Diversified Soil Types Differentially Regulated the Peanut (Arachis hydropoaea L.) Growth and Rhizosphere Bacterial Community Structure
Abstract
1. Introduction
2. Results
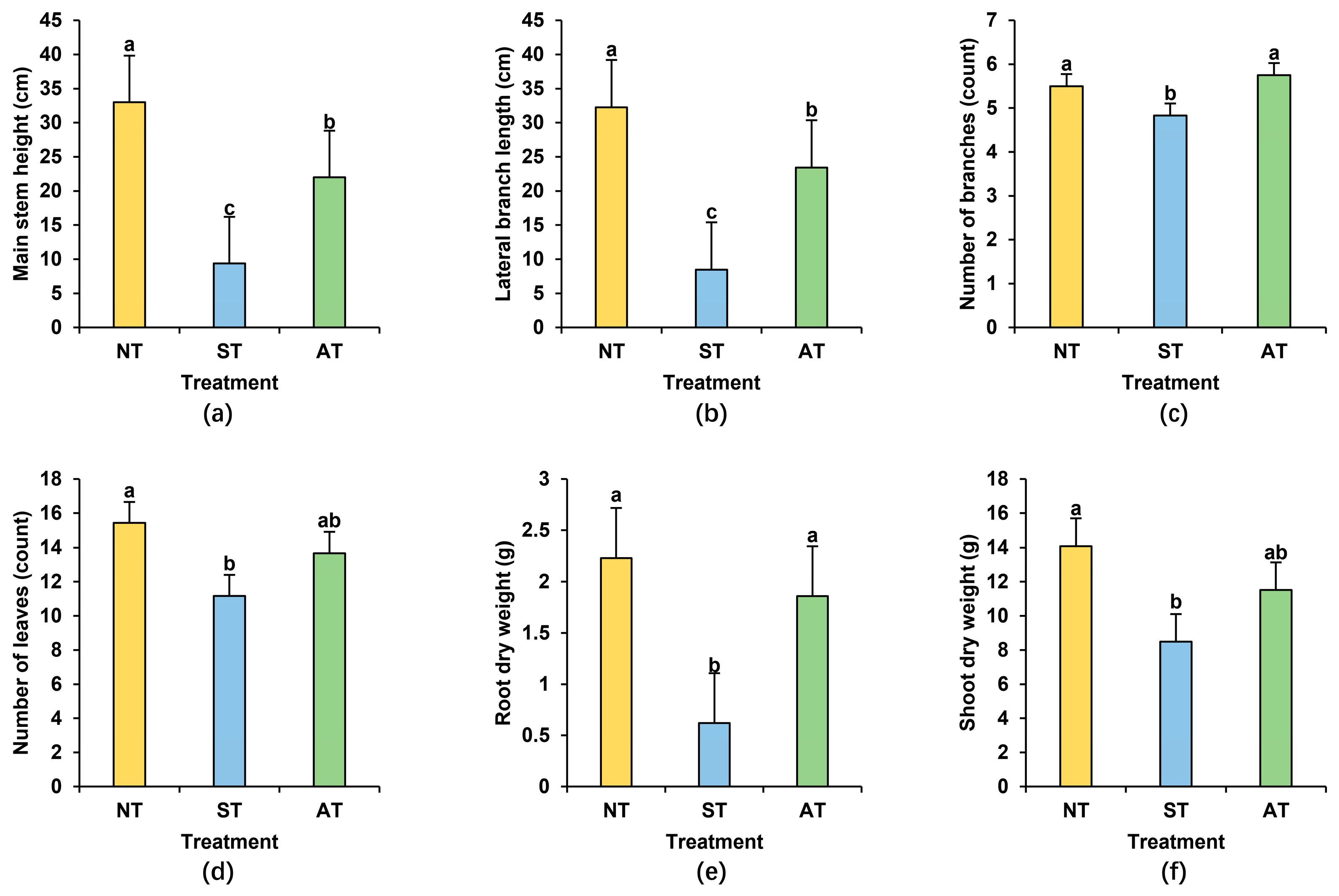
2.1. Effects of Different Soil Types on Peanut Agronomic Traits
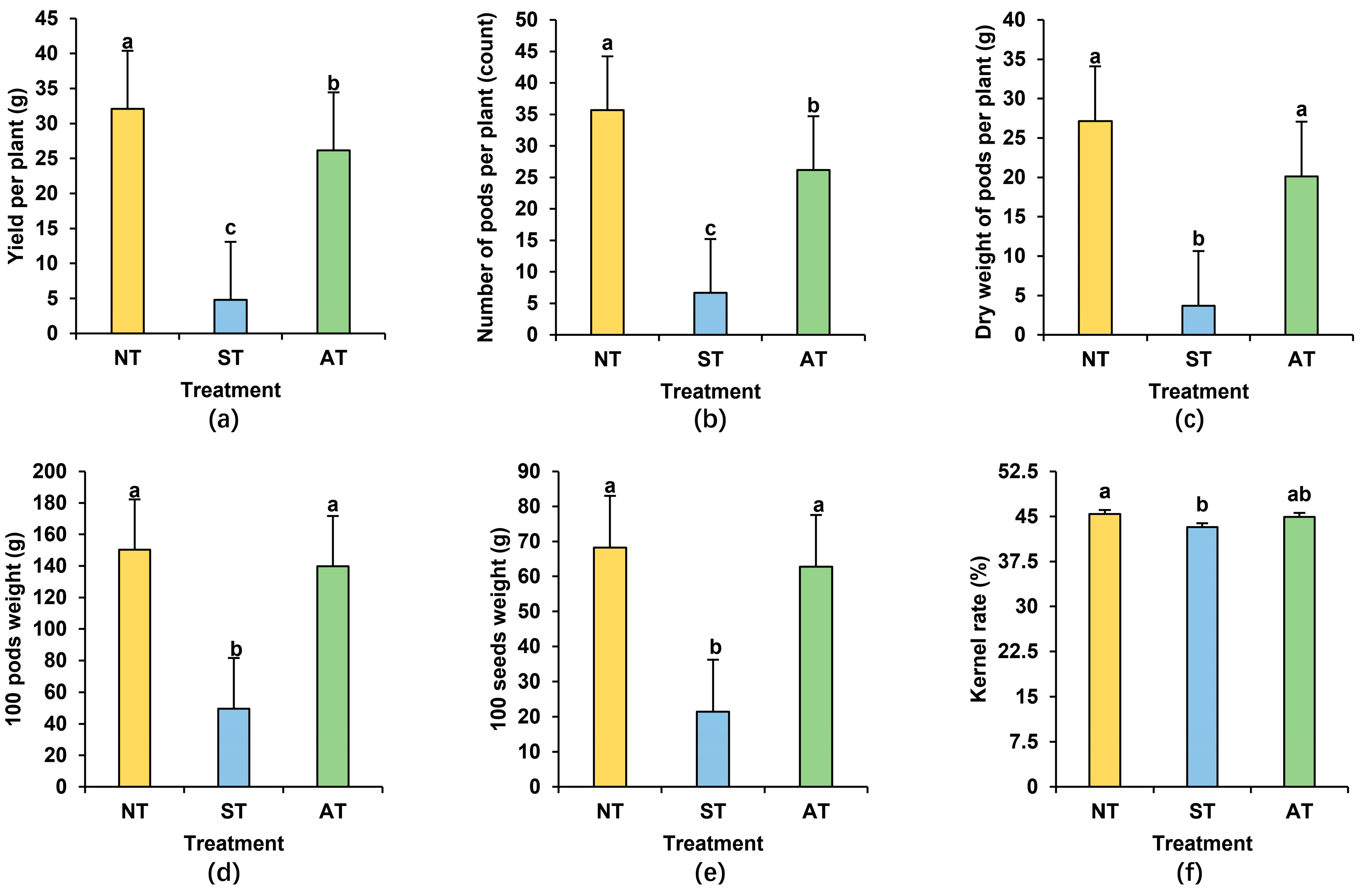
2.2. Differential Peanut Yield in Diverse Soil Types
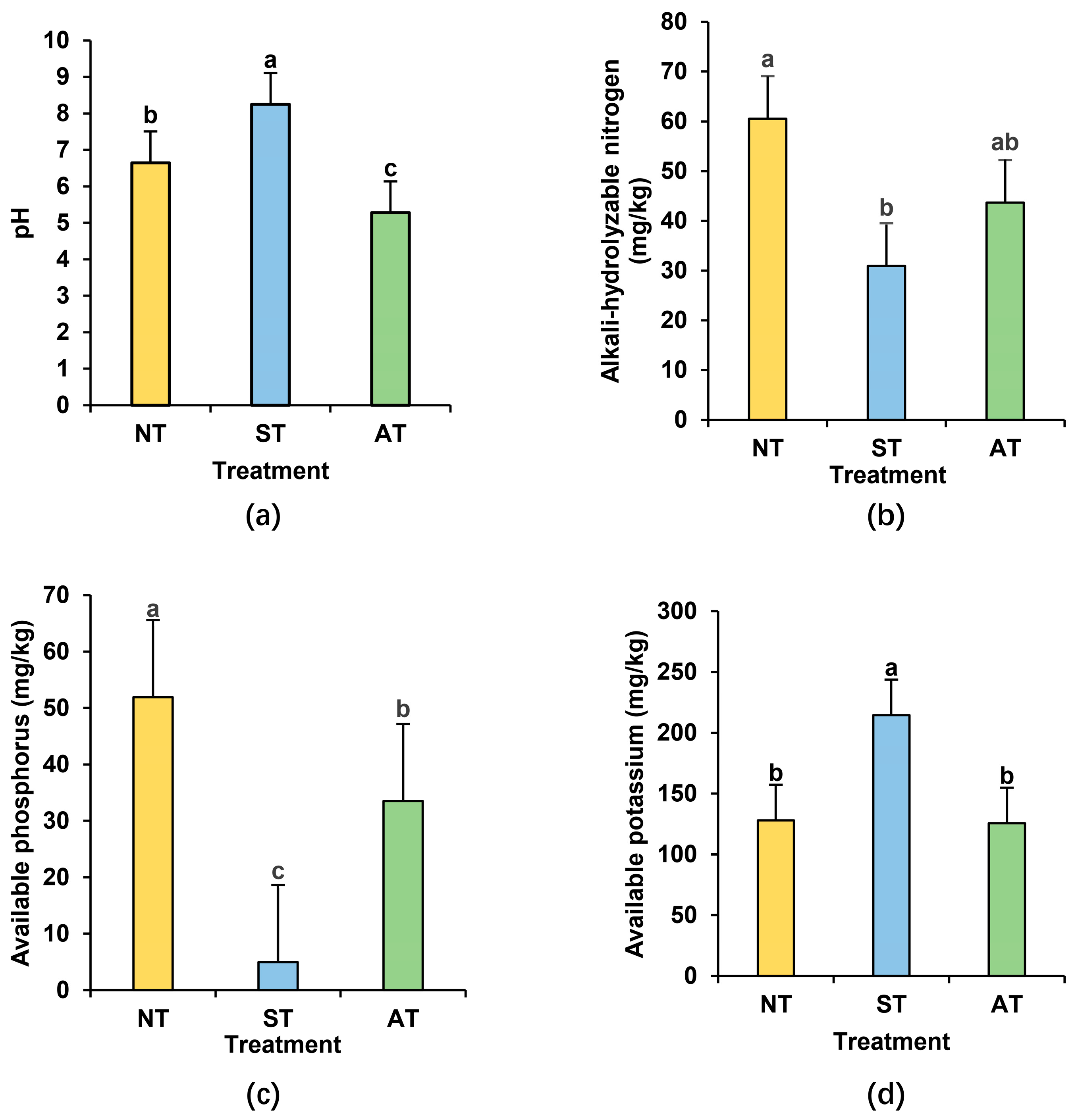
2.3. Rhizosphere Soil Physicochemical Properties Analysis in Different Soil Types
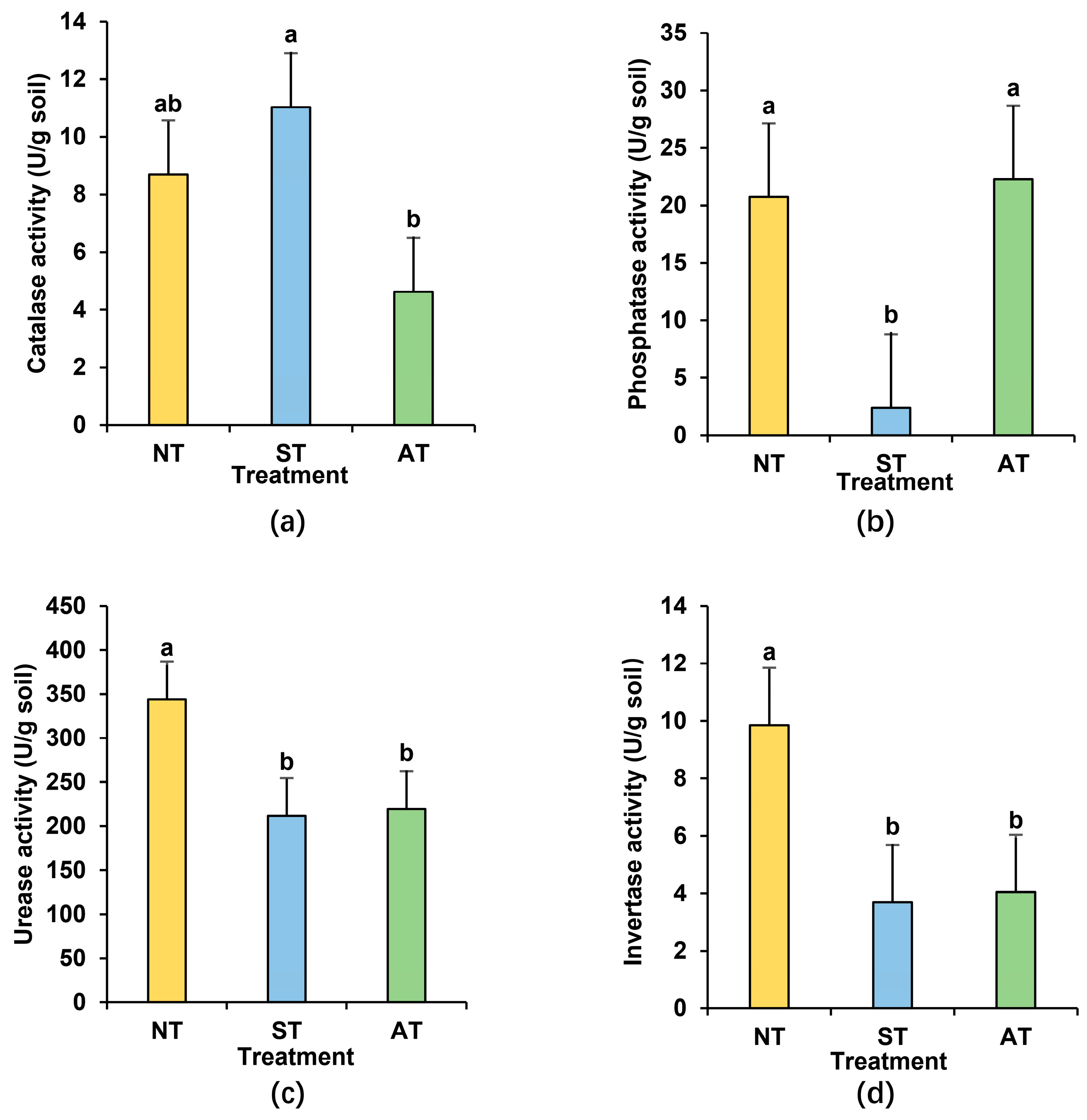
2.4. Soil Types Altered Rhizosphere Soil Enzyme Activities
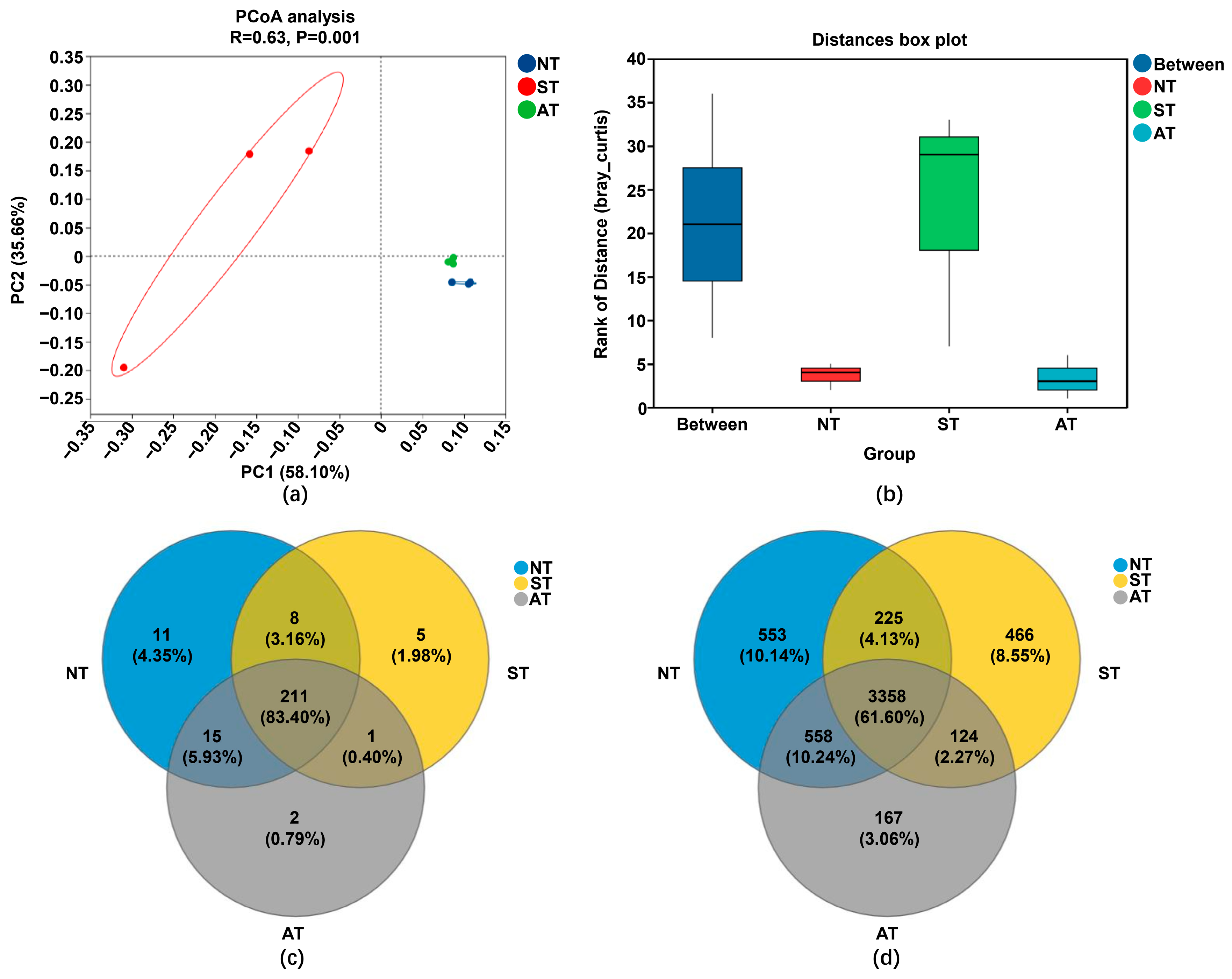
2.5. Alpha and Beta Diversity of the Peanut Rhizosphere Bacterial Community in Different Soil Types
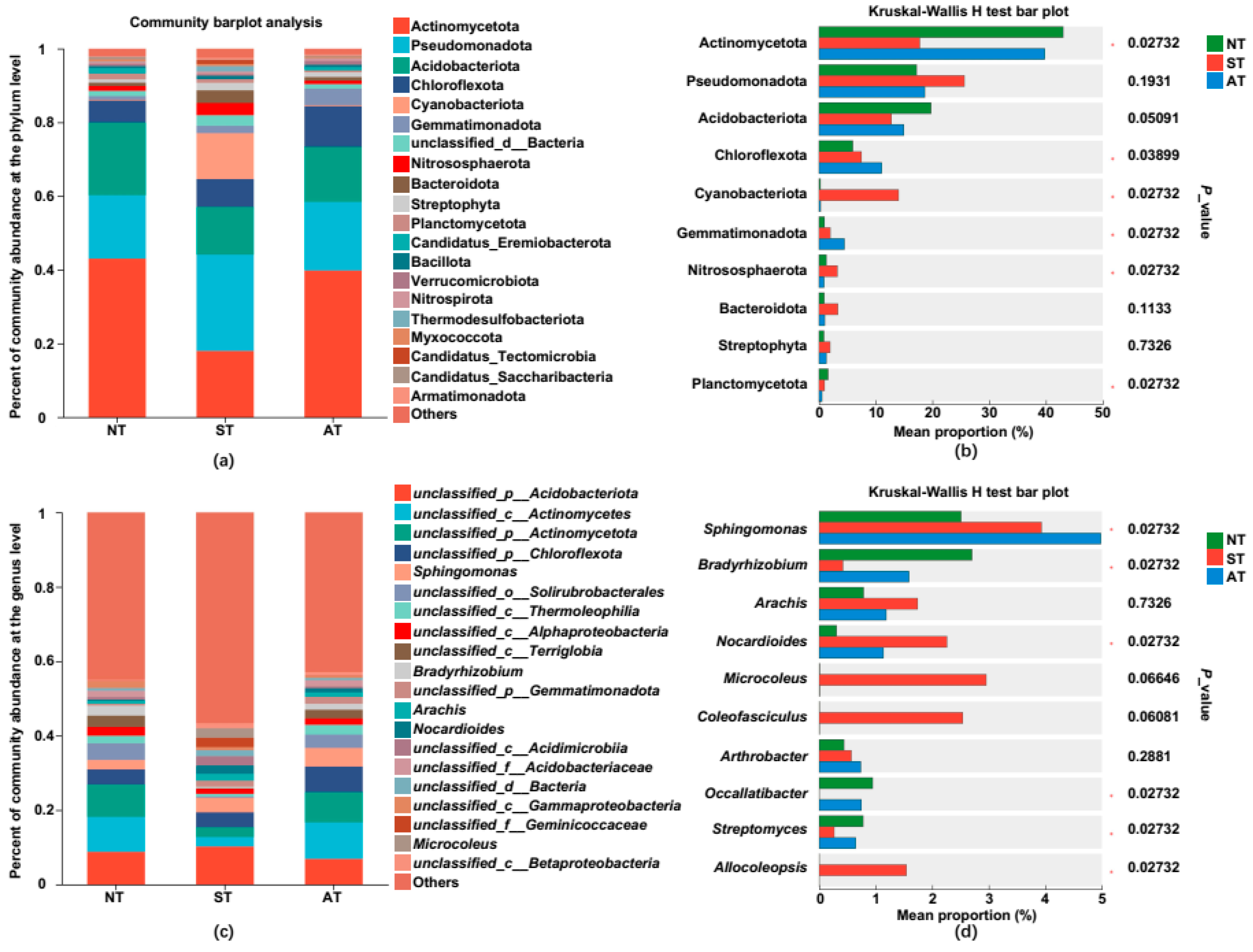
2.6. Diversified Soil Types Differentially Regulated the Peanut Rhizosphere Bacterial Community Structure
2.7. Linear Discriminant Analysis Effect Size (LEfSe) Analysis
2.8. Environmental Factor Analysis
2.9. KEGG Functional Enrichment Analysis of Microorganisms
3. Discussion
4. Materials and Methods
4.1. Experiment Site and Design
4.2. Sample Collection
4.3. Soil Physicochemical Properties and Enzyme Activity Assays
4.4. Metagenomic Sequencing and Data Analysis
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NT | Neutral soil |
| ST | Saline–alkali soil |
| AT | Acidic soil |
| ORFs | Open reading frames |
| PCoA | Principal coordinates analysis |
| ANOSIM | Analysis of similarities |
| LEfSe | Linear discriminant analysis effect size |
| LDA | Linear discriminant analysis |
| RDA | Redundancy analysis |
| AN | Alkali-hydrolyzable nitrogen |
| AP | Available phosphorus |
| AK | Available potassium |
References
- Mingrou, L.; Guo, S.; Ho, C.T.; Bai, N. Review on chemical compositions and biological activities of peanut (Arachis hypogeae L.). J. Food Biochem. 2022, 46, e14119. [Google Scholar] [CrossRef] [PubMed]
- Ci, D.; Tang, Z.; Ding, H.; Cui, L.; Zhang, G.; Li, S.; Dai, L.; Qin, F.; Zhang, Z.; Yang, J.; et al. The synergy effect of arbuscular mycorrhizal fungi symbiosis and exogenous calcium on bacterial community composition and growth performance of peanut (Arachis hypogaea L.) in saline alkali soil. J. Microbiol. 2021, 59, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.X.; Jia, L.H.; Wang, Y.F.; Wang, M.L.; Mcgiffen, M.E. Effects of Different Soil Texture on Peanut Growth and Development. Commun. Soil Sci. Plant Anal. 2015, 46, 2249–2257. [Google Scholar] [CrossRef]
- Steiner, F.; da Queiroz, L.F.M.; Zuffo, A.M.; da Silva, K.C.; Lima, I.M.D. Peanut response to co-inoculation of spp. and Azospirillum brasilense and molybdenum application in sandy soil of the Brazilian Cerrado. Agron. J. 2021, 113, 623–632. [Google Scholar] [CrossRef]
- Yu, Z.P.; Duan, X.B.; Luo, L.; Dai, S.J.; Ding, Z.J.; Xia, G.M. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, X.; Gao, J.; Wang, Y.; Zhu, D. Comparative Study on the Nutritional Quality of Peanut in Saline and Non-Saline Land. Foods 2024, 13, 3751. [Google Scholar] [CrossRef]
- Yao, T.X.; Zhang, W.T.; Gulaqa, A.; Cui, Y.F.; Zhou, Y.M.; Weng, W.N.; Wang, X.; Liu, Q.T.; Jin, F. Effects of Peanut Shell Biochar on Soil Nutrients, Soil Enzyme Activity, and Rice Yield in Heavily Saline-Sodic Paddy Field. J. Soil Sci. Plant Nutr. 2021, 21, 655–664. [Google Scholar] [CrossRef]
- Qin, F.F.; Xin, Z.H.; Wang, J.G.; Zhang, J.L.; Yang, J.S.; Guo, F.; Tang, Z.H.; Ci, D.W. Peanut production in saline-alkali land of Yellow River Delta: Influence of spatiotemporal changes of meteorological conditions and soil properties. BMC Plant Biol. 2024, 24, 1029. [Google Scholar] [CrossRef]
- Pasricha, N.S.; Aulakh, M.S.; Vempati, R.K. Evaluation of available phosphorus soil test methods for peanut in neutral and alkaline soils. Commun. Soil Sci. Plant Anal. 2002, 33, 3593–3601. [Google Scholar] [CrossRef]
- Basu, M.; Bhadoria, P.; Mahapatra, S. Influence of soil ameliorants, manures and fertilizers on bacterial populations, enzyme activities, N fixation and P solubilization in peanut rhizosphere under lateritic soil. Br. Microbiol. Res. J. 2011, 1, 10–25. [Google Scholar]
- Wang, T.; Liu, M.Q.; Li, H.X. Inoculation of phosphate-solubilizing bacteria B1 increases available phosphorus and growth of peanut in acidic soil. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2014, 64, 252–259. [Google Scholar] [CrossRef]
- Xia, H.; Riaz, M.; Liu, B.; Li, Y.X.; El-Desouki, Z.; Jiang, C.C. Peanut shell biochar in acidic soil increases nitrogen absorption and photosynthesis characteristics of maize under different nitrogen levels. Environ. Dev. Sustain. 2023, 25, 8957–8974. [Google Scholar] [CrossRef]
- Yu, T.Y.; Lu, Y.; Rouzi, M.; Zhang, Y.; Alimu, Y.; Remutula, M.; Sun, Q.Q.; Wu, Z.F. Root Morphology, Leaf Photosynthesis and Chloroplast Ultrastructure Responses of Peanut Seedlings to Soil Acidification. J. Soil. Sci. Plant Nutr. 2025, 25, 1453–1465. [Google Scholar] [CrossRef]
- Li, Y.; Fang, F.; Wei, J.L.; Wu, X.B.; Cui, R.Z.; Li, G.S.; Zheng, F.L.; Tan, D.S. Humic Acid Fertilizer Improved Soil Properties and Soil Microbial Diversity of Continuous Cropping Peanut: A Three-Year Experiment. Sci. Rep. 2019, 9, 12014. [Google Scholar] [CrossRef]
- Ahsan, T.; Tian, P.C.; Gao, J.; Wang, C.; Liu, C.; Huang, Y.Q. Effects of microbial agent and microbial fertilizer input on soil microbial community structure and diversity in a peanut continuous cropping system. J. Adv. Res. 2024, 64, 1–13. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, G.; Yu, Z.; Ding, H.; Xu, Y.; Zhang, Z. Effect of Drought Stress and Developmental Stages on Microbial Community Structure and Diversity in Peanut Rhizosphere Soil. Int. J. Mol. Sci. 2019, 20, 2265. [Google Scholar] [CrossRef]
- Zhang, C.L.; Yu, Z.P.; Zhang, M.Y.; Li, X.X.; Wang, M.J.; Li, L.X.; Li, X.G.; Ding, Z.J.; Tian, H.Y. PLR enhances lateral root formation through supplying PLR-derived auxin and enhancing auxin biosynthesis in Arabidopsis. J. Exp. Bot. 2022, 73, 3711–3725. [Google Scholar] [CrossRef]
- Schreiter, S.; Ding, G.C.; Heuer, H.; Neumann, G.; Sandmann, M.; Grosch, R.; Kropf, S.; Smalla, K. Effect of the soil type on the microbiome in the rhizosphere of field-grown lettuce. Front. Microbiol. 2014, 5, 144. [Google Scholar] [CrossRef]
- Wang, P.; Nie, J.; Yang, L.; Zhao, J.; Wang, X.; Zhang, Y.; Zang, H.; Yang, Y.; Zeng, Z. Plant growth stages covered the legacy effect of rotation systems on microbial community structure and function in wheat rhizosphere. Environ. Sci. Pollut. Res. Int. 2023, 30, 59632–59644. [Google Scholar] [CrossRef]
- Yan, M.; Xiong, T.; Yang, J.; Wu, T.; Mao, J.; Tang, X.; Hu, G. Effects of Rotary and Deep Tillage on Soil Environment and Melon Root Development. Plants 2024, 13, 2611. [Google Scholar] [CrossRef]
- Hirpara, K.R.; Hinsu, A.T.; Kothari, R.K. Metagenomic evaluation of peanut rhizosphere microbiome from the farms of Saurashtra regions of Gujarat, India. Sci. Rep. 2024, 14, 10525. [Google Scholar] [CrossRef] [PubMed]
- Obayomi, O.; Seyoum, M.M.; Ghazaryan, L.; Tebbe, C.C.; Murase, J.; Bernstein, N.; Gillor, O. Soil texture and properties rather than irrigation water type shape the diversity and composition of soil microbial communities. Appl. Soil. Ecol. 2021, 161, 103834. [Google Scholar] [CrossRef]
- Shu, X.; Zhang, K.; Zhang, Q.; Wang, W. Changes in the composition of rhizosphere bacterial communities in response to soil types and acid rain. J. Environ. Manag. 2023, 325, 116493. [Google Scholar] [CrossRef]
- Jiao, F.; Zhang, D.D.; Chen, Y.; Wu, J.H.; Zhang, J.Y. Effects of Long-Term Straw Returning and Nitrogen Fertilizer Reduction on Soil Microbial Diversity in Black Soil in Northeast China. Agronomy 2023, 13, 2036. [Google Scholar] [CrossRef]
- Ceja-Navarro, J.A.; Wang, Y.; Ning, D.L.; Arellano, A.; Ramanculova, L.; Yuan, M.M.; Byer, A.; Craven, K.D.; Saha, M.C.; Brodie, E.L.; et al. Protist diversity and community complexity in the rhizosphere of switchgrass are dynamic as plants develop. Microbiome 2021, 9, 96. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, Z.; Peijnenburg, W.; Liu, W.; Lu, T.; Hu, B.; Chen, J.; Chen, J.; Lin, Z.; Qian, H. Rhizosphere Microbiome Assembly and Its Impact on Plant Growth. J. Agric. Food Chem. 2020, 68, 5024–5038. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Shen, H.; He, X.H.; Thomas, B.; Lupwayi, N.Z.; Hao, X.Y.; Thomas, M.C.; Shi, X.J. Fertilization Shapes Bacterial Community Structure by Alteration of Soil pH. Front. Microbiol. 2017, 8, 1325. [Google Scholar] [CrossRef]
- Bartram, A.K.; Jiang, X.P.; Lynch, M.D.J.; Masella, A.P.; Nicol, G.W.; Dushoff, J.; Neufeld, J.D. Exploring links between pH and bacterial community composition in soils from the Craibstone Experimental Farm. FEMS Microbiol. Ecol. 2014, 87, 403–415. [Google Scholar] [CrossRef]
- Zheng, Y.; Cao, X.; Zhou, Y.; Ma, S.; Wang, Y.; Li, Z.; Zhao, D.; Yang, Y.; Zhang, H.; Meng, C. Purines enrich root-associated Pseudomonas and improve wild soybean growth under salt stress. Nat. Commun. 2024, 15, 3520. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Ding, H.; Wen, S.; Zhang, G.; Qin, F.; Dai, L. Comprehensive effects of salt stress and peanut cultivars on the rhizosphere bacterial community diversity of peanut. Arch. Microbiol. 2021, 204, 15. [Google Scholar] [CrossRef]
- Halo, B.A.; Aljabri, Y.A.S.; Yaish, M.W. Drought-induced microbial dynamics in cowpea rhizosphere: Exploring bacterial diversity and bioinoculant prospects. PLoS ONE 2025, 20, e0320197. [Google Scholar] [CrossRef] [PubMed]
- Lindgreen, S.; Adair, K.L.; Gardner, P.P. An evaluation of the accuracy and speed of metagenome analysis tools. Sci. Rep. 2016, 6, 19233. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, K.; Abdulaha-Al Baquy, M.; Xu, R.K. Influence of nitrogen fertilizer forms and crop straw biochars on soil exchange properties and maize growth on an acidic Ultisol. Arch. Agron. Soil Sci. 2018, 64, 834–849. [Google Scholar] [CrossRef]
- Shi, R.Y.; Hong, Z.N.; Li, J.Y.; Jiang, J.; Kamran, M.A.; Xu, R.K.; Qian, W. Peanut straw biochar increases the resistance of two Ultisols derived from different parent materials to acidification: A mechanism study. J. Environ. Manag. 2018, 210, 171–179. [Google Scholar] [CrossRef]
- Wang, J.G.; Geng, Y.; Zhang, J.L.; Li, L.; Guo, F.; Yang, S.; Zou, J.; Wan, S.B. Increasing Calcium and Decreasing Nitrogen Fertilizers Improves Peanut Growth and Productivity by Enhancing Photosynthetic Efficiency and Nutrient Accumulation in Acidic Red Soil. Agronomy 2023, 13, 1924. [Google Scholar] [CrossRef]
- Jiang, H.H.; Qi, P.S.; Wang, T.; Chi, X.Y.; Wang, M.A.; Chen, M.N.; Chen, N.; Pan, L.J. Role of halotolerant phosphate-solubilising bacteria on growth promotion of peanut (Arachis hypogaea) under saline soil. Ann. Appl. Biol. 2019, 174, 20–30. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, G.; Ding, H.; Ci, D.; Dai, L.; Zhang, Z. Influence of salt stress on the rhizosphere soil bacterial community structure and growth performance of groundnut (Arachis hypogaea L.). Int. Microbiol. 2020, 23, 453–465. [Google Scholar] [CrossRef]
- Li, M.; Li, Q.; Wang, S.; Wang, X.; Li, Q.; Liu, W.; Yu, J.; Zhang, G.; Wang, J.; Wu, Q.L.; et al. The diversity and biogeography of bacterial communities in lake sediments across different climate zones. Environ. Res. 2024, 263, 120028. [Google Scholar] [CrossRef]
- Shi, X.L.; Guo, P.; Chen, Y.X.; Liu, C.; Liu, C.J.; Yu, H.Q.; Zhou, Y.F.; Zou, H.T. Integrated Analysis of Soil Metagenome and Soil Metabolome Reveals the Differential Responses of Sorghum and Peanut Rhizosphere Microbes to Salt Stress. J. Soil Sci. Plant Nutr. 2024, 24, 2959–2971. [Google Scholar] [CrossRef]
- Lu, Q.; Ge, G.; Sa, D.; Wang, Z.; Hou, M.; Jia, Y.S. Effects of salt stress levels on nutritional quality and microorganisms of alfalfa-influenced soil. PeerJ 2021, 9, e11729. [Google Scholar] [CrossRef]
- Chen, J.Y.; Gu, J.; Wang, E.T.; Ma, X.X.; Kang, S.T.; Huang, L.Z.; Cao, X.P.; Li, L.B.; Wu, Y.L. Wild peanut Arachis duranensis are nodulated by diverse and novel Bradyrhizobium species in acid soils. Syst. Appl. Microbiol. 2014, 37, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Sarao, S.K.; Boothe, V.; Das, B.K.; Gonzalez-Hernandez, J.L.; Brözel, V.S. and the soybean rhizosphere: Species level bacterial population dynamics in established soybean fields, rhizosphere and nodules. Plant Soil 2024, 508, 515–530. [Google Scholar] [CrossRef]
- Klepa, M.S.; Ferraz Helene, L.C.; O’Hara, G.; Hungria, M. Bradyrhizobium agreste sp. nov., Bradyrhizobium glycinis sp. nov. and Bradyrhizobium diversitatis sp. nov., isolated from a biodiversity hotspot of the genus Glycine in Western Australia. Int. J. Syst. Evol. Microbiol. 2019, 71, 004742. [Google Scholar] [CrossRef]
- Alam, K.; Mazumder, A.; Sikdar, S.; Zhao, Y.M.; Hao, J.; Song, C.; Wang, Y.; Sarkar, R.; Islam, S.; Zhang, Y.; et al. Streptomyces: The biofactory of secondary metabolites. Front. Microbiol. 2022, 13, 968053. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications and interactions in plant growth promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef]
- Sorouri, B.; Scales, N.C.; Gaut, B.S.; Allison, S.D. Sphingomonas clade and functional distribution with simulated climate change. Microbiol. Spectr. 2024, 12, e0023624. [Google Scholar] [CrossRef]
- Ghosh, I.; Mukherji, S. Substrate interaction effects during pyrene biodegradation by RS1. J. Environ. Chem. Eng. 2017, 5, 1791–1800. [Google Scholar] [CrossRef]
- Dindar, E.; Sagban, F.O.; Baskaya, H.S. Evaluation-of soil enzyme activities as soil quality indicators in sludge-amended soils. J. Environ. Biol. 2015, 36, 919–926. [Google Scholar]
- Yu, H.; Si, P.; Shao, W.; Qiao, X.; Yang, X.; Gao, D.; Wang, Z. Response of enzyme activities and microbial communities to soil amendment with sugar alcohols. Microbiologyopen 2016, 5, 604–615. [Google Scholar] [CrossRef]
- Liang, H.; Wang, X.; Yan, J.; Luo, L. Characterizing the Intra-Vineyard Variation of Soil Bacterial and Fungal Communities. Front. Microbiol. 2019, 10, 1239. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, S.; Han, P.; Hu, X.; Xie, L.; Li, Y.; Brooks, M.; Liao, X.; Qin, L. Soil Enzyme Activity in Soils Subjected to Flooding and the Effect on Nitrogen and Phosphorus Uptake by Oilseed Rape. Front. Plant Sci. 2019, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, S.; Chen, C.; Tang, Y.; Liu, B. Multi-omics analysis to reveal key pathways involved in low C/N ratio stress response in Pseudomonas sp. LW60 with superior nitrogen removal efficiency. Bioresour. Technol. 2023, 389, 129812. [Google Scholar] [CrossRef]
- Wen, X.Y.; Dubinsky, E.; Wu, Y.; Yu, R.; Chen, F. Wheat, maize and sunflower cropping systems selectively influence bacteria community structure and diversity in their and succeeding crop’s rhizosphere. J. Integr. Agric. 2016, 15, 1892–1902. [Google Scholar] [CrossRef]
- Imam, A.; Suman, S.K.; Kanaujia, P.K.; Ray, A. Biological machinery for polycyclic aromatic hydrocarbons degradation: A review. Bioresour. Technol. 2022, 343, 126121. [Google Scholar] [CrossRef]
- Phale, P.S.; Malhotra, H.; Shah, B.A. Degradation strategies and associated regulatory mechanisms/features for aromatic compound metabolism in bacteria. Adv. Appl. Microbiol. 2020, 112, 1–65. [Google Scholar] [CrossRef]
- Chen, W.W.; He, P.J.; Zhang, H.; Lü, F. Effects of volatile fatty acids on soil properties, microbial communities, and volatile metabolites in wheat rhizosphere of loess. J. Clean. Prod. 2024, 476, 143798. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, H.; Yang, Y.F.; Liang, Y.T.; Zhou, J.Z.; He, Z.L.; Chen, F.L.; Ouyang, Z.Y. Microbial functional gene diversity in natural secondary forest Ultisols. Acta Oecologica-Int. J. Ecol. 2020, 105, 103575. [Google Scholar] [CrossRef]
- Ren, R.; Cao, Z.; Ma, X.; Li, Z.; Zhao, K.; Cao, D.; Ma, Q.; Hou, M.; Zhao, K.; Zhang, L.; et al. Multi-Omics Analysis Reveals That AhNHL Contributes to Melatonin-Mediated Cadmium Tolerance in Peanut Plants. J. Pineal Res. 2025, 77, e70035. [Google Scholar] [CrossRef]
- Karapareddy, S.; Anche, V.C.; Tamatamu, S.R.; Janga, M.R.; Lawrence, K.; Nyochembeng, L.M.; Todd, A.; Walker, L.T.; Sripathi, V.R. Profiling of rhizosphere-associated microbial communities in North Alabama soils infested with varied levels of reniform nematodes. Front. Plant Sci. 2025, 16, 1521579. [Google Scholar] [CrossRef]
- Mohale, P.M.; Manyevere, A.; Parwada, C.; Zerizghy, M.G. Effect of eucalyptus wood-based compost application rates on soil chemical properties in semi-organic avocado plantations, Limpopo province, South Africa. PLoS ONE 2023, 18, e0265728. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, Y.; Wang, Y.; Gao, F.; Zhao, J.; Li, Y.; Li, X. Effects of Soil Tillage, Management Practices, and Mulching Film Application on Soil Health and Peanut Yield in a Continuous Cropping System. Front. Microbiol. 2020, 11, 570924. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, Y.; Zheng, Y.; Wang, L.; Zhang, Y.; Sun, Y.; Ji, J.; Hao, X.; Liu, S.; Sun, N. Effect of Different Fertilization on Soil Fertility, Biological Activity, and Maize Yield in the Albic Soil Area of China. Plants 2025, 14, 810. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ding, H.; Zhang, G.; Li, Z.; Guo, Q.; Feng, H.; Qin, F.; Dai, L.; Zhang, Z. Green manure increases peanut production by shaping the rhizosphere bacterial community and regulating soil metabolites under continuous peanut production systems. BMC Plant Biol. 2023, 23, 69. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef]
- Beghini, F.; McIver, L.J.; Blanco-Miguez, A.; Dubois, L.; Asnicar, F.; Maharjan, S.; Mailyan, A.; Manghi, P.; Scholz, M.; Thomas, A.M.; et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. eLife 2021, 10, e65088. [Google Scholar] [CrossRef]


| Sample | Ace | Shannon | Simpson | |
|---|---|---|---|---|
| Phylum | NT | 234.00 ± 1.00 a | 1.78 ± 0.04 b | 0.28 ± 0.01 a |
| ST | 202.67 ± 0.58 c | 2.14 ± 0.29 a | 0.20 ± 0.07 a | |
| AT | 220.00 ± 1.00 b | 1.87 ± 0.03 ab | 0.24 ± 0.01 a | |
| Class | NT | 439.33 ± 10.07 a | 2.73 ± 0.03 a | 0.12 ± 0.00 a |
| ST | 375.33 ± 2.89 c | 2.95 ± 0.44 a | 0.12 ± 0.08 a | |
| AT | 392.67 ± 5.03 b | 2.81 ± 0.04 a | 0.10 ± 0.01 a | |
| Order | NT | 814.67 ± 27.79 a | 3.63 ± 0.02 a | 0.05 ± 0.00 a |
| ST | 674.67 ± 15.04 c | 3.82 ± 0.31 a | 0.05 ± 0.02 a | |
| AT | 720.00 ± 7.55 b | 3.62 ± 0.01 a | 0.05 ± 0.00 a | |
| Family | NT | 1543.67 ± 65.43 a | 3.94 ± 0.02 a | 0.04 ± 0.00 a |
| ST | 1256.00 ± 40.51 c | 4.23 ± 0.32 a | 0.04 ± 0.02 a | |
| AT | 1353.67 ± 10.07 b | 3.94 ± 0.01 a | 0.04 ± 0.00 a | |
| Genus | NT | 4087.33 ± 100.11 a | 4.39 ± 0.02 b | 0.04 ± 0.00 a |
| ST | 3599.33 ± 136.36 b | 4.69 ± 0.23 a | 0.03 ± 0.01 a | |
| AT | 3723.00 ± 53.84 b | 4.34 ± 0.03 b | 0.04 ± 0.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, W.; Ding, H.; Zhang, Z.; Li, F.; Feng, H.; Guo, Q.; Qin, F.; Zhang, G.; Xu, M.; Xu, Y. Diversified Soil Types Differentially Regulated the Peanut (Arachis hydropoaea L.) Growth and Rhizosphere Bacterial Community Structure. Plants 2025, 14, 1169. https://doi.org/10.3390/plants14081169
Lan W, Ding H, Zhang Z, Li F, Feng H, Guo Q, Qin F, Zhang G, Xu M, Xu Y. Diversified Soil Types Differentially Regulated the Peanut (Arachis hydropoaea L.) Growth and Rhizosphere Bacterial Community Structure. Plants. 2025; 14(8):1169. https://doi.org/10.3390/plants14081169
Chicago/Turabian StyleLan, Wenfei, Hong Ding, Zhimeng Zhang, Fan Li, Hao Feng, Qing Guo, Feifei Qin, Guanchu Zhang, Manlin Xu, and Yang Xu. 2025. "Diversified Soil Types Differentially Regulated the Peanut (Arachis hydropoaea L.) Growth and Rhizosphere Bacterial Community Structure" Plants 14, no. 8: 1169. https://doi.org/10.3390/plants14081169
APA StyleLan, W., Ding, H., Zhang, Z., Li, F., Feng, H., Guo, Q., Qin, F., Zhang, G., Xu, M., & Xu, Y. (2025). Diversified Soil Types Differentially Regulated the Peanut (Arachis hydropoaea L.) Growth and Rhizosphere Bacterial Community Structure. Plants, 14(8), 1169. https://doi.org/10.3390/plants14081169






