Evaluation of Luffa Rootstocks to Improve Resistance in Bitter Gourd (Momordica charantia L.) Against Fusarium Wilt
Abstract
1. Introduction
2. Results
2.1. Pathogenicity Test of Fomh16 and Fomo33 Isolates
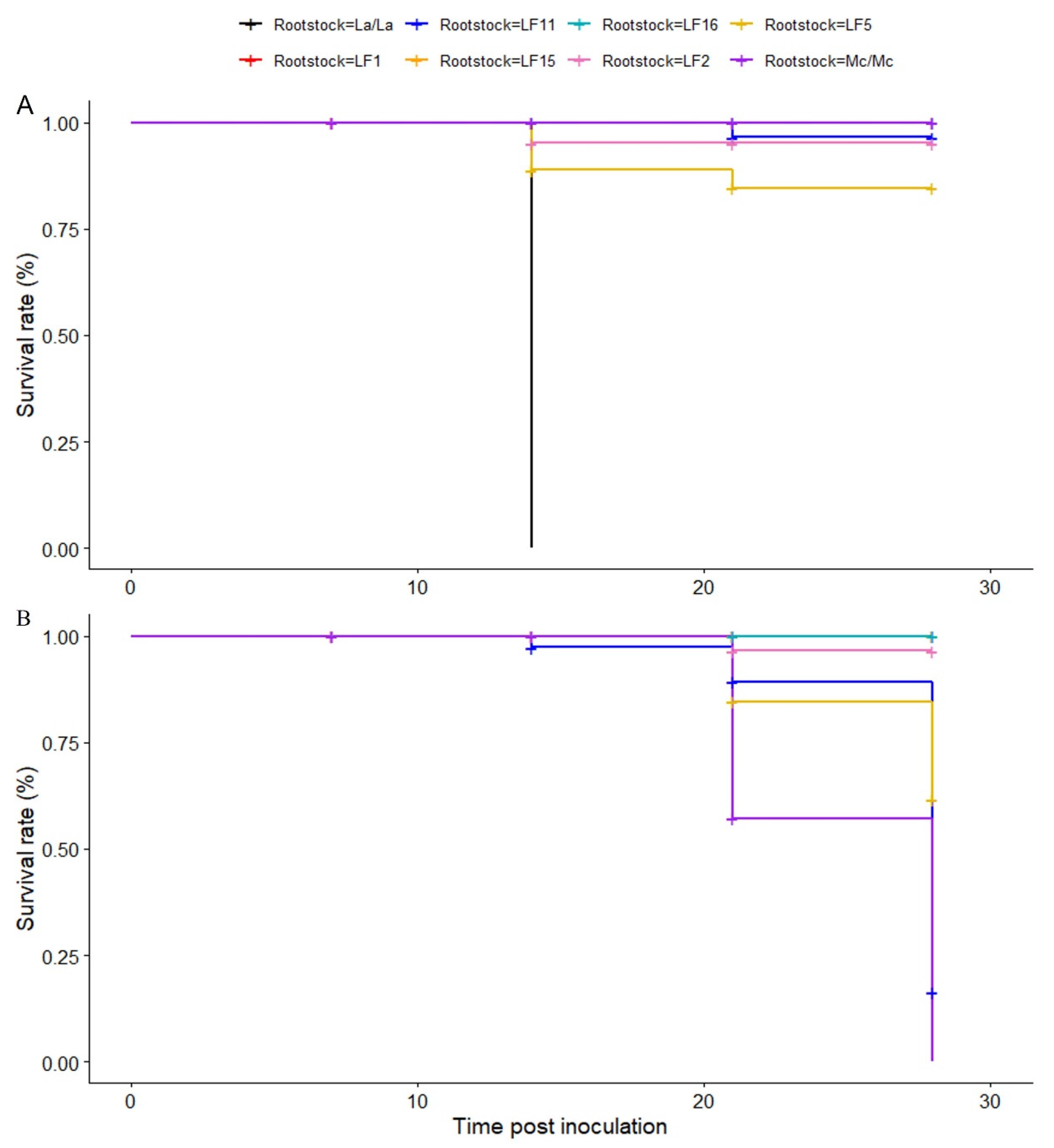
2.2. Evaluation of Luffa Hybrids to Fusarium Wilt Resistance
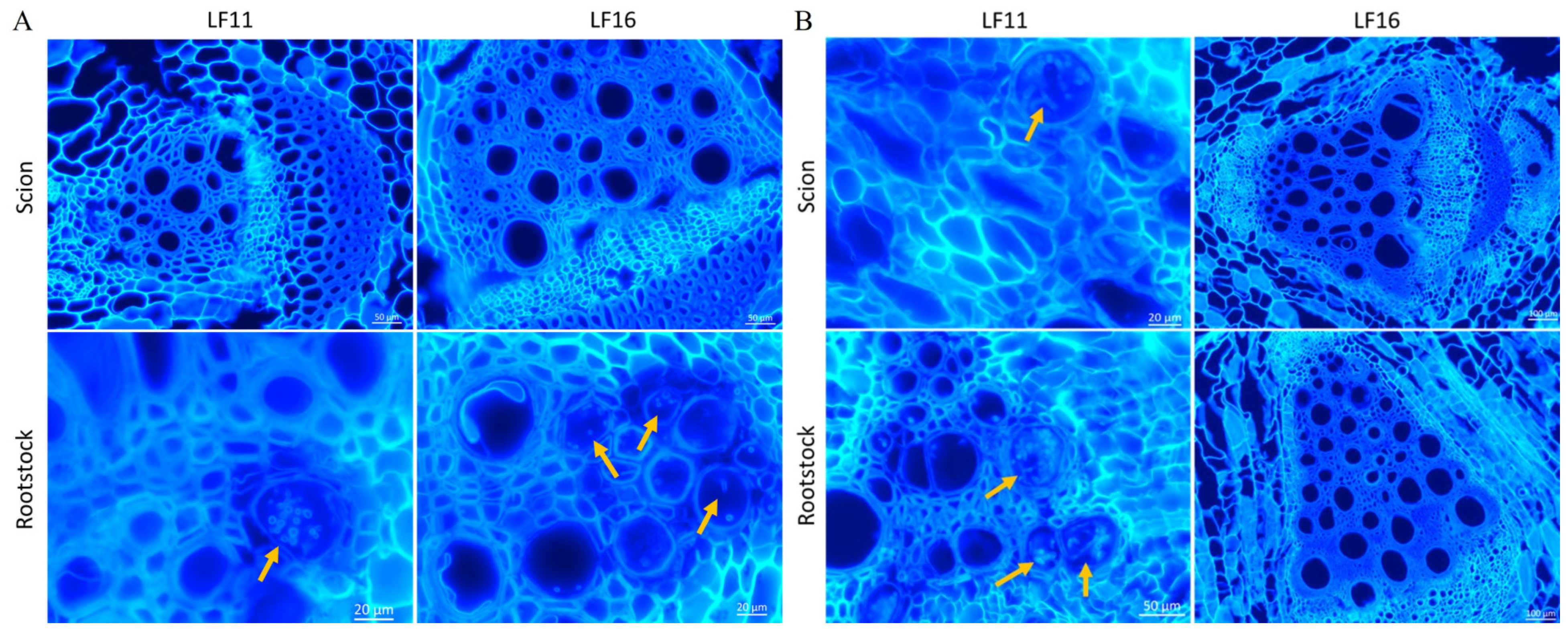
2.3. Colonization of Grafted Plants by Fomh16 and Fomo33 Isolates
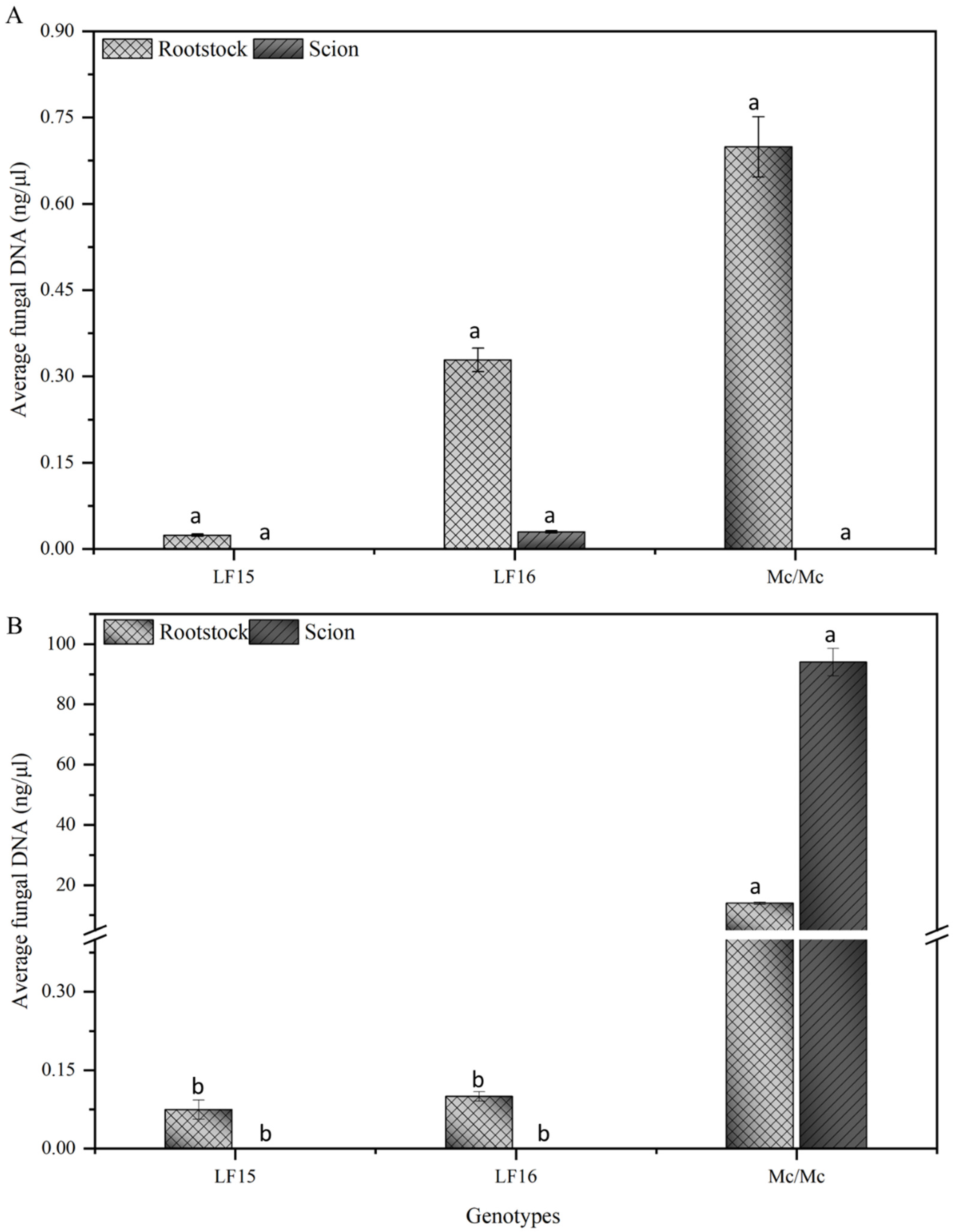
2.4. Quantification of Fomh16 and Fomo33 Isolates in Grafted Plants
3. Discussion
4. Materials and Methods
4.1. Plant Material and Grafting Procedure
4.2. Fungal Isolates and Pathogenicity Test
4.3. Inoculum Preparation and Inoculation Assay
4.4. Plant Assessed
4.5. Pathogen Recovery and Molecular Confirmation
4.6. Biomass Quantification of Fomh16 and Fomo33 Isolates in Grafted Plants
4.7. Microscopic Examinations
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCreight, J.; Staub, J.; Wehner, T.; Dhillon, N. Gone Global: Familiar and exotic cucurbits have asian origins. HortScience 2013, 48, 1078–1089. [Google Scholar] [CrossRef]
- Schaefer, H.; Renner, S.S. A three-genome phylogeny of Momordica (Cucurbitaceae) suggests seven returns from dioecy to monoecy and recent long-distance dispersal to Asia. Mol. Phylogenet. Evol. 2010, 54, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Muronga, M.; Quispe, C.; Tshikhudo, P.P.; Msagati, T.A.M.; Mudau, F.N.; Martorell, M.; Salehi, B.; Abdull Razis, A.F.; Sunusi, U.; Kamal, R.M.; et al. Three selected edible crops of the genus Momordica as potential sources of phytochemicals: Biochemical, nutritional, and medicinal values. Front. Pharmacol. 2021, 12, 625546. [Google Scholar] [CrossRef] [PubMed]
- Krawinkel, M.B.; Keding, G.B. Bitter gourd (Momordica charantia): A dietary approach to hyperglycemia. Nutr. Rev. 2006, 64, 331–337. [Google Scholar] [CrossRef]
- Mahwish; Saeed, F.; Sultan, M.T.; Riaz, A.; Ahmed, S.; Bigiu, N.; Amarowicz, R.; Manea, R. Bitter melon (Momordica charantia L.) fruit bioactives charantin and vicine potential for diabetes prophylaxis and treatment. Plants 2021, 10, 730. [Google Scholar] [CrossRef]
- Sheu, Z.M.; Cheng, H.C.; Chiu, M.S.; Yu, C.C.; Huang, H.Y.; Barchenger, D.W.; Kenyon, L. Evaluation of cucurbit rootstocks and screening of bitter gourd genotypes for resistance to Fusarium wilt. ActaHortic 2019, 1257, 57–62. [Google Scholar] [CrossRef]
- Sun, S.K.; Huang, J.W. A new Fusarium wilt of bitter gourd in Taiwan. Plant Dis. 1983, 67, 226–227. [Google Scholar] [CrossRef]
- Chen, Z.-D.; Huang, R.-K.; Li, Q.-Q.; Wen, J.-L.; Yuan, G.-Q. Development of pathogenicity and AFLP to characterize Fusarium oxysporum f.sp. momordicae isolates from bitter gourd in China. J. Phytopathol. 2015, 163, 202–211. [Google Scholar]
- Zang, R.; Zhao, Y.; Guo, K.; Hong, K.; Xi, H.; Wen, C. Population genetic variation analysis of bitter gourd wilt caused by Fusarium oxysporum f. sp. momordicae in China using inter simple sequence repeats (ISSR) molecular markers. J. Plant Pathol. 2021, 103, 787–797. [Google Scholar]
- Tian, Y.; Fu, X.; Yan, X.; Li, X.; Peng, H.; Gao, K. The control efficacy and mechanism of Talaromyces purpurogenus on Fusarium wilt of bitter gourd. Biol. Control 2022, 165, 104804. [Google Scholar] [CrossRef]
- Guo, T.X.; Mo, J. Resistance to the blight disease (Fusarium oxysporum f. sp. momordicae) of several bitter gourd varieties. Guangxi Agric. Sci. 2007, 38, 408–410. [Google Scholar]
- Yadeta, K.; Thomma, B. The xylem as battleground for plant hosts and vascular wilt pathogens. Front. Plant Sci. 2013, 4, 97. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Ghag, S.B.; Shekhawat, U.K.S.; Ganapathi, T.R. Characterization of Fusarium wilt resistant somaclonal variants of banana cv. Rasthali by cDNA-RAPD. Mol. Biol. Rep. 2014, 41, 7929–7935. [Google Scholar] [CrossRef]
- Ayala-Doñas, A.; Cara-García, M.D.; Talavera-Rubia, M.; Verdejo-Lucas, S. Management of soil-borne fungi and root-knot nematodes in cucurbits through breeding for resistance and grafting. Agronomy 2020, 10, 1641. [Google Scholar] [CrossRef]
- de Lamo, F.J.; Takken, F.L.W. Biocontrol by Fusarium oxysporum using endophyte-mediated resistance. Front. Plant Sci. 2020, 11, 37. [Google Scholar] [CrossRef]
- Namisy, A.; Huang, J.-H.; Rakha, M.; Hong, C.-F.; Chung, W.-H. Resistance to Fusarium oxysporum f. sp. luffae in luffa germplasm despite hypocotyl colonization. Plant Dis. 2023, 107, 1993–2001. [Google Scholar] [CrossRef]
- Jha, U.C.; Bohra, A.; Pandey, S.; Parida, S.K. Breeding, genetics, and genomics approaches for improving Fusarium wilt resistance in major grain Legumes. Front. Genet. 2020, 11, 1001. [Google Scholar] [CrossRef]
- Murata, J.; Ohara, K. Prevention of watermelon Fusarium wilt by grafting Lagenaria. Jpn. J. Phytopathol. 1936, 6, 183–189. [Google Scholar]
- Sakata, Y.; Ohara, T.; Sugiyama, M. The history of melon and cucumber grafting in Japan. Acta Hortic. 2008, 767, 217–228. [Google Scholar] [CrossRef]
- Davis, A.R.; Perkins-Veazie, P.; Sakata, Y.; López-Galarza, S.; Maroto, J.V.; Lee, S.-G.; Huh, Y.-C.; Sun, Z.; Miguel, A.; King, S.R.; et al. Cucurbit grafting. Crit. Rev. Plant Sci. 2008, 27, 50–74. [Google Scholar] [CrossRef]
- Guan, W.; Zhao, X.; Hassell, R.; Thies, J. Defense mechanisms involved in disease resistance of grafted vegetables. HortScience 2012, 47, 164–170. [Google Scholar] [CrossRef]
- King, S.; Davis, A.; Zhang, X.; Crosby, K. Genetics, breeding and selection of rootstocks for Solanaceae and Cucurbitaceae. Sci. Hortic. 2010, 127, 106–111. [Google Scholar] [CrossRef]
- Louws, F.J.; Rivard, C.L.; Kubota, C. Grafting fruiting vegetables to manage soilborne pathogens, foliar pathogens, arthropods and weeds. Sci. Hortic. 2010, 127, 127–146. [Google Scholar] [CrossRef]
- Kumar, P.; Rouphael, Y.; Cardarelli, M.; Colla, G. Vegetable grafting as a tool to improve drought resistance and water use efficiency. Front. Plant Sci. 2017, 8, 1130. [Google Scholar] [CrossRef]
- Guo, Z.; Qin, Y.; Lv, J.; Wang, X.; Dong, H.; Dong, X.; Zhang, T.; Du, N.; Piao, F. Luffa rootstock enhances salt tolerance and improves yield and quality of grafted cucumber plants by reducing sodium transport to the shoot. Environ. Pollut. 2023, 316, 120521. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Lv, X.; Ahammed, G.J.; Xia, X.; Zhou, J.; Shi, K.; Asami, T.; Yu, J.; Zhou, Y. Grafting cucumber onto luffa improves drought tolerance by increasing ABA biosynthesis and sensitivity. Sci. Rep. 2016, 6, 20212. [Google Scholar] [CrossRef]
- Edelstein, M.; Burger, Y.; Horev, C.; Porat, A.; Meir, A.; Cohen, R. Assessing the effect of genetic and anatomic variation of Cucurbita rootstocks on vigour, survival and yield of grafted melons. J. Hortic. Sci. Biotechnol. 2004, 79, 370–374. [Google Scholar] [CrossRef]
- PT2004-36; Grafting techniques for controlling fusarium wilt of bitter gourd. Fruits and Fertilizer Technology Center: Taipei, Taiwan, 2004.
- Li, W.M.; Dita, M.; Wu, W.; Hu, G.B.; Xie, J.H.; Ge, X.J. Resistance sources to Fusarium oxysporumf. sp. cubensetropical race 4 in banana wild relatives. Plant Pathol. 2015, 64, 1061–1067. [Google Scholar] [CrossRef]
- Chung, C.-C.; Wang, C.-J.; Chen, Y.-J.; Chung, W.-H. Pathogenicity and phylogeny of Fusarium oxysporum causing cucurbit wilting in Taiwan. In Proceedings of the International Congress of Plant Pathology (ICPP) 2018: Plant Health in A Global Economy, Boston, MA, USA, 29 July–3 August 2018. [Google Scholar]
- Zuo, C.; Deng, G.; Li, B.; Huo, H.; Li, C.; Hu, C.; Kuang, R.; Yang, Q.; Dong, T.; Sheng, O.; et al. Germplasm screening of Musa spp. for resistance to Fusarium oxysporum f. sp. cubense tropical race 4 (Foc TR4). Eur. J. Plant Pathol. 2018, 151, 723–734. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, T.; Wang, Y.; Zhang, D.; Bai, T.; Xu, S.; Wang, Y.; Tang, W.; Zheng, S.-J. Identification and evaluation of resistance to Fusarium oxysporum f. sp. cubense tropical race 4 in Musa acuminata Pahang. Euphytica 2018, 214, 106. [Google Scholar] [CrossRef]
- Santhosha, H.M.; Indiresh, K.M.; Gopalakrishnan, C.; Singh, T.H. Evaluation of brinjal genotypes against bacterial wilt caused by Ralstonia solanacearum. J. Hortic. Sci. 2015, 10, 74–78. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, J.; Ren, R.; Liu, G.; Yao, X.; Lou, L.; Xu, J.; Yang, X. Proteomic analysis of Fusarium oxysporum-induced mechanism in grafted watermelon seedlings. Front. Plant Sci. 2021, 12, 632758. [Google Scholar] [CrossRef]
- Vanlay, M.; Samnang, S.; Jung, H.-J.; Choe, P.; Kang, K.K.; Nou, I.-S. Interspecific and Intraspecific hybrid rootstocks to improve horticultural traits and soil-borne disease resistance in tomato. Genes 2022, 13, 1468. [Google Scholar] [CrossRef]
- King, S.; Davis, A.; Wenge, L.; Levi, A. Grafting for disease resistance. HortScience 2008, 43, 1673–1676. [Google Scholar] [CrossRef]
- Yetışır, H.; Sari, N.; Yücel, S. Rootstock resistance to Fusarium wilt and effect on watermelon fruit yield and quality. Phytoparasitica 2003, 31, 163–169. [Google Scholar] [CrossRef]
- Mudge, K.; Janick, J.; Scofield, S.; Goldschmidt, E.E. A history of grafting. Hortic. Rev. 2009, 35, 437–493. [Google Scholar]
- Herman, R.; Perl-Treves, R. Characterization and inheritance of a new source of resistance to Fusarium oxysporum f. sp. melonis race 1.2 in Cucumis melo. Plant Dis. 2007, 91, 1180–1186. [Google Scholar] [CrossRef]
- Muramatsu, Y. Problems on vegetable grafting. Shisetu Engei 1981, 10, 48–53. [Google Scholar]
- Ren, Y.; Xu, Q.; Wang, L.; Guo, S.; Shu, S.; Lu, N.; Sun, J. Involvement of metabolic, physiological and hormonal responses in the graft-compatible process of cucumber/pumpkin combinations was revealed through the integrative analysis of mRNA and miRNA expression. Plant Physiol. Biochem. 2018, 129, 368–380. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, S.-R.; Li, H.; Du, N.-S.; Shu, S.; Sun, J. Physiological aspects of compatibility and incompatibility in grafted cucumber seedlings. J. Am. Soc. Hortic. Sci. 2015, 140, 299–307. [Google Scholar] [CrossRef]
- Lü, G.; Guo, S.; Zhang, H.; Geng, L.; Martyn, R.D.; Xu, Y. Colonization of Fusarium wilt-resistant and susceptible watermelon roots by a green-fluorescent-protein-tagged isolate of Fusarium oxysporum f.sp. niveum. J. Phytopathol. 2014, 162, 228–237. [Google Scholar] [CrossRef]
- Martyn, R.; Netzer, D. Resistance to Races 0, 1, and 2 of Fusarium wilt of watermelon in Citrullus sp. PI-296341-FR. HortScience 1991, 26, 429–432. [Google Scholar] [CrossRef]
- Li, J.; Fokkens, L.; van Dam, P.; Rep, M. Related mobile pathogenicity chromosomes in Fusarium oxysporum determine host range on cucurbits. Mol. Plant Pathol. 2020, 21, 761–776. [Google Scholar] [CrossRef]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef]
- Upasani, M.L.; Gurjar, G.S.; Kadoo, N.Y.; Gupta, V.S. Dynamics of colonization and expression of pathogenicity related genes in Fusarium oxysporum f.sp. ciceri during chickpea vascular wilt disease progression. PLoS ONE 2016, 11, e0156490. [Google Scholar] [CrossRef]
- Namisy, A.; Chen, S.Y.; Huang, J.H.; Unartngam, J.; Thanarut, C.; Chung, W.H. Histopathology and quantification of green fluorescent protein-tagged Fusarium oxysporum f. sp. luffae isolate in resistant and susceptible Luffa germplasm. Microbiol. Spectr. 2024, 12, e0312723. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Pathology; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Hilaire, E.; Young, S.A.; Willard, L.H.; McGee, J.D.; Sweat, T.; Chittoor, J.; Guikema, J.A.; Leach, J.E. Vascular defense responses in rice: Peroxidase accumulation in xylem parenchyma cells and xylem wall thickening. Mol. Plant Microbe Interact. 2001, 14, 1411–1419. [Google Scholar] [CrossRef]
- Rioux, D.; Ouellette, G. Barrier zone formation in host and nonhost trees inoculated with Ophiostoma ulmi. I. Anatomy and histochemistry. Can. J. Bot. 1991, 69, 2055–2073. [Google Scholar] [CrossRef]
- Keinath, A.; Hassell, R. Control of Fusarium wilt of watermelon by grafting onto bottle gourd or interspecific hybrid squash despite colonization of rootstocks by Fusarium. Plant Dis. 2014, 98, 255–266. [Google Scholar] [CrossRef]
- Rasool, A.; Mansoor, S.; Bhat, K.; Hassan, G.; Baba, T.R.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; Paray, B.A.; Ahmad, P. Mechanisms underlying graft union formation and rootstock scion interaction in horticultural plants. Front. Plant Sci. 2020, 11, 590847. [Google Scholar] [CrossRef] [PubMed]
- Toscano-Morales, R.; Xoconostle-Cázares, B.; Martínez-Navarro, A.C.; Ruiz-Medrano, R. AtTCTP2 mRNA and protein movement correlates with formation of adventitious roots in tobacco. Plant Signal. Behav. 2016, 11, e1071003. [Google Scholar] [CrossRef] [PubMed]
- Bani, M.; Rubiales, D.; Rispail, N. A detailed evaluation method to identify sources of quantitative resistance to Fusarium oxysporum f. sp. pisi race 2 within a Pisum spp. germplasm collection. Plant Pathol. 2012, 61, 532–542. [Google Scholar] [CrossRef]
- van Dam, P.; de Sain, M.; Ter Horst, A.; van der Gragt, M.; Rep, M. Use of comparative genomics-based markers for discrimination of host specificity in Fusarium oxysporum. Appl. Environ. Microbiol. 2018, 84, e01868-17. [Google Scholar] [CrossRef]
- Flores-Félix, J.D.; Menendez, E.; Mateos, P.; Celador Lera, L.; Rivas, R. Calcofluor white, an alternative to propidium iodide for plant tissues staining in studies of root colonization by fluorescent-tagged Rhizobia. J. Adv. Biol. Biotechnol. 2015, 2, 65–70. [Google Scholar] [CrossRef]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 12: Survival analysis. Crit. Care 2004, 8, 389–394. [Google Scholar] [CrossRef]

| Rootstock Code | Luffa Species | Fomh16 | ||
|---|---|---|---|---|
| MDR x | AUDPC y | Reaction z | ||
| LF1 | L. acutangula | 0.0 ± 0.0 d | 0.0 ± 0.0 c | R |
| LF2 | L. acutangula | 0.0 ± 0.0 d | 0.0 ± 0.0 c | R |
| LF3 | L. acutangula | 0.0 ± 0.0 d | 0.0 ± 0.0 c | R |
| LF5 | L. acutangula | 1.2 ± 0.24 c | 20.5 ± 4.1 b | MR |
| LF6 | L. acutangula | 2.5 ± 0.26 b | 45.9 ± 4.8 b | MS |
| LF10 | L. acutangula | 0.9 ± 0.05 cd | 17.8 ± 0.9 bc | R |
| LF11 | L. acutangula | 1.6 ± 0.18 c | 29.0 ± 3.1 b | MR |
| LF15 | L. acutangula | 0.0 ± 0.0 d | 0.0 ± 0.0 c | R |
| LF16 | L. acutangula | 0.0 ± 0.0 d | 0.0 ± 0.0 c | R |
| La | L. aegyptiaca | 5.0 ± 0.0 a | 87.5 ± 1.4 a | S |
| Rootstock Code | Luffa Species | Fomh16 | Fomo33 | ||||
|---|---|---|---|---|---|---|---|
| MDR x | AUDPC y | Reaction z | MDR | AUDPC | Reaction | ||
| LF1 | L. acutangula | 0.4 ± 0.0 b | 5.4 ± 1.5 cd | R | 0.3 ± 0.3 bc | 4.9 ± 4.9 c | R |
| LF2 | L. acutangula | 0.7 ± 0.7 b | 11.4 ± 11.4 c | R | 0.5 ± 0.3 bc | 7.9 ± 4.0 c | R |
| LF3 | L. acutangula | ND | ND | ND | 0.4 ± 0.7 bc | 5.4 ± 5.4 c | R |
| LF5 | L. acutangula | 1.6 ± 0.3 b | 25.9 ± 5.2 b | MR | 1.7 ± 0.1 b | 17.3 ± 0.2 b | MR |
| LF11 | L. acutangula | 0.3 ± 0.3 b | 4.7 ± 4.7 cd | R | 3.8 ± 0.4 a | 36.2 ± 3.1 a | S |
| LF15 | L. acutangula | 0.0 ± 0.0 b | 0.0 ± 0.0 d | R | 0.0 ± 0.0 c | 0.0 ± 0.0 c | R |
| LF16 | L. acutangula | 0.0 ± 0.0 b | 0.0 ± 0.0 d | R | 0.0 ± 0.0 c | 0.0 ± 0.0 c | R |
| Mc/Mc | M. charantia | 0.0 ± 0.0 b | 0.0 ± 0.0 d | R | 4.3 ± 0.4 a | 38.3 ± 4.9 a | S |
| La/La | L. aegyptiaca | 5.0 ± 0.0 a | 92.2 ± 0.0 a | S | 0.0 ± 0.0 c | 0.0 ± 0.0 c | R |
| Rootstock Code | Fomh16 | Fomo33 | ||
|---|---|---|---|---|
| Rootstock | Scion | Rootstock | Scion | |
| LF1 | + | − | + | − |
| LF2 | + | − | + | − |
| LF3 | + | − | + | − |
| LF5 | + | − | + | + |
| LF11 | + | − | + | + |
| LF15 | +/− | − | +/− | − |
| LF16 | +/− | − | +/− | − |
| Mc/Mc | + | − | + | + |
| La/La | + | + | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namisy, A.; Chen, S.-Y.; Sritongkam, B.; Unartngam, J.; Thanarut, C.; Chung, W.-H. Evaluation of Luffa Rootstocks to Improve Resistance in Bitter Gourd (Momordica charantia L.) Against Fusarium Wilt. Plants 2025, 14, 1168. https://doi.org/10.3390/plants14081168
Namisy A, Chen S-Y, Sritongkam B, Unartngam J, Thanarut C, Chung W-H. Evaluation of Luffa Rootstocks to Improve Resistance in Bitter Gourd (Momordica charantia L.) Against Fusarium Wilt. Plants. 2025; 14(8):1168. https://doi.org/10.3390/plants14081168
Chicago/Turabian StyleNamisy, Ahmed, Shu-Yun Chen, Benjapon Sritongkam, Jintana Unartngam, Chinnapan Thanarut, and Wen-Hsin Chung. 2025. "Evaluation of Luffa Rootstocks to Improve Resistance in Bitter Gourd (Momordica charantia L.) Against Fusarium Wilt" Plants 14, no. 8: 1168. https://doi.org/10.3390/plants14081168
APA StyleNamisy, A., Chen, S.-Y., Sritongkam, B., Unartngam, J., Thanarut, C., & Chung, W.-H. (2025). Evaluation of Luffa Rootstocks to Improve Resistance in Bitter Gourd (Momordica charantia L.) Against Fusarium Wilt. Plants, 14(8), 1168. https://doi.org/10.3390/plants14081168







