Evaluation of Cannabis sativa L. Callus Extract as a Novel Cosmetic Ingredient with Dual Anti-Inflammatory and Antioxidant Effects
Abstract
1. Introduction
2. Materials and Methods
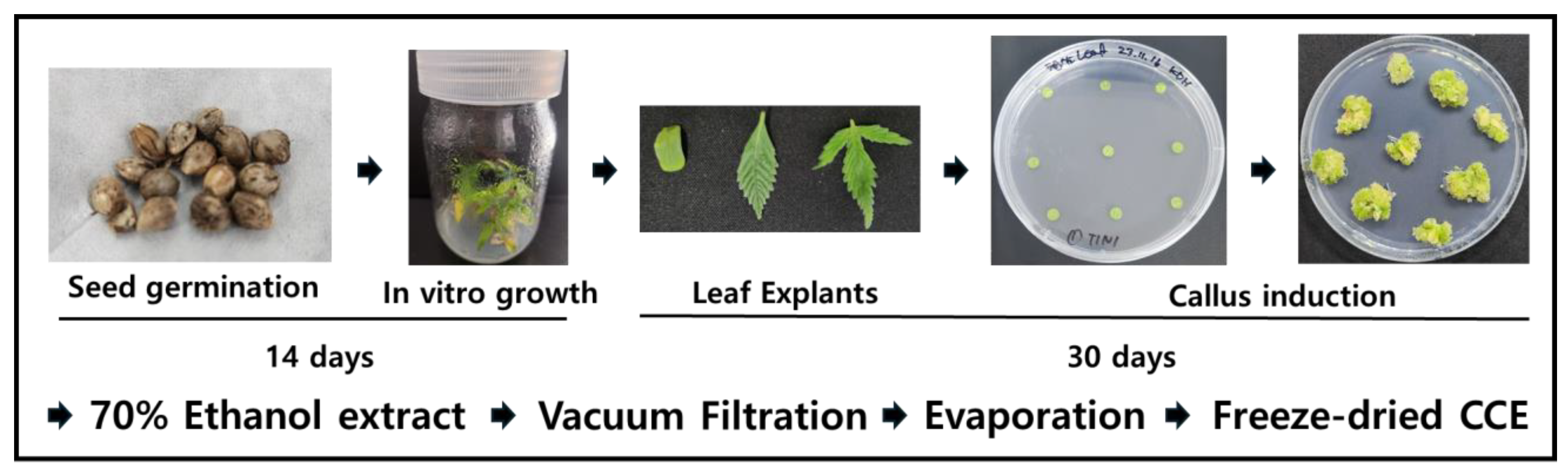
2.1. Preparation of Callus Induction Medium
2.2. Cultivation of Aseptic C. sativa Plants In Vitro
2.3. Callus Induction Conditions
2.4. Preparation of the Callus Extract
2.5. DPPH Antioxidant Assay
2.6. Cell Culture and Treatment
2.7. Cell Viability Assay
2.8. Nitrite Determination
2.9. Western Blot
2.10. Immunofluorescence Staining
2.11. Quantitative Real-Time Polymerase Chain Reaction
2.12. ELISA Analysis
2.13. HPLC Profile Finerprinting Analysis of CCE
2.14. Statistical Analysis
3. Results
3.1. DPPH Radical Scavenging Activity of C. sativa Callus Extract
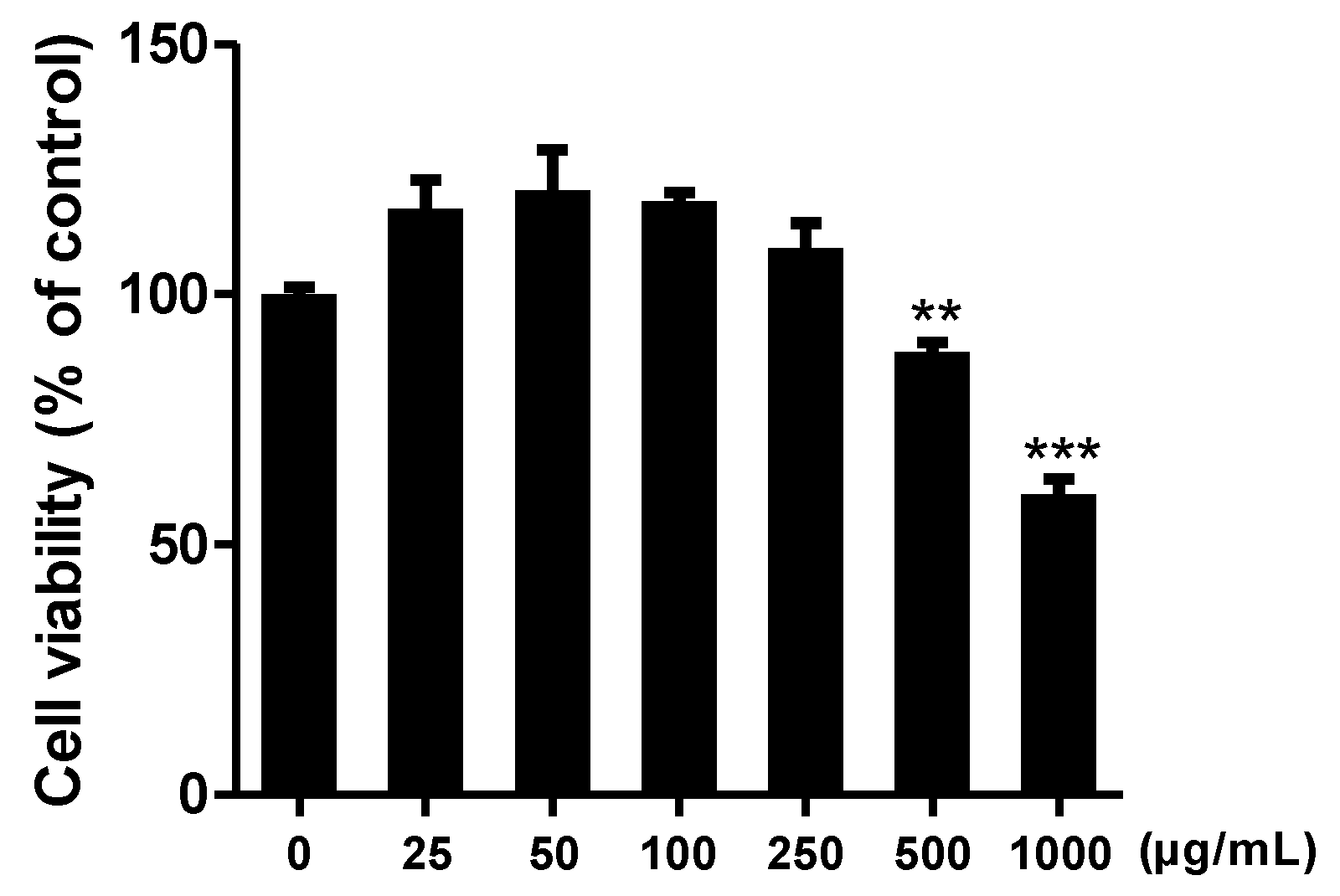
3.2. Effect of C. sativa Callus Extract on Raw 264.7 Cell Viability
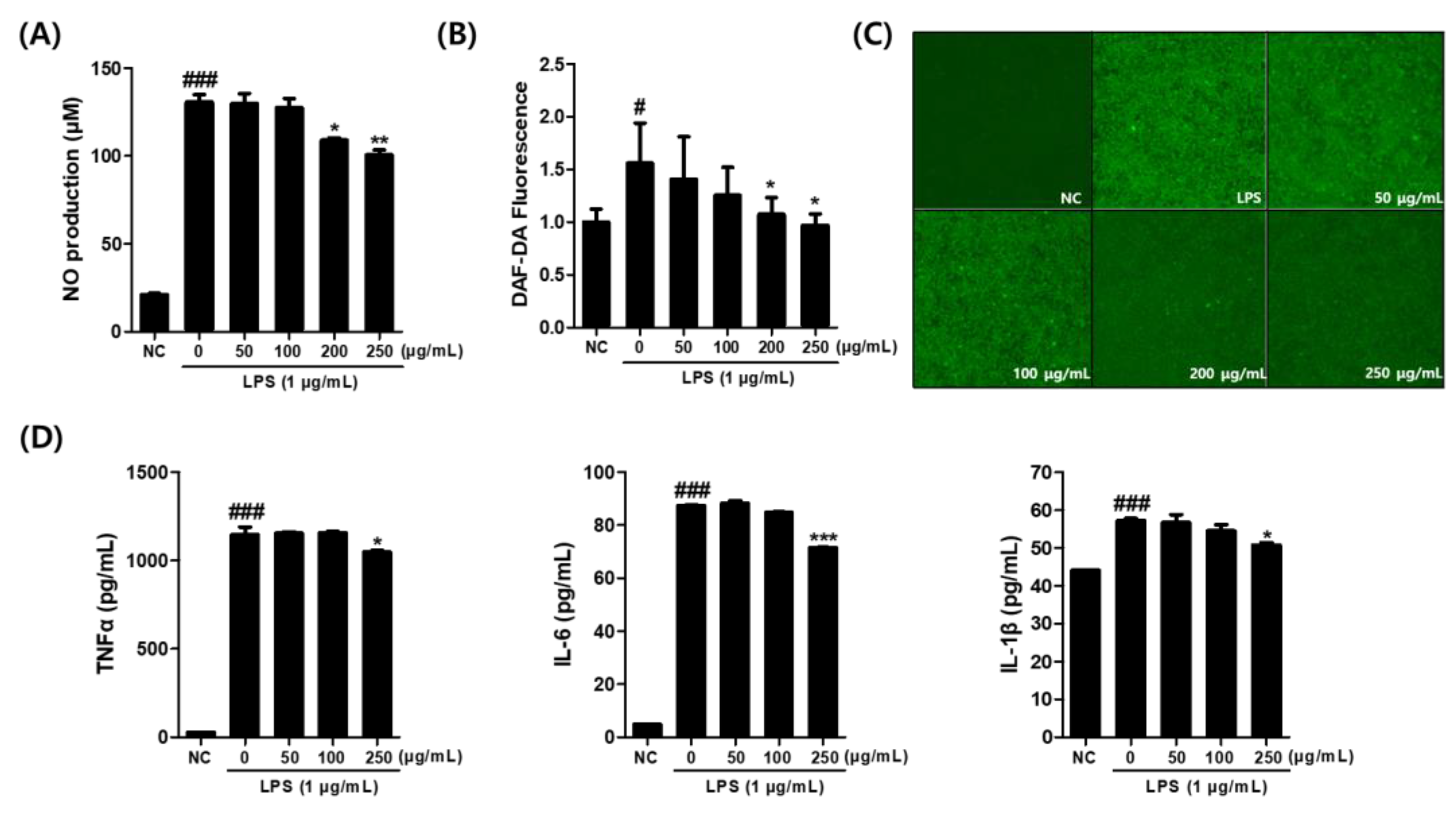
3.3. Effects of C. sativa Callus Extract on the NO and Pro-Inflammatory Cytokine Production in LPS-Stimulated Macrophages
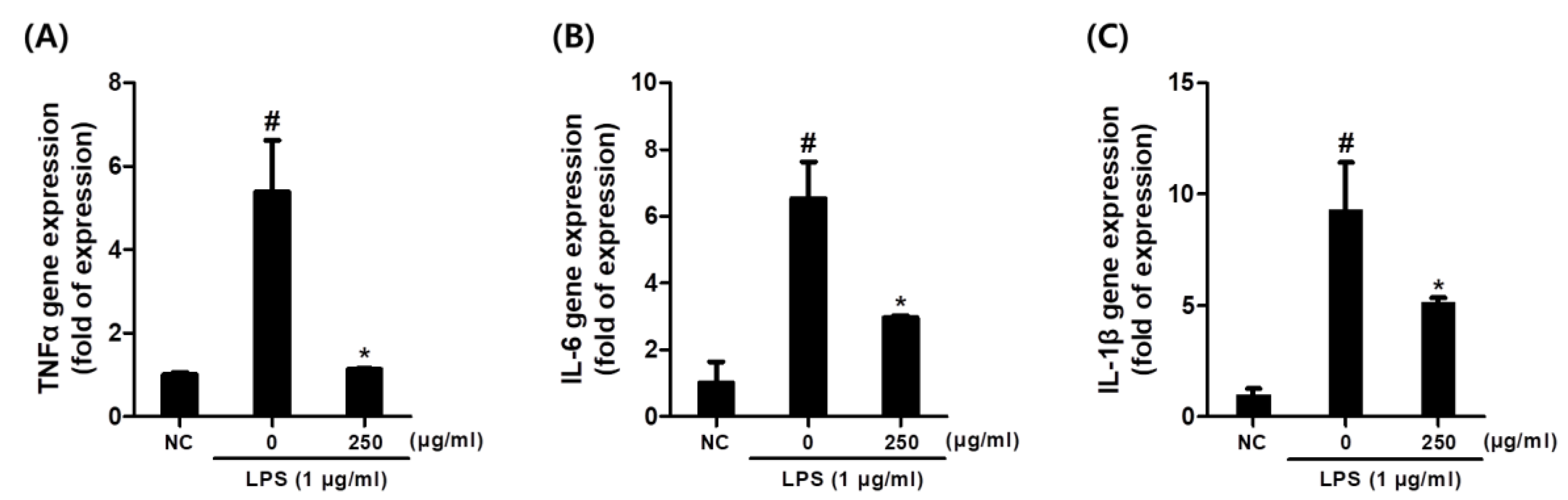
3.4. Effects of C. sativa Callus Extract on the Expression of Inflammatory Cytokine Gene Expression Levels in LPS-Stimulated Macrophages
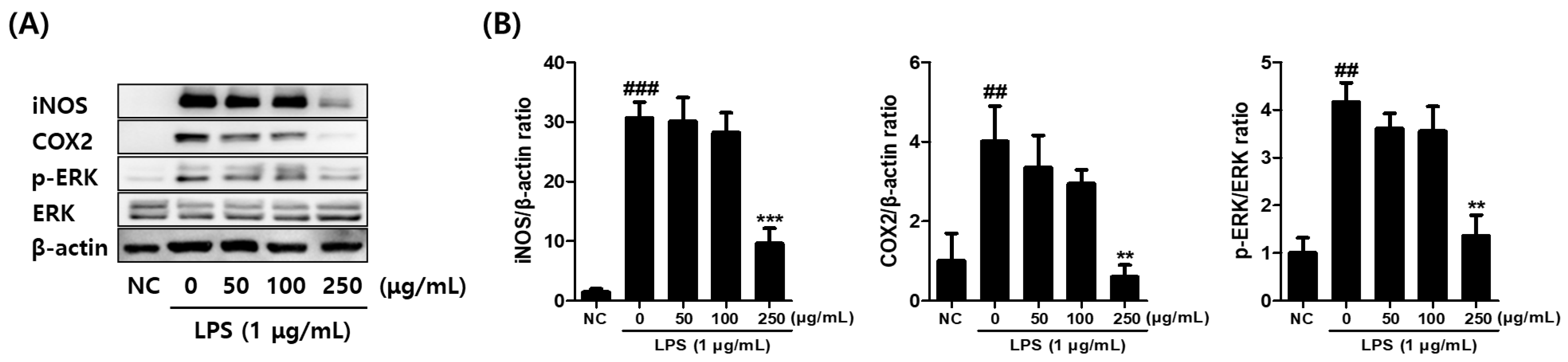
3.5. Effects of C. sativa Callus Extract on the Inflammatory Mediator Protein Levels in LPS-Stimulated Macrophages
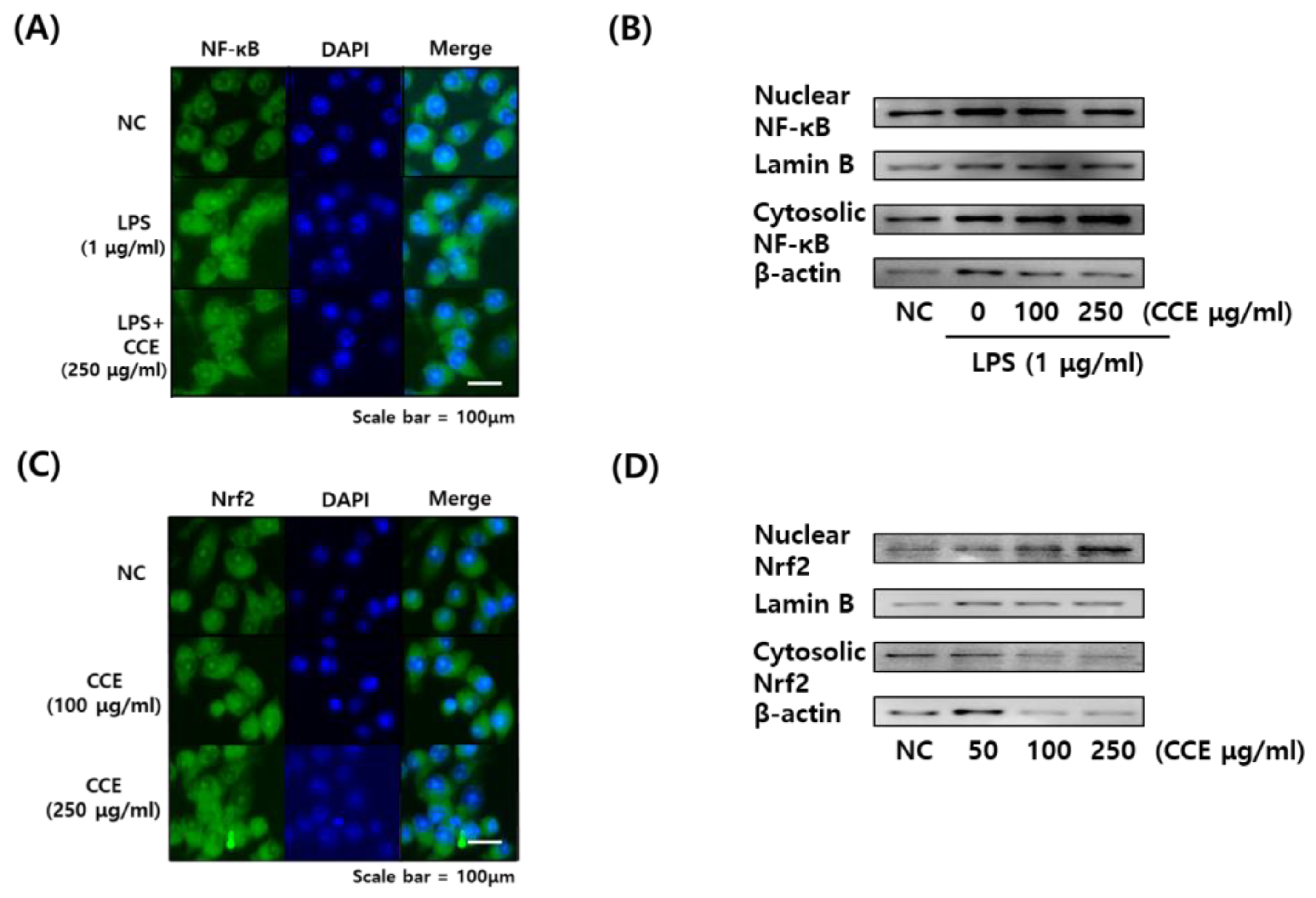
3.6. Effects of C. sativa Callus Extract on the NF-κB and NRF2 Translocation in LPS-Stimulated Macrophages
3.7. HPLC Fingerprinting Analysis of CCE with Cannabinoids
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yeoman, M.; Aitchison, P. Growth patterns in tissue (callus) cultures. In Plant Tissue and Cell Culture; University of California Press: Oakland, CA, USA, 1973; Volume 11. [Google Scholar]
- Neumann, K.-H.; Kumar, A.; Imani, J.; Neumann, K.-H.; Kumar, A.; Imani, J. Callus cultures. In Plant Cell and Tissue Culture—A Tool in Biotechnology: Basics and Application; Springer: Berlin/Heidelberg, Germany, 2020; pp. 25–59. [Google Scholar]
- Malik, S.I.; Rashid, H.; Yasmin, T.; Minhas, N.M. Plant regeneration by somatic embryogenesis from callus of mature seed explants of bread wheat (Triticum aestivum L.). Pak. J. Bot. 2004, 36, 629–634. [Google Scholar]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant callus: Mechanisms of induction and repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [PubMed]
- Mohajer, S.; Taha, R.M.; Khorasani, A.; Yaacob, J.S. Induction of different types of callus and somatic embryogenesis in various explants of Sainfoin (‘Onobrychis sativa’). Aust. J. Crop Sci. 2012, 6, 1305–1313. [Google Scholar]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Sonkar, N.; Shukla, P.K.; Misra, P. Plant hairy roots as biofactory for the production of industrial metabolites. In Plants as Bioreactors for Industrial Molecules; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 273–297. [Google Scholar]
- Salih, A.M.; Al-Qurainy, F.; Khan, S.; Tarroum, M.; Nadeem, M.; Shaikhaldein, H.O.; Alabdallah, N.M.; Alansi, S.; Alshameri, A. Mass propagation of Juniperus procera Hoechst. Ex Endl. From seedling and screening of bioactive compounds in shoot and callus extract. BMC Plant Biol. 2021, 21, 1–13. [Google Scholar]
- Sánchez-Ramos, M.; Bahena, S.M.; Romero-Estrada, A.; Bernabé-Antonio, A.; Cruz-Sosa, F.; Gonzálesssz-Christen, J.; Acevedo-Fernández, J.J.; Perea-Arango, I.; Alvarez, L. Establishment and phytochemical analysis of a callus culture from Ageratina pichinchensis (Asteraceae) and its anti-inflammatory activity. Molecules 2018, 23, 1258. [Google Scholar] [CrossRef]
- Ali, A.M.A.; El-Nour, M.E.M.; Yagi, S.M. Total phenolic and flavonoid contents and antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. J. Genet. Eng. Biotechnol. 2018, 16, 677–682. [Google Scholar]
- Menbari, A.; Bahramnejad, B.; Abuzaripoor, M.; Shahmansouri, E.; Zarei, M.A. Establishment of callus and cell suspension cultures of Granny Smith apple fruit and antityrosinase activity of their extracts. Sci. Hortic. 2021, 286, 110222. [Google Scholar]
- Asadollahi, L.; Abbaspour-Ravasjani, S.; Kim, K.A.; Maghsoodi, M.; Hamishehkar, H.; Kosari-Nasab, M.; Kim, K.H. Rice (Oryza sativa) Stem Cells as a Novel Promising Active Ingredient with Anti-Proliferative Effects for Potential Skin Cancer Prevention and Skin Whitening Activity. Foods 2024, 13, 2803. [Google Scholar] [CrossRef]
- Wang, J.W.; Wu, J.Y. Effective elicitors and process strategies for enhancement of secondary metabolite production in hairy root cultures. In Biotechnology of Hairy Root Systems; Springer: Berlin/Heidelberg, Germany, 2013; pp. 55–89. [Google Scholar]
- Georgiev, V.; Slavov, A.; Vasileva, I.; Pavlov, A. Plant cell culture as emerging technology for production of active cosmetic ingredients. Eng. Life Sci. 2018, 18, 779–798. [Google Scholar]
- Jiao, Q.; Zhi, L.; You, B.; Wang, G.; Wu, N.; Jia, Y. Skin homeostasis: Mechanism and influencing factors. J. Cosmet. Dermatol. 2024, 23, 1518–1526. [Google Scholar]
- Wagener, F.A.; Carels, C.E.; Lundvig, D.M. Targeting the redox balance in inflammatory skin conditions. Int. J. Mol. Sci. 2013, 14, 9126–9167. [Google Scholar] [CrossRef]
- Condrò, G.; Guerini, M.; Castello, M.; Perugini, P. Acne vulgaris, atopic dermatitis and rosacea: The Role of the Skin Microbiota—A Review. Biomedicines 2022, 10, 2523. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [PubMed]
- Thiele, J.J.; Dreher, F.; Packer, L. Antioxidant defense systems in skin. J. Toxicol. Cutan. Ocul. Toxicol. 2002, 21, 119–160. [Google Scholar]
- Bell, S.; Degitz, K.; Quirling, M.; Jilg, N.; Page, S.; Brand, K. Involvement of NF-κB signalling in skin physiology and disease. Cell. Signal. 2003, 15, 1–7. [Google Scholar] [PubMed]
- Boo, Y.C. Natural Nrf2 modulators for skin protection. Antioxidants 2020, 9, 812. [Google Scholar] [CrossRef]
- Hu, X.; Chen, M.; Nawaz, J.; Duan, X. Regulatory Mechanisms of Natural Active Ingredients and Compounds on Keratinocytes and Fibroblasts in Mitigating Skin Photoaging. Clin. Cosmet. Investig. Dermatol. 2024, 17, 1943–1962. [Google Scholar]
- Fuller, B. Role of PGE-2 and other inflammatory mediators in skin aging and their inhibition by topical natural anti-inflammatories. Cosmetics 2019, 6, 6. [Google Scholar] [CrossRef]
- Barbulova, A.; Colucci, G.; Apone, F. New trends in cosmetics: By-products of plant origin and their potential use as cosmetic active ingredients. Cosmetics 2015, 2, 82–92. [Google Scholar] [CrossRef]
- Xie, Z.; Mi, Y.; Kong, L.; Gao, M.; Chen, S.; Chen, W.; Meng, X.; Sun, W.; Chen, S.; Xu, Z. Cannabis sativa: Origin and history, glandular trichome development, and cannabinoid biosynthesis. Hortic. Res. 2023, 10, uhad150. [Google Scholar] [CrossRef] [PubMed]
- Ryz, N.R.; Remillard, D.J.; Russo, E.B. Cannabis roots: A traditional therapy with future potential for treating inflammation and pain. Cannabis Cannabinoid Res. 2017, 2, 210–216. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, C.M.L.; Caetano, T.T.V.; Campos, F.K.; Gandra, V.M.; Alves, F.H.F.; Stein, V.C. Cannabis sativa L. in the cosmeceutical industry: Prospects and biotechnological approaches for metabolite improvement. S. Afr. J. Bot. 2023, 161, 171–179. [Google Scholar] [CrossRef]
- Raihan, A.; Bijoy, T.R. A review of the industrial use and global sustainability of Cannabis sativa. Glob. Sustain. Res. 2023, 2, 1–29. [Google Scholar] [CrossRef]
- Martins, A.M.; Gomes, A.L.; Vilas Boas, I.; Marto, J.; Ribeiro, H.M. Cannabis-based products for the treatment of skin inflammatory diseases: A timely review. Pharmaceuticals 2022, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Mnekin, L.; Ripoll, L. Topical use of Cannabis sativa L. Biochemicals. Cosmetics 2021, 8, 85. [Google Scholar] [CrossRef]
- Manosroi, A.; Chankhampan, C.; Kietthanakorn, B.O.; Ruksiriwanich, W.; Chaikul, P.; Boonpisuttinant, K.; Sainakham, M.; Manosroi, W.; Tangjai, T.; Manosroi, J. Pharmaceutical and cosmeceutical biological activities of hemp (Cannabis sativa L. var. sativa) leaf and seed extracts. Chiang Mai J. Sci 2019, 46, 180–195. [Google Scholar]
- Lee, K.W.; Park, S.; Park, S.I.; Shin, M.S. Cosmetic Efficacy of Supercritical Cannabis sativa Seed Extracts and Enhancement of Skin Permeation. J. Convergence Cult. Technol. 2021, 7, 683–691. [Google Scholar]
- Zagórska-Dziok, M.; Bujak, T.; Ziemlewska, A.; Nizioł-Łukaszewska, Z. Positive effect of Cannabis sativa L. herb extracts on skin cells and assessment of cannabinoid-based hydrogels properties. Molecules 2021, 26, 802. [Google Scholar] [CrossRef]
- Choi, W.-S.; Shin, P.-G.; Lee, J.-H.; Kim, G.-D. The regulatory effect of veratric acid on NO production in LPS-stimulated RAW264. 7 macrophage cells. Cell. Immunol. 2012, 280, 164–170. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, W.; Zhang, X.; Chen, X. Neocryptotanshinone inhibits lipopolysaccharide-induced inflammation in RAW264. 7 macrophages by suppression of NF-κB and iNOS signaling pathways. Acta Pharm. Sin. B 2015, 5, 323–329. [Google Scholar] [PubMed]
- Espinosa-Leal, C.A.; Garcia-Lara, S. Current methods for the discovery of new active ingredients from natural products for cosmeceutical applications. Planta Med. 2019, 85, 535–551. [Google Scholar]
- Berthon, J.-Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.-P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free. Radic. Res. 2017, 51, 555–567. [Google Scholar]
- Barbulova, A.; Apone, F.; Colucci, G. Plant cell cultures as source of cosmetic active ingredients. Cosmetics 2014, 1, 94–104. [Google Scholar] [CrossRef]
- Moon, S.H.; Kim, E.; Kim, H.-I.; Kim, S.-Y.; Seo, H.-H.; Lee, J.H.; Lee, M.-S.; Lee, S.-K.; Moh, S.H.; Bae, S. Skin-whitening effect of a callus extract of Nelumbo nucifera isolate Haman. Plants 2023, 12, 3923. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Tollenaere, M.D.; Sennelier, B.; Lambert, C.; Durduret, A.; Kim, S.-Y.; Seo, H.-H.; Lee, J.-H.; Scandolera, A.; Reynaud, R. Analysis of Active Components and Transcriptome of Freesia refracta Callus Extract and Its Effects against Oxidative Stress and Wrinkles in Skin. Int. J. Mol. Sci. 2024, 25, 8150. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.T.; Jung, H.S.; Cho, M.J.; Song, M.Y.; Moh, S.H.; Seo, H.H. Effect of Artemisia annua Linne callus induced by plant cell culture technology on wound healing. J. Korea Acad. Ind. Coop. Soc. 2014, 15, 5628–5636. [Google Scholar]
- di Giacomo, V.; Recinella, L.; Chiavaroli, A.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Politi, M.; Antolini, M.D.; Acquaviva, A. Metabolomic profile and antioxidant/anti-inflammatory effects of industrial hemp water extract in fibroblasts, keratinocytes and isolated mouse skin specimens. Antioxidants 2021, 10, 44. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Fumagalli, M.; Pacchetti, B.; Piazza, S.; Magnavacca, A.; Khalilpour, S.; Melzi, G.; Martinelli, G.; Dell’Agli, M. Cannabis sativa L. extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phytother. Res. 2019, 33, 2083–2093. [Google Scholar]
- Jeong, S.; Kim, M.S.; Lee, S.H.; Park, B.D. Epidermal endocannabinoid system (EES) and its cosmetic application. Cosmetics 2019, 6, 33. [Google Scholar] [CrossRef]
- Cho, W.K.; Kim, S.-Y.; Jang, S.J.; Lee, S.; Kim, H.-I.; Kim, E.; Lee, J.H.; Choi, S.S.; Moh, S.H. Comparative analysis of water extracts from roselle (Hibiscus sabdariffa L.) plants and callus cells: Constituents, effects on human skin cells, and transcriptome profiles. Int. J. Mol. Sci. 2023, 24, 10853. [Google Scholar] [CrossRef] [PubMed]
- Kikowska, M.A.; Chmielewska, M.; Włodarczyk, A.; Studzińska-Sroka, E.; Żuchowski, J.; Stochmal, A.; Kotwicka, M.; Thiem, B. Effect of pentacyclic triterpenoids-rich callus extract of Chaenomeles japonica (Thunb.) Lindl. ex Spach on viability, morphology, and proliferation of normal human skin fibroblasts. Molecules 2018, 23, 3009. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Tsuruta, D. What are reactive oxygen species, free radicals, and oxidative stress in skin diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Wen, X.; Hao, D.; Zhang, N.; He, G.; Jiang, X. NF-κB signaling in skin aging. Mech. Ageing Dev. 2019, 184, 111160. [Google Scholar] [CrossRef]
- Tran, H.G.; Shuayprom, A.; Kueanjinda, P.; Leelahavanichkul, A.; Wongsinkongman, P.; Chaisomboonpan, S.; Tawatsin, A.; Ruchusatsawat, K.; Wongpiyabovorn, J. Oxyresveratrol attenuates inflammation in human keratinocyte via regulating NF-kB signaling and ameliorates eczematous lesion in DNCB-induced dermatitis mice. Pharmaceutics 2023, 15, 1709. [Google Scholar] [CrossRef]
- Liu, T.; Xia, Q.; Lv, Y.; Wang, Z.; Zhu, S.; Qin, W.; Yang, Y.; Wang, X.; Zhao, Z.; Ma, H. ErZhiFormula prevents UV-induced skin photoaging by Nrf2/HO-1/NQO1 signaling: An in vitro and in vivo studies. J. Ethnopharmacol. 2023, 309, 115935. [Google Scholar] [CrossRef]
- Ekiner, S.A.; Gęgotek, A.; Skrzydlewska, E. The molecular activity of cannabidiol in the regulation of Nrf2 system interacting with NF-κB pathway under oxidative stress. Redox Biol. 2022, 57, 102489. [Google Scholar]

| Sample | Concentration (mg/mL) | Inhibition Rate (%) |
|---|---|---|
| CCE (C. sativa callus extract) | 2 | 19.398 ± 0.32 |
| 1 | 15.034 ± 2.10 | |
| 0.2 | 11.031 ± 1.86 | |
| 0.1 | 10.008 ± 1.82 | |
| 0.02 | 7.329 ± 4.97 | |
| Positive Control (Trolox) | 2 | 91.482 ± 0.15 |
| 1 | 91.783 ± 0.15 | |
| 0.2 | 91.693 ± 0.10 | |
| 0.1 | 91.452 ± 0.10 | |
| 0.02 | 53.198 ± 2.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.-R.; Kim, D.-H.; Kim, H.; Lim, D.-W. Evaluation of Cannabis sativa L. Callus Extract as a Novel Cosmetic Ingredient with Dual Anti-Inflammatory and Antioxidant Effects. Plants 2025, 14, 1148. https://doi.org/10.3390/plants14071148
Yu G-R, Kim D-H, Kim H, Lim D-W. Evaluation of Cannabis sativa L. Callus Extract as a Novel Cosmetic Ingredient with Dual Anti-Inflammatory and Antioxidant Effects. Plants. 2025; 14(7):1148. https://doi.org/10.3390/plants14071148
Chicago/Turabian StyleYu, Ga-Ram, Da-Hoon Kim, Hyuck Kim, and Dong-Woo Lim. 2025. "Evaluation of Cannabis sativa L. Callus Extract as a Novel Cosmetic Ingredient with Dual Anti-Inflammatory and Antioxidant Effects" Plants 14, no. 7: 1148. https://doi.org/10.3390/plants14071148
APA StyleYu, G.-R., Kim, D.-H., Kim, H., & Lim, D.-W. (2025). Evaluation of Cannabis sativa L. Callus Extract as a Novel Cosmetic Ingredient with Dual Anti-Inflammatory and Antioxidant Effects. Plants, 14(7), 1148. https://doi.org/10.3390/plants14071148






