Abstract
Cancer is a complex disease due to its high variability and resistance to conventional treatments. The search for new therapies has prompted the study of less invasive natural sources, such as endophytic bacteria from medicinal plants. Bacillus subtilis is known to produce bioactive metabolites with promising pharmacological properties. This study evaluated the antitumor activity of the endophyte B. subtilis from Ibervillea sonorae against murine L5178Y-R lymphoma cells within in vitro and in vivo models. B. subtilis methanol extract was fractionated in hexane, chloroform, and methanol, with the chloroform partition showing the highest tumor cell growth inhibition (IC50 = 34.62 ± 0.180 µg/mL) and the highest selectivity index (SI = 15.53) when compared with the hexane and methanol partitions. The in vivo study showed that mice treated with 10 mg/kg of the chloroform partition significantly (p < 0.01) reduced the tumor volume and weight without affecting tumor-free body weight. The maximum tolerated dose test indicated that 10 mg/kg was safe and well tolerated. These results indicate that B. subtilis may be a promising source of selective antitumor compounds.
1. Introduction
Cancer continues to represent a major public health problem worldwide, with an estimated morbidity rate of 18.1 million and a mortality rate of 9.6 million per year. Some of the main characteristics of cancer include uncontrolled cell proliferation, the potential to evade control mechanisms, and cellular dimorphism [1,2]. In particular, lymphoma is a group of malignant neoplasms of lymphocytes with more than 90 subtypes, currently classified into Hodgkin’s and non-Hodgkin’s lymphoma [3]. Surgery, chemotherapy, and radiotherapy are the conventional treatments for this type of disease. However, they remain expensive and negatively impact the patient’s health due to their side effects, myelosuppression, and neurological, cardiac, pulmonary, and renal toxicity, reducing patients’ quality of life and discouraging them from undergoing current treatments, favoring the progression of cancer [4,5]. Therefore, there is a need to design new strategies that promote the control and elimination of this disease, which are more effective, selective, and less toxic than conventional therapies. The search for new drugs to treat this type of pathology has led to the discovery of new sources of bioactive molecules with selectivity and specificity characteristics [6,7].
In the last two decades, various endophytic bacteria associated with different plants have produced a wide range of bioactive compounds with therapeutic effects, especially antitumor activity [6,8]. Endophytic bacteria in cucurbits, such as Ibervillea sonorae, produce bioactive compounds with antitumor effects, specifically against in vitro lymphoma models [9]. This represents a source of new drugs with biological activity, especially antitumor activity. Therefore, in the present work, the antitumor potential of the chloroform partition of B. subtilis (ISE-B27), an endophyte of I. sonorae, was evaluated against murine L5178Y-R lymphoma cells (ATCC CRL-1722) in an in vitro and in vivo model using BALB/c mice.
2. Results
2.1. In Vitro Antitumor Activity of B. subtilis Extracts Against L5178Y-R Cells
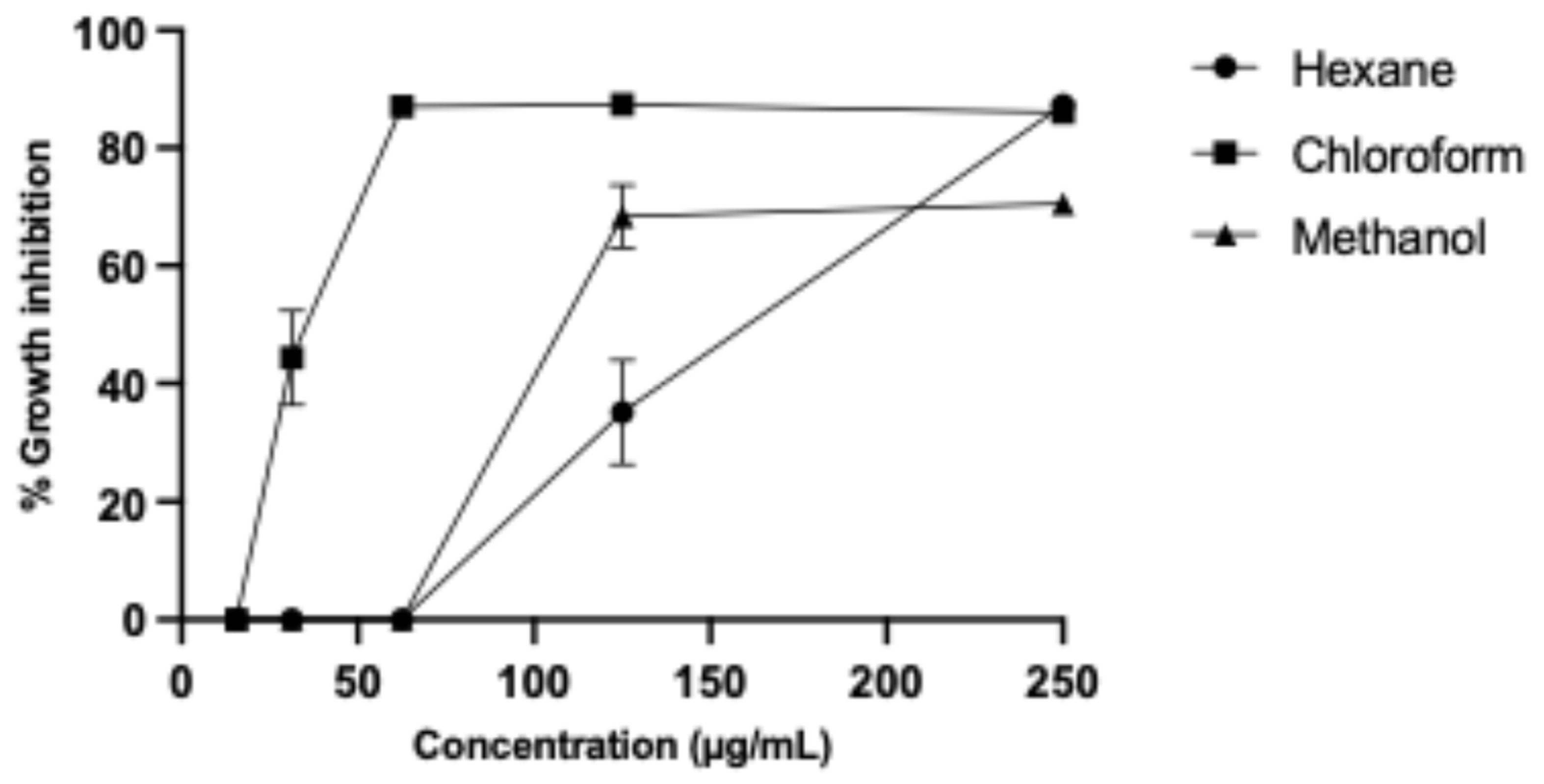
We obtained hexane, chloroform, and methanol partitions from B. subtilis (ISE-B27) crude methanol extract, resulting in the highest yield (1187 mg) for the chloroform partition, followed by the methanol partition with 1011 mg and the hexane partition with 124 mg. The partitions were evaluated against murine L5178Y-R lymphoma and human peripheral blood mononuclear cells (PBMCs), obtaining the highest IC50 and SI for the chloroform partition of 34.62 ± 0.180 µg/mL and 15.53, respectively (Table 1), whereas the IC50 of the hexane and methanol partitions were 148.1 ± 0.330 µg/mL and 117.7 ± 0.310 µg/mL; however, their respective SIs were 3.37 and 4.24 (Figure 1).

Table 1.
In vitro performance and antitumor activity of B. subtilis methanol extract partitions.
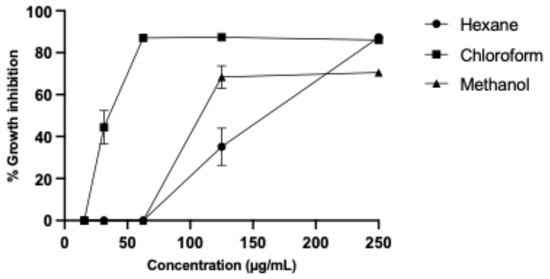
Figure 1.
L5178Y-R lymphoma cell growth inhibition by B. subtilis partitions.
2.2. Biological Activity of B. subtilis Extracts
We evaluated the hemolytic and anti-hemolytic effect on erythrocytes, using the most effective and selective extract partitions against L5178Y-R cells (Table 1). The extract partition that showed the lowest hemolytic activity was chloroform with an IC50 of 1443 ± 0.490 µg/mL, followed by methanol-based partitioning with an IC50 of 223.2 ± 0.370 µg/mL, and the hexane-based partition with an IC50 of 74.01 ± 0.270 µg/mL. In addition, evaluation of the anti-hemolytic activity demonstrated that the methanol-based partition possessed the highest protective potential with an IC50 of 89.35 ± 0.290 µg/mL, whereas the hexane and chloroform partitions presented IC50 > 500 µg/mL (606.5 ± 0.440 and 11,847 ± 0.610 µg/mL, respectively (Table 2)). However, the partitions did not show antioxidant activity (Table 2).

Table 2.
Hemolytic, anti-hemolytic, and antioxidant activity of ISE-B27 extract partitions.
2.3. Phytochemical Analysis of B. subtilis Extract
For the characterization of the main chemical groups present in the chloroform partition, a series of chemical tests were performed, revealing the presence and absence of various families of compounds. Table 3 shows the results of the chemical tests of the ISE-B27 (B. subtilis) extract chloroform partition. The test was positive for triterpenes, coumarins, tannins, and alkaloids, and negative for sesquiterpene lactones, quinones, saponins, flavonoids, and carbohydrates (Table 3).

Table 3.
Phytochemical composition of chloroform-based partition.
2.4. Maximum Tolerated Dose of B. subtilis Extract in Tumor-Free Mice
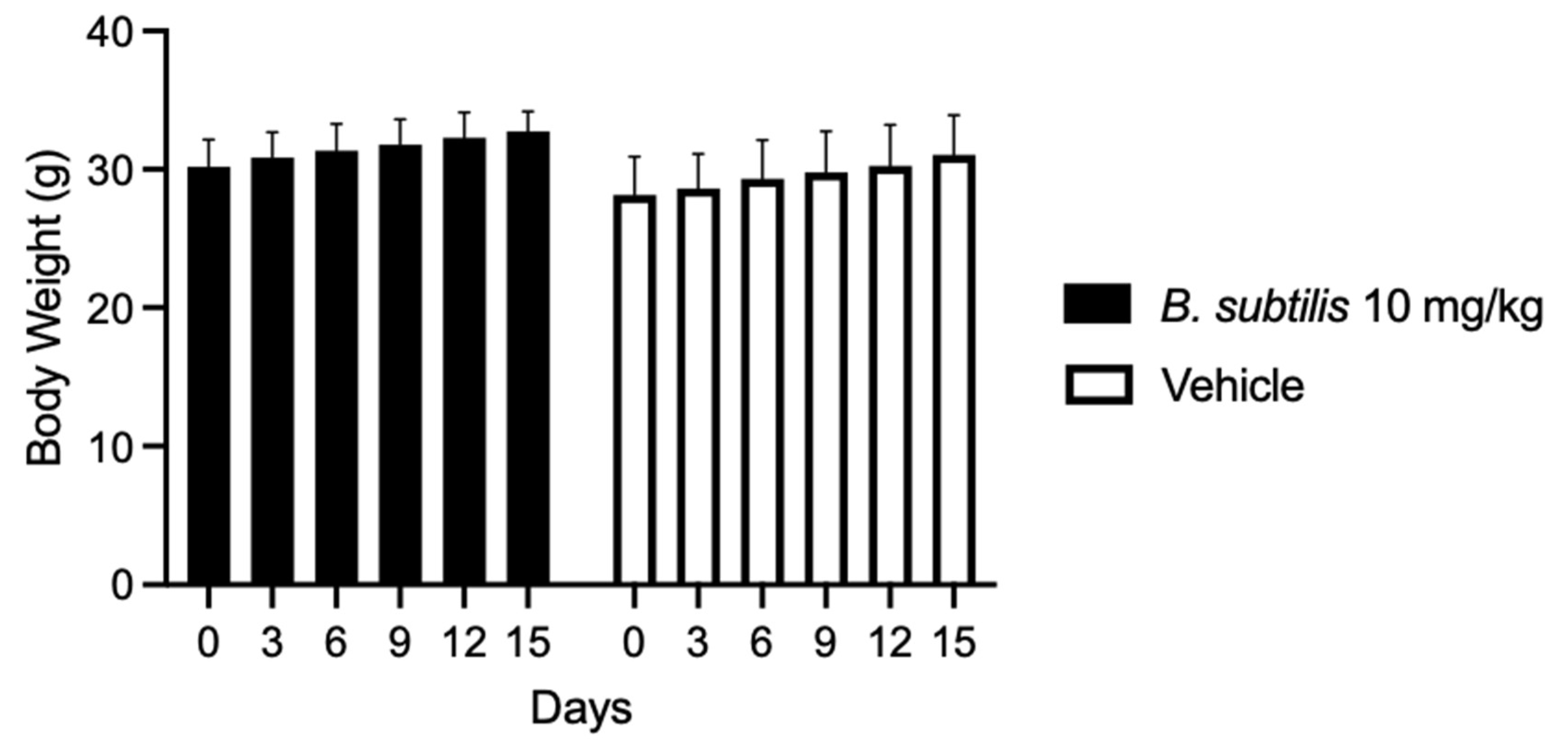
To determine the dose to be used in the in vivo model the maximum tolerated dose test was performed, in which the extract was administered at different concentrations. This resulted in a 100% survival of the treated group at 10 mg/kg chloroform-based partition of the ISE-B27 bacterial extract and vehicle. Furthermore, the group treated at 50 mg/kg had a survival rate of 33.3%, whereas the highest concentrations of 100 mg/kg and 1000 mg/kg were significantly toxic, resulting in mice death. Mice treated with the B. subtilis extract at 10 mg/kg and vehicles did not show significant differences in weight loss (Figure 2). To determine whether B. subtilis extract treatment does not cause liver damage, liver function was evaluated by clinical chemical analysis of the group treated with 10 mg/kg, showing no liver damage (Table 4).
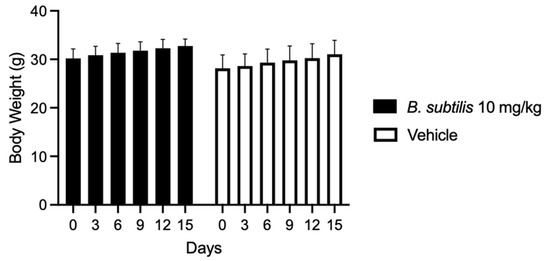
Figure 2.
Body weight variation in mice treated with B. subtilis extract and vehicle for 15 days.

Table 4.
Liver function at 15 days following administration of treatments at the maximum tolerated dose test in tumor-free mice.
2.5. In Vivo Effect of B. subtilis Extract Against L5178Y-R Cells
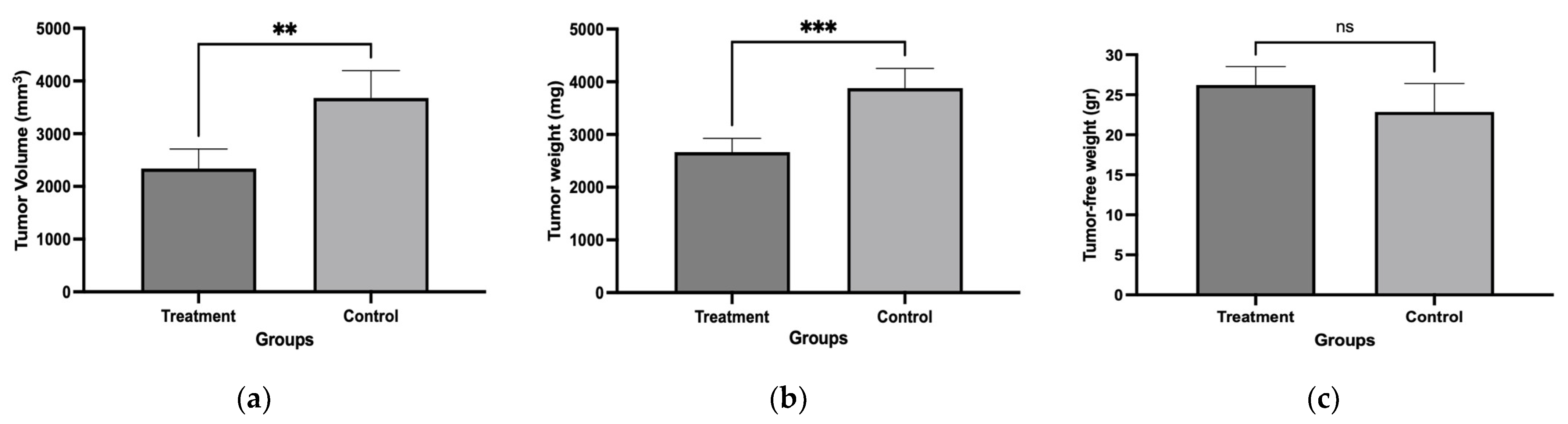
Mice were implanted with L5178Y-R cells and treated with the chloroform-based partition (10 mg/kg) of the bacteria ISE-B27 for 14 d after tumor implantation. We observed a significant reduction in tumor volume (p < 0.01) and tumor weight (p < 0.001) in mice treated with the chloroform partition compared to untreated tumor-bearing mice (Figure 3), without affecting tumor-free weight (Figure 4). In addition, as shown in Table 5, liver function was not affected by this treatment in tumor-bearing mice in the maximum tolerated dose test.
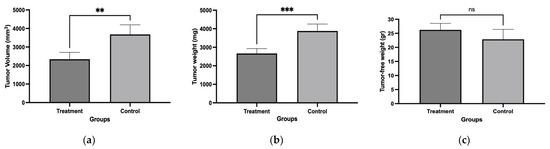
Figure 3.
Tumor volume, tumor weight, and tumor-free weight of female BALB/c mice treated with chloroform-based partition (10 mg/kg) from ISE-B27 (B. subtilis) bacterial extract. (a) Tumor volume, (b) tumor weight, and (c) tumor-free weight. ** p < 0.01; *** p < 0.001; ns: not significant.
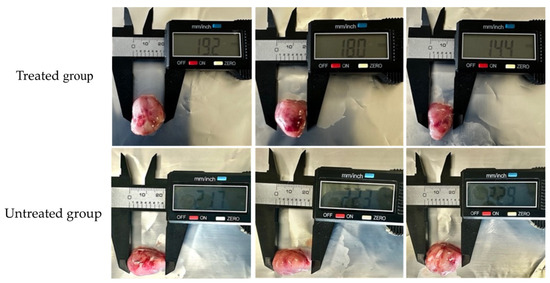
Figure 4.
Effect of chloroform-based partition (10 mg/kg) from ISE-B27 (B. subtilis) bacterial extract on tumor size. Tumors of the untreated group were significantly larger compared to those of the treated group.

Table 5.
In vivo liver function.
3. Discussion
Cancer represents one of the main causes of death worldwide [11]. Despite advances in cancer therapies they still have disadvantages, such as drug resistance and side effects [12], which has prompted researchers to discover new selective antitumor agents of high therapeutic efficacy and with marginal side effects [13]. Gram-positive bacteria, such as actinomycetes and endophytic Bacillus from medicinal plants, synthesize compounds for pharmaceutical application against cancer, such as aromatic compounds, polysaccharides, and lipopeptides [13,14,15,16].
Results of the present study demonstrated the in vitro and in vivo antitumor activity of B. subtilis, which is an endophyte of I. sonorae, a cucurbit native to northern Mexico, whose methanol and ethyl acetate extracts contain cucurbitacins, with cytotoxic properties against the tumor cell lines HepG2, HeLa, L5178Y-R, A549, M12A, LS180, and MDA-MB-231 [17,18,19,20].
Currently, the cancer incidence and mortality rate are increasing worldwide and lymphoma is one of the eleven most prevalent types of cancer [11]. Our study showed significant tumor cell growth inhibition by chloroform-based partitions of the endophytic bacterium B. subtilis (ISE-B27) methanol extract, exerting an IC50 of 34.62 ± 0.180 µg/mL against L5178Y-R cells. This result is comparable with other studies, such as one isolating a dipeptide from Bacillus pumilus AMK1 using chloroform which had significant cytotoxic and apoptotic activity against HepG2 liver cancer cells [21]. Another study found soluble proteins from Bacillus thuringiensis strains GM1, GM18, and HD-512 with cytotoxic activity against L5178Y-R cells [22].
In addition, the evaluation of hemolytic activity in the present study showed that the partition of chloroform from the bacterial extract exerted an IC50 of 443 ± 0.490 µg/mL, which is consistent with results obtained by others where ethyl acetate fractions did not possess hemolytic activity in human erythrocytes [23]. We also found that the chloroform partition had in vivo antitumor potential, which agrees with recent studies using a mouse xenograft model with sarcoma 180 [24]. Another study reported a reduction in tumor size and weight using a mouse xenograft of human colon cancer treated with compounds synthesized from B. polyfermenticus [25]. Moreover, iturin A, extracted from B. subtilis methanol extract, was shown to reduce 58% of the tumor weight at a dose of 3 mg/kg/day in a xenograft model of HepG2 cells [26].
Marginal information has been reported on the interaction that bacteria play in the production of bioactive compounds in medicinal plants. This study contributes to the knowledge of the relationship that these bacteria may have with the plant’s biological activity. It is then essential to investigate the compounds that exert this activity, as well as their tumor selectivity and cell death mechanisms.
4. Materials and Methods
4.1. Bacillus Strain
We used the bacterial strain B. subtillis ISE-B27, an endophyte of I. sonorae [9]. It was activated on malt extract agar (ISP2) (Sigma-Aldrich, St. Louis, MO, USA) for three days at 28 °C.
4.2. Preparation of Extracts
B. subtillis ISE-B27 was fermented in 120 mL of ISP2 broth (Sigma-Aldrich, St. Louis, MO, USA) for three days under shaking (120 rpm) at 28 ± 2 °C. Cultures were then centrifuged at 4500 rpm for 10 min, separating the biomass from the supernatant. Methanol extracts were prepared by macerating for 48 h and then suspending 50 g of the biomass in 100 mL of methanol [27]. Next, the solvent was filtered and evaporated in a rotary evaporator (Buchi R-3000; Brinkman Instruments, Inc., Westbury, Long Island, NY, USA). Fractionation was performed from the crude methanol extract in a Soxhlet apparatus with 500 mL of hexane, chloroform, and methanol for 24 h with each solvent. Once the fractionated extracts were obtained, they were evaporated and reconstituted with dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) and stored at −20 °C until use. Extract yields were analyzed using the following formula: % yield = [(gram of dry extract/gram of biomass) (100)].
4.3. Cell Lines
We used the murine L5178Y-R lymphoma cell line (ATCC CRL-1722) (ATCC, Manassas, VA, USA) and human peripheral blood mononuclear cells (PBMCs) as controls, which were obtained from 20 mL of blood from a healthy volunteer donor. Blood was diluted at a ratio of 1:9 with phosphate-buffered saline (PBS) and placed in a tube containing 15 mL of Ficoll-Paque PLUS (GE Healthcare Life Sciences, Pittsburgh, PA, USA), taking care that the phases did not mix. It was then centrifuged at 400× g for 30 min at 20 °C, after which the lymphocyte layer was carefully collected and washed with PBS at 100× g for 10 min at 20 °C, the supernatant was discarded, and PBMCs were suspended in RPMI 1640 culture medium (Life Technologies, Inc., Grand Island, NY, USA) supplemented with 10% inactivated fetal bovine serum (Life Technologies, Inc., Grand Island, NY, USA) and 1% antibiotic/antimycotic solution (Life Technologies, Inc., Grand Island, NY, USA). Cells were then incubated at 37 °C in an atmosphere of 5% CO2 in 95% air.
4.4. Effect of Extracts on L5178Y-R Cell Growth
In 96-well flat-bottom plates (Corning Inc., Corning, NY, USA) in complete RPMI 1640 culture medium, L5178Y-R cell suspensions (100 μL) were cultured at a density of 1 × 104 cells/well and PBMCs were cultured at a density of 1 × 105 cells/well. After 24 h, cells were incubated in triplicate for 48 h at 37 °C in 5% CO2 with 1:2 serial dilutions of 1 mg/mL stock extracts (final concentrations ranging from 15.6 μg/mL to 500 μg/mL), in a final volume of 200 µL. Tumor cell growth was then assessed by the colorimetric 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide reduction assay (MTT; Affymetrix, Cleveland, OH, USA), adding 15 µL/well MTT (final concentration 0.5 mg/mL). Formazan crystals were dissolved in DMSO, and optical densities (ODs) were measured at 570 nm on a MULTISKAN GO microplate reader (Thermo Fisher Scientific, Waltham, MA, USA) [28]. The percentage of cell growth inhibition was calculated as follows: % growth inhibition = 100 − ((OD570 in extract-treated cells/OD570 in untreated cells) (100)). The positive control was 0.05 µg/mL vincristine sulfate (VC; Hospira, Warwickshire, UK). Concentrations were plotted on a log scale against the percentage of growth inhibition to determine IC50 values, which were used to determine the selectivity index (SI). This index was calculated by dividing the IC50 of normal cells by that of tumor cells [29].
4.5. Antioxidant Activity
The antioxidant activity of each extract partition was evaluated by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method [30]. In a 96-well plate, we incubated 100 μL of extract at different concentrations and 100 μL of DPPH (Sigma-Aldrich, St. Louis, MO, USA) for 30 min at room temperature in the dark, using DMSO as a negative control and ascorbic acid as a standard at concentrations ranging from 10 µg/mL to 100 µg/mL. ODs were then read at 517 nm in a MULTISKAN GO microplate reader (Thermo Fisher Scientific, Waltham, MA, USA), and the percentage of DPPH radical inhibition was calculated.
4.6. Hemolytic and Anti-Hemolytic Activity
The hemolytic and anti-hemolytic activity of the extracts was determined as previously described [31]. We obtained 20 mL of blood from a healthy volunteer (approved by the Ethics Committee of the Facultad de Ciencias Biológicas, UANL protocol CI-09-2022; informed consent obtained in accordance with NOM-253-SSA1-2012) in tubes with EDTA anticoagulant, after which the erythrocytes were washed three times with PBS at pH 7.2 and 5% of erythrocytes were suspended in sterile PBS. Next, we incubated the extracts (15.625 µg/mL to 250 µg/mL) plus the erythrocyte suspension in 2 mL tubes in triplicate, using distilled water as a positive control and PBS as a negative control, at 37 °C for 30 min, after which we centrifuged at 4 °C for 5 min at 13,000 rpm. To assess the anti-hemolytic activity, we incubated a suspension of red blood cells with 150 mM of 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) (Sigma-Aldrich, St. Louis, MO, USA) plus extracts, using PBS as a negative control and erythrocytes with AAPH as a positive control, at 37 °C for 5 h at 200 rpm and centrifuged under the conditions described above. In both cases, once the sample was centrifuged, 200 μL of the supernatant was obtained and placed in a 96-well microplate to measure the OD at 540 nm. The percentage of hemolysis and anti-hemolysis was calculated as follows: % hemolysis or % AAPH inhibition (anti-hemolysis) = [(OD540 treatment − OD540 negative control)/(OD540 positive control − OD540 negative control)] × 100.
4.7. Phytochemical Analysis of the Extract
The phytochemical profile of the extract was analyzed by qualitative tests to detect the presence of different compounds, using each extract dissolved in methanol. For the detection of xanthophylls, 0.2 mL of HCl was added to 0.5 mL of extract: a green or purple coloration indicated a positive result. To identify unsaturated carbon–carbon bonds we used the Baeyer test, in which 5 drops of 1% KMnO4 were added to 10 drops of each extract. The test is positive if a color changes from purple to reddish brown occurs together with a precipitate. Carbohydrates were detected by the Molisch test. For this, the Molisch reagent was added to 10 drops of extract and concentrated H2SO4; the presence of a purple ring at the interface was interpreted as positive. The presence of flavonoids was detected by the Shinoda test, in which 10 drops of HCl and a small piece of Mg were added to 20 drops of extracts; the presence of an orange, red, or purple color indicated a positive result. Alkaloids were detected by the Dragendorff test, in which five drops of Dragendorff reagent were added to five drops of extract: the presence of an orange-brown precipitate indicated a positive test. The phenolic hydroxyl group was determined by the FeCl3 test, in which two drops of reagent were added to three drops of extract; the test is positive if a red, blue, or purple color or precipitate appears. For sterols and triterpenes, the Liebermann–Burchard test was used. A total of 10 drops of the Liebermann–Burchard reagent were added to 20 drops of extract; the presence of green or blue colors was positive for sterols, whereas a purple or pink color was positive for triterpenes. Coumarins were detected by adding 10 drops of 10% NaOH to the extract, which produced a color change, followed by slowly adding 1 drop of concentrated HCl until a discoloration was observed, indicating a positive test. To determine the presence of lactones, the Baljet test was used, in which five drops of Baljet reagent were added to five drops of extract; the presence of a red, orange, or violet precipitate was a positive result [32].
4.8. Animals
Female BALB/c mice aged 6 to 7 wk were provided by the Bioterium of the Immunology and Virology Laboratory of the School of Biological Sciences at the Autonomous University of Nuevo Leon, Mexico. The animal study protocol was approved by the Research Ethics and Animal Welfare Committee of the Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León (protocol code CEIBA-2021-011). They were housed in microventilated cages enriched with cardboard tubes for recreational purposes, access to water and food ad libitum, in a stress- and pathogen-free environment, and at a temperature of 22 °C, with light and dark cycles of 12 h and a relative humidity of 45%. Established guidelines for the care and welfare of animals in cancer research were followed [33]. To determine the evaluation criteria of the animals, a clinical score was used that evaluated body weight, hair condition, posture, and activity [34].
4.9. Maximum Tolerated Dose Test
We performed the maximum tolerated dose experiment following the protocol described above [34]. Extracts were suspended in a vehicle composed of 90% PEG-300 (Sigma-Aldrich), 5% DMSO, and 5% of 96% ethanol [35]. Three doses of 10 mg/kg of the chloroform-based extract were intraperitoneally administered to each mouse, in addition to maintaining a vehicle-treated group and the control group. The observation period was 15 d, during which the weight and clinical score of each mouse were recorded. For blood studies, we intraperitoneally administered 25 mg/kg of sodium pentobarbital (Aranda Salud Animal, Querétaro, Mexico) as an anesthetic. A cardiac puncture was then performed to obtain a blood sample, followed by euthanasia of the animal. The blood samples were stored in tubes without heparin and centrifuged at 3000 rpm for five minutes to separate the serum and perform liver function tests. Animals were euthanized by cervical dislocation if a weight loss ≥ 20% or a clinical score ≥ 3 occurred.
4.10. In Vivo Antitumor Activity
L5178Y-R lymphoma was maintained by intraperitoneally inoculating 0.2 mL of L5178Y-R cells (5 × 106 cells/mouse) into 4- to 6-week-old female BALB/c mice as previously described [36]. Ascites cell suspensions were washed twice in PBS by centrifugation at 2000 rpm for 10 min and adjusted to 2 × 105 cells/mL in PBS, after which 6- to 7-week-old female BALB/c mice received a subcutaneous administration (upper right thigh) of 0.2 mL of this lymphoma suspension. After 7 days of tumor inoculation, mice bearing the L5178Y-R tumor were selected and treated with B. subtilis extract. Controls were tumor-bearing mice not treated with extracts (untreated), tumor-bearing mice treated with vehicle (a solution containing 90% PEG-300, 5% DMSO, and 5% of 96% ethanol) (vehicle), and untreated normal mice (untreated control without tumor). Overall survival and tumor volume (length) (width)2/2 were then determined. Tumor measurements (length and width) were obtained with a vernier caliper.
4.11. Statistical Analysis
Results were expressed as mean ± SD of three replicate determinations per treatment (in vitro study) and five mice (in vivo study) per experimental group. Statistical analyses were performed using GraphPad Prism 9 software (GraphPad Software Inc., San Diego, CA, USA). Statistical analysis determined the distribution of the data obtained for each variable (in vivo and in vitro data) studied using the Kolmogorov–Smirnov test, and subsequently a comparison was made by analysis of variance (ANOVA). For data with a predominant normal distribution, the one-way test was used. In cases where a difference was found between the groups, the Student t test was used to evaluate the level of significance of the difference in the mean ± SD of the control group against each of the treatment concentrations (p < 0.05).
5. Conclusions
Results of the present study demonstrated the pharmacological potential of chloroform partitions of Bacillus subtilis ISE-B27 extracts, an endophyte of Ibervillea sonorae, and the its potential to become a promising source of compounds with therapeutic potential against cancer by it significantly reducing tumor growth without affecting the general health of mice.
Author Contributions
Conceptualization, R.R.-A. and C.I.R.-S.; methodology, R.R.-A., D.F.-R. and R.G.-F.; software, D.F.-R. and C.M.Q.-F.; validation, C.I.R.-S., P.T.-G. and O.P.-G.; formal analysis, C.A.-Q., J.H.E.-L. and N.E.R.-G.; investigation, R.R.-A., L.P.L.H. and R.G.-F.; resources, C.I.R.-S. and R.G.-F.; data curation, R.R.-A.; writing—original draft preparation, R.R.-A. and C.I.R.-S.; writing—review and editing, R.G.-F.; visualization, R.R.-A., L.P.L.H. and R.G.-F.; supervision, C.I.R.-S.; project administration, C.I.R.-S. and R.G.-F.; funding acquisition, C.I.R.-S. and R.G.-F. All authors have read and agreed to the published version of the manuscript.
Funding
We received financial support through SNII-445572 to C.I.R.-S. and SNII-9942 to R.G.-F.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors thank CONAHCYT-MEXICO for scholarships CVU: 3367959 to R.R.-A., CVU: 708487 to D.F.-R., CVU: 418935 to J.H.E-L., CVU: 1119878 to L.P.L.-H., and CVU: 1179478 to N.E.R.-G.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the title, changing “GreeneEndophytic” to “Greene Endophytic”. This change does not affect the scientific content of the article.
References
- Stewart, B.W.; Kleihues, P. (Eds.) World Cancer Report; IARC Publications: Lyon, France, 2003. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Molyneux, E.M.; Rochford, R.; Griffin, B.; Newton, R.; Jackson, G.; Menon, G.; Harrison, C.J.; Israels, T.; Bailey, S. Burkitt’s lymphoma. Lancet 2012, 379, 1234–1244. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA A Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Cavalcanti, I.D.L.; Soares, J.C.S.; Cavalcanti, I.D.L.; Soares, J.C.S. Conventional cancer treatment. In Advances in Cancer Treatment: From Systemic Chemotherapy to Targeted Therapy; Springer Nature: Berlin/Heidelberg, Germany, 2021; pp. 29–56. [Google Scholar]
- Singh, A.; Singh, D.K.; Kharwar, R.N.; White, J.F.; Gond, S.K. Fungal endophytes as efficient sources of plant-derived bioactive compounds and their prospective applications in natural product drug discovery: Insights, avenues, and challenges. Microorganisms 2021, 9, 197. [Google Scholar] [CrossRef]
- Raimi, A.; Adeleke, R. Bioprospecting of endophytic microorganisms for bioactive compounds of therapeutic importance. Arch. Microbiol. 2021, 203, 1917–1942. [Google Scholar] [CrossRef]
- Numan, M.; Shah, M.; Asaf, S.; Ur Rehman, N.; Al-Harrasi, A. Bioactive compounds from endophytic bacteria Bacillus subtilis strain EP1 with their antibacterial activities. Metabolites 2022, 12, 1228. [Google Scholar] [CrossRef]
- Romero-Arguelles, R.; Romo-Sáenz, C.I.; Morán-Santibáñez, K.; Tamez-Guerra, P.; Quintanilla-Licea, R.; Orozco-Flores, A.A.; Gomez-Flores, R. In vitro antitumor activity of endophytic and rhizosphere gram-positive bacteria from Ibervillea sonidoe (S. Watson) Greene against L5178Y-R lymphoma cells. Int. J. Environ. Res. Public Health 2022, 19, 894. [Google Scholar] [CrossRef]
- Hedrich, H. (Ed.) The Laboratory Mouse; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Raguz, S.; Yagüe, E. Resistance to chemotherapy: New treatments and novel insights into an old problem. Br. J. Cancer 2008, 99, 387–391. [Google Scholar] [CrossRef]
- Zheng, L.P.; Zou, T.; Ma, Y.J.; Wang, J.W.; Zhang, Y.Q. Antioxidant and DNA damage protecting activity of exopolysaccharides from the endophytic bacterium Bacillus Cereus SZ1. Molecules 2016, 21, 174. [Google Scholar] [CrossRef]
- Ghosh, A.; Sutradhar, S.; Baishya, D. Delineating thermophilic xylanase from Bacillus licheniformis DM5 towards its potential application in xylooligosaccharides production. World J. Microbiol. Biotechnol. 2019, 35, 34. [Google Scholar] [CrossRef]
- Nafie, M.S.; Awad, N.M.; Tag, H.M.; Abd El-Salam, I.M.; Diab, M.K.; El-Shatoury, S.A. Micromonospora species from rarely-exploited Egyptian habitats: Chemical profile, antimicrobial, and antitumor activities through antioxidant properties. Appl. Microbiol. Biotechnol. 2021, 105, 2427–2439. [Google Scholar] [CrossRef]
- Ek-Ramos, M.J.; Gomez-Flores, R.; Orozco-Flores, A.A.; Rodríguez-Padilla, C.; González-Ochoa, G.; Tamez-Guerra, P. Bioactive products from plant-endophytic Gram-positive bacteria. Front. Microbiol. 2019, 10, 463. [Google Scholar] [CrossRef]
- Alarcon-Aguilar, F.J.; Calzada-Bermejo, F.; Hernandez-Galicia, E.; Ruiz-Angeles, C.; Roman-Ramos, R. Acute and chronic hypoglycemic effect of Ibervillea sonidoe root extracts-II. J. Ethnopharmacol. 2005, 97, 447–452. [Google Scholar] [CrossRef]
- Torres-Moreno, H.; Marcotullio, M.C.; Velázquez, C.; Ianni, F.; Garibay-Escobar, A.; Robles-Zepeda, R.E. Cucurbitacin IIb, a steroidal triterpene from Ibervillea sonidoe induces antiproliferative and apoptotic effects on cervical and lung cancer cells. Steroids 2020, 157, 108597. [Google Scholar] [CrossRef]
- Vidal-Gutiérrez, M.; Torres-Moreno, H.; Hernández-Gutiérrez, S.; Velazquez, C.; Robles-Zepeda, R.E.; Vilegas, W. Antiproliferative activity of standardized phytopreparations from Ibervillea sonidoe (S. Watson) Greene. Steroids 2021, 169, 108824. [Google Scholar] [CrossRef]
- Torres-Moreno, H.; Velázquez, C.A.; Garibay-Escobar, A.; Curini, M.; Marcotullio, M.C.; Robles-Zepeda, R.E. Antiproliferative induction and apoptosis of cucurbitacin-type triterpenes from Ibervillea sonorae. Crops Ind. Prod. 2015, 77, 895–900. [Google Scholar] [CrossRef]
- Karanam, G.; Arumugam, M.K.; Sirpu Natesh, N. Anticancer effect of marine sponge-associated Bacillus pumilus AMK1 derived dipeptide Cyclo (-Pro-Tyr) in human liver cancer cell line through apoptosis and G2/M phase arrest. Int. J. Pept. Res. Ther. 2020, 26, 445–457. [Google Scholar] [CrossRef]
- Molina, M.A.F.; Gamboa, E.M.; Calderon, M.E.R.; Benavides, P.Z.; Morales, L.G.R.; Monsivais, L.Z.; Cerda, E.E.C.N.; Rivera, C.A.S.; Guerra, R.T.; Padilla, C.R.I.G. In vitro antitumor activity of soluble protein extracts from Bacillus thuringiensis. Afr. J. Microbiol. Res. 2016, 10, 324–329. [Google Scholar] [CrossRef]
- Nighat, F.; Mushtaq, Z.; Maqsood, M.; Shahid, M.; Hanif, M.A.; Jamil, A. Cytotoxic, a-amylase inhibitory and thrombolytic activities of organic and aqueous extracts of Bacillus clausii KP10. Pak. J. Pharm. Sci. 2020, 33, 135. [Google Scholar]
- Dey, G.; Bharti, R.; Banerjee, I.; Das, A.K.; Das, C.K.; Das, S.; Jena, B.C.; Misra, M.; Sen, R.; Mandal, M. Pre-clinical risk assessment and therapeutic potential of antitumor lipopeptide ‘Iturin A’ in an in vivo and in vitro model. RSC Adv. 2016, 6, 71612–71623. [Google Scholar] [CrossRef]
- Ma, E.L.; Choi, Y.J.; Choi, J.; Pothoulakis, C.; Rhee, S.H.; Im, E. The anticancer effect of probiotic Bacillus polyfermenticus on human colon cancer cells is mediated through ErbB2 and ErbB3 inhibition. Int. J. Cancer 2010, 127, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yan, L.; Guo, L.; Sun, H.; Huang, Q.; Shao, D.; Jiang, C.; Shi, J. Effects of Bacillus subtilis iturin A on HepG2 cells in vitro and vivo. AMB Express 2021, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Prihanto, A.A.; Firdaus, M.; Nurdiani, R. Endophytic Fungi Isolated from Mangrove (Rhyzopora mucronata) and Its Antibacterial Activity on Staphylococcus aureus and Escherichia coli. J. Food Sci. Eng. 2011, 1, 386–389. [Google Scholar]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ramírez-Villalobos, J.M.; Romo-Sáenz, C.I.; Morán-Santibañez, K.S.; Tamez-Guerra, P.; Quintanilla-Licea, R.; Orozco-Flores, A.A.; Romero-Arguelles, R.; Tamez-Guerra, R.; Rodríguez-Padilla, C.; Gomez-Flores, R. In vitro tumor cell growth inhibition induced by Lophocereus marginatus (Dc.) S. Arias and Terrazas endophytic fungi extracts. Int. J. Environ. Public Health Res. 2021, 18, 9917. [Google Scholar] [CrossRef]
- Kačániová, M.; Vukovic, N.L.; Čmiková, N.; Bianchi, A.; Garzoli, S.; Ben Saad, R.; Ben Hsouna, A.; Elizondo-Luévano, J.H.; Ahl, H.A.H.S.-A.; Hikal, W.M.; et al. Biological activity and phytochemical characteristics of star anise (Illicium verum) essential oil and its anti-Salmonella activity on sous vide pumpkin model. Foods 2024, 13, 1505. [Google Scholar] [CrossRef]
- Elizondo-Luévano, J.H.; Castro-Ríos, R.; Vicente, B.; Fernández-Soto, P.; López-Aban, J.; Antonio, M.U.R.O.; Chávez-Montes, A. In vitro antischistosomal activity of the Argemone Mexicana methanolic extract and its main component berberine. Iran. J. Parasitol. 2021, 16, 91. [Google Scholar] [CrossRef]
- Rodríguez-Garza, N.E.; Quintanilla-Licea, R.; Romo-Sáenz, C.I.; Elizondo-Luevano, J.H.; Tamez-Guerra, P.; Rodríguez-Padilla, C.; Gomez-Flores, R. In Vitro Biological Activity and Lymphoma Cell Growth Inhibition by Selected Mexican Medicinal Plants. Life 2023, 13, 958. [Google Scholar] [CrossRef]
- Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.H.; Glennie, M.J.; et al. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef]
- Clark-Pérez, D.L.; Romo-Sáenz, C.I.; Ramírez-Villalobos, J.M.; Tamez-Guerra, P.; Caballero-Hernández, D.; Delgado-Miranda, A.L.; García, A.; Gomez-Flores, R. In Vitro and In Vivo Antitumor Activity of Lophocereus marginatus (DC.) S. Arias & Terrazas Endophytic Aspergillus versicolor and Metarhizium anisopliae Extracts Against the Murine Lymphoma L5178Y-R. Microorganisms 2024, 12, 2310. [Google Scholar] [CrossRef] [PubMed]
- Matias, M.; Silvestre, S.; Falcao, A.; Alves, G. Considerations and Pitfalls in Selecting the Drug Vehicles for Evaluation of New Drug Candidates: Focus on in Vivo Pharmaco-Toxicological Assays Based on the Rotarod Performance Test. J. Pharm. Pharm. Sci. 2018, 21, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Flores, R.; Quintanilla-Licea, R.; Hernandez-Martinez, C.H.; Samaniego-Escamilla, M.; Tamez-Guerra, P.; Monreal-Cuevas, E.; Tamez-Guerra, R.; Rodriguez-Padilla, C. Survival of Lymphoma-Bearing Mice by Pachycereus Marginatus Cactus Extracts and Elucidation of Bioactive Compounds. Nat. Prod. Commun. 2019, 14, 1934578X19845814. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).