Light Adaptations of Ipomoea purpurea (L.) Roth: Functional Analysis of Leaf and Petal Interfaces
Abstract
1. Introduction
2. Results
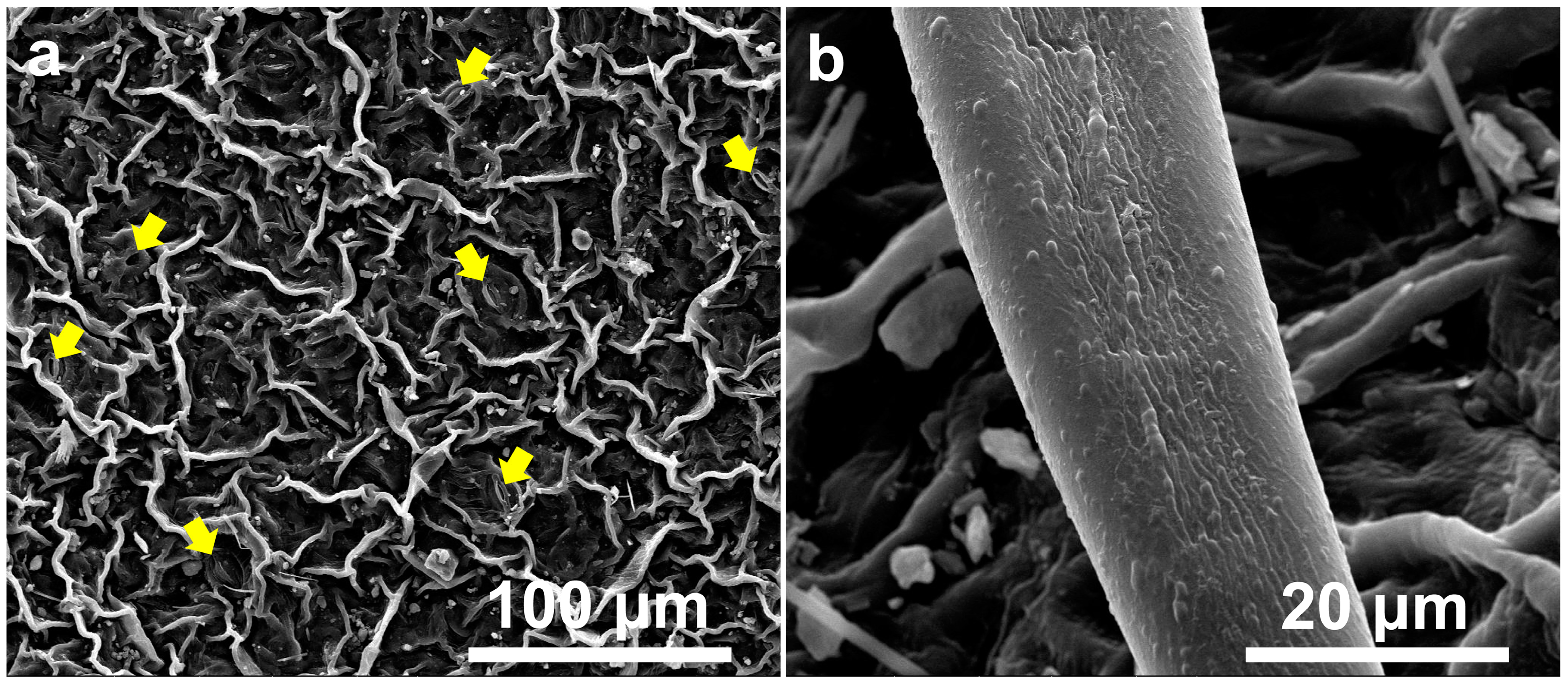
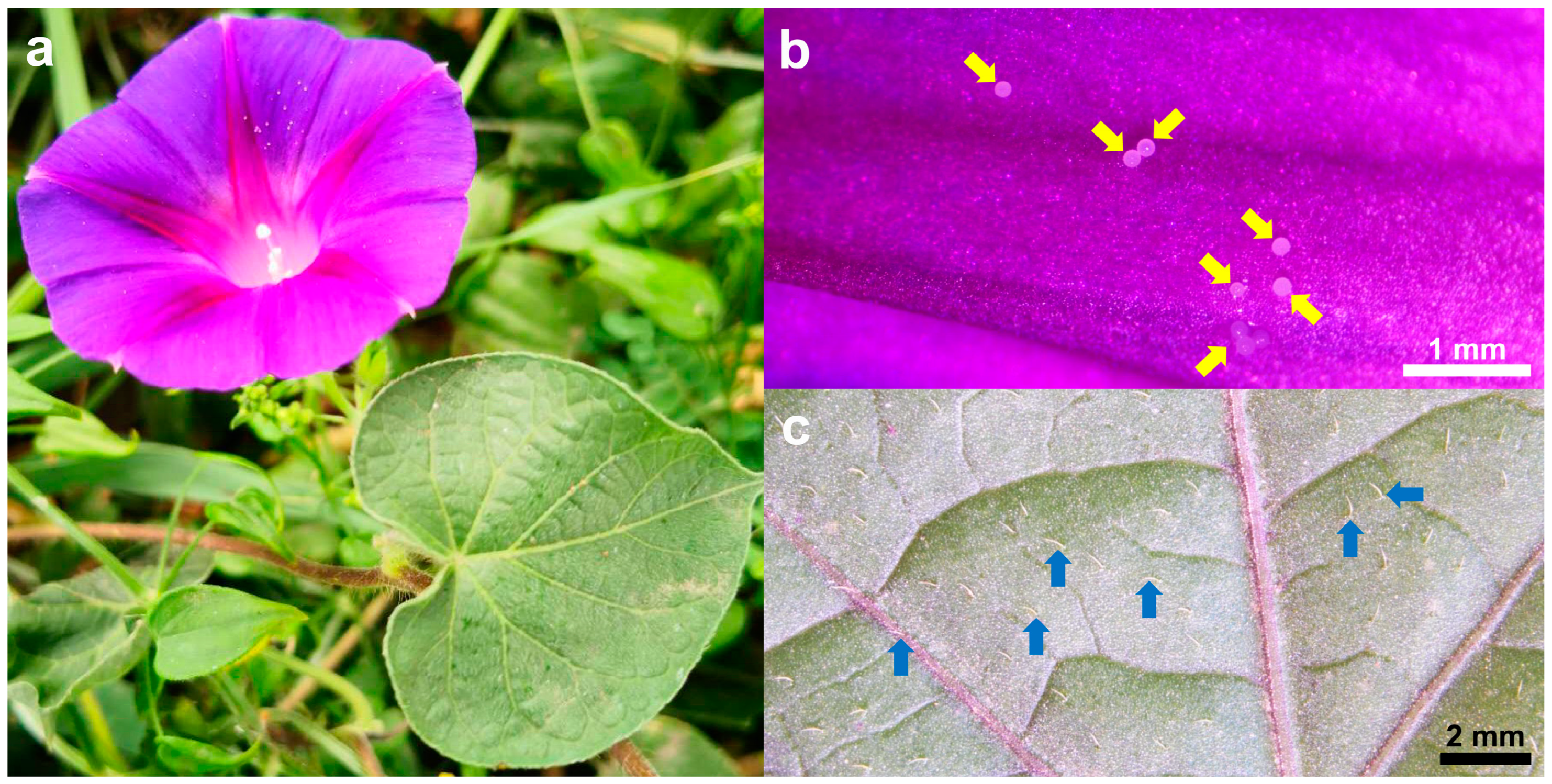
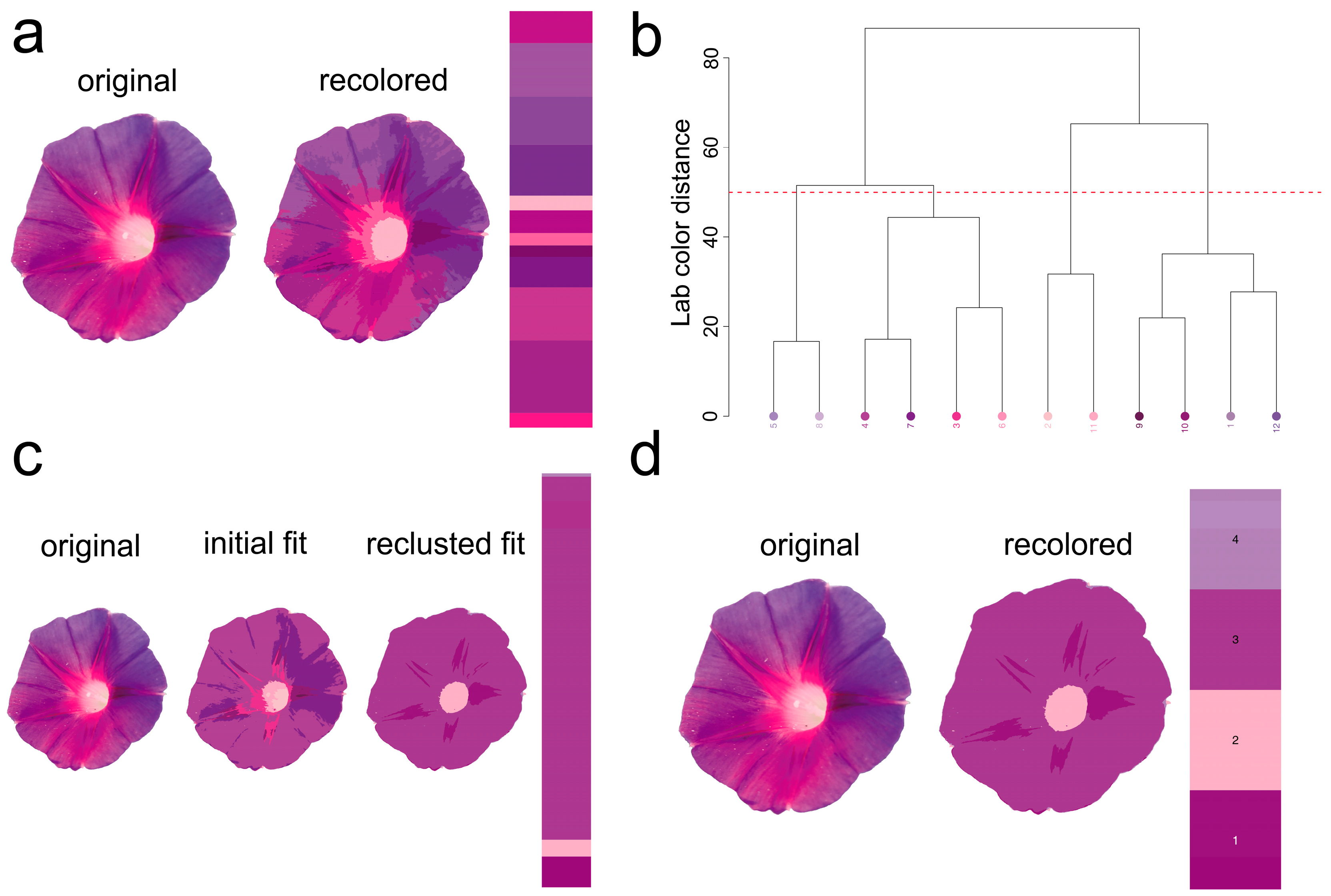
2.1. Morphological Characteristics of Leaves and Petals
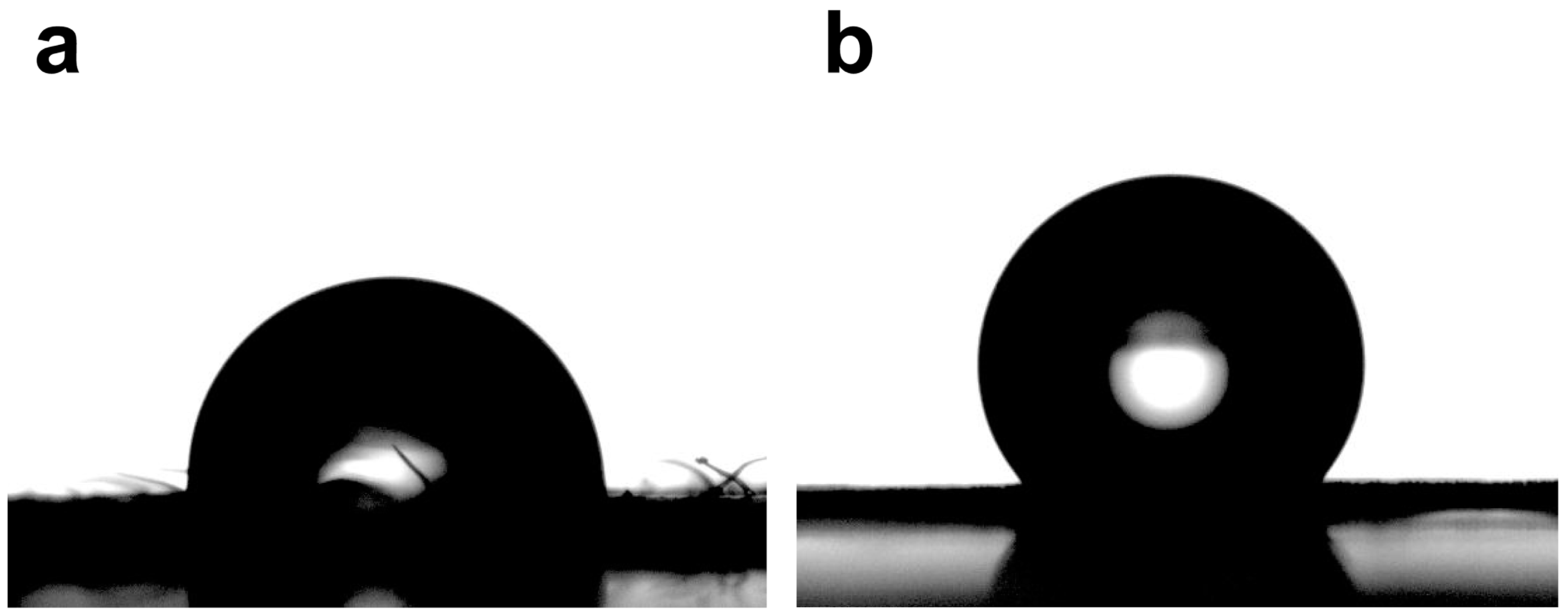
2.2. Contact Angle of Leaves and Petals Interfaces
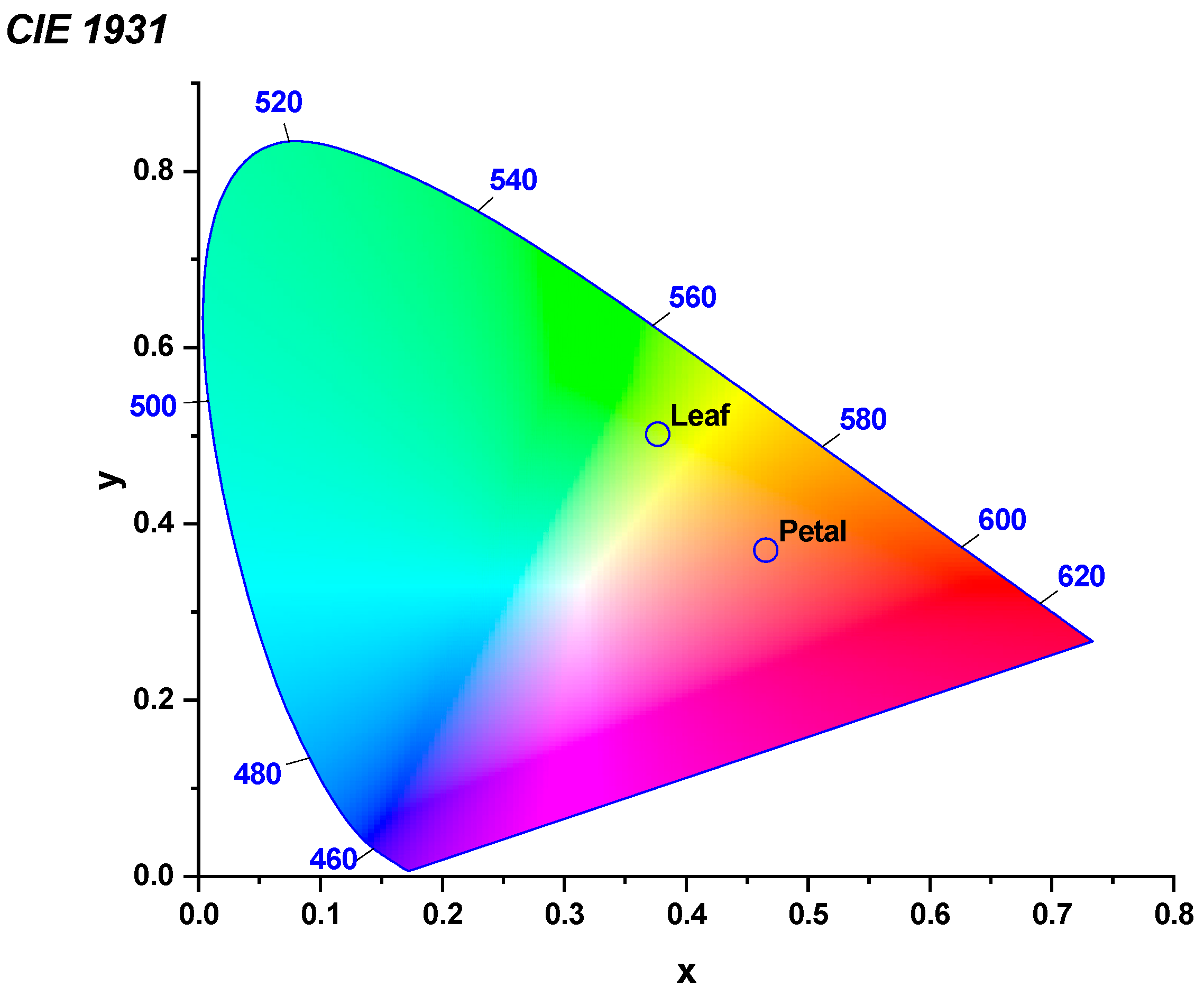
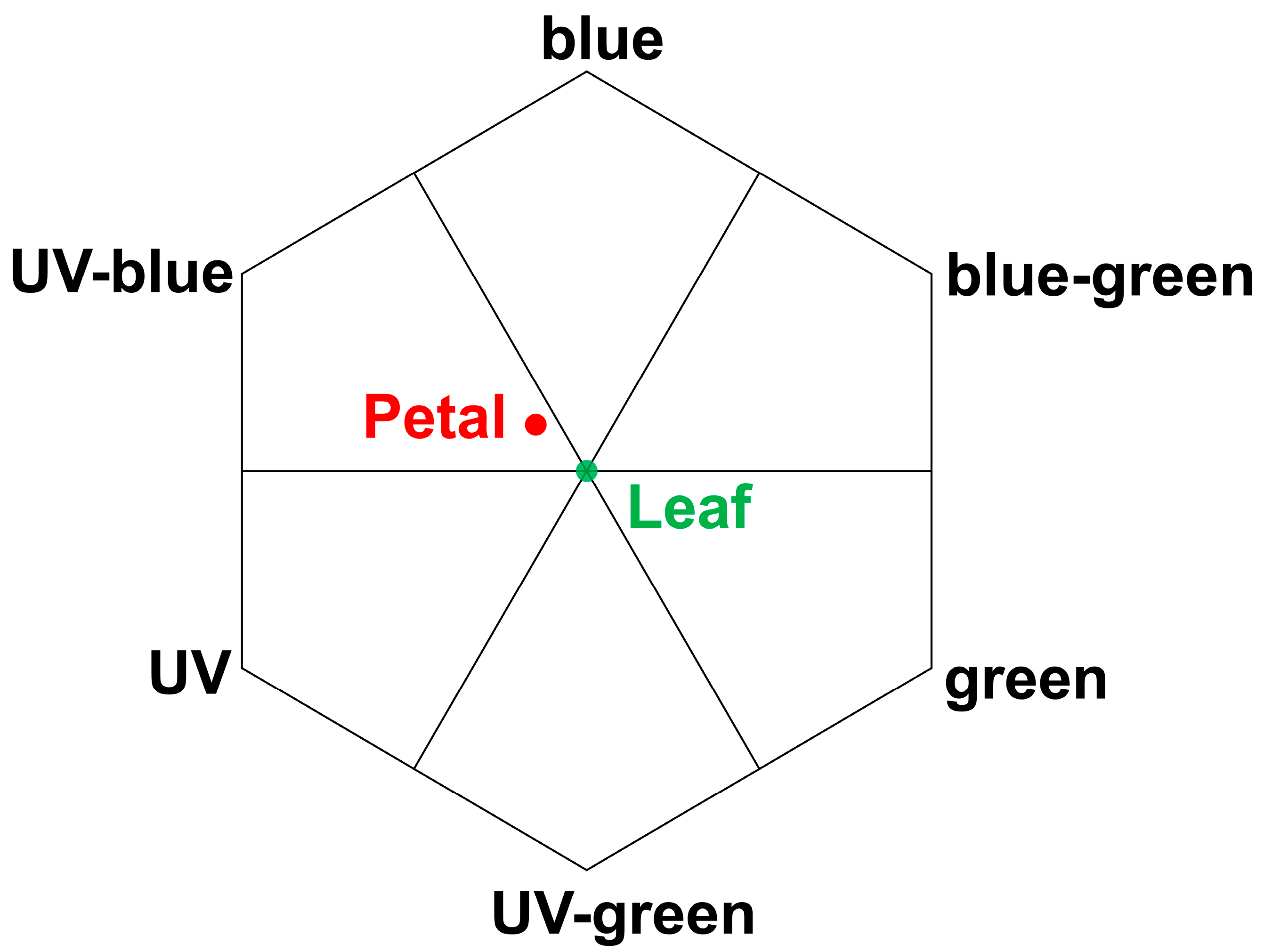
2.3. Reflection Spectra of Leaf and Petal Interfaces
3. Discussion
4. Materials and Methods
4.1. Leaf and Petal Collection
4.2. Observation of Petal and Leaf Morphology
4.3. Reflectance Spectroscopy Testing
4.4. Wetting Test
4.5. Establishment of Bee Vision Color Hexagon Model
4.6. Flexible Color Segmentation of Biological Images
4.7. Data Statistics and Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smith, E.L. Photosynthesis in relation to light and carbon dioxide. Proc. Natl. Acad. Sci. USA 1936, 22, 504–511. [Google Scholar] [CrossRef]
- Ruban, A.V. Evolution under the sun: Optimizing light harvesting in photosynthesis. J. Exp. Bot. 2015, 66, 7–23. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef]
- Vandenbrink, J.P.; Brown, R.I.; Harmer, S.L.; Blackman, B.K. Turning heads: The biology of solar tracking in sunflower. Plant Sci. 2014, 224, 20–26. [Google Scholar] [CrossRef]
- Dodd, A.N.; Salathia, N.; Hall, A.; Kévei, E.; Tóth, R.; Nagy, F.; Hibberd, J.M.; Millar, A.J.; Webb, A.A. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 2005, 309, 630–633. [Google Scholar] [CrossRef]
- Bei, Z.; Zhang, X.; Tian, X. The mechanism by which umbrella-shaped ratchet trichomes on the Elaeagnus angustifolia leaf surface collect water and reflect light. Biology 2023, 12, 1024. [Google Scholar] [CrossRef]
- Waseem, M.; Nie, Z.F.; Yao, G.Q.; Hasan, M.; Xiang, Y.; Fang, X.W. Dew absorption by leaf trichomes in Caragana korshinskii: An alternative water acquisition strategy for withstanding drought in arid environments. Physiol. Plant. 2021, 172, 528–539. [Google Scholar] [CrossRef]
- Bei, Z.; Zhang, X.; Zhang, F.; Yan, X. The Response of Oxytropis aciphylla Ledeb. Leaf Interface to Water and Light in Gravel Deserts. Plants 2023, 12, 3922. [Google Scholar] [CrossRef]
- Galen, C.; Stanton, M.L. Sunny-side up: Flower heliotropism as a source of parental environmental effects on pollen quality and performance in the snow buttercup, Ranunculus adoneus (Ranunculaceae). Am. J. Bot. 2003, 90, 724–729. [Google Scholar] [CrossRef]
- van der Kooi, C.J.; Kevan, P.G.; Koski, M.H. The thermal ecology of flowers. Ann. Bot. 2019, 124, 343–353. [Google Scholar] [CrossRef]
- Fiorucci, A.-S.; Fankhauser, C. Plant strategies for enhancing access to sunlight. Curr. Biol. 2017, 27, R931–R940. [Google Scholar] [CrossRef]
- Atamian, H.S.; Creux, N.M.; Brown, R.I.; Garner, A.G.; Blackman, B.K.; Harmer, S.L. Circadian regulation of sunflower heliotropism, floral orientation, and pollinator visits. Science 2016, 353, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Ehleringer, J.; Forseth, I. Solar tracking by plants. Science 1980, 210, 1094–1098. [Google Scholar] [CrossRef]
- Stoochnoff, J.; Johnston, M.; Hoogenboom, J.; Graham, T.; Dixon, M. Intracanopy lighting strategies to improve green bush bean (Phaseolus vulgaris) compatibility with vertical farming. Front. Agron. 2022, 4, 905286. [Google Scholar] [CrossRef]
- Henry, H.A.; Aarssen, L.W. On the relationship between shade tolerance and shade avoidance strategies in woodland plants. Oikos 1997, 80, 575–582. [Google Scholar] [CrossRef]
- Bone, R.A.; Lee, D.W.; Norman, J. Epidermal cells functioning as lenses in leaves of tropical rain-forest shade plants. Appl. Opt. 1985, 24, 1408–1412. [Google Scholar] [CrossRef]
- Franklin, K.A. Shade avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef]
- Van Der Kooi, C.J.; Stavenga, D.G.; Arikawa, K.; Belušič, G.; Kelber, A. Evolution of insect color vision: From spectral sensitivity to visual ecology. Annu. Rev. Entomol. 2021, 66, 435–461. [Google Scholar] [CrossRef]
- Suárez-Cáceres, G.P.; Mejía-Sampedro, D.K.; Fernández-Cañero, R.; Loges, V.; Pérez-Urrestarazu, L. Establishment and development of ornamental grasses on green roofs and living walls. Landsc. Ecol. Eng. 2023, 19, 123–136. [Google Scholar] [CrossRef]
- Li, L.; Cheng, J.; Liu, Z.; Li, Q.; Yu, L.; Zhou, X.; Pang, Y. The Impact of Coverage Forms of Exterior Vertical Greening Walls on the Thermal Environmental Benefits of Buildings in Hot and Humid Regions. Buildings 2024, 14, 3840. [Google Scholar] [CrossRef]
- Austin, D.F. Convolvulaceae morning glory family. J. Ariz.-Nev. Acad. Sci. 1998, 30, 61–83. [Google Scholar]
- Brown, B.A.; Clegg, M.T. Influence of flower color polymorphism on genetic transmission in a natural population of the common morning glory, Ipomoea purpurea. Evolution 1984, 38, 796–803. [Google Scholar] [CrossRef]
- Liang, D.; Huang, G. Influence of Urban Tree Traits on Their Ecosystem Services: A Literature Review. Land 2023, 12, 1699. [Google Scholar] [CrossRef]
- WANG, L.; GUO, L. Exploring the Cultural Connotations of “Purple” and “Zi.”. Can. Soc. Sci. 2019, 15, 57–61. [Google Scholar]
- Perini, K.; Ottelé, M.; Fraaij, A.; Haas, E.; Raiteri, R. Vertical greening systems and the effect on air flow and temperature on the building envelope. Build. Environ. 2011, 46, 2287–2294. [Google Scholar] [CrossRef]
- Liu, S.-K.; Cai, S.; Chen, Y.; Xiao, B.; Chen, P.; Xiang, X.-D. The effect of pollutional haze on pulmonary function. J. Thorac. Dis. 2016, 8, E41. [Google Scholar]
- Perini, K.; Ottelé, M.; Giulini, S.; Magliocco, A.; Roccotiello, E. Quantification of fine dust deposition on different plant species in a vertical greening system. Ecol. Eng. 2017, 100, 268–276. [Google Scholar] [CrossRef]
- Lohr, V.I.; Pearson-Mims, C.H. Particulate matter accumulation on horizontal surfaces in interiors: Influence of foliage plants. Atmos. Environ. 1996, 30, 2565–2568. [Google Scholar] [CrossRef]
- Xu, L.; Yan, Q.; He, P.; Zhen, Z.; Jing, Y.; Duan, Y.; Chen, X. Combined effects of different leaf traits on foliage dust-retention capacity and stability. Air Qual. Atmos. Health 2022, 15, 1263–1274. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Z. Recent advances of bioinspired functional materials with specific wettability: From nature and beyond nature. Nanoscale Horiz. 2019, 4, 52–76. [Google Scholar] [CrossRef] [PubMed]
- Crick, C.R.; Parkin, I.P. Preparation and characterisation of super-hydrophobic surfaces. Chem. A Eur. J. 2010, 16, 3568–3588. [Google Scholar] [CrossRef]
- Pakdel, E.; Naebe, M.; Sun, L.; Wang, X. Advanced functional fibrous materials for enhanced thermoregulating performance. ACS Appl. Mater. Interfaces 2019, 11, 13039–13057. [Google Scholar] [CrossRef]
- Bilger, H.-W.; Schreiber, U.; Lange, O. Determination of leaf heat resistance: Comparative investigation of chlorophyll fluorescence changes and tissue necrosis methods. Oecologia 1984, 63, 256–262. [Google Scholar] [CrossRef]
- Henrion, W.; Tributsch, H. Optical solar energy adaptations and radiative temperature control of green leaves and tree barks. Sol. Energy Mater. Sol. Cells 2009, 93, 98–107. [Google Scholar] [CrossRef]
- Brooks, C.J.; Atamian, H.S.; Harmer, S.L. Multiple light signaling pathways control solar tracking in sunflowers. PLoS Biol. 2023, 21, e3002344. [Google Scholar] [CrossRef]
- Dudareva, L.; Tarasenko, V.; Rudikovskaya, E. Involvement of photoprotective compounds of a phenolic nature in the response of Arabidopsis thaliana leaf tissues to low-Intensity laser radiation. Photochem. Photobiol. 2020, 96, 1243–1250. [Google Scholar] [CrossRef]
- Ilias, I.F.; Rajapakse, N. The Effects of End-of-the-day Red and Far-red Light on Growth and Flowering of Petunia × hybrida ’Countdown Burgundy’ Grown under Photoselective Films. HortScience 2005, 40, 131–133. [Google Scholar] [CrossRef]
- Yamazaki, K.; Ishii, Y.; Tanaka, I. Spectral sensitivity of the promotion and inhibition of flowering in morning glory (Pharbitis nil). Environ. Control. Biol. 2007, 45, 75–83. [Google Scholar] [CrossRef]
- Ozanne, S.; Hales, C. Olfaction: Scent-triggered navigation in honeybees. Nat. Phys. Sci. 2004, 427, 411. [Google Scholar] [CrossRef]
- Dyer, A.G.; Whitney, H.M.; Arnold, S.E.; Glover, B.J.; Chittka, L. Mutations perturbing petal cell shape and anthocyanin synthesis influence bumblebee perception of Antirrhinum majus flower colour. Arthropod-Plant Interact. 2007, 1, 45–55. [Google Scholar] [CrossRef]
- Dafni, A.; Lehrer, M.; Kevan, P. Spatial flower parameters and insect spatial vision. Biol. Rev. 1997, 72, 239–282. [Google Scholar] [CrossRef]
- Peitsch, D.; Fietz, A.; Hertel, H.; de Souza, J.; Ventura, D.F.; Menzel, R. The spectral input systems of hymenopteran insects and their receptor-based colour vision. J. Comp. Physiol. A 1992, 170, 23–40. [Google Scholar] [CrossRef]
- Chittka, L.; Beier, W.; Hertel, H.; Steinmann, E.; Menzel, R. Opponent colour coding is a universal strategy to evaluate the photoreceptor inputs in Hymenoptera. J. Comp. Physiol. A 1992, 170, 545–563. [Google Scholar] [CrossRef]
- Lunau, K. Visual ecology of flies with particular reference to colour vision and colour preferences. J. Comp. Physiol. A 2014, 200, 497–512. [Google Scholar] [CrossRef]
- Majetic, C.J.; Raguso, R.A.; Tonsor, S.J.; Ashman, T.-L. Flower color–flower scent associations in polymorphic Hesperis matronalis (Brassicaceae). Phytochemistry 2007, 68, 865–874. [Google Scholar] [CrossRef]
- Fineblum, W.L.; Rausher, M.D. Tradeoff between resistance and tolerance to herbivore damage in a morning glory. Nature 1995, 377, 517–520. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, M.; Wang, X.; Guo, R.; Sun, Y.; Gui, M.; Li, J.; Wang, T.; Zhang, L. Morphological characterization and transcriptional regulation of corolla closure in Ipomoea purpurea. Front. Plant Sci. 2021, 12, 697764. [Google Scholar] [CrossRef]
- Ennos, R.; Clegg, M.T. Flower color variation in the morning glory, Ipomoea purpurea. J. Hered. 1983, 74, 247–250. [Google Scholar] [CrossRef]
- Ismail, A.; Yeok, F.S.; Samad, M.H.A.; Rahman, A.M.A. Significant effect of Beach Morning Glory in improving the Quality of Life. Procedia-Soc. Behav. Sci. 2015, 170, 340–349. [Google Scholar] [CrossRef][Green Version]
- Ismail, W.; Abdullah, M.; Asmoni, M. A review of methods for estimating carbon sequestration at intensive green roofs. Int. J. Real Estate Stud. 2019, 13, 30–39. [Google Scholar]
- Roth, A.W. Botanische Abhandlungen und Beobachtungen; Winterschmidt: Nuremberg, Germany, 1787. [Google Scholar]
- Chittka, L. The colour hexagon: A chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. J. Comp. Physiol. A 1992, 170, 533–543. [Google Scholar] [CrossRef]
- Maia, R.; Gruson, H.; Endler, J.A.; White, T.E. pavo 2: New tools for the spectral and spatial analysis of colour in R. Methods Ecol. Evol. 2019, 10, 1097–1107. [Google Scholar] [CrossRef]
- Weller, H.I.; Hiller, A.E.; Lord, N.P.; Van Belleghem, S.M. recolorize: An R package for flexible colour segmentation of biological images. Ecol. Lett. 2024, 27, e14378. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bei, Z.; Lu, L.; Amar, Z.; Zhang, X. Light Adaptations of Ipomoea purpurea (L.) Roth: Functional Analysis of Leaf and Petal Interfaces. Plants 2025, 14, 862. https://doi.org/10.3390/plants14060862
Bei Z, Lu L, Amar Z, Zhang X. Light Adaptations of Ipomoea purpurea (L.) Roth: Functional Analysis of Leaf and Petal Interfaces. Plants. 2025; 14(6):862. https://doi.org/10.3390/plants14060862
Chicago/Turabian StyleBei, Zhanlin, Lulu Lu, Zubayda Amar, and Xin Zhang. 2025. "Light Adaptations of Ipomoea purpurea (L.) Roth: Functional Analysis of Leaf and Petal Interfaces" Plants 14, no. 6: 862. https://doi.org/10.3390/plants14060862
APA StyleBei, Z., Lu, L., Amar, Z., & Zhang, X. (2025). Light Adaptations of Ipomoea purpurea (L.) Roth: Functional Analysis of Leaf and Petal Interfaces. Plants, 14(6), 862. https://doi.org/10.3390/plants14060862





