First Record of Flower Bud Galls in Senega (Fabales: Polygalaceae): The Case of S. salasiana and Their Effect on Plant Reproduction
Abstract
1. Introduction
2. Results
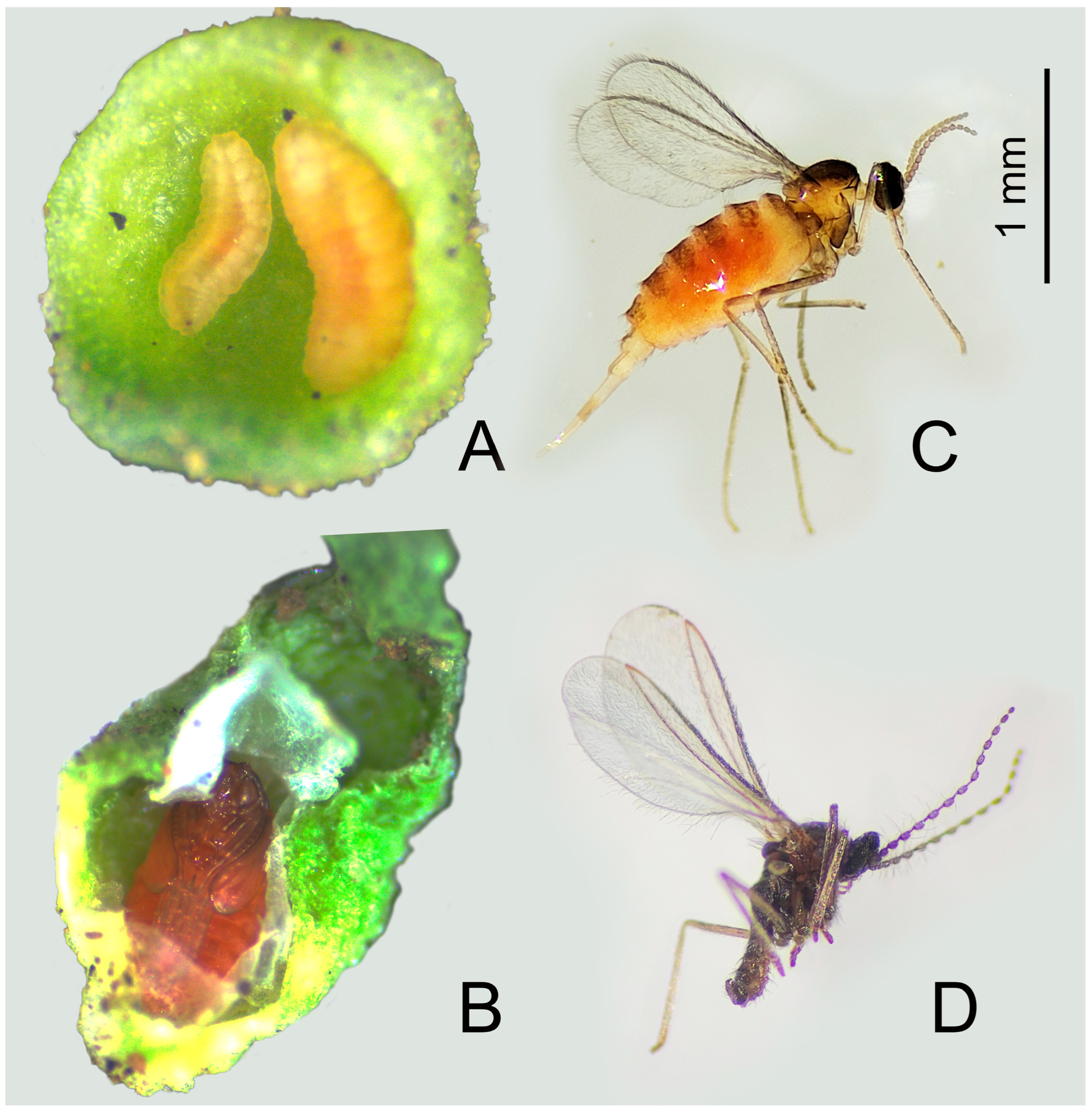
2.1. Galls Description
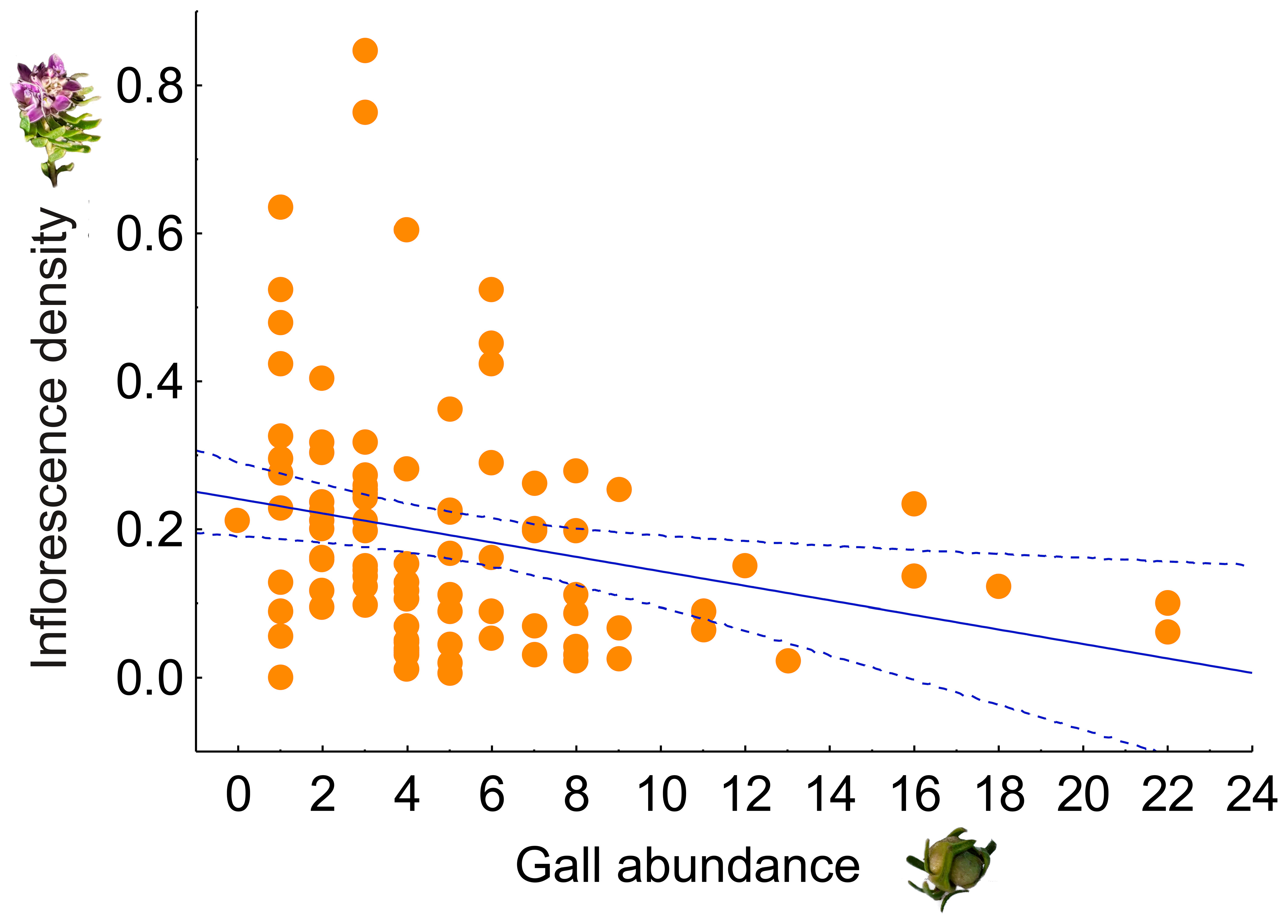
2.2. Effect on the Number of Inflorescences
3. Discussion
4. Materials and Methods
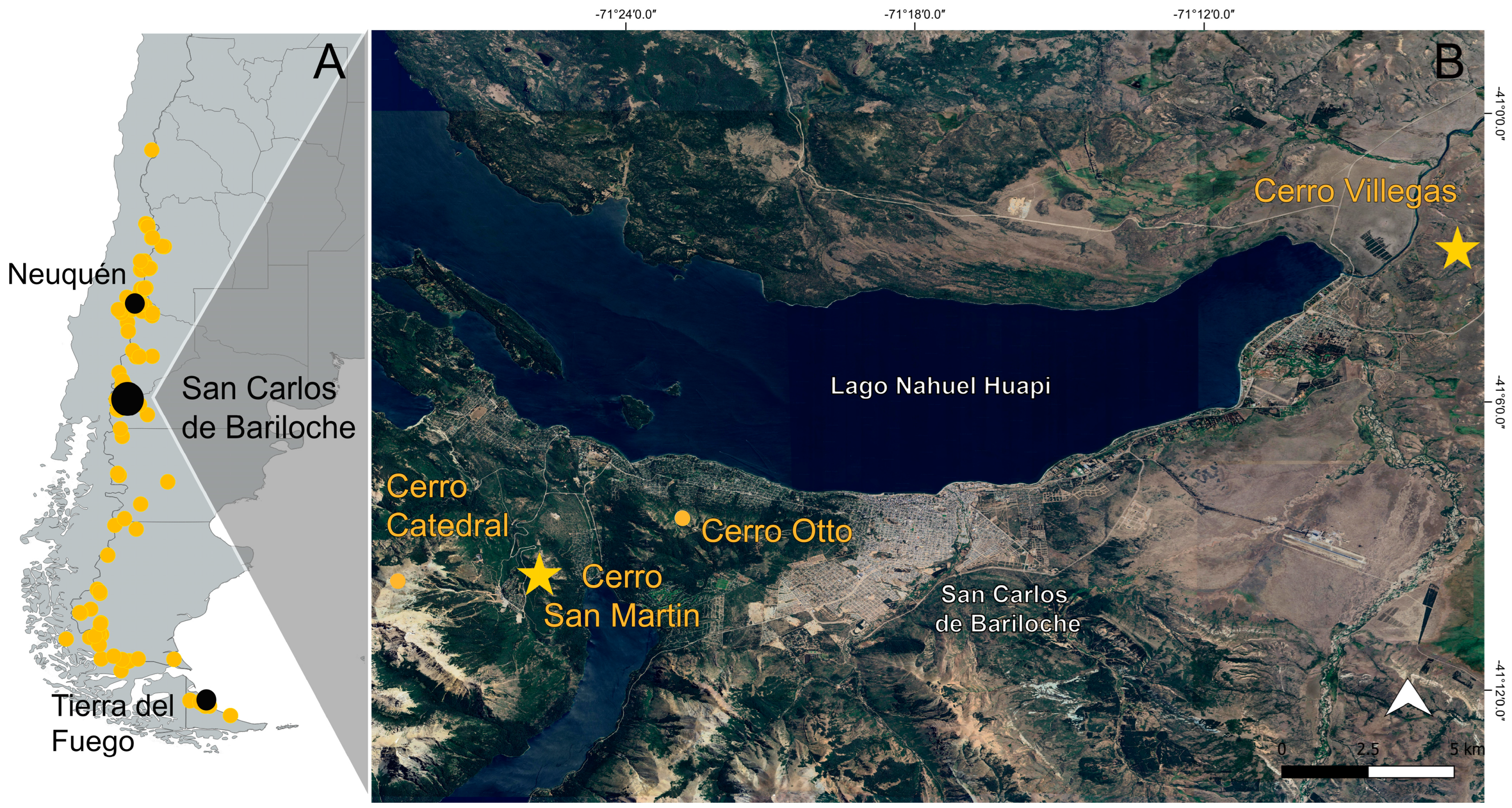
4.1. Study Area
- Cerro Villegas 41°02′51″ S, 71°06′44″ W, 1175 to1202 m a.s.l., measured 28 November 2024. The site vegetation showed a predominance of Acaena sp. and grasses, with the presence of Astragalus sp., Adesmia sp., and Viola maculata. The population area measured was approximately 3240 m2;
- Cerro San Martin, 41°9′36″ S, 71°25′45″ W, 1260 to 1266 m a.s.l., measured 8 and 17 December 2024. The site vegetation showed a predominance of Anemone multifida, Quinchamalium sp., Acaena sp., Alstroemeria aurea, and Berberis microphylla. The population area measured was approximately 3080 m2.
4.2. Methodology
4.3. Data Collection
4.3.1. Gall Abundance per Plant, Number of Inflorescences, and Plant Cover
4.3.2. Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marquis, R.J. Leaf Herbivores Decrease Fitness of a Tropical Plant. Science 1984, 226, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Viana, L.R.; Silveira, F.A.O.; Santos, J.C.; Rosa, L.H.; Cares, J.E.; Café-Filho, A.C.; Fernandes, G.W. Nematode-induced galls in Miconia albicans: Effect of host plant density and correlations with performance. Plant Species Biol. 2013, 28, 63–69. [Google Scholar] [CrossRef]
- Silva, I.M.; Andrade, G.I.; Fernandes, G.W.; Lemos Filho, J.P. Parasitic Relationships between a Gall-forming Insect Tomoplagia rudolphi (Diptera: Tephritidae) and its Host Plant (Vernonia polyanthes, Asteraceae). Ann. Bot. 1996, 78, 45–48. [Google Scholar] [CrossRef]
- Petro, R.; Madoffe, S.S.; Iddi, S.; Mugasha, W.A. Impact of Eucalyptus gall wasp, Leptocybe invasa infestation on growth and biomass production of Eucalyptus grandis and E. saligna seedlings in Tanzania. Int. J. Pest Manag. 2015, 61, 220–227. [Google Scholar] [CrossRef]
- Strydom, M.; Veldtman, R.; Ngwenya, M.Z.; Esler, K.J. Questioning the effectiveness of seed-reducing agents on invasive Acacia: Pod production relative to gall abundance of classical biological control agents. Perspect. Plant Ecol. Evol. Syst. 2024, 64, 125813. [Google Scholar] [CrossRef]
- Shorthouse, J.D.; Wool, D.; Raman, A. Gall-inducing insects—Nature’s most sophisticated herbivores. Basic Appl. Ecol. 2005, 6, 407–411. [Google Scholar] [CrossRef]
- Barrancos, M.L.; Moncaglieri, R.; Farji-Brener, A. Infección por agallas y producción de inflorescencias en el arbusto Schinus patagonicus. Ecol. Austral. 2008, 18, 133–137. [Google Scholar]
- Mani, M.S. Ecology of Plant Galls; Monographiae Biologicae; Weisbach, W.W., Van Oye, P., Eds.; Springer: Dordrecht, The Netherlands, 1964. [Google Scholar] [CrossRef]
- Kuzmanich, N.; Altamirano, A.A.; Salvo, A. Agallas de insectos de la región Rioplatense, Buenos Aires. Rev. Soc. Entomol. Argent 2015, 74, 47–56. [Google Scholar]
- Kuzmanich, N.; Giorgis, M.A.; Salvo, A. Insect galls from Córdoba, Argentina: A case where stem galls predominate. Rev. Biol. Trop. 2018, 66, 1135–1148. [Google Scholar] [CrossRef]
- Medicinal Plant Names Services Portal, Royal Botanic Gardens, Kew, Version 14. 2025. Available online: http://mpns.kew.org/mpns-portal (accessed on 28 April 2025).
- Pastore, J.F.B.; Abbott, J.R.; Neubig, K.M.; Van Den Berg, C.; Mota, M.C.D.A.; Cabral, A.; Whitten, W.M. Phylogeny and biogeography of Polygala (Polygalaceae). Taxon 2019, 68, 673–691. [Google Scholar] [CrossRef]
- Pastore, J.F.B.; Mota, M.; Amano, E.; Martinez, A. Disentangling Polygala obovata Complex (Polygalaceae), with a Description of Three New Species for Brazil. Syst. Bot. 2021, 46, 985–997. [Google Scholar] [CrossRef]
- Pastore, J.F.B. Revision of the ‘Polygala herbiola group’ (Polygalaceae): A new species and a new variety. Kew Bull. 2022, 77, 221–232. [Google Scholar] [CrossRef]
- Pastore, J.F.B.; Martinez, A.; Abbott, J.R.; Neubig, K. Toward New Generic Delimitations in Polygalaceae II: Senega. Ann. Mo. Bot. Gard. 2023, 108, 126–249. [Google Scholar] [CrossRef]
- Martinez, A.; Acosta, J.M.; Ferrero, M.A.; Pastore, F.B.; Aagesen, L. Evolutionary patterns within the New World Clade Polygala sections Clinclinia and Monninopsis (Polygalaceae). Perspect. Plant Ecol. Evol. Syst. 2022, 55, 125673. [Google Scholar] [CrossRef]
- Martinez, A. Revisión de Senega subgénero Clinclinia (Polygalaceae). Darwiniana Nueva Ser. 2023, 11, 541–642. [Google Scholar] [CrossRef]
- Urso-Guimarães, M.V.; Scareli-Santos, C. Galls and gall makers in plants from the Pé-de-Gigante Cerrado Reserve, Santa Rita do Passa Quatro, SP, Brazil. Braz. J. Biol. 2006, 66, 357–369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramos Rodrigues, A.; Maia, V.C.; Souto Couri, M. Insect galls of restinga areas of Ilha da Marambaia, Rio de Janeiro, Brazil. Rev. Bras. Entomol. 2014, 58, 173–197. [Google Scholar] [CrossRef]
- Nishida, K.; Adamski, D. Two new gall-inducing Saphenista Walsingham (Lepidoptera: Tortricidae: Cochylini) from Costa Rica. Proc. Entomol. Soc. Wash. 2004, 106, 133–139. [Google Scholar]
- Adair, R.J.; Neser, S.; Stajsic, V. Phytophagous Organisms Associated with the Woody Shrub “Polygala myrtifolia” (Polygalaceae) and Their Potential for Classical Biological Control in Australia. Plant Prot. Q. 2011, 26, 72–80. [Google Scholar]
- Smith Meyer, M.K.P.; Ueckermann, E.A. Three new species of Aceria (Acari: Eriophyidae) from South African Polygalaceae and Polygonaceae. Int. J. Acarol. 1996, 22, 17–22. [Google Scholar] [CrossRef]
- Martini, V.C.; Raymundo, D.; Prado-Junior, J.; Oliveira, D.C. Bottom-up and top-down forces in plant-gall relationships: Testing the hypotheses of resource concentration, associational resistance, and host fitness reduction. Ecol. Entomol. 2021, 46, 1072–1081. [Google Scholar] [CrossRef]
- Leege, L.M. The relationship between psyllid leaf galls and redbay (Persea borbonia) fitness traits in sun and shade. Plant Ecol. 2006, 184, 203–212. [Google Scholar] [CrossRef]
- iNaturalist. 2025. Available online: https://www.inaturalist.org/home (accessed on 28 April 2025).
- Castillo, C. Observation of Senega salasiana. In iNaturalist. 2021. Available online: https://www.inaturalist.org/observations/102924729 (accessed on 28 April 2025).
- Sersic, A. Observation of Senega salasiana. In iNaturalist. 2007. Available online: https://www.inaturalist.org/observations/103823852 (accessed on 28 April 2025).
- Gagné, R.J.; Jaschof, M. A Catalog of the Cecidomyiidae (Diptera) of the World, 5th ed.; Memoirs of the Entomological Society of Washington; Entomological Society of Washington: Washington, DC, USA, 2021; 816p. Available online: https://www.ars.usda.gov/ARSUserFiles/80420580/Gagne_Jaschhof_2021_World_Cat_5th_Ed.pdf (accessed on 28 April 2025).
- Kolesik, P.; McFadyen, R.E.C.; Wapshere, A.J. New gall midges (Diptera: Cecidomyiidae) infesting native and introduced Solanum spp. (Solanaceae) in Australia. Trans. R Soc. S Aust. Inc. 2000, 124, 31–36. [Google Scholar]
- Kolesik, P.; Sutton, G.F.; Steenderen, C.J.M.; Martins, D.J.; Plowes, R.; Paterson, I.D. A new genus and two new species of gall midges (Diptera: Cecidomyiidae) feeding on Guinea grass Megathyrsus maximus (Poaceae) in Africa. Austral. Entomol. 2025, 64, 12719. [Google Scholar] [CrossRef]
- Kolesik, P.; Sutherland, R.; Gillard, K.; Gresham, B.; Withers, T.M. A new species of Mycodiplosis gall midge (Diptera: Cecidomyiidae) feeding on myrtle rust Austropuccinia psidii. N. Z. Entomol. 2021, 44, 121–129. [Google Scholar] [CrossRef]
- Kolesik, P.; Adair, R.J.; Eick, G. Nine new species of Dasineura (Diptera: Cecidomyiidae) from flowers of Australian Acacia (Mimosaceae). Syst. Entomol. 2005, 30, 454–479. [Google Scholar] [CrossRef]
- Flechtmann, C.H.W.; De Queiroz, D.L. New taxa in the Eriophyidae (Acari, Prostigmata) from forest trees in southern Brazil. Zootaxa 2010, 2337, 18–30. [Google Scholar] [CrossRef]
- Chetverikov, P.E.; Desnitskiy, A.G.; Letukhova, V.Y.; Ozman-sullivan, S.K.; Romanovich, A.E.; Sarratt, J.V.; Sukhareva, S.I. A new species, new records, and DNA barcodes of eriophyine mites (Eriophyidae, Eriophyinae) from southeast Crimea and remarks on ability to form galls in conspecific eriophyoids. Syst. Appl. Acarol. 2021, 26, 1721–1734. [Google Scholar] [CrossRef]
- Khaustov, A.A.; Fjellberg, A.; Lindquist, E.E. A new genus and species of Pseudotarsonemoidini (Acari: Heterostigmata: Tarsonemidae) associated with xylophagous gall midges in Norway. Syst. Appl. Acarol. 2022, 27, 1020–1034. [Google Scholar] [CrossRef]
- Situngu, S.; Elhalawany, A.S.; Ngubane-Ndhlovu, N.P.; Chetverikov, P.E. New species and records of gall mites of the genus Aceria (Eriophyoidea, Eriophyidae) associated with Tamarix in Egypt and South Africa. Acarologia 2023, 63, 1271–1303. [Google Scholar] [CrossRef]
- Safenraiter, M.E.; Campos-Soldini, M.P.; Fernández, E.E.N.; Del Rio, M.G. Escarabajos vesicantes Sudamericanos (Coleoptera: Meloidae). Aportes al estado del conocimiento del género andino Pseudomeloe Fairmaire y Germain. Idesia Arica 2019, 37, 101–113. [Google Scholar] [CrossRef]
- Manson, D.C.M. Eriophyinae (Arachnida: Acari: Eriophyoidea). Fauna N. Z. 1984, 5, 128. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, A.; Kuzmanich, N.; Farji-Brener, A. First Record of Flower Bud Galls in Senega (Fabales: Polygalaceae): The Case of S. salasiana and Their Effect on Plant Reproduction. Plants 2025, 14, 1337. https://doi.org/10.3390/plants14091337
Martinez A, Kuzmanich N, Farji-Brener A. First Record of Flower Bud Galls in Senega (Fabales: Polygalaceae): The Case of S. salasiana and Their Effect on Plant Reproduction. Plants. 2025; 14(9):1337. https://doi.org/10.3390/plants14091337
Chicago/Turabian StyleMartinez, Agustina, Nicolás Kuzmanich, and Alejandro Farji-Brener. 2025. "First Record of Flower Bud Galls in Senega (Fabales: Polygalaceae): The Case of S. salasiana and Their Effect on Plant Reproduction" Plants 14, no. 9: 1337. https://doi.org/10.3390/plants14091337
APA StyleMartinez, A., Kuzmanich, N., & Farji-Brener, A. (2025). First Record of Flower Bud Galls in Senega (Fabales: Polygalaceae): The Case of S. salasiana and Their Effect on Plant Reproduction. Plants, 14(9), 1337. https://doi.org/10.3390/plants14091337





