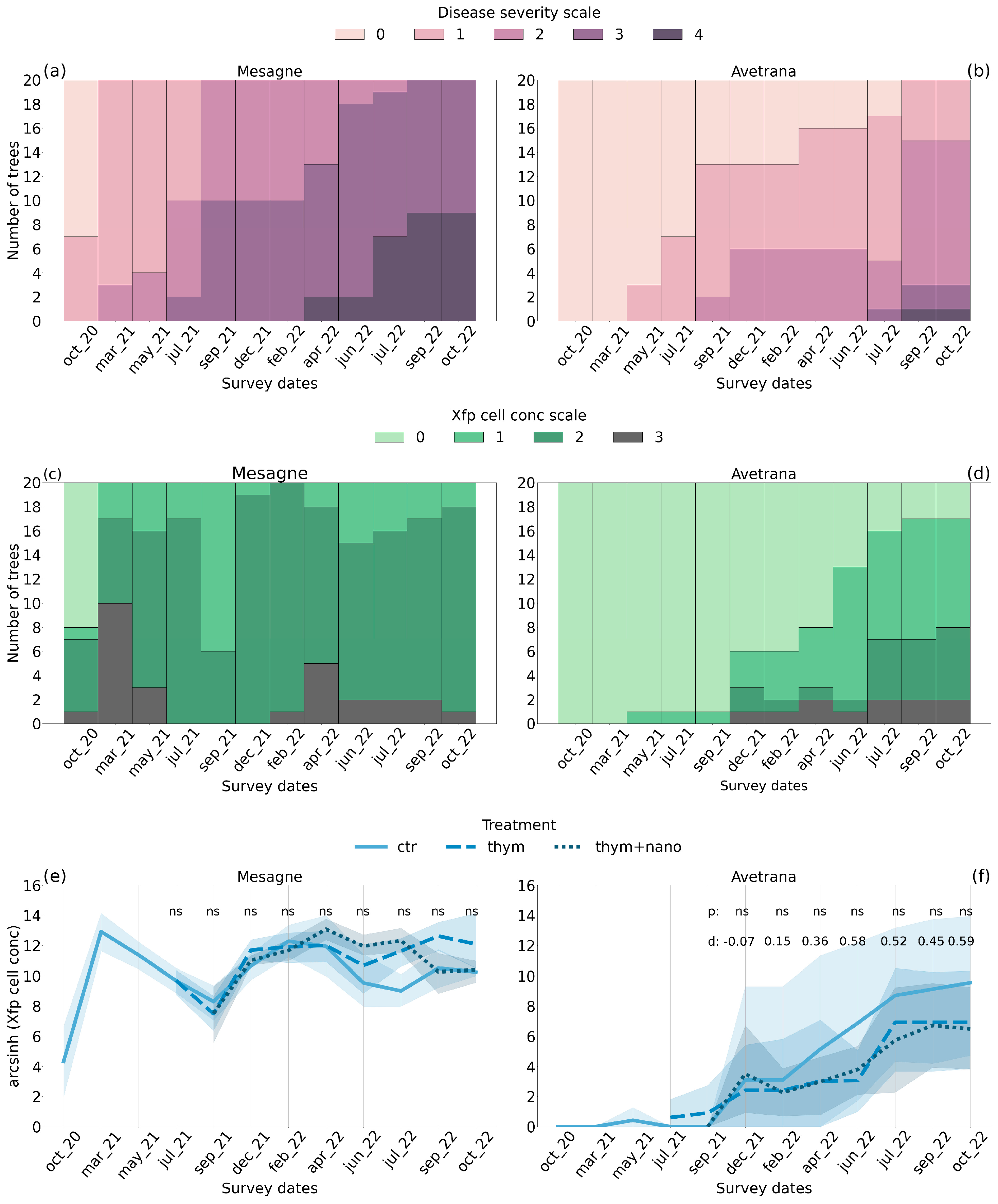
2.1. Xfp Disease Patterns
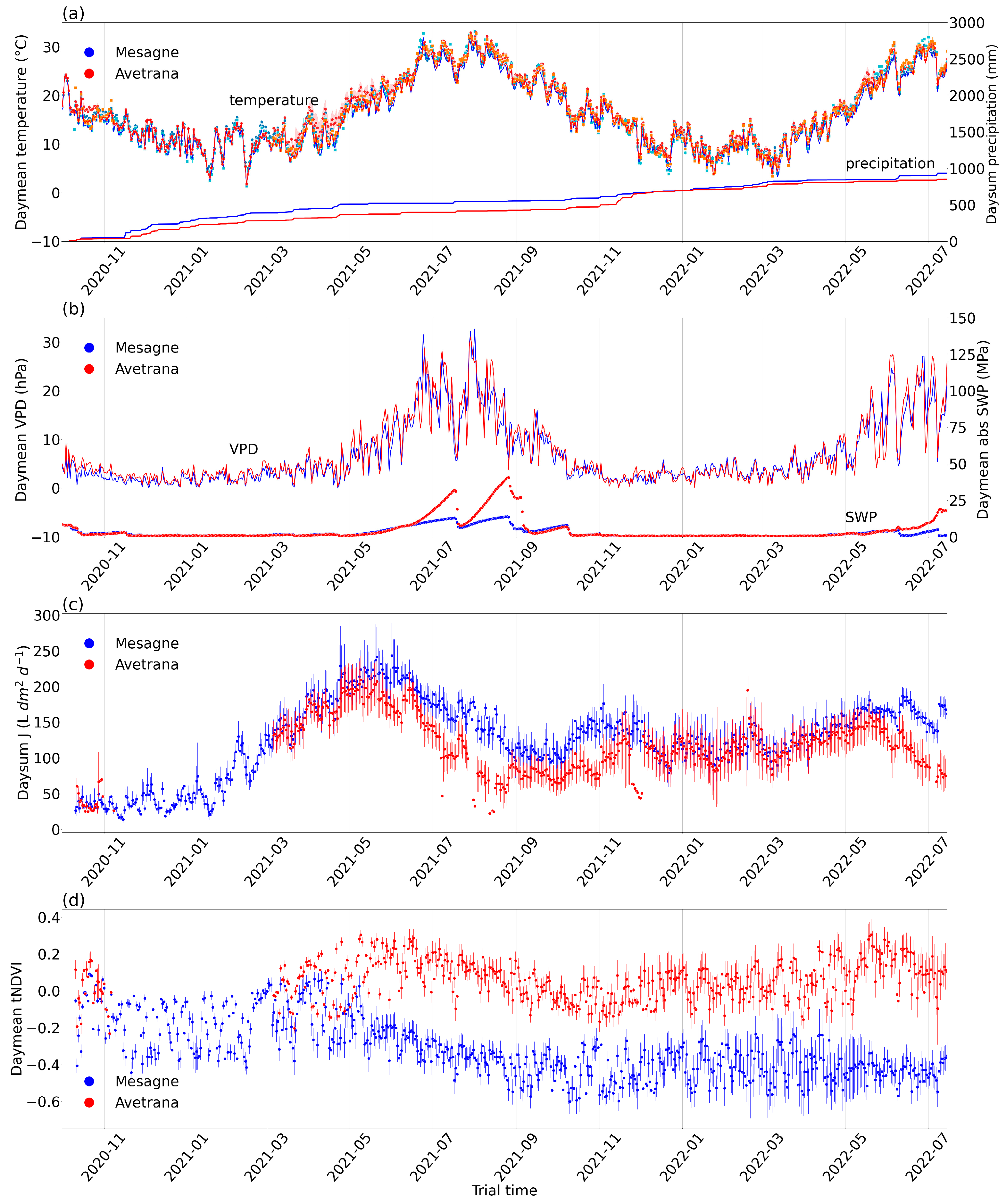
2.2. Climate and Tree Physiology Time Trends
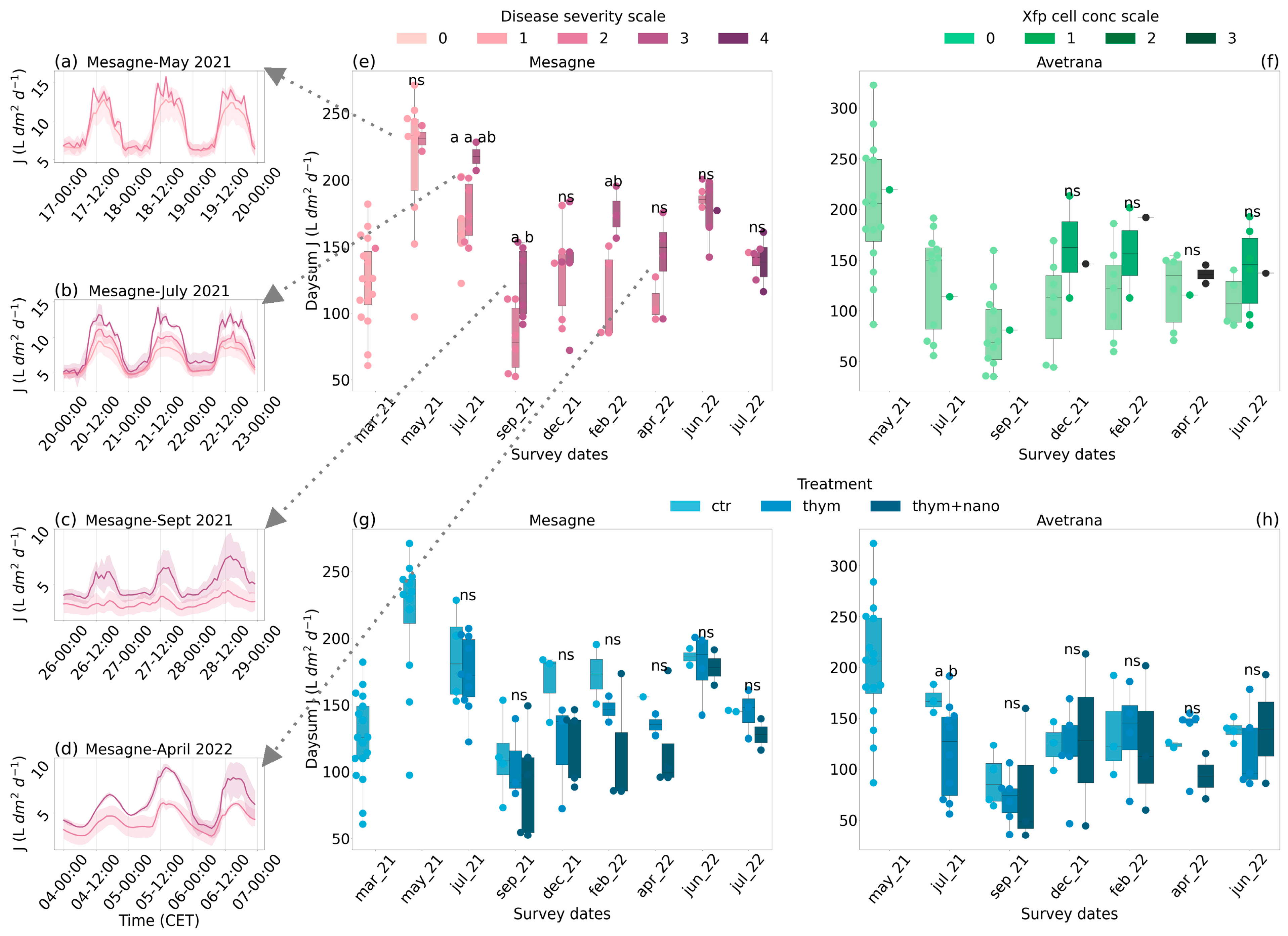
2.3. Sap Flux Density
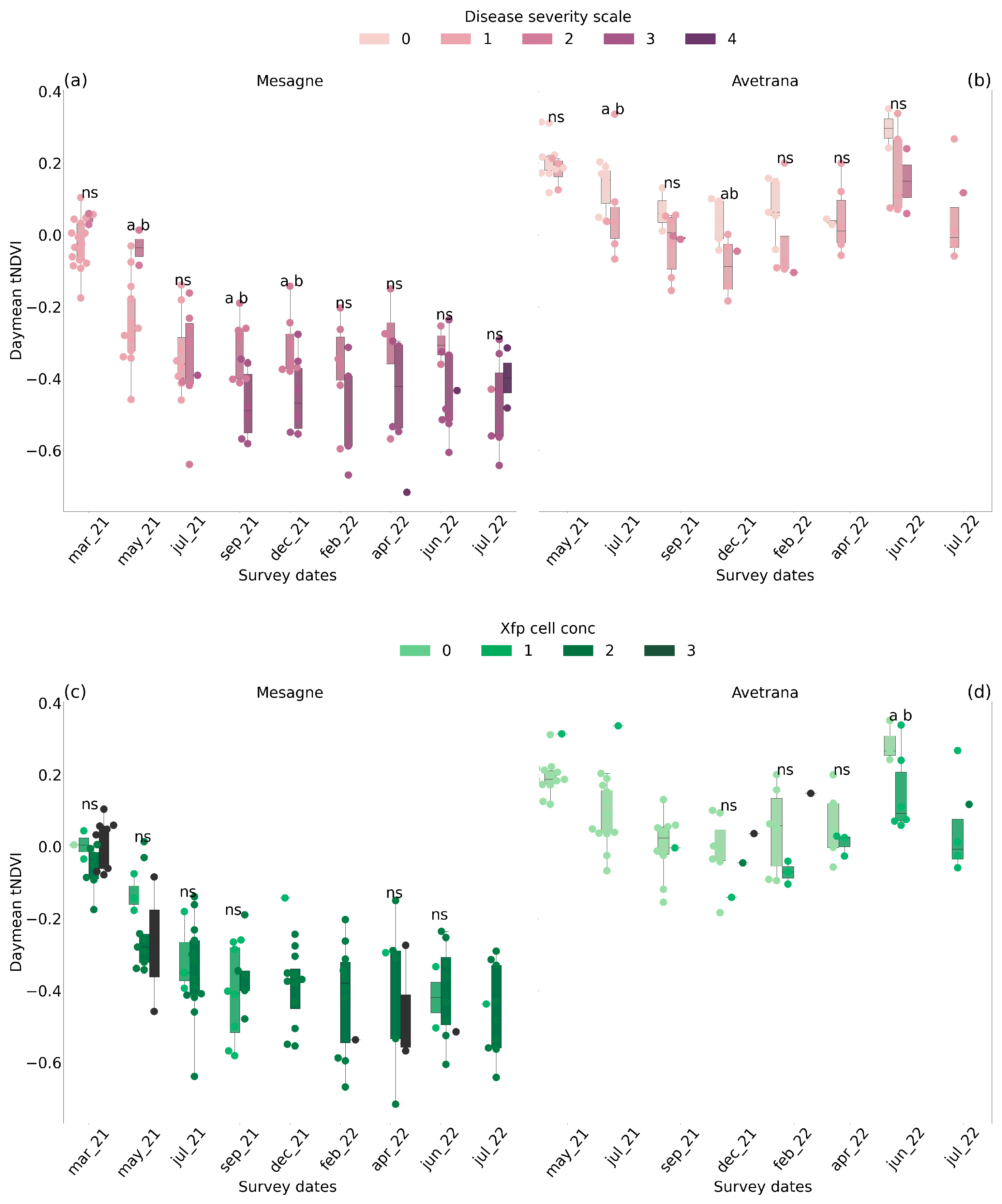
2.4. Leaf Traits
2.5. Canopy Transmitted Radiation
3. Discussion
3.1. Short-Term Efficacy of the Thymol-Extract Therapies
3.2. Tree’s Physiological Response to Xfp
3.3. Xfp Monitoring Through Transmitted Radiation
3.4. Limitations and Perspectives for Improvement
4. Materials and Methods
4.1. Study Sites
4.2. Field Experiment
4.3. TreeTalker Sensors
4.4. TreeTalker Data Processing
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (Southern Italy). J. Plant Pathol. 2013, 95, 668. [Google Scholar] [CrossRef]
- Saponari, M.; Giampetruzzi, A.; Loconsole, G.; Boscia, D.; Saldarelli, P. Xylella fastidiosa in olive in apulia: Where we stand. Phytopathology 2019, 109, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Saponari, M.; Boscia, D.; Altamura, G.; D’Attoma, G.; Cavalieri, V.; Zicca, S.; Morelli, M.; Tavano, D.; Loconsole, G.; Susca, L.; et al. Pilot project on Xylella fastidiosa to reduce risk assessment uncertainties. EFSA Support. Publ. 2017, 13, 1013E. [Google Scholar] [CrossRef]
- De Pascali, M.; Vergine, M.; Sabella, E.; Aprile, A.; Nutricati, E.; Nicolì, F.; Buja, I.; Negro, C.; Miceli, A.; Rampino, P.; et al. Molecular effects of Xylellan fastidiosa and drought combined stress in olive trees. Plants 2019, 8, 437. [Google Scholar] [CrossRef]
- De Pascali, M.; Vergine, M.; Negro, C.; Greco, D.; Vita, F.; Sabella, E.; De Bellis, L.; Luvisi, A. Xylella fastidiosa and Drought Stress in Olive Trees: A Complex Relationship Mediated by Soluble Sugars. Biology 2022, 11, 112. [Google Scholar] [CrossRef]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Simonetto, A.; Saladini, M.A.; Plazio, E.; Gilioli, G.; Molinatto, G.; Saponari, M.; Bosco, D. Dispersal of Philaenus spumarius (Hemiptera: Aphrophoridae), a Vector of Xylella fastidiosa, in Olive Grove and Meadow Agroecosystems. Environ. Entomol. 2021, 50, 267–279. [Google Scholar] [CrossRef]
- Morelli, M.; García-Madero, J.M.; Jos, Á.; Saldarelli, P.; Dongiovanni, C.; Kovacova, M.; Saponari, M.; Baños Arjona, A.; Hackl, E.; Webb, S.; et al. Xylella fastidiosa in olive: A review of control attempts and current management. Microorganisms 2021, 9, 1771. [Google Scholar] [CrossRef]
- Scortichini, M.; Loreti, S.; Pucci, N.; Scala, V.; Tatulli, G.; Verweire, D.; Oehl, M.; Widmer, U.; Codina, J.M.; Hertl, P.; et al. Progress towards sustainable control of Xylella fastidiosa subsp. Pauca in olive groves of salento (apulia, italy). Pathogens 2021, 10, 668. [Google Scholar] [CrossRef]
- Muranaka, L.S.; Giorgiano, T.E.; Takita, M.A.; Forim, M.R.; Silva, L.F.; Coletta-Filho, H.D.; Machado, M.A.; de Souza, A.A. N-Acetylcysteine in Agriculture, a Novel Use for an Old Molecule: Focus on Controlling the Plant-Pathogen Xylella fastidiosa. PLoS ONE. 2013, 8, e72937. [Google Scholar] [CrossRef]
- de Souza, A.; Coletta-Filho, H.D.; Dongiovanni, C.; Saponari, M. N-acetyl-cysteine for controlling Xylella fastidiosa in citrus and olive: Understanding the differences to improve management. In Book of Abstracts, Proceedings of the Proceedings of the 2nd European Conference on Xylella fastidiosa 2019, Ajaccio, France, 29–30 October 2019; EFSA Supporting Publications on Wiley Online Library: Parma, Italy, 2019; p. 42. [Google Scholar]
- Bruno, G.L.; Cariddi, C.; Botrugno, L. Exploring a sustainable solution to control Xylella fastidiosa subsp. pauca on olive in the Salento Peninsula, Southern Italy. Crop Prot. 2021, 139, 105288. [Google Scholar] [CrossRef]
- Rongai, D.; Pucci, N.; Cesari, E.; di Marco, C.; Valentini, F. Potential of endotherapeutic treatments with pomegranate peel extract to control the olive quick decline syndrome (OQDS) caused by Xylella fastidiosa subsp. pauca. Eur. J. Plant Pathol. 2023, 170, 805–817. [Google Scholar] [CrossRef]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Galotto, M.J. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 2011, 146, 144–150. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- D’onghia, A.M.; Santoro, F.; Minutillo, S.A.; Frasheri, D.; Gallo, M.; Gualano, S.; Cavallo, G.; Valentini, F. Optimisation of sampling and testing for asymptomatic olive trees infected by Xylella fastidiosa in Apulia region, Italy. Phytopathol. Mediterr. 2022, 61, 439–449. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Camino, C.; Beck, P.S.A.; Calderon, R.; Hornero, A.; Hernández-Clemente, R.; Kattenborn, T.; Montes-Borrego, M.; Susca, L.; Morelli, M.; et al. Previsual symptoms of Xylella fastidiosa infection revealed in spectral plant-trait alterations. Nat. Plants 2018, 4, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Blonda, P.; Tarantino, C.; Scortichini, M.; Maggi, S.; Tarantino, M.; Adamo, M. Satellite monitoring of bio-fertilizer restoration in olive groves affected by Xylella fastidiosa subsp. pauca. Sci. Rep. 2023, 13, 5695. [Google Scholar] [CrossRef]
- Tognetti, R.; Valentini, R.; Marchesini, L.B.; Gianelle, D.; Panzacchi, P.; Marshall, J.D. Continuous Monitoring of Tree Responses to Climate Change for Smart Forestry: A Cybernetic Web of Trees. In Climate-Smart Forestry in Mountain Regions; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Valentini, R.; Belelli Marchesini, L.; Gianelle, D.; Sala, G.; Yarovslavtsev, A.; Vasenev, V.; Castaldi, S. New tree monitoring systems: From industry 4.0 to nature 4.0. Ann. Silvic. Res. 2019, 43, 84–88. [Google Scholar] [CrossRef]
- Saponari, M.; Altamura, G.; Abou Kubaa, R.; Montilon, V.; Saldarelli, P.; Specchia, F.; Palmisano, F.; Silletti, M.R.; Pollastro, P.; Zicca, S. Further acquisition on the response of a large number of olive cultivars to infections caused by Xylella fastidiosa subsp. pauca, ST53. In Proceedings of the 2nd European Conference on Xylella fastidiosa (How Research Can Support Solutions), Ajaccio, France, 29–30 October 2019. [Google Scholar]
- Savoia, M.A.; Fanelli, V.; Miazzi, M.M.; Taranto, F.; Procino, S.; Susca, L.; Montilon, V.; Potere, O.; Nigro, F.; Montemurro, C. Apulian Autochthonous Olive Germplasm: A Promising Resource to Restore Cultivation in Xylella fastidiosa-Infected Areas. Agriculture 2023, 13, 1746. [Google Scholar] [CrossRef]
- Cardinale, M.; Luvisi, A.; Meyer, J.B.; Sabella, E.; De Bellis, L.; Cruz, A.C.; Ampatzidis, Y.; Cherubini, P. Specific fluorescence in situ hybridization (Fish) test to highlight colonization of xylem vessels by Xylella fastidiosa in naturally infected olive trees (Olea europaea L.). Front. Plant Sci. 2018, 9, 431. [Google Scholar] [CrossRef]
- Montilon, V.; De Stradis, A.; Saponari, M.; Abou Kubaa, R.; Giampetruzzi, A.; D’Attoma, G.; Saldarelli, P. Xylella fastidiosa subsp. pauca ST53 exploits pit membranes of susceptible olive cultivars to spread systemically in the xylem. Plant Pathol. 2023, 72, 144–153. [Google Scholar] [CrossRef]
- Petit, G.; Bleve, G.; Gallo, A.; Mita, G.; Montanaro, G.; Nuzzo, V.; Zambonini, D.; Pitacco, A. Susceptibility to Xylella fastidiosa and functional xylem anatomy in Olea europaea: Revisiting a tale of plant-pathogen interaction. AoB Plants. 2021, 13, plab027. [Google Scholar] [CrossRef] [PubMed]
- Sabella, E.; Aprile, A.; Genga, A.; Siciliano, T.; Nutricati, E.; Nicolì, F.; Vergine, M.; Negro, C.; De Bellis, L.; Luvisi, A. Xylem cavitation susceptibility and refilling mechanisms in olive trees infected by Xylella fastidiosa. Sci. Rep. 2019, 9, 9602. [Google Scholar] [CrossRef]
- Walker, N.C.; White, S.M.; McKay Fletcher, D.; Ruiz, S.A.; Rankin, K.E.; De Stradis, A.; Saponari, M.; Williams, K.A.; Petroselli, C.; Roose, T. The impact of xylem geometry on olive cultivar resistance to Xylella fastidiosa: An image-based study. Plant Pathology 2023, 72, 521–535. [Google Scholar] [CrossRef]
- Surano, A.; Abou Kubaa, R.; Nigro, F.; Altamura, G.; Losciale, P.; Saponari, M.; Saldarelli, P. Susceptible and resistant olive cultivars show differential physiological response to Xylella fastidiosa infections. Front. Plant Sci. 2022, 13, 968934. [Google Scholar] [CrossRef]
- Surano, A.; Del Grosso, C.; Musio, B.; Todisco, S.; Giampetruzzi, A.; Altamura, G.; Saponari, M.; Gallo, V.; Mastrorilli, P.; Boscia, D.; et al. Exploring the xylem-sap to unravel biological features of Xylella fastidiosa subspecies pauca ST53 in immune, resistant and susceptible crop species through metabolomics and in vitro studies. Front. Plant Sci. 2023, 14, 1343876. [Google Scholar] [CrossRef]
- Baldassarre, F.; Schiavi, D.; Ciarroni, S.; Tagliavento, V.; De Stradis, A.; Vergaro, V.; Suranna, G.P.; Balestra, G.M.; Ciccarella, G. Thymol-Nanoparticles as Effective Biocides against the Quarantine Pathogen Xylella fastidiosa. Nanomaterials 2023, 13, 1285. [Google Scholar] [CrossRef]
- Baldassarre, F.; De Stradis, A.; Altamura, G.; Vergaro, V.; Citti, C.; Cannazza, G.; Capodilupo, A.L.; Dini, L.; Ciccarella, G. Application of calcium carbonate nanocarriers for controlled release of phytodrugs against Xylella fastidiosa pathogen. Pure Appl. Chem. 2020, 92, 429–444. [Google Scholar] [CrossRef]
- Aouada, F.A.; de Moura, M.R. Nanotechnology applied in agriculture: Controlled release of agrochemicals. In Nanotechnologies in Food and Agriculture; Springer: Cham, Switzerland, 2015; pp. 103–118. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Torres, F.; Commeaux, S.; Troncoso, O. Biocompatibility of Bacterial Cellulose Based Biomaterials. J. Funct. Biomater. 2012, 3, 864–878. [Google Scholar] [CrossRef]
- Román-Écija, M.; Landa, B.B.; Testi, L.; Navas-Cortés, J.A. Modelling temperature response of Xylella fastidiosa strains, and xylem vessel temperature in woody plants. In Abstracts of Invited, Proceedings of the Oral and Poster Papers of the 16th Congress of the Mediterranean Phytopathological Union, Limassol, Cyprus, 4–8 April 2022; Mugnai, L., Ed.; Firenze University Press: Florence, Italy, 2022; pp. 181–239. [Google Scholar]
- Do, F.C.; Isarangkool Na Ayutthaya, S.; Rocheteau, A. Transient thermal dissipation method for xylem sap flow measurement: Implementation with a single probe. Tree Physiol. 2011, 31, 369–380. [Google Scholar] [CrossRef]
- López-Bernal, Á.; Alcántara, E.; Testi, L.; Villalobos, F.J. Spatial sap flow and xylem anatomical characteristics in olive trees under different irrigation regimes. Tree Physiol. 2010, 30, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Almeida, R.P.P.; Lindow, S. Living in two worlds: The plant and insect lifestyles of Xylella fastidiosa. Annu. Rev. Phytopathol. 2008, 46, 243–271. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, L.; Hoch, G.; Kahmen, A.; Körner, C. Losing half the conductive area hardly impacts the water status of mature trees. Sci. Rep. 2018, 8, 15006. [Google Scholar] [CrossRef] [PubMed]
- Laurin, G.V.; Cotrina-Sanchez, A.; Belelli-Marchesini, L.; Tomelleri, E.; Battipaglia, G.; Cocozza, C.; Niccoli, F.; Kabala, J.P.; Gianelle, D.; Vescovo, L.; et al. Comparing ground below-canopy and satellite spectral data for an improved and integrated forest phenology monitoring system. Ecol. Indic. 2024, 158, 111328. [Google Scholar] [CrossRef]
- Chiriacò, M.V.; Samad, N.; Magnani, F.; Vianello, G.; Vittori-Antisari, L.; Mazzoli, I.; Ranieri, R.; Valentini, R. Ecophysiology of Mediterranean Chestnut (Castanea sativa Mill.) Forests: Effects of Pruning Studied through an Advanced IoT System. Forests 2024, 15, 1579. [Google Scholar] [CrossRef]
- Baranoski, G.V.G.; Rokne, J.G. Efficiently simulating scattering of light by leaves. Vis. Comput. 2001, 17, 491–505. [Google Scholar] [CrossRef]
- Baranoski, G.V.G.; Rokne, J.G. An algorithmic reflectance and transmittance model for plant tissue. In Computer Graphics Forum; Blackwell Publishers Ltd.: Oxford, UK, 1997; p. 16. [Google Scholar] [CrossRef]
- Brakke, T.W.; Smith, J.A.; Harnden, J.M. Bidirectional scattering of light from tree leaves. Remote Sens. Environ. 1989, 29, 175–183. [Google Scholar] [CrossRef]
- Hovi, A.; Forsström, P.; Mõttus, M.; Rautiainen, M. Evaluation of accuracy and practical applicability of methods for measuring leaf reflectance and transmittance spectra. Remote Sens. 2018, 10, 25. [Google Scholar] [CrossRef]
- Volterrani, M.; Minelli, A.; Gaetani, M.; Grossi, N.; Magni, S.; Caturegli, L. Reflectance, absorbance and transmittance spectra of bermudagrass and manilagrass turfgrass canopies. PLoS ONE. 2017, 12, e0188080. [Google Scholar] [CrossRef]
- Poblete, T.; Camino, C.; Beck, P.S.A.; Hornero, A.; Kattenborn, T.; Saponari, M.; Boscia, D.; Navas-Cortes, J.A.; Zarco-Tejada, P.J. Detection of Xylella fastidiosa infection symptoms with airborne multispectral and thermal imagery: Assessing bandset reduction performance from hyperspectral analysis. ISPRS J. Photogramm. Remote Sens. 2020, 162, 27–40. [Google Scholar] [CrossRef]
- Oerke, E.C.; Mahlein, A.K.; Steiner, U. Proximal sensing of plant diseases. In Detection and Diagnostics of Plant Pathogens; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Zubler, A.V.; Yoon, J.Y. Proximal Methods for Plant Stress Detection Using Optical Sensors and Machine Learning. Biosensors 2020, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.J.; Ward, L.I.; Clover, G.R.G. Development of LAMP and real-time PCR methods for the rapid detection of Xylella fastidiosa for quarantine and field applications. Phytopathology 2010, 100, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Scortichini, M.; Saponari, M.; Loconsole, G.; Legendre, B.; Olivier, V.; Poliakoff, F.; Bergsma-Vlami, M.; Gottsberger, R.; Dreo, T.; Loretti, S.; et al. PM 7/24 (5) Xylella fastidiosa. EPPO Bull. 2023, 53, 205–276. [Google Scholar] [CrossRef]
- de la Rosa, J.M.; Campos, P.; Diaz-Espejo, A. Soil Biochar Application: Assessment of the Effects on Soil Water Properties, Plant Physiological Status, and Yield of Super-Intensive Olive Groves under Controlled Irrigation Conditions. Agronomy 2022, 12, 2321. [Google Scholar] [CrossRef]
- Dbara, S.; Haworth, M.; Emiliani, G.; Ben Mimoun, M.; Gómez-Cadenas, A.; Centritto, M. Partial root-zone drying of olive (Olea europaea var. ’chetoui’) induces reduced yield under field conditions. PLoS ONE. 2016, 11, e0157089. [Google Scholar] [CrossRef]
- Long, S.P.; Bernacchi, C.J. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 2003, 54, 2393–2401. [Google Scholar] [CrossRef]
- Liberati, D.; Guidolotti, G.; de Dato, G.; De Angelis, P. Enhancement of ecosystem carbon uptake in a dry shrubland under moderate warming: The role of nitrogen-driven changes in plant morphology. Glob. Change Biol. 2021, 27, 5629–5642. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Singsaas, E.L.; Pimentel, C.; Portis, A.R., Jr.; Long, S.P. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ. 2001, 24, 253–259. [Google Scholar] [CrossRef]
- McMurtrie, R.E.; Wang, Y.P. Mathematical models of the photosynthetic response of tree stands to rising CO2 concentrations and temperatures. Plant Cell Environ. 1993, 16, 1–13. [Google Scholar] [CrossRef]
- Granier, A. Sap Flow Measurements in Douglas-Fir Tree Trunks by Means of a New Thermal Method. Ann. For. Sci. 1987, 44, 1–14. [Google Scholar] [CrossRef]
- Do, F.; Rocheteau, A. Influence of natural temperature gradients on measurements of xylem sap flow with thermal dissipation probes. 1. Field observations and possible remedies. Tree Physiol. 2002, 22, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Tomelleri, E.; Belelli Marchesini, L.; Yaroslavtsev, A.; Asgharinia, S.; Valentini, R. Toward a Unified TreeTalker Data Curation Process. Forests 2022, 13, 855. [Google Scholar] [CrossRef]
- Wutzler, T.; Lucas-Moffat, A.; Migliavacca, M.; Knauer, J.; Sickel, K.; Šigut, L.; Menzer, O.; Reichstein, M. Basic and extensible post-processing of eddy covariance flux data with REddyProc. Biogeosciences 2018, 15, 5015–5030. [Google Scholar] [CrossRef]
- Kabala, J.P.; Niccoli, F.; Battipaglia, G. A customizable and use friendly R package to process big data from the Tree Talker system. In Proceedings of the 2022 IEEE Workshop on Metrology for Agriculture and Forestry (Metroagrifor), Perugia, Italy, 3–5 November 2022; pp. 70–74. [Google Scholar] [CrossRef]
- Papale, D.; Reichstein, M.; Aubinet, M.; Canfora, E.; Bernhofer, C.; Kutsch, W.; Longdoz, B.; Rambal, S.; Valentini, R.; Vesala, T.; et al. Towards a standardized processing of Net Ecosystem Exchange measured with eddy covariance technique: Algorithms and uncertainty estimation. Biogeosciences 2006, 3, 571–583. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010. [Google Scholar] [CrossRef]
- Pfautsch, S.; Keitel, C.; Turnbull, T.L.; Braimbridge, M.J.; Wright, T.E.; Simpson, R.R.; O’Brien, J.A.; Adams, M.A. Diurnal patterns of water use in Eucalyptus victrix indicate pronounced desiccation-rehydration cycles despite unlimited water supply. Tree Physiol. 2011, 31, 1041–1051. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]

| Property | Mesagne Mean (sd) | Avetrana Mean (sd) | Significance |
|---|---|---|---|
| vcmax (µmol CO2 m−2 s−1) | 69 (17) | 71 (15) | ns |
| Jmax (µmol m−2 s−1) | 198 (37) | 156 (34) | * |
| Jmax/vcmax | 3 (0.5) | 2.2 (0.2) | *** |
| LMA (g m−2) | 230 (47) | 216 (40) | ns |
| N (%) | 1.6 (0.6) | 1.6 (0.2) | ns |
| Narea (g m−2) | 3.8 (1.5) | 3.6 (0.5) | ns |
| Carea (g m−2) | 112 (24.1) | 111 (18.9) | ns |
| Property | Mesagne Mean (sd) | Avetrana Mean (sd) | Significance |
|---|---|---|---|
| gsw (mol H2O m−2 s−1) before noon | 0.164 (0.072) | 0.098 (0.039) | *** |
| gsw (mol H2O m−2 s−1) after noon | 0.098 (0.045) | 0.094 (0.046) | ns |
| E (mmol H2O m−2 s−1) before noon | 3.26 (1.45) | 2.62 (1.11) | *** |
| E (mmol H2O m−2 s−1) after noon | 2.81 (1.38) | 2.98 (1.55) | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cagnarini, C.; De Angelis, P.; Liberati, D.; Valentini, R.; Falanga, V.; Valentini, F.; Dongiovanni, C.; Carrieri, M.; Chiriacò, M.V. Physiological Response of Olive Trees Under Xylella fastidiosa Infection and Thymol Therapy Monitored Through Advanced IoT Sensors. Plants 2025, 14, 1380. https://doi.org/10.3390/plants14091380
Cagnarini C, De Angelis P, Liberati D, Valentini R, Falanga V, Valentini F, Dongiovanni C, Carrieri M, Chiriacò MV. Physiological Response of Olive Trees Under Xylella fastidiosa Infection and Thymol Therapy Monitored Through Advanced IoT Sensors. Plants. 2025; 14(9):1380. https://doi.org/10.3390/plants14091380
Chicago/Turabian StyleCagnarini, Claudia, Paolo De Angelis, Dario Liberati, Riccardo Valentini, Valentina Falanga, Franco Valentini, Crescenza Dongiovanni, Mauro Carrieri, and Maria Vincenza Chiriacò. 2025. "Physiological Response of Olive Trees Under Xylella fastidiosa Infection and Thymol Therapy Monitored Through Advanced IoT Sensors" Plants 14, no. 9: 1380. https://doi.org/10.3390/plants14091380
APA StyleCagnarini, C., De Angelis, P., Liberati, D., Valentini, R., Falanga, V., Valentini, F., Dongiovanni, C., Carrieri, M., & Chiriacò, M. V. (2025). Physiological Response of Olive Trees Under Xylella fastidiosa Infection and Thymol Therapy Monitored Through Advanced IoT Sensors. Plants, 14(9), 1380. https://doi.org/10.3390/plants14091380











