Tessaria absinthioides (Hook. & Arn.) DC. Determines Inhibition of Tumor Growth and Metastasis In Vitro and In Vivo in Murine Melanoma
Abstract
1. Introduction
2. Results
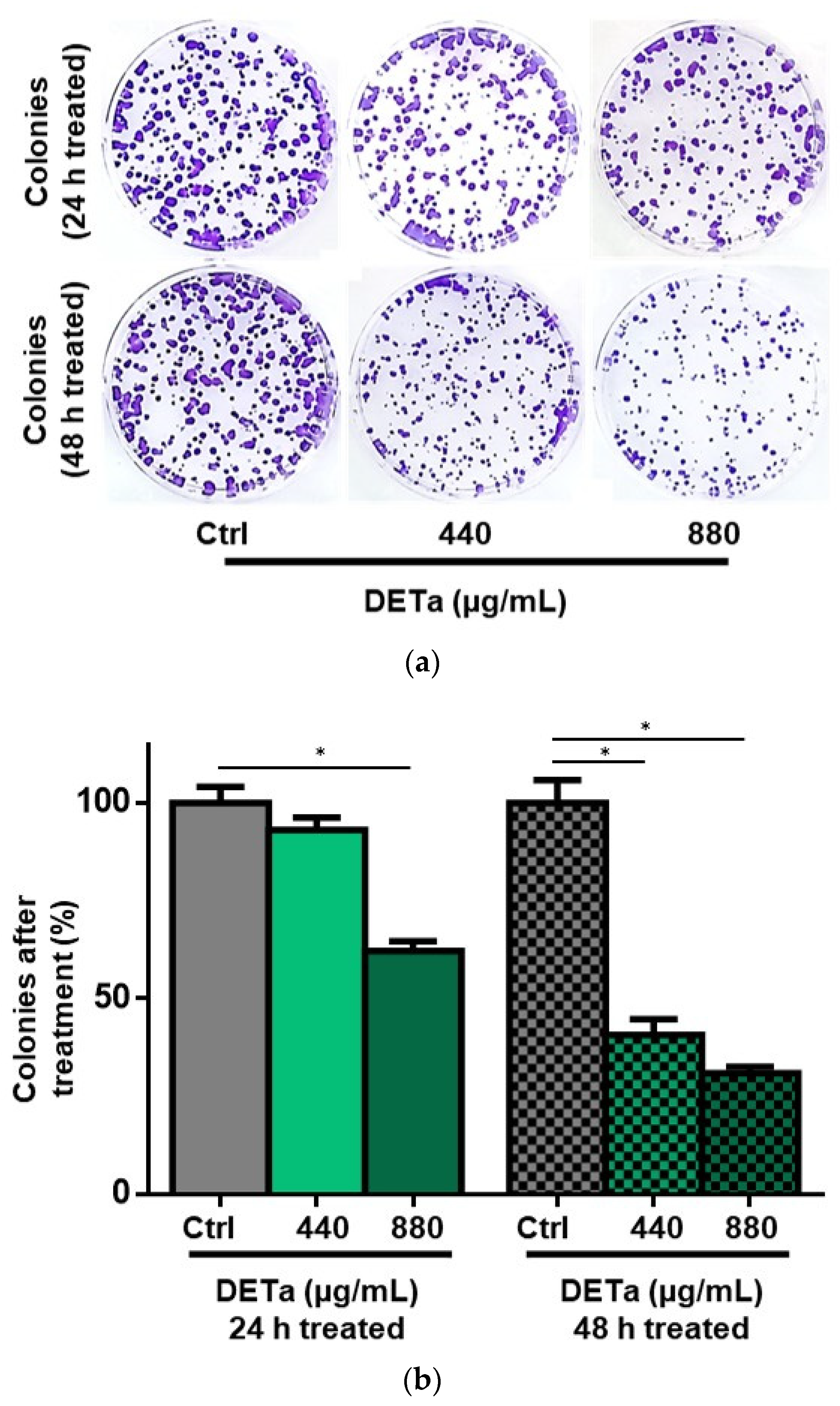
2.1. In Vitro Study of DETa Cytotoxicity on Melanoma Non-Metastatic Cell Line
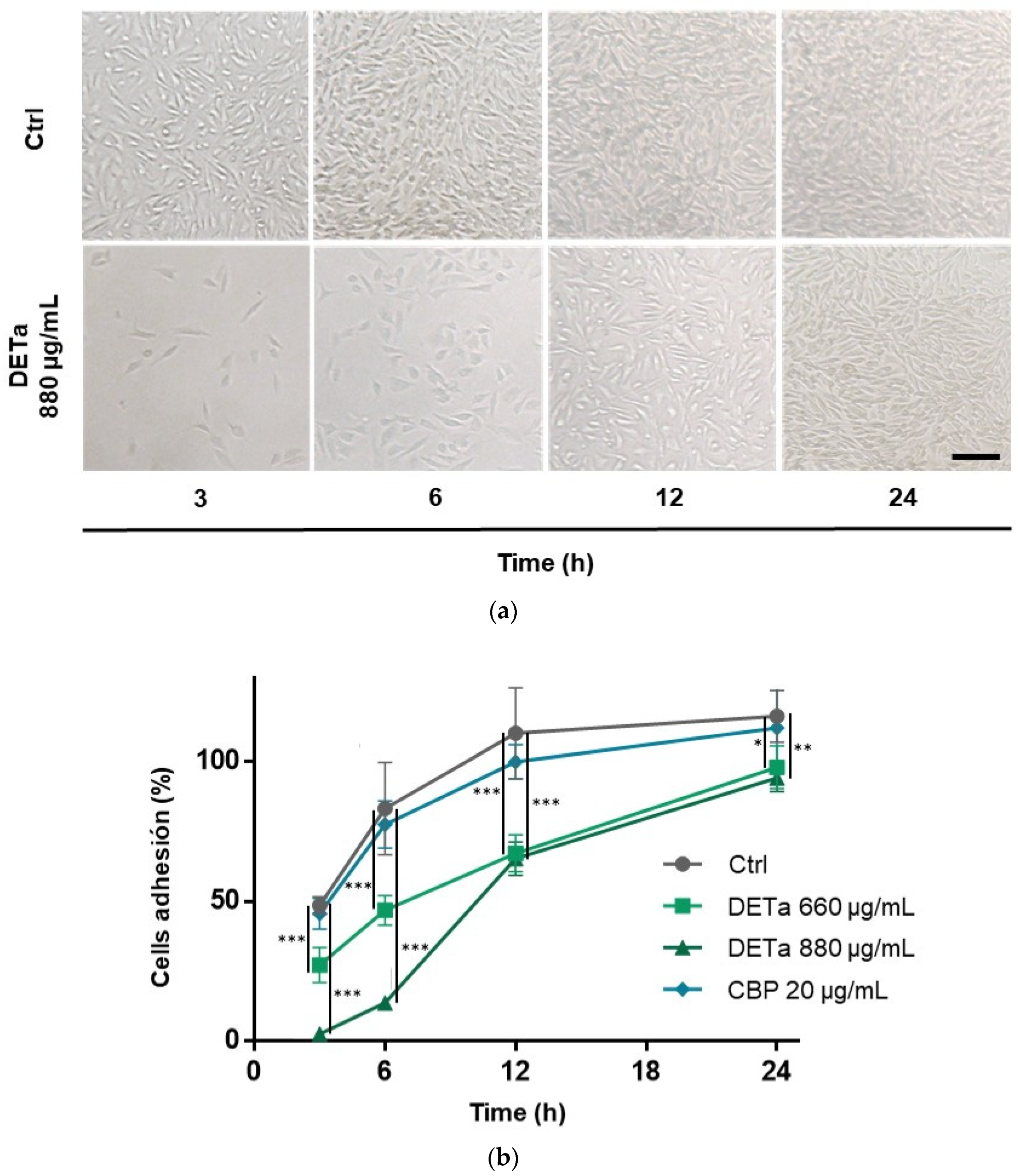
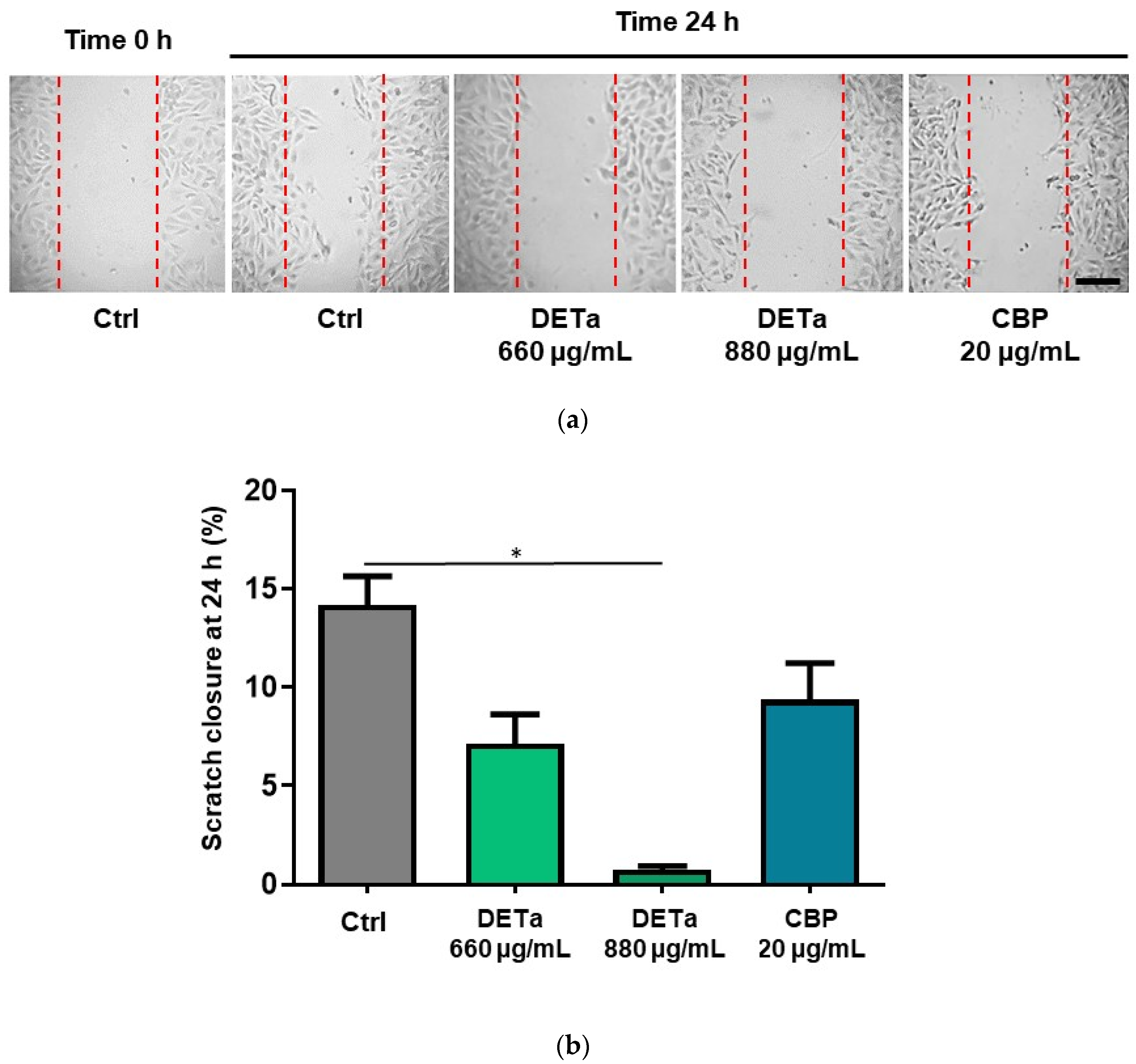
2.2. In Vitro Study of DETa Effects on Melanoma Metastatic Cell Line Adhesion, Migration and Invasion
2.3. Intestinal Permeability of DETa Phenolic Compounds
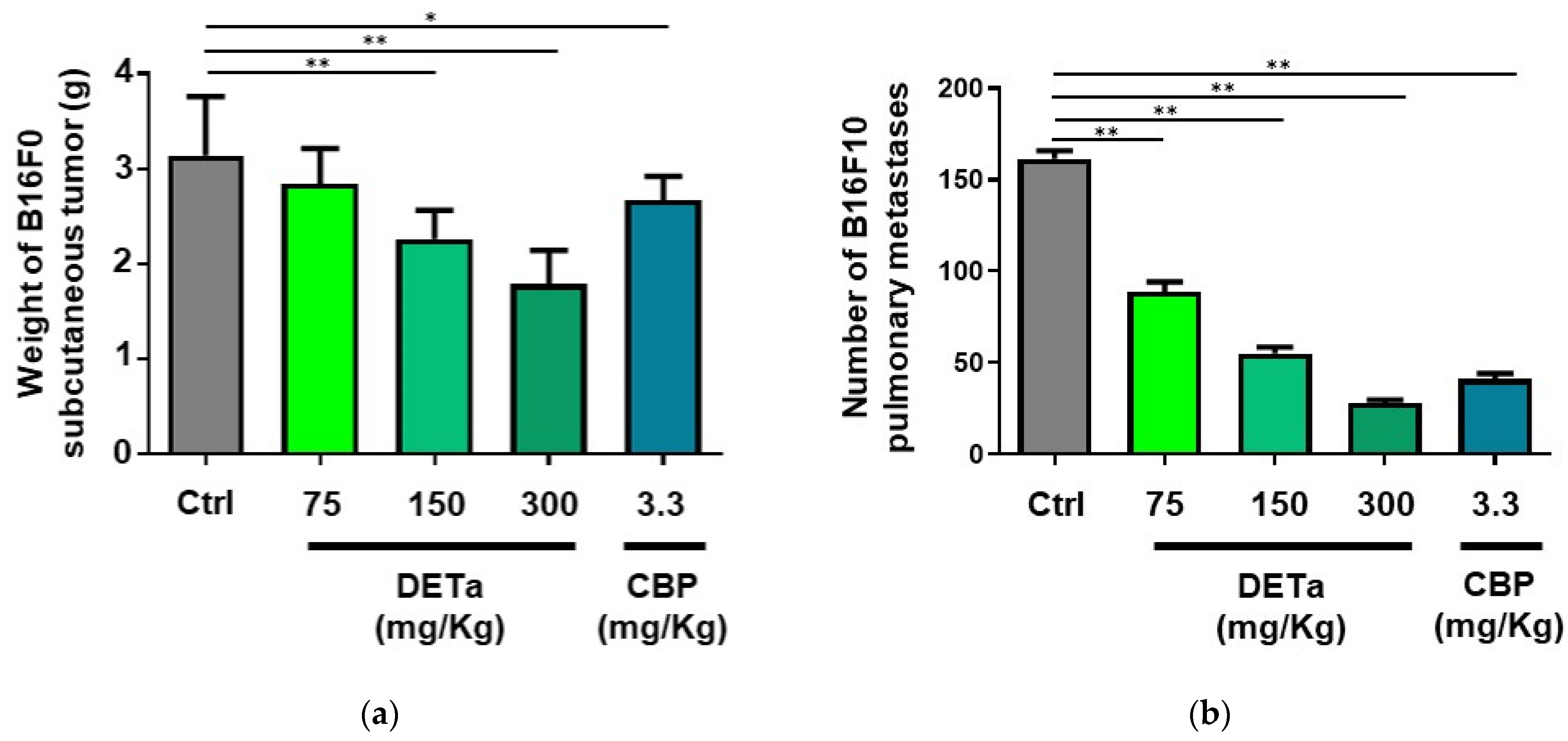
2.4. In Vivo Demonstration of DETa Antitumor and Antimetastatic Effects
2.4.1. Oral Administration of DETa Determines Antitumoral Effects on Induced Subcutaneous Melanoma
2.4.2. Oral Administration of DETa Reduces Pulmonary Metastasis of Melanoma
3. Discussion
4. Materials and Methods
4.1. Plant Material and Aqueous Extract Preparation
4.2. Cell Culture and In Vitro Treatment
4.3. Cytotoxicity, Proliferation and Viability Determinations
4.4. Clonogenic Survival Assay
4.5. Cell Adhesion Assay
4.6. Wound Healing Cell Migration Assay
4.7. Cell Invasion Assay
4.8. Intestinal Absorption of Phenolic Compounds
4.9. Animals and In Vivo Treatment
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous melanoma. Lancet 2023, 402, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Waseh, S.; Lee, J.B. Advances in melanoma: Epidemiology, diagnosis, and prognosis. Front. Med. 2023, 10, 1268479. [Google Scholar] [CrossRef]
- Toma, A.O.; Prodan, M.; Reddyreddy, A.R.; Seclaman, E.; Crainiceanu, Z.; Bloanca, V.; Bratosin, F.; Dumitru, C.; Pilut, C.N.; Alambaram, S.; et al. The Epidemiology of Malignant Melanoma during the First Two Years of the COVID-19 Pandemic: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 20, 305. [Google Scholar] [CrossRef]
- Tyagi, A.; Wu, S.Y.; Watabe, K. Metabolism in the progression and metastasis of brain tumors. Cancer Lett. 2022, 539, 215713. [Google Scholar] [CrossRef] [PubMed]
- Isacescu, E.; Chiroi, P.; Zanoaga, O.; Nutu, A.; Budisan, L.; Pirlog, R.; Atanasov, A.G.; Berindan-Neagoe, I. Melanoma Cellular Signaling Transduction Pathways Targeted by Polyphenols Action Mechanisms. Antioxidants 2023, 12, 407. [Google Scholar] [CrossRef]
- Hidalgo, L.; Saldías-Fuentes, C.; Carrasco, K.; Halpern, A.C.; Mao, J.J.; Navarrete-Dechent, C. Complementary and alternative therapies in skin cancer a literature review of biologically active compounds. Dermatol. Ther. 2022, 35, e15842. [Google Scholar] [CrossRef] [PubMed]
- An, S.; An, J.; Lee, D.; Kang, H.N.; Kang, S.; Ahn, C.-H.; Syahputra, R.A.; Ribeiro, R.I.M.A.; Kim, B. Natural Products for Melanoma Therapy: From Traditional Medicine to Modern Drug Discovery. Plants 2025, 14, 951. [Google Scholar] [CrossRef]
- Danciu, C.; Zupko, I.; Bor, A.; Schwiebs, A.; Radeke, H.; Hancianu, M.; Cioanca, O.; Alexa, E.; Oprean, C.; Bojin, F.; et al. Botanical Therapeutics: Phytochemical Screening and Biological Assessment of Chamomile, Parsley and Celery Extracts against A375 Human Melanoma and Dendritic Cells. Int. J. Mol. Sci. 2018, 19, 3624. [Google Scholar] [CrossRef]
- Thaichinda, S.; Tancharoen, S.; Kanekura, T.; Higashi, Y.; Dararat, P.; Kikuchi, K.; Nararatwanchai, T. Pinus maritima Extract Induces Apoptosis in Human Malignant Melanoma Cells via ROS/Caspase-3 Signaling. Nat. Product. Commun. 2020, 15, 1934578X20926889. [Google Scholar] [CrossRef]
- Sipos, S.; Moacă, E.A.; Pavel, I.Z.; Avram, Ş.; Crețu, O.M.; Coricovac, D.; Racoviceanu, R.M.; Ghiulai, R.; Pană, R.D.; Şoica, C.M.; et al. Melissa officinalis L. Aqueous Extract Exerts Antioxidant and Antiangiogenic Effects and Improves Physiological Skin Parameters. Molecules 2021, 26, 2369. [Google Scholar] [CrossRef]
- Persia, F.A.; Troncoso, M.E.; Rinaldini, E.; Simirgiotis, M.; Tapia, A.; Bórquez, J.; Mackern-Oberti, J.P.; Hapon, M.B.; Gamarra-Luques, C. UHPLC–Q/Orbitrap/MS/MS fingerprinting and antitumoral effects of Prosopis strombulifera (LAM.) BENTH. queous extract on allograft colorectal and melanoma cancer models. Heliyon 2020, 6, e03353. [Google Scholar]
- Sosa-Lochedino, A.; Hapon, M.B.; Gamarra-Luques, C. A systematic review about the contribution of the genus Tessaria (Asteraceae) to cancer study and treatment. Uniciencia 2022, 36, 467–483. [Google Scholar] [CrossRef]
- Gómez, J.; Simirgiotis, M.J.; Lima, B.; Gamarra-Luques, C.; Bórquez, J.; Caballero, D.; Feresin, G.E.; Tapia, A. UHPLC–Q/Orbitrap/MS/MS fingerprinting, free radical scavenging, and antimicrobial activity of Tessaria absinthiodes (Hook. & arn.) DC.(Asteraceae) lyophilized decoction from Argentina and Chile. Antioxidants 2019, 8, 593. [Google Scholar] [CrossRef]
- Pascual, L.I.; Luna, L.; González, R.E.; Ortiz, J.E.; Gomez-Gomez, L.; Donadel, O.J.; Hapon, M.B.; Feresin, G.E.; Gamarra-Luques, C. Correlation Analysis Among the Chemical Composition and Cytotoxic and Antioxidative Activities of a Tessaria absinthioides Decoction for Endorsing Its Potential Application in Oncology. Plants 2024, 13, 3062. [Google Scholar] [CrossRef]
- Persia, F.A.; Rinaldini, E.; Carrión, A.; Hapon, M.B.; Gamarra-Luques, C. Evaluation of cytotoxic and antitumoral properties of Tessaria absinthioides (Hook & Arn) DC,” pájaro bobo”, aqueous extract. Medicina 2017, 77, 283–290. [Google Scholar] [PubMed]
- Rey, M.; Kruse, M.S.; Magrini-Huamán, R.N.; Gómez, J.; Simirgiotis, M.J.; Tapia, A.; Feresin, G.E.; Coirini, H. Tessaria absinthioides (Hook. & arn.) DC.(Asteraceae) decoction improves the hypercholesterolemia and alters the expression of LXRs in rat liver and hypothalamus. Metabolites 2021, 11, 579. [Google Scholar] [CrossRef]
- Quesada, I.; de Paola, M.; Alvarez, M.S.; Hapon, M.B.; Gamarra-Luques, C.; Castro, C. Antioxidant and anti-atherogenic properties of Prosopis strombulifera and Tessaria absinthioides aqueous extracts: Modulation of NADPH oxidase-derived reactive oxygen species. Front. Physiol. 2021, 12, 662833. [Google Scholar] [CrossRef] [PubMed]
- Shalinsky, D.R.; Brekken, J.; Zou, H.; McDermott, C.D.; Forsyth, P.; Edwards, D.; Margosiak, S.; Bender, S.; Truitt, G.; Wood, A.; et al. Broad antitumor and antiangiogenic activities of AG3340, a potent and selective MMP inhibitor undergoing advanced oncology clinical trials. Ann. N. Y. Acad. Sci. 1999, 878, 236–270. [Google Scholar] [CrossRef]
- Woo, J.; Choo, G.; Yoo, E.; Kim, S.; Lee, J.; Han, S.; Kim, H.; Soo-Hyun, H.K.; Jung, S.; Park, Y.; et al. Apigenin induces apoptosis by regulating Akt and MAPK pathways in human melanoma cell A375SM. Mol. Med. Rep. 2020, 22, 4877–4889. [Google Scholar] [CrossRef]
- Cao, H.; Chu, J.; Kwan, H.; Su, T.; Yu, H.; Cheng, C.; Fu, X.; Guo, H.; Li, T.; Tse, A.K.; et al. Inhibition of the STAT3 signaling pathway contributes to Apigenin-mediated anti-metastatic effect in melanoma. Sci. Rep. 2016, 6, 21731. [Google Scholar] [CrossRef]
- Kimsa-Dudek, M.; Krawczyk, A.; Synowiec-Wojtarowicz, A.; Dudek, S.; Pawłowska-Góral, K. The impact of the co-exposure of melanoma cells to Chlorogenic acid and a moderate-strength static magnetic field. J. Food Biochem. 2020, 44, e13512. [Google Scholar] [CrossRef]
- Li, X.; Zhu, S.; Yin, P.; Zhang, S.; Xu, J.; Zhang, Q.; Shi, S.; Zhang, T. Combination immunotherapy of Chlorogenic acid liposomes modified with sialic acid and PD-1 blockers effectively enhances the anti-tumor immune response and therapeutic effects. Drug Deliv. 2021, 28, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yang, Z.; da Silva, F.; Liu, W.; Pan, Z.; Zhang, X.; Li, M.; Li, D. The anti-tumor effects of p-Coumaric acid on melanoma A375 and B16 cells. Front. Oncol. 2020, 10, 558414. [Google Scholar] [CrossRef]
- Pelinson, L.P.; Assmann, C.E.; Palma, T.V.; Manica da Cruz, I.B.; Pillat, M.M.; Manica, A.; Stefanello, N.; Cezimbra Weis, G.C.; de Oliveira Alves, A.; Melazzo de Andrade, C.; et al. Antiproliferative and apoptotic effects of Caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol. Biol. Rep. 2019, 46, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Kimsa-Dudek, M.; Synowiec-Wojtarowicz, A.; Krawczyk, A.; Kosowska, A.; Kimsa-Furdzik, M.; Francuz, T. The Apoptotic Effect of Caffeic or Chlorogenic Acid on the C32 Cells That Have Simultaneously Been Exposed to a Static Magnetic Field. Int. J. Mol. Sci. 2022, 23, 3859. [Google Scholar] [CrossRef]
- Li, H.R.; Habasi, M.; Xie, L.Z.; Aisa, H.A. Effect of Chlorogenic acid on melanogenesis of B16 melanoma cells. Molecules 2014, 19, 12940–12948. [Google Scholar] [CrossRef]
- Wang, F.; Chen, J.; Xiang, D.; Lian, X.; Wu, C.; Quan, J. Ellagic acid inhibits cell proliferation, migration, and invasion in melanoma via EGFR pathway. Am. J. Transl. Res. 2020, 12, 2295–2304. [Google Scholar] [PubMed]
- Jensen, J.D.; Dunn, J.H.; Luo, Y.; Liu, W.; Fujita, M.; Dellavalle, R.P. Ellagic acid inhibits melanoma growth in vitro. Dermatol. Rep. 2011, 3, e36. [Google Scholar] [CrossRef]
- Du, B.; Lin, P.; Lin, J. EGCG and ECG induce apoptosis and decrease autophagy via the AMPK/mTOR and PI3K/AKT/mTOR pathway in human melanoma cells. Chin. J. Nat. Med. 2022, 20, 290–300. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, Y.; Hui, Q.; Wang, H.; Tao, K. Naringin suppresses the metabolism of A375 cells by inhibiting the phosphorylation of c-Src. Tumor Biol. 2016, 37, 3841–3850. [Google Scholar] [CrossRef]
- Seo, S.Y.; Ju, W.S.; Kim, K.; Kim, J.; Yu, J.O.; Ryu, J.S.; Kim, J.S.; Lee, H.A.; Koo, D.-B.; Choo, Y.-K. Quercetin Induces Mitochondrial Apoptosis and Downregulates Ganglioside GD3 Expression in Melanoma Cells. Int. J. Mol. Sci. 2024, 25, 5146. [Google Scholar] [CrossRef]
- Cao, H.-H.; Tse, A.K.-W.; Kwan, H.-Y.; Yu, H.; Cheng, C.-Y.; Su, T.; Yu, Z.-L. Quercetin exerts anti-melanoma activities and inhibits STAT3 signaling. Biochem. Pharmacol. 2014, 87, 424–434. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.B.; Manica, D.; da Silva, A.P.; Marafon, F.; Moreno, M.; Bagatini, M.D. Rosmarinic acid decreases viability, inhibits migration and modulates expression of apoptosis-related CASP8/CASP3/NLRP3 genes in human metastatic melanoma cells. Chem.-Biol. Interact. 2023, 375, 110427. [Google Scholar] [CrossRef]
- Dumitraș, D.A.; Andrei, S. Recent Advances in the Antiproliferative and Proapoptotic Activity of Various Plant Extracts and Constituents against Murine Malignant Melanoma. Molecules 2022, 27, 2585. [Google Scholar] [CrossRef]
- Mustapha, N.; Mokdad-Bzéouich, I.; Maatouk, M.; Ghedira, K.; Hennebelle, T.; Chekir-Ghedira, L. Antitumoral, antioxidant, and antimelanogenesis potencies of Hawthorn, a potential natural agent in the treatment of melanoma. Melanoma Res. 2016, 26, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, F.; Mustapha, N.; Mokdad-Bzeouich, I.; Sassi, A.; Kilani-Jaziri, S.; Dijoux Franca, M.G.; Michalet, S.; Fathallah, M.; Krifa, M.; Ghedira, K.; et al. In vitro and in vivo anti-melanoma effects of Daphne gnidium aqueous extract via activation of the immune system. Tumor Biol. 2016, 37, 6511–6517. [Google Scholar] [CrossRef]
- Yun, J.; Kim, B.G.; Kang, J.S.; Park, S.K.; Lee, K.; Hyun, D.H.; Kim, H.M.; In, M.J.; Kim, D.C. Lipid-soluble ginseng extract inhibits invasion and metastasis of B16F10 melanoma cells. J. Med. Food 2015, 18, 102–108. [Google Scholar] [CrossRef]
- Chen, M.H.; Lee, C.H.; Liang, H.K.; Huang, S.C.; Li, J.P.; Lin, C.J.; Chen, J.K. Integrating the microneedles with carboplatin to facilitate the therapeutic effect of radiotherapy for skin cancers. Biomater. Adv. 2022, 141, 213113. [Google Scholar] [CrossRef] [PubMed]
- van der Westhuizen, A.; Knoblauch, N.; Graves, M.C.; Levy, R.; Vilain, R.E.; Bowden, N.A. Pilot early phase II study of de-citabine and carboplatin in patients with advanced melanoma. Medicine 2020, 99, e20705. [Google Scholar] [CrossRef] [PubMed]
- van der Westhuizen, A.; Lyle, M.; Graves, M.C.; Zhu, X.; Wong, J.W.H.; Cornall, K.; Ren, S.; Pugliese, L.; Levy, R.; Majid, A.; et al. Repurposing Azacitidine and Carboplatin to Prime Immune Checkpoint Blockade-resistant Melanoma for Anti-PD-L1 Rechallenge. Cancer Res. Commun. 2022, 2, 814–826. [Google Scholar] [CrossRef]
- Ruan, L.P.; Chen, S.; Yu, B.Y.; Zhu, D.N.; Cordell, G.A.; Qiu, S.X. Prediction of human absorption of natural compounds by the non-everted rat intestinal sac model. Eur. J. Med. Chem. 2006, 41, 605–610. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual authors and contributors and not of MDPI and/or the editors. MDPI and/or the editors disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascual, L.I.; Real, S.; Sosa-Lochedino, A.; Campo Verde Arbocco, F.; Hapon, M.B.; Gamarra-Luques, C. Tessaria absinthioides (Hook. & Arn.) DC. Determines Inhibition of Tumor Growth and Metastasis In Vitro and In Vivo in Murine Melanoma. Plants 2025, 14, 1379. https://doi.org/10.3390/plants14091379
Pascual LI, Real S, Sosa-Lochedino A, Campo Verde Arbocco F, Hapon MB, Gamarra-Luques C. Tessaria absinthioides (Hook. & Arn.) DC. Determines Inhibition of Tumor Growth and Metastasis In Vitro and In Vivo in Murine Melanoma. Plants. 2025; 14(9):1379. https://doi.org/10.3390/plants14091379
Chicago/Turabian StylePascual, Lourdes Inés, Sebastián Real, Arianna Sosa-Lochedino, Fiorella Campo Verde Arbocco, María Belén Hapon, and Carlos Gamarra-Luques. 2025. "Tessaria absinthioides (Hook. & Arn.) DC. Determines Inhibition of Tumor Growth and Metastasis In Vitro and In Vivo in Murine Melanoma" Plants 14, no. 9: 1379. https://doi.org/10.3390/plants14091379
APA StylePascual, L. I., Real, S., Sosa-Lochedino, A., Campo Verde Arbocco, F., Hapon, M. B., & Gamarra-Luques, C. (2025). Tessaria absinthioides (Hook. & Arn.) DC. Determines Inhibition of Tumor Growth and Metastasis In Vitro and In Vivo in Murine Melanoma. Plants, 14(9), 1379. https://doi.org/10.3390/plants14091379








