Exploring the Interplay of Explant Origin and Culture Density on Olive Micropropagation Efficiency
Abstract
1. Introduction
2. Results
2.1. Morphological Parameters
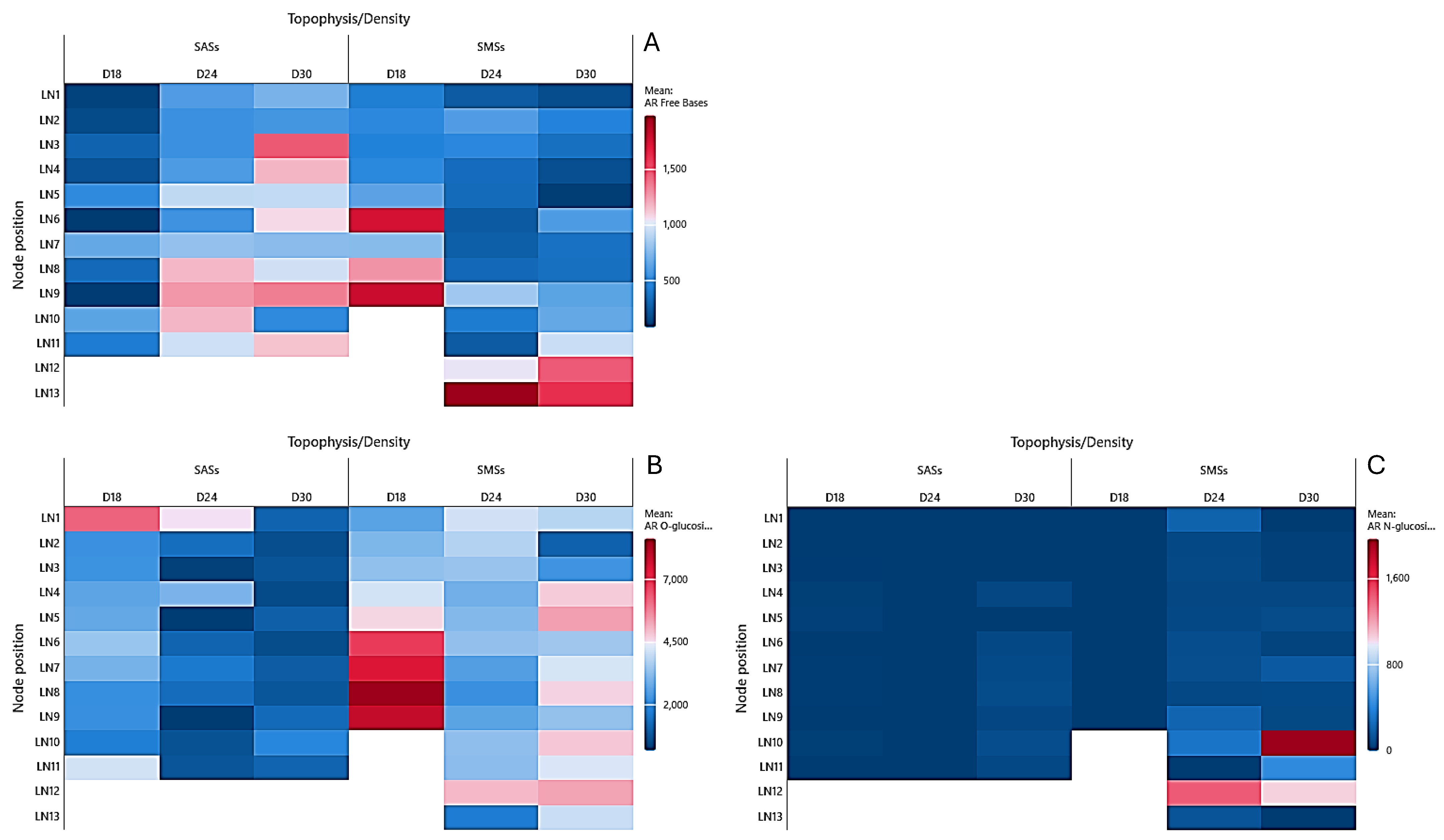
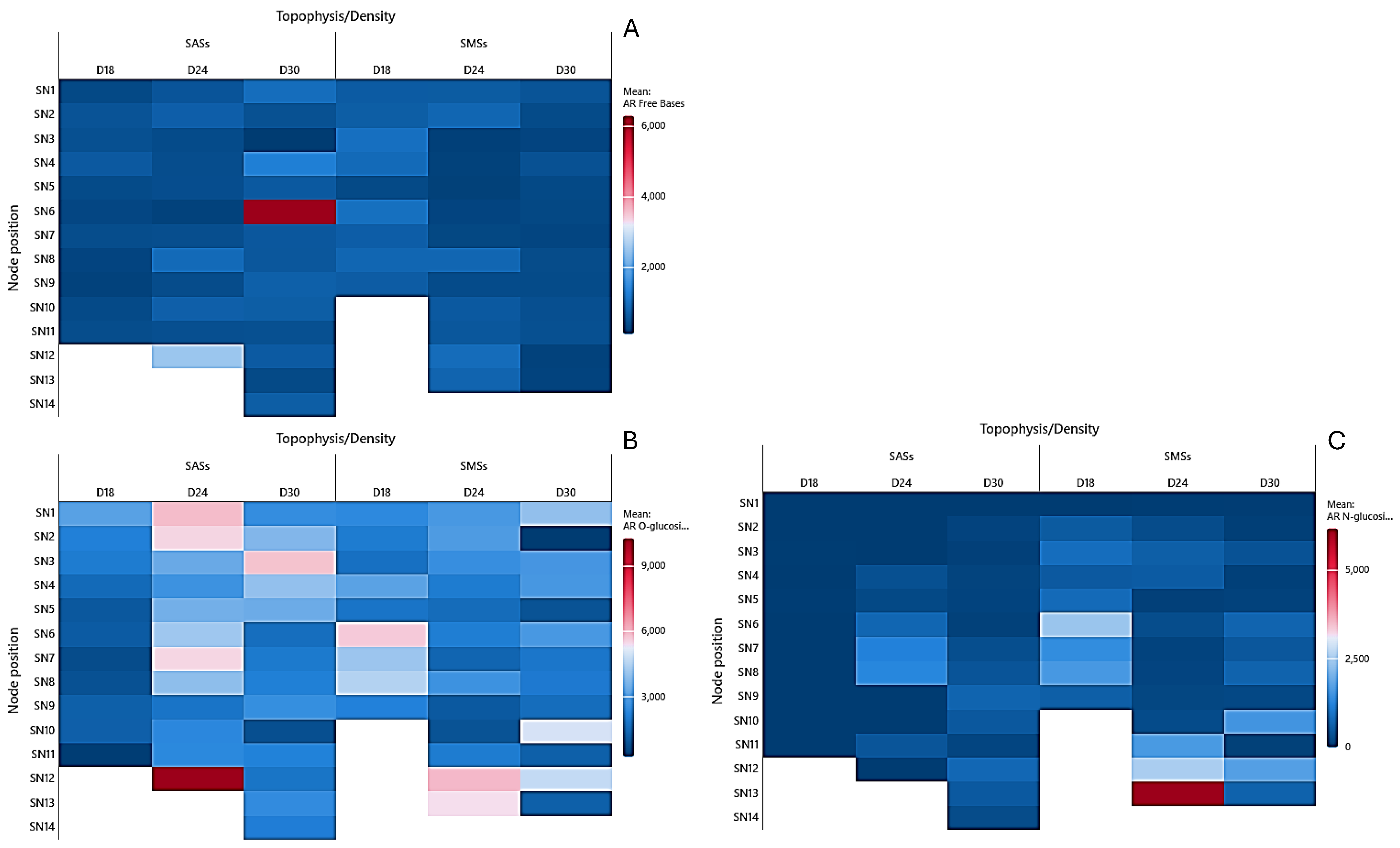
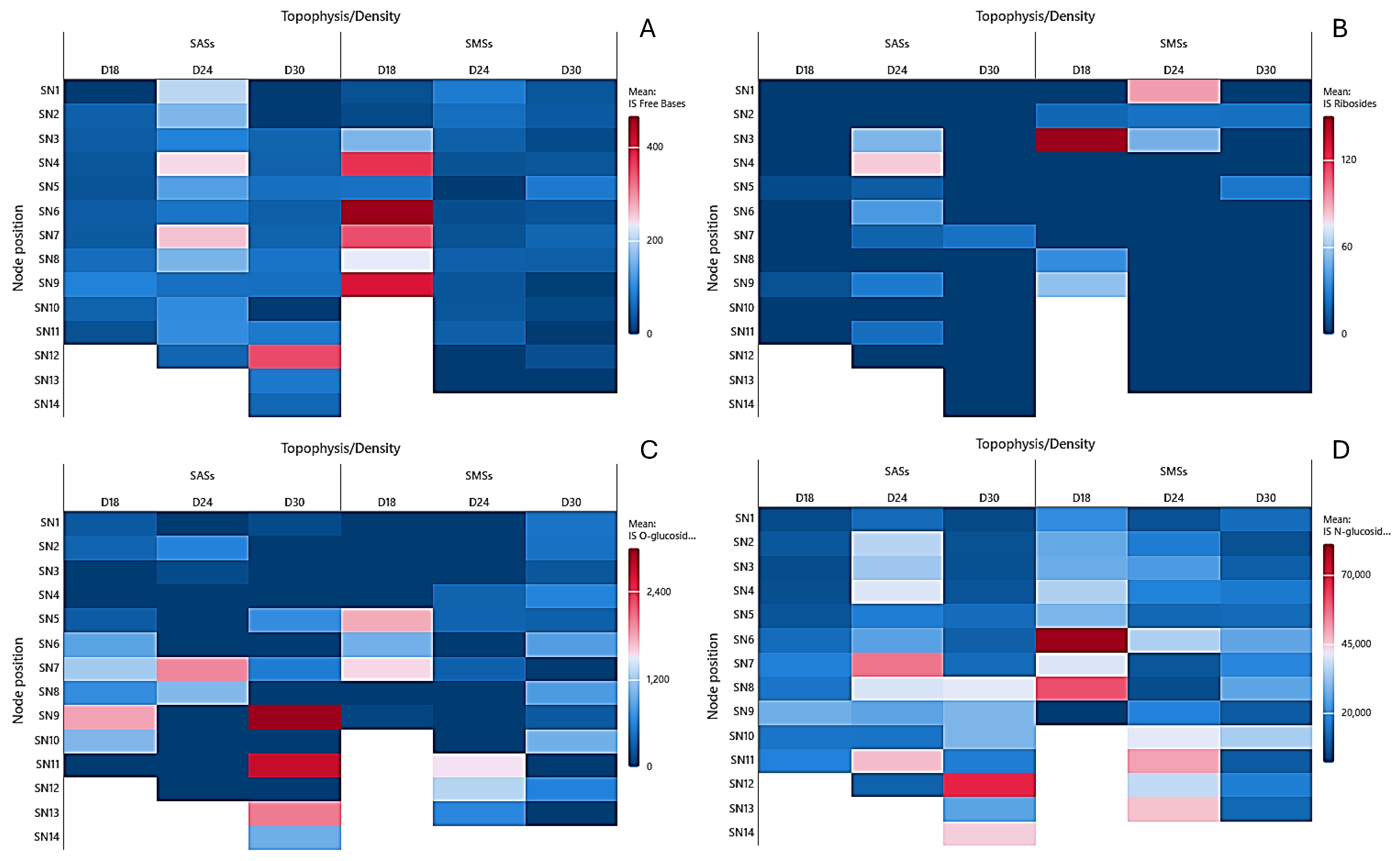
2.2. Cytokinin Analysis
2.2.1. Aromatic Cytokinins
2.2.2. Isoprenoid Cytokinins
3. Discussion
3.1. Morphological Parameters
3.2. Cytokinin Analysis
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dabbou, S.; Dabbou, S.; Selvaggini, R.; Urbani, S.; Taticchi, A.; Servili, M.; Hammami, M. Comparison of the chemical composition and the organoleptic profile of virgin olive oil from two wild and two cultivated Tunisian Olea europaea. Chem. Biodivers. 2011, 8, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Baccouri, B.; Guerfel, M.; Zarrouk, W.; Taamalli, W.; Daoud, D. Wild olive (Olea europaea L.) selection for quality oil production. J. Food Biochem. 2011, 35, 161–176. [Google Scholar]
- Bouarroudj, K.; Tamendjari, A.; Larbat, R. Quality, composition and antioxidant activity of Algerian wild olive (Olea europaea L. subsp. Oleaster) oil. Ind. Crops Prod. 2016, 83, 484–491. [Google Scholar]
- Gagour, J.; El Ghailassi, K.; Ibourki, M.; Sakar, E.H.; Gharby, S. Physicochemical traits of olive fruit and oil from eight Moroccan wild olive (Olea europaea L. Subsp. Oleaster) populations. Biocatal. Agric. Biotechnol. 2024, 56, 103021. [Google Scholar]
- Fanelli, V.; Mascio, I.; Falek, W.; Miazzi, M.M.; Montemurro, C. Current Status of Biodiversity Assessment and Conservation of Wild Olive (Olea europaea L. subsp. europaea var. sylvestris). Plants 2022, 11, 480. [Google Scholar]
- Iraki, M.; Samara, T.; Bouloumpasi, E.; Kadoglidou, K.; Chatzopoulou, P.; Spanos, I. Valuable Nutrients, Aroma Profile, and Functional Bioactives Extracted by Eco-Friendly Extraction Techniques from Wild Olive Fruits (Olea europaea L. var. sylvestris). Processes 2024, 12, 1181. [Google Scholar] [CrossRef]
- Kayel, I.; Essghaier, B.; Benabderrahim, M.A.; Rodríguez-Arcos, R.; Jiménez-Araujo, A.; Guillén-Bejarano, R.; Hannachi, H. Three Mediterranean species from natural plant communities (Ceratonia siliqua, Pistacia lentiscus, and Olea europaea var. sylvestris): Phenolic acids, flavonoids, and biological activities. South Afr. J. Bot. 2024, 175, 620–627. [Google Scholar]
- Baccouri, B.; Mechi, D.; Rajhi, I.; Vertedor, D.M. Tunisian wild olive leaves: Phenolic compounds and antioxidant activity as an important step toward their valorization. Food Anal. Methods 2023, 16, 436–444. [Google Scholar]
- Anwar, P.; Bendini, A.; Gulfraz, M.; Qureshi, R.; Valli, E.; Di Lecce, G.; Saqlan Naqvi, S.M.; Toschi, T.G. Characterization of olive oils obtained from wild olive trees (Olea ferruginea Royle) in Pakistan. Food Res. Int. 2013, 54, 1965–1971. [Google Scholar]
- Bouarroudj-Hamici, K.; Metouchi, S.; Medjkouh-Rezzak, L.; Larbat, R.; Tamendjari, A. Antibacterial Activity of the Phenolic Extract of Wild Virgin Olive Oil In Vitro. Curr. Bioact. Compd. 2022, 18, 45–52. [Google Scholar]
- Lafka, T.-I.; Lazou, A.E.; Sinanoglou, V.J.; Lazos, E.S. Phenolic extracts from wild olive leaves and their potential as edible oils antioxidants. Foods 2013, 2, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Hannachi, H.; Elfalleh, W.; Marzouk, S. Oil, protein, antioxidants and free radical scavenging activity of stone from wild olive trees (Olea europaea L.). Pak. J. Pharm. Sci. 2013, 26, 503–510. [Google Scholar] [PubMed]
- Ghorbel, A.; Wedel, S.; Kallel, I.; Cavinato, M.; Sakavitsi, M.E.; Fakhfakh, J.; Halabalaki, M.; Jansen-Durr, P.; Allouche, N. Extraction yield optimization of Oleaster (Olea europaea var. sylvestris) fruits using response surface methodology, LC/MS profiling and evaluation of its effects on antioxidant activity and autophagy in HFF cells. J. Food Meas. Charact. 2021, 15, 4946–4959. [Google Scholar]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—A review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Carew, J.S.; Kelly, K.R.; Nawrocki, S.T. Autophagy as a target for cancer therapy: New developments. Cancer Manag. Res. 2012, 4, 357–365. [Google Scholar]
- Kamle, M.; Bajpai, A.; Chandra, R.; Kalim, S.; Kumar, R. Somatic embryogenesis for crop improvement. GERF Bull. Biosci. 2011, 2, 54–59. [Google Scholar]
- Shukla, S.; Shukla, S.K. Micropropagation for crop improvement and it’s commercialization potential. In The Potential of Microbes for a Circular Economy; Academic Press: Cambridge, MA, USA, 2024; pp. 271–287. [Google Scholar]
- Allied Market Research. Olive Oil Market by Type and Application: Global Opportunity Analysis and Industry Forecast, 2023–2032. 2024. Available online: https://www.alliedmarketresearch.com/olive-oil-market (accessed on 30 January 2025).
- Belaj, A.; León, L.; Satovic, Z.; de la Rosa, R. Variability of wild olives (Olea europaea subsp. europaea var. sylvestris) analyzed by agro-morphological traits and SSR markers. Sci. Hortic. 2011, 129, 561–569. [Google Scholar]
- Debbabi, O.S.; Amar, F.B.; Rahmani, S.M.; Taranto, F.; Montemurro, C.; Miazzi, M.M. The status of genetic resources and olive breeding in Tunisia. Plants 2022, 11, 1759. [Google Scholar] [CrossRef]
- Brito, G.; Lopes, T.; Loureiro, J.; Rodriguez, E.; Santos, C. Assessment of genetic stability of two micropropagated wild olive species using flow cytometry and microsatellite markers. Trees 2010, 24, 723–732. [Google Scholar] [CrossRef]
- Zacchini, M.; De Agazio, M. Micropropagation of a local olive cultivar for germplasm preservation. Biol. Plant. 2004, 48, 589–592. [Google Scholar]
- Mousavi, S.; Mariotti, R.; Regni, L.; Nasini, L.; Bufacchi, M.; Pandolfi, S.; Baldoni, L.; Proietti, P. The first molecular identification of an olive collection applying standard simple sequence repeats and novel expressed sequence tag markers. Front. Plant Sci. 2017, 8, 1283. [Google Scholar] [CrossRef] [PubMed]
- Lambardi, M.; Fabbri, A.; Micheli, M.; Vitale, A.; Baldoni, L.; Caruso, T.; Famiani, F. Olive Propagation and Nursery. In The Olive Botany and Production; Fabbri, A., Baldoni, L., Caruso, T., Famiani, F., Eds.; CABI: Wallingford, UK, 2023; pp. 228–256. [Google Scholar]
- Bhojwani, S.S.; Dantu, P.K. Plant Tissue Culture: An Introductory Text; Springer: London, UK, 2013. [Google Scholar]
- Rugini, E.; Baldoni, L.; Silvestri, C.; Narváez, I.; Cultrera, N.; Cristofori, M.; Bashir, M.A.; Mousavi, E.; Palomo-Rios, J.; Mercado, F.; et al. Olea europaea olive. In Biotechnology of Fruit and Nut Crops; CAB International: Wallingford, UK, 2020; pp. 343–376. [Google Scholar]
- Antonopoulou, C.; Dimassi, K.; Therios, I.; Chatzissavvidis, C. Does dikegulac affect in vitro shoot proliferation and hyperhydricity incidence in olive explants? Hort. Sci. 2018, 45, 125–130. [Google Scholar] [CrossRef]
- Hamooh, B.T.; Shah, Z.H. In vitro evaluation of shoot induction and proliferation protocol for olive cultivars by assessing morpho-physiologic effects of pre-cooling and growth regulators. Int. J. Biol. 2017, 11, 126–139. [Google Scholar]
- Rkhis, A.C.; Maalej, M.; Drira, N.; Standardi, A. Micropropagation of olive tree Olea europaea L.‘Oueslati’. Turk. J. Agric. For. 2011, 35, 403–412. [Google Scholar] [CrossRef]
- Miller, C.O.; Skoog, F.; Von Saltza, M.H.; Strong, F.M. Kinetin, a cell division factor from deoxyribonucleic acid1. J. Am. Chem. Soc. 1955, 77, 1392. [Google Scholar] [CrossRef]
- Kamínek, M.; Vaněk, T.; Motyka, V. Cytokinin activities of N 6-benzyladenosine derivatives hydroxylated on the side-chain phenyl ring. J. Plant Growth Regul. 1987, 6, 113–120. [Google Scholar] [CrossRef]
- Nisler, J. Cytokinin Properties of Meta-Topolin and Related Compounds. In Meta-Topolin: A Growth Regulator for Plant Biotechnology and Agriculture; Springer: Berlin/Heidelberg, Germany, 2021; pp. 23–30. [Google Scholar]
- Zürcher, E.; Müller, B. Cytokinin synthesis, signaling, and function—Advances and new insights. Int. Rev. Cell Mol. Biol. 2016, 324, 1–38. [Google Scholar]
- Hussain, S.; Chang, J.; Li, J.; Chen, L.; Ahmad, S.; Song, Z.; Zhang, B.; Chen, X. Multifunctional Role of Cytokinin in Horticultural Crops. Int. J. Mol. Sci. 2025, 26, 1037. [Google Scholar] [CrossRef]
- Werner, T.; Schmülling, T. Cytokinin action in plant development. Curr. Opin. Plant Biol. 2009, 12, 527–538. [Google Scholar] [CrossRef]
- Wybouw, B.; De Rybel, B. Cytokinin–a developing story. Trends Plant Sci. 2019, 24, 177–185. [Google Scholar] [CrossRef]
- Mok, M.C. Cytokinins and plant development—An overview. In Cytokinins; Taylor & Francis Group: Milton Park, UK, 2019; pp. 155–166. [Google Scholar]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. In Vitro Cell. Dev. Biol. Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Vylíčilová, H.; Bryksová, M.; Matušková, V.; Doležal, K.; Plíhalová, L.; Strnad, M. Naturally occurring and artificial N9-cytokinin conjugates: From synthesis to biological activity and back. Biomolecules 2020, 10, 832. [Google Scholar] [CrossRef] [PubMed]
- Svolacchia, N.; Sabatini, S. Cytokinins. Curr. Biol. 2023, 33, R10–R13. [Google Scholar] [CrossRef] [PubMed]
- Oshchepkov, M.S.; Kalistratova, A.V.; Savelieva, E.M.; Romanov, G.A.; Bystrova, N.A.; Kochetkov, K.A. Natural and synthetic cytokinins and their applications in biotechnology, agrochemistry and medicine. Russ. Chem. Rev. 2020, 89, 787. [Google Scholar] [CrossRef]
- Letham, D.S. Cytokinins as phytohormones—Sites of biosynthesis, translocation, and function of translocated cytokinin. In Cytokinins; CRC Press: Boca Raton, FL, USA, 2019; pp. 57–80. [Google Scholar]
- Strnad, M. The aromatic cytokinins. Physiol. Plant 1997, 101, 674–688. [Google Scholar] [CrossRef]
- Mok, D.W.S.; Mok, M.C. Cytokinin metabolism and action. Annu. Rev. Plant Biol. 2001, 52, 89–118. [Google Scholar] [CrossRef]
- Lomin, S.N.; Krivosheev, D.M.; Steklov, M.Y.; Arkhipov, D.V.; Osolodkin, D.I.; Schmülling, T.; Romanov, G.A. Plant membrane assays with cytokinin receptors underpin the unique role of free cytokinin bases as biologically active ligands. J. Exp. Bot. 2015, 66, 1851–1863. [Google Scholar] [CrossRef]
- Hoyerová, K.; Hošek, P. New insights into the metabolism and role of cytokinin N-glucosides in plants. Front. Plant Sci. 2020, 11, 741. [Google Scholar] [CrossRef]
- Hou, D.-X.; Fujii, M.; Terahara, N.; Yoshimoto, M. Molecular mechanisms behind the chemopreventive effects of anthocyanidins. BioMed Res. Int. 2004, 2004, 321–325. [Google Scholar] [CrossRef]
- Bajguz, A.; Piotrowska, A. Conjugates of auxin and cytokinin. Phytochemistry 2009, 70, 957–969. [Google Scholar] [CrossRef]
- Argueso, C.T.; Ferreira, F.J.; Kieber, J.J. Environmental perception avenues: The interaction of cytokinin and environmental response pathways. Plant Cell Environ. 2009, 32, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.J.; Hussain, A.; Hamayun, M.; El-Sheikh, M.A.; Elansary, H.O.; Kim, H.Y. Impact of cis-zeatin and lovastatin on antioxidant systems and growth parameters in Zea mays seedlings under phytohormonal crosstalks. J. Plant Interact. 2024, 19, 2327378. [Google Scholar] [CrossRef]
- Teixeira, L.S.; Mota, T.A.; Lopez, D.J.C.; Amorim, V.A.; Almeida, C.S.; Souza, G.A.; Ribeiro, D.M. Cytokinin Biosynthesis Is Affected by Selenium and Nitrate Availabilities to Regulate Shoot and Root Growth in Rice Seedlings. Nitrogen 2024, 5, 191–201. [Google Scholar] [CrossRef]
- Esen, P.O.O. On cyclophysis and topophysis. Silvae Genet. 1978, 27, 5. [Google Scholar]
- Mitchell, R.G.; Zwolinski, J.; Jones, N.B. A review on the effects of donor maturation on rooting and field performance of conifer cuttings. South. Afr. For. J. 2004, 201, 53–63. [Google Scholar] [CrossRef]
- Hung, C.D.; Trueman, S.J. Topophysic effects differ between node and organogenic cultures of the eucalypt Corymbia torelliana × C. citriodora. Plant Cell Tissue Organ Cult. 2011, 104, 69–77. [Google Scholar] [CrossRef]
- Power, A.B.; Dodd, R.S.; Libby, W.J. Cyclophysis and topophysis in coast redwood stecklings. Silvae Genet. 1988, 37, 8–14. [Google Scholar]
- Peer, K.R.; Greenwood, M.S. Maturation, topophysis and other factors in relation to rooting in Larix. Tree Physiol. 2001, 21, 267–272. [Google Scholar] [CrossRef][Green Version]
- Hallé, F.; Oldeman, R.A.A.; Tomlinson, P.B. Tropical Trees and Forests: An Architectural Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Wendling, I.; Brooks, P.R.; Trueman, S.J. Topophysis in Corymbia torelliana × C. citriodora seedlings: Adventitious rooting capacity, stem anatomy, and auxin and abscisic acid concentrations. New For. 2015, 46, 107–120. [Google Scholar] [CrossRef]
- Zalewska, M.; Miler, N.; Wenda-Piesik, A. Effect of in vitro topophysis on the growth, development, and rooting of chrysanthemum explants (Chrysanthemum grandiflorum/Ramat./Kitam). J. Hortic. Sci. Biotechnol. 2010, 85, 362–366. [Google Scholar] [CrossRef]
- Pitekelabou, R.; Aidam, A.V.; Kokou, K. In vitro micropropagation of Nauclea diderrichii (de wild &t. Durand) Merrill: Effect of nodes position on plantlets growth and rooting. Eur. Sci. J. 2015, 11, 21. [Google Scholar]
- Liu, J.; Ke, M.; Sun, Y.; Niu, S.; Zhang, W.; Li, Y. Epigenetic regulation and epigenetic memory resetting during plant rejuvenation. J. Exp. Bot. 2024, 75, 733–745. [Google Scholar] [CrossRef]
- Prabhuling, G.; Sathyanarayana, B.N. Liquid medium culture method for rapid multiplication of banana (Musa acuminata) cv.‘GRAND NAINE’ through tissue culture. Int. J. Plant Sci. 2017, 12, 85–89. [Google Scholar] [CrossRef]
- Hamad, A.M. Effect of Explants Length and Density on In Vitro Rooting of Pineapple Ananas comosus L Merr. Lybian J. Basic Sci. 2021, 5, 75–80. [Google Scholar] [CrossRef]
- Aycan, M.; Kayan, M.; Yildiz, M. The effect of ‘in vitro’ spacing competition on shoot regeneration from cotyledon node explants of Lathyrus chrysanthus Boiss. Aust. J. Crop Sci. 2014, 8, 1019–1023. [Google Scholar]
- Mohamed, F.H.; Abdel-Hamid, K.E.; Omar, G.F.; El-Safty, B.A. In Vitro Microtuberization of Potato: Effect of Explant Density, Source, and Genotype. Arab Univ. J. Agric. Sci. 2017, 25, 243–257. [Google Scholar] [CrossRef]
- Yildiz, M.; Sağlık, Ç.; Kahramanoğulları, C.T.; Erkiliç, E.G. The effect of in vitro competition on shoot regeneration from hypocotyl explants of Linum usitatissimum. Turk. J. Bot. 2011, 35, 211–218. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Kozai, T. Environmental effects on the growth of plantlets in micropropagation. Environ. Control Biol. 1998, 36, 59–75. [Google Scholar] [CrossRef]
- El Boullani, R.; Lagram, K.; El Mousadik, A.; Serghini, M.A. Effect of explants density and size on the in vitro proliferation and growth of separated shoots of globe artichoke (Cynara cardunculus var. scolymus L.). J. Mater. Environ. Sci. 2017, 8, 2469–2473. [Google Scholar]
- Chun, Y.W.; Hall, R.B.; Stephens, L.C. Influences of medium consistency and shoot density on in vitro shoot proliferation of Populus alba × P. grandidentata. Plant Cell Tissue Organ Cult. 1986, 5, 179–185. [Google Scholar] [CrossRef]
- Yildiz, M. Evaluation of the effect of in vitro stress and competition on tissue culture response of flax. Biol. Plant. 2011, 55, 541–544. [Google Scholar]
- Tábori, K.; Ferenczy, A.; Dobránszki, J. Effects of culture density on growth and in vitro tuberization capacity of potato plantlets. Acta Agron. Hung. 2000, 48, 185–189. [Google Scholar]
- Li, W.; Pang, S.; Lu, Z.; Jin, B. Function and mechanism of WRKY transcription factors in abiotic stress responses of plants. Plants 2020, 9, 1515. [Google Scholar] [CrossRef] [PubMed]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ. 2019, 42, 998–1018. [Google Scholar]
- Mughal, N.; Shoaib, N.; Chen, J.; He, Y.; Fu, M.; Li, X.; He, Y.; Gua, J.; Deng, J.; Yang, W.; et al. Adaptive roles of cytokinins in enhancing plant resilience and yield against environmental stressors. Chemosphere 2024, 364, 143189. [Google Scholar]
- Caers, M.; Rudelsheim, P.; Van Onckelen, H.; Horemans, S. Effect of heat stress on photosynthetic activity and chloroplast ultrastructure in correlation with endogenous cytokinin concentration in maize seedlings. Plant Cell Physiol. 1985, 26, 47–52. [Google Scholar]
- Liu, X.; Huang, B. Cytokinin effects on creeping bentgrass response to heat stress: II. Leaf senescence and antioxidant metabolism. Crop Sci. 2002, 42, 466–472. [Google Scholar]
- Chen, C.; Chen, J.-J. Measurement of gas exchange rates in plant tissue culture vessels. Plant Cell Tissue Organ Cult. 2002, 71, 103–109. [Google Scholar]
- Jackson, M.B.; Abbott, A.J.; Belcher, A.R.; Hall, K.C.; Butler, R.; Cameron, J. Ventilation in plant tissue cultures and effects of poor aeration on ethylene and carbon dioxide accumulation, oxygen depletion and explant development. Ann. Bot. 1991, 67, 229–237. [Google Scholar]
- Kumar, P.P.; Reid, D.M.; Thorpe, T.A. The role of ethylene and carbon dioxide in differentiation of shoot buds in excised cotyledons of Pinus radiata in vitro. Physiol. Plant. 1987, 69, 244–252. [Google Scholar]
- Heuser, C.W.; Heinemann, P.H. Micropropagation: Effects of ventilation and carbon dioxide level on Rhododendron ‘PJM’. Trans. ASAE 1989, 32, 348–0352. [Google Scholar]
- Lentini, Z.; Mussell, H.; Mutschler, M.A.; Earle, E.D. Ethylene generation and reversal of ethylene effects during development in vitro of rapid-cycling Brassica campestris L. Plant Sci. 1988, 54, 75–81. [Google Scholar] [CrossRef]
- Vanderschaeghe, A.M.; Debergh, P.C. Technical aspects of the control of the relative humidity in tissue culture containers. Meded. Van. Fac. Landbouwwet. Rijksuniv. Gent 1987, 52, 1429–1437. [Google Scholar]
- Chen, C. Development of a heat transfer model for plant tissue culture vessels. Biosyst. Eng. 2003, 85, 67–77. [Google Scholar] [CrossRef]
- Majada, J.P.; Sierra, M.I.; Sanchez-Tames, R. Air exchange rate affects the in vitro developed leaf cuticle of carnation. Sci. Hortic. 2001, 87, 121–130. [Google Scholar] [CrossRef]
- Fatma, M.; Asgher, M.; Iqbal, N.; Rasheed, F.; Sehar, Z.; Sofo, A.; Khan, N.A. Ethylene signaling under stressful environments: Analyzing collaborative knowledge. Plants 2022, 11, 2211. [Google Scholar] [CrossRef]
- Dubois, M. Time After Time: The Chronology of Photosynthesis Inhibition by Ethylene; Oxford University Press USA: New York, NY, USA, 2024. [Google Scholar]
- Love, J.; Björklund, S.; Vahala, J.; Hertzberg, M.; Kangasjärvi, J.; Sundberg, B. Ethylene is an endogenous stimulator of cell division in the cambial meristem of Populus. Proc. Natl. Acad. Sci. USA 2009, 106, 5984–5989. [Google Scholar] [CrossRef]
- Zwack, P.J.; Rashotte, A.M. Interactions between cytokinin signalling and abiotic stress responses. J. Exp. Bot. 2015, 66, 4863–4871. [Google Scholar] [CrossRef]
- Hansen, H.; Dörffling, K. Root-derived trans-zeatin riboside and abscisic acid in drought-stressed and rewatered sunflower plants: Interaction in the control of leaf diffusive resistance? Funct. Plant Biol. 2003, 30, 365–375. [Google Scholar] [CrossRef]
- Pospisilova, J.; Vagner, M.; Malbeck, J.; Travnickova, A.; Batkova, P. Interactions between abscisic acid and cytokinins during water stress and subsequent rehydration. Biol. Plant. 2005, 49, 533–540. [Google Scholar] [CrossRef]
- Alvarez, S.; Marsh, E.L.; Schroeder, S.G.; Schachtman, D.P. Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ. 2008, 31, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Kudoyarova, G.R.; Vysotskaya, L.B.; Cherkozyanova, A.; Dodd, I.C. Effect of partial rootzone drying on the concentration of zeatin-type cytokinins in tomato (Solanum lycopersicum L.) xylem sap and leaves. J. Exp. Bot. 2007, 58, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Albacete, A.; Ghanem, M.E.; Martínez-Andújar, C.; Acosta, M.; Sánchez-Bravo, J.; Martínez, V.; Lutts, S.; Dodd, I.; Pérez-Alfocea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.E.; Albacete, A.; Smigocki, A.C.; Frébort, I.; Pospíšilová, H.; Martínez-Andújar, C.; Acosta, M.; Sanchez-Bravo, J.; Lutts, S.; Dodd, I.; et al. Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2011, 62, 125–140. [Google Scholar] [CrossRef]
- Tekaya, M.; Dabbaghi, O.; Guesmi, A.; Attia, F.; Chehab, H.; Khezami, L.; Algathami, F.K.; Ben Hamadi, N.; Hammami, M.; Els Prinsen, E.; et al. Arbuscular mycorrhizas modulate carbohydrate, phenolic compounds and hormonal metabolism to enhance water deficit tolerance of olive trees (Olea europaea). Agric. Water Manag. 2022, 274, 107947. [Google Scholar] [CrossRef]
- Grira, M.; Prinsen, E.; Werbrouck, S.P.O. The effect of topophysis on the in vitro development of Handroanthus guayacan and on its metabolism of meta-topolin riboside. Plants 2023, 12, 3577. [Google Scholar] [CrossRef]
- Werbrouck, S.P.O.; Strnad, M.; Van Onckelen, H.A.; Debergh, P. Meta-topolin, an alternative to benzyladenine in tissue culture? Physiol. Plant. 1996, 98, 291–297. [Google Scholar] [CrossRef]





| Number of Shoots | Length of Shoots (cm) | Number of Nodes/Plant | Weight of Callus (g) | Length of Internodes (cm) | |
|---|---|---|---|---|---|
| D18 | 1.5 ± 0.2 b | 2.1 ± 0.08 a | 7.2 ± 0.2 b | 0.18 ± 0.01 b | 0.4 ± 0.09 a |
| D24 | 2.0 ± 0.1 a | 2 ± 0.05 a | 10.4 ± 0.01 a | 0.22 ± 0.01 ab | 0.35 ± 0.1 b |
| D30 | 2.1 ± 0.1 a | 1.9 ± 0.02 a | 11.3 ± 0.01 a | 0.26 ± 0.01 a | 0.34 ± 0.09 b |
| Number of Shoots | Length of Shoots (cm) | Number of Nodes/Plant | Weight of Callus (g) | Length of Internodes (cm) | |
|---|---|---|---|---|---|
| D18 | 2.2 ± 0.02 b | 1.5 ± 0.05 b | 10.7 ± 1.1 c | 0.16 ± 0.02 c | 0.3 ± 0.08 a |
| D24 | 3 ± 0.01 a | 1.6 ± 0.06 ab | 14.5 ± 1.1 b | 0.27 ± 0.025 b | 0.3 ± 0.06 a |
| D30 | 3.5 ± 0.01 a | 1.7 ± 0.01 a | 17.3 ± 0.9 a | 0.38 ± 0.03 a | 0.3 ± 0.07 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grira, M.; Rabaaoui, A.; Prinsen, E.; Werbrouck, S. Exploring the Interplay of Explant Origin and Culture Density on Olive Micropropagation Efficiency. Plants 2025, 14, 1170. https://doi.org/10.3390/plants14081170
Grira M, Rabaaoui A, Prinsen E, Werbrouck S. Exploring the Interplay of Explant Origin and Culture Density on Olive Micropropagation Efficiency. Plants. 2025; 14(8):1170. https://doi.org/10.3390/plants14081170
Chicago/Turabian StyleGrira, Maroua, Amal Rabaaoui, Els Prinsen, and Stefaan Werbrouck. 2025. "Exploring the Interplay of Explant Origin and Culture Density on Olive Micropropagation Efficiency" Plants 14, no. 8: 1170. https://doi.org/10.3390/plants14081170
APA StyleGrira, M., Rabaaoui, A., Prinsen, E., & Werbrouck, S. (2025). Exploring the Interplay of Explant Origin and Culture Density on Olive Micropropagation Efficiency. Plants, 14(8), 1170. https://doi.org/10.3390/plants14081170






