Low Caffeine Concentrations Induce Callus and Direct Organogenesis in Tissue Cultures of Ornithogalum dubium
Abstract
1. Introduction
2. Results
2.1. Chemistry of the Compounds
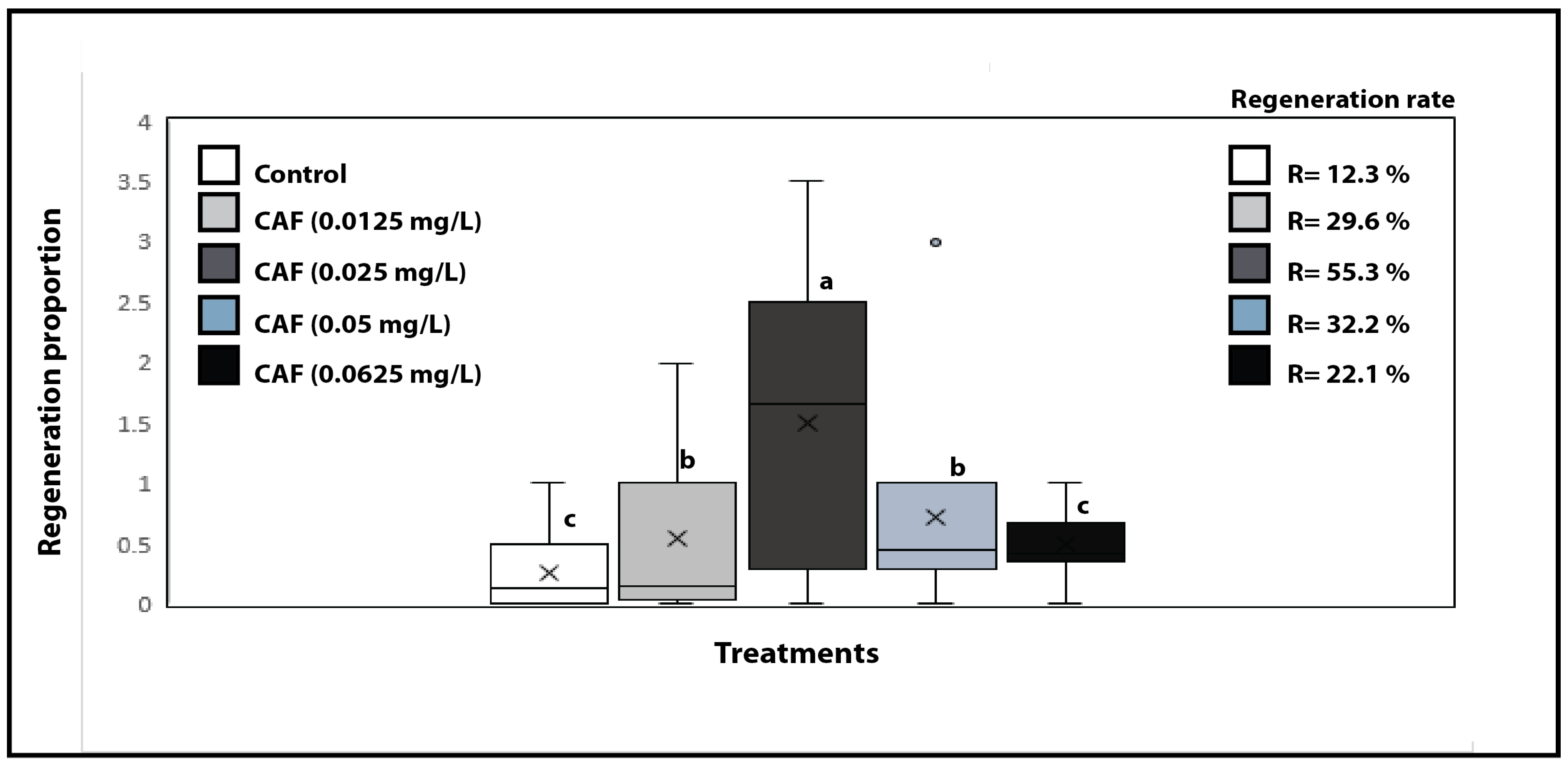
2.2. Evaluation of Caffeine’s Ability to Stimulate Cellular Development in Ornithogalum dubium Tissue Culture
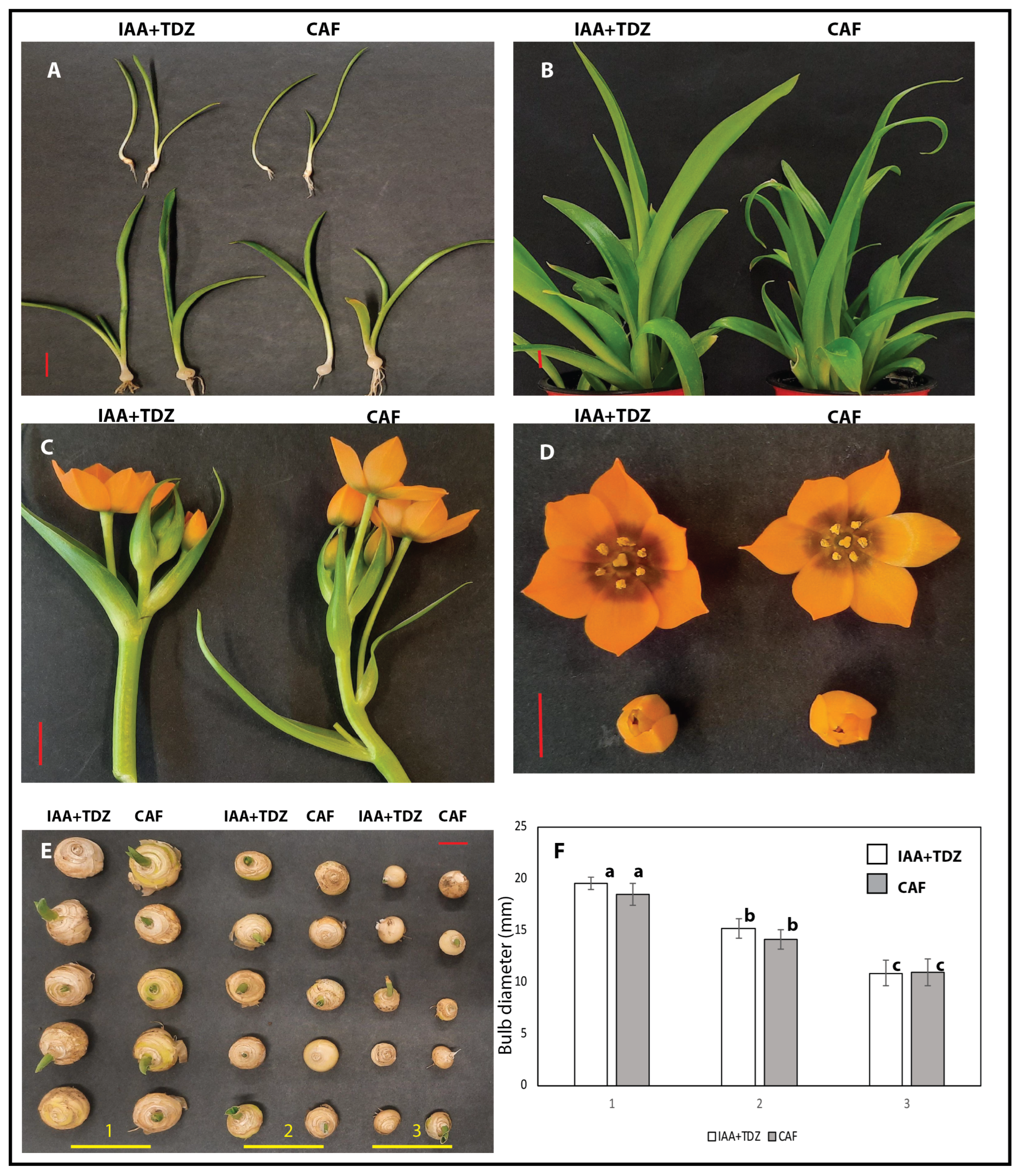
2.3. Evaluation of Caffeine Compared to Standard Phytohormones
2.4. IAA Inhibitory Studies
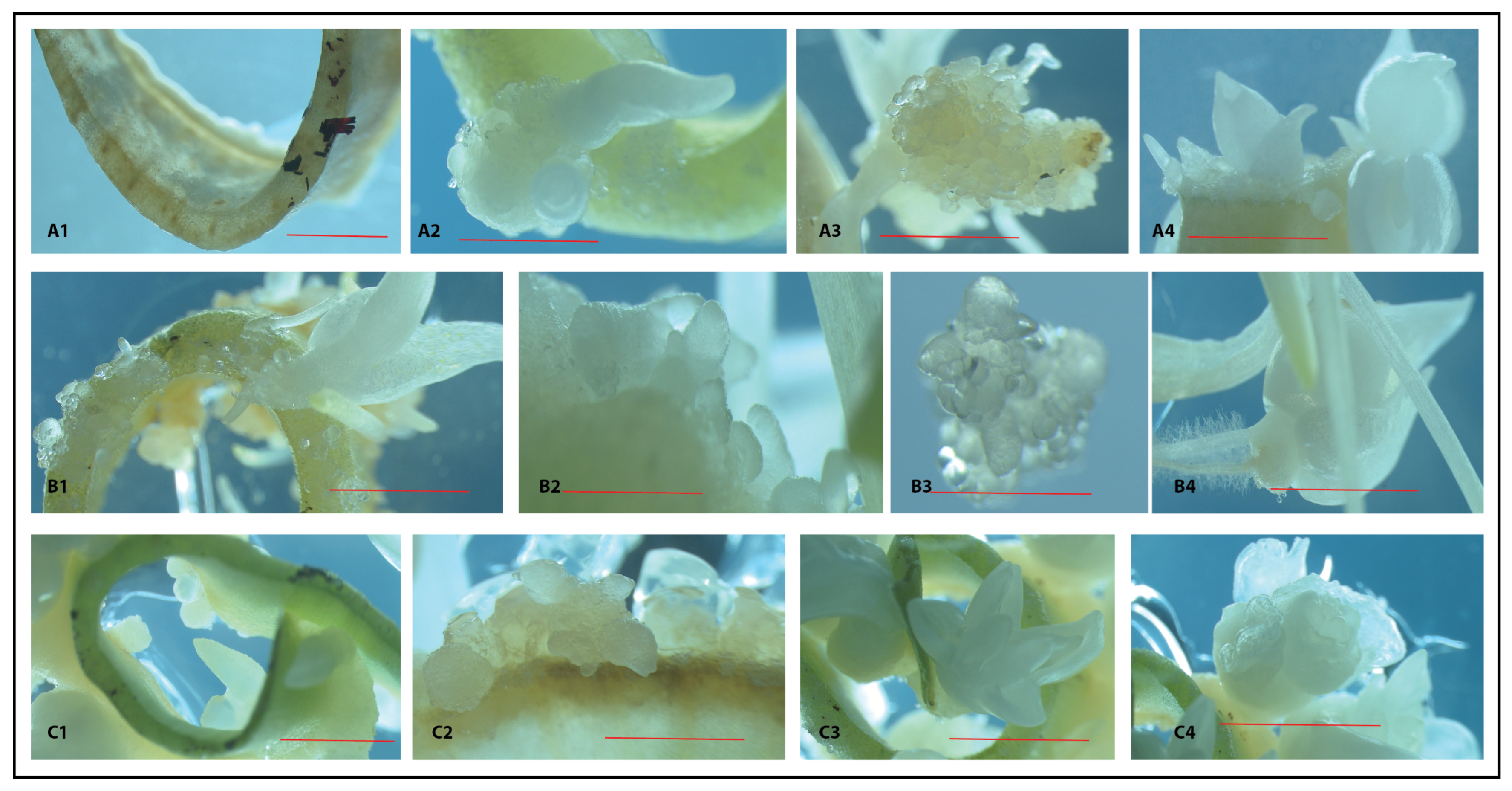
2.5. Macro- and Micro-Morphologies
3. Discussion
4. Materials and Methods
4.1. Plant Maintenance and Tissue Culture
4.2. Chemical Stocks
4.3. Media Preparation
4.4. Evaluation of Caffeine Concentrations on O. dubium Regeneration Potentials
4.5. Evaluation of Caffeine Effect on Treatments with Phytohormones
4.6. Evaluation of Indole Acetic Acid Inhibitor
4.7. Morphological Evaluation
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashihara, H.; Suzuki, T. Distribution and biosynthesis of caffeine in plants. Front. Biosci. 2004, 9, 1864–1876. [Google Scholar] [PubMed]
- Kretschmar, J.A.; Baumann, T.W. Caffeine in Citrus flowers. Phytochemistry 1999, 52, 19–23. [Google Scholar]
- Ashihara, H.; Crozier, A. Caffeine: A well known but little mentioned compound in plant science. Trends Plant Sci. 2001, 6, 407–413. [Google Scholar] [PubMed]
- Hewavitharanage, P.; Karunaratne, S.; Kumar, N.S. Effect of caffeine on shot-hole borer beetle (Xyleborusfornicatus) of tea (Camellia sinensis). Phytochemistry 1999, 51, 35–41. [Google Scholar] [CrossRef]
- Guerreiro Filho, O.; Mazzafera, P. Caffeine and resistance of coffee to the berry borer Hypothenemus hampei (Coleoptera: Scolytidae). J. Agric. Food Chem. 2003, 51, 6987–6991. [Google Scholar] [CrossRef]
- Phankaen, Y.; Manaprasertsak, A.; Pluempanupat, W.; Koul, O.; Kainoh, Y.; Bullangpoti, V. Toxicity and repellent action of Coffea arabica against Tribolium castaneum (Herbst) adults under laboratory conditions. J. Stored Prod. Res. 2017, 71, 112–118. [Google Scholar]
- Jurić, S.; Vinceković, M.; Marijan, M.; Vlahoviček-Kahlina, K.; Galešić, M.A.; Orešković, M.; Lemic, D.; Čirjak, D.; Pajač Živković, I. Effectiveness of aqueous coffee extract and caffeine in controlling phytophagous heteropteran species. Appl. Ecol. Environ. Res. 2023, 21, 1499–1513. [Google Scholar]
- Chou, C.H.; Waller, G.R. Possible allelopathic constituents of Coffea arabica. J. Chem. Ecol. 1980, 6, 643–654. [Google Scholar]
- Waller, G.R. Biochemical frontiers of allelopathy. Biol. Plant. 1989, 31, 418–447. [Google Scholar]
- Silva, R.M.; Brigatti, J.G.; Santos, V.H.; Mecina, G.F.; Silva, L.P. Allelopathic effect of the peel of coffee fruit. Sci. Hortic. 2013, 158, 39–44. [Google Scholar]
- Tanti, A.; Bhattacharyya, P.N.; Sandilya, S.P.; Dutta, P. Allelopathic potential of caffeine as growth and germination inhibitor to popular tea weed, Boreria hispida L. Curr. Life Sci. 2016, 2, 114–117. [Google Scholar]
- Pham, V.T.T.; Ismail, T.; Mishyna, M.; Appiah, K.S.; Oikawa, Y.; Fujii, Y. Caffeine: The allelochemical responsible for the plant growth inhibitory activity of Vietnamese tea (Camellia sinensis L. Kuntze). Agronomy 2019, 9, 396. [Google Scholar] [CrossRef]
- da Rosa, S.D.V.F.; de Oliveira Vilela, A.L.; Alves, M.V.P.; Cardoso, M.D.G.; Vieira, L.F.A.; Ferreira, A.M.O.; da Silva, L.M. Allelopathic activity of coffee extracts: Implications for germination and initial growth in select weeds and polyploidy in Lactuca sativa L. J. Toxicol. Environ. Health Part A 2025, 88, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wei, J.; Hu, Y.; Pi, D.; Jiang, M.; Lang, T. Caffeine synthesis and its mechanism and application by microbial degradation, A review. Foods 2023, 12, 2721. [Google Scholar] [CrossRef]
- Huang, R.; O’Donnell, A.J.; Barboline, J.J.; Barkman, T.J. Convergent evolution of caffeine in plants by co-option of exapted ancestral enzymes. Proc. Natl. Acad. Sci. USA 2016, 113, 10613–10618. [Google Scholar] [PubMed]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The safety of ingested caffeine: A comprehensive review. Front. Psychiatry 2017, 8, 80. [Google Scholar]
- Wikoff, D.; Welsh, B.T.; Henderson, R.; Brorby, G.P.; Britt, J.; Myers, E.; Goldberger, J.; Lieberman, H.R.; O’Brien, C.; Peck, J.; et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem. Toxicol. 2017, 109, 585–648. [Google Scholar]
- Camargo, M.A.F.; Camargo, C.A.C.M. Effects of Caffeine on the Organism—Literature Review. Open Access Libr. J. 2019, 6, 1–7. [Google Scholar]
- Ibrahim, S.A.; Salameh, M.M.; Phetsomphou, S.; Yang, H.; Seo, C.W. Application of caffeine, 1, 3, 7-trimethylxanthine, to control Escherichia coli O157: H7. Food Chem. 2006, 99, 645–650. [Google Scholar]
- Lele, O.H.; Maniar, J.A.; Chakravorty, R.L.; Vaidya, S.P.; Chowdhary, A.S. Assessment of biological activities of caffeine. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 45–53. [Google Scholar]
- Sledz, W.; Los, E.; Paczek, A.; Rischka, J.; Motyka, A.; Zoledowska, S.; Piosik, J.; Lojkowska, E. Antibacterial activity of caffeine against plant pathogenic bacteria. Acta Biochim. Pol. 2015, 62, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Maghsoudloo, M.; Maroufi, M. Antioxidant, antineurodegenerative, anticancer, and antimicrobial activities of caffeine and its derivatives: Micro and nano aspects. Micro Nano Bio Asp. 2023, 2, 32–37. [Google Scholar]
- Kim, Y.S.; Uefuji, H.; Ogita, S.; Sano, H. Transgenic tobacco plants producing caffeine: A potential new strategy for insect pest control. Transgenic Res. 2006, 15, 667–672. [Google Scholar] [CrossRef]
- Kim, Y.S.; Sano, H. Pathogen resistance of transgenic tobacco plants producing caffeine. Phytochemistry 2008, 69, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Choi, Y.E.; Sano, H. Plant vaccination: Stimulation of defense system by caffeine production in planta. Plant Signal. Behav. 2010, 5, 489–493. [Google Scholar] [CrossRef]
- Sano, H.; Kim, Y.S.; Choi, Y.E. Like cures like: Caffeine immunizes plants against biotic stresses. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 1 January 2013; Volume 68, pp. 273–300. [Google Scholar]
- Roy, S.C. Comparative effects of colchicine, caffeine. Biol. Plant. 1973, 15, 383–390. [Google Scholar] [CrossRef]
- Lim, K.B.; Gonzalez, R.B.; Zhou, S.; Ramanna, M.S.; Van Tuyl, J.M. Meiotic polyploidization with homoeologous recombination induced by caffeine treatment in interspecific lily hybrids. Korean J. Genet. 2005, 27, 219–226. [Google Scholar]
- Broughton, S.; Castello, M.; Liu, L.; Killen, J.; Hepworth, A.; O’Leary, R. The effect of caffeine and trifluralin on chromosome doubling in wheat anther culture. Plants 2020, 9, 105. [Google Scholar] [CrossRef]
- Muratova, S.A.; Papikhin, R.V.; Khoroshkova, Y.V. The effect of caffeine in a nutrient medium on rhizogenesis of the Rubus genus plants. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2020; Volume 23, p. 03013. [Google Scholar]
- Manning, J.C.; Forest, F.; Devey, D.S.; Fay, M.F.; Goldblatt, P. A molecular phylogeny and a revised classification of Ornithogaloideae (Hyacinthaceae) based on an analysis of four plastid DNA regions. Taxon 2009, 58, 77–107. [Google Scholar] [CrossRef]
- Reinten, E.Y.; Coetzee, J.H.; Van Wyk, B.E. The potential of South African indigenous plants for the international cut flower trade. S. Afr. J. Bot. 2011, 77, 934–946. [Google Scholar] [CrossRef]
- Griesbach, R.J.; Meyer, F.; Koopowitz, H. Creation of new flower colors in Ornithogalum via interspecific hybridization. J. Am. Soc. Hortic. Sci. 1993, 118, 409–414. [Google Scholar]
- Littlejohn, G.M. Star of Bethlehem: Ornithogalum. In Flower Breeding and Genetics: Issues, Challenges and Opportunities for the 21st Century; Springer: Dordrecht, The Netherlands, 2007; pp. 741–754. [Google Scholar]
- Luria, G.; Watad, A.A.; Cohen-Zhedek, Y.; Borochov, A. Growth and flowering of Ornithogalum dubium. In Proceedings of the VIII International Symposium on Flowerbulbs, Cape Town, South Africa, 28–31 August 2000; Volume 570, pp. 113–119. [Google Scholar]
- Niederwieser, J.G.; Van De Venter, H.A.; Robbertse, P.J. Embryo rescue in Ornithogalum. HortScience 1990, 25, 565–566. [Google Scholar]
- Lee, J.; Gómez, M.I.; Miller, W.B. Paclobutrazol and flurprimidol control stem elongation of potted star of Bethlehem. HortTechnology 2015, 25, 480–486. [Google Scholar] [CrossRef]
- Lee, J.; Miller, W.B. Preplant storage and greenhouse temperature influence flowering of Ornithogalum. HortScience 2015, 50, 1784–1790. [Google Scholar]
- Joshi, J.R.; Yedidia, I. Breeding for resistance to soft rot disease in Ornithogalum. In Proceedings of the XII International Symposium on Flower Bulbs and Herbaceous Perennials, Kunming, China, 28 June–2 July 2016; Volume 1171, pp. 279–284. [Google Scholar]
- Lipsky, A.; Joshi, J.R.; Carmi, N.; Yedidia, I. Expression levels of antimicrobial peptide tachyplesin I in transgenic Ornithogalum lines affect the resistance to Pectobacterium infection. J. Biotechnol. 2016, 238, 22–29. [Google Scholar]
- De Villiers, S.M.; Kamo, K.; Thomson, J.A.; Bornman, C.H.; Berger, D.K. Biolistic transformation of chincherinchee (Ornithogalum) and regeneration of transgenic plants. Physiol. Plant. 2000, 109, 450–455. [Google Scholar] [CrossRef]
- Cohen, A.; Lipsky, A.; Arazi, T.; Ion, A.; Stav, R.; Sandler-Ziv, D.; Fintea, C.; Gaba, V.; Gera, A. Particle bombardment-mediated transformation of Ornithogalum dubium for Ornithogalum mosaic virus resistance. In Proceedings of the IX International Symposium on Flower Bulbs, Niigata, Japan, 19–22 April 2004; Volume 673, pp. 183–190. [Google Scholar]
- Van Emmenes, L.; Veale, A.; Cohen, A.; Arazi, T. Agrobacterium-mediated transformation of the bulbous flower Ornithogalum. In Proceedings of the XXVII International Horticultural Congress-IHC2006: International Symposium on Ornamentals, Now! Seoul, Republic of Korea, 13–19 August 2006; Volume 766, pp. 477–484. [Google Scholar]
- Tripathi, P.K.; Ayzenshtat, D.; Kumar, M.; Zemach, H.; Yedidia, I.; Bocobza, S.E. An efficient and reproducible Agrobacterium-mediated genetic transformation method for the ornamental monocotyledonous plant Ornithogalum dubium Houtt. Plant Growth Regul. 2023, 101, 201–214. [Google Scholar]
- Ziv, M.; Lilien-Kipnis, H. Bud regeneration from inflorescence explants for rapid propagation of geophytes in vitro. Plant Cell Rep. 2000, 19, 845–850. [Google Scholar]
- Malabadi, R.B.; Van Staden, J.; Bornman, C.H. Regeneration of Ornithogalum in vitro. S. Afr. J. Bot. 2004, 70, 618–621. [Google Scholar]
- López-Marín, J.; González, A.; Cos, J. In Vitro Multiplication of Four Species of the Genus Ornithogalum. In Proceedings of the III International Symposium on Acclimatization and Establishment of Micropropagated Plants, Faro, Portugal, 12–15 September 2007; Volume 812, pp. 161–164. [Google Scholar]
- Suh, J.K.; Lee, W.H.; Lee, A.K. New plantlet proliferation and bulbing promotion in in vitro culture of Ornithogalum hybrid. In Proceedings of the V International Symposium on New Floricultural Crops, Paranà, Brazil, 26–31 August 2003; Volume 683, pp. 155–164. [Google Scholar]
- Petti, C. Phloroglucinol Mediated Plant Regeneration of Ornithogalum dubium as the Sole “Hormone-Like Supplement” in Plant Tissue Culture Long-Term Experiments. Plants 2020, 9, 929. [Google Scholar] [CrossRef]
- Tun, O.M.; Lipsky, A.; Luzzatto Knaan, T.; Kerem, Z.; Yedidia, I. The plant activator BTH promotes Ornithogalum dubium and O. thyrsoides differentiation and regeneration in vitro. Biol. Plant. 2013, 57, 41–48. [Google Scholar] [CrossRef]
- da Silva, J.A.T.; Dobránszki, J.; Ross, S. Phloroglucinol in plant tissue culture. In Vitro Cell. Dev. Biology. Plant 2013, 49, 1–16. [Google Scholar]
- Mohanpuria, P.; Yadav, S.K. Retardation in seedling growth and induction of early senescence in plants upon caffeine exposure is related to its negative effect on Rubisco. Photosynthetica 2009, 47, 293–297. [Google Scholar]
- He, W.; Brumos, J.; Li, H.; Ji, Y.; Ke, M.; Gong, X.; Zeng, Q.; Li, W.; Zhang, X.; An, F.; et al. A small-molecule screen identifies L-kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell 2011, 23, 3944–3960. [Google Scholar] [CrossRef] [PubMed]
- Batish, D.R.; Singh, H.P.; Kaur, M.; Kohli, R.K.; Yadav, S.S. Caffeine affects adventitious rooting and causes biochemical changes in the hypocotyl cuttings of mung bean (Phaseolus aureus Roxb.). Acta Physiol. Plant. 2008, 30, 401–405. [Google Scholar] [CrossRef]
- Smyth, D.A. Effect of methylxanthine treatment on rice seedling growth. J. Plant Growth Regul. 1992, 11, 125–128. [Google Scholar] [CrossRef]
- Sivak, A.; Rudenko, L.; Teague, L.G. Variations among species and cell types in the effects of caffeine on mutagen-induced cytotoxicity and postreplication repair of DNA. Environ. Mutagen. 1982, 4, 143–162. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, A.; Hernandez, P.; Lopez-Saez, J.F. Effect of caffeine and adenosine on G2 repair: Mitotic delay and chromosome damage. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1985, 149, 275–281. [Google Scholar] [CrossRef]
- Lock, R.B.; Galperina, O.V.; Feldhoff, R.C.; Rhodes, L.J. Concentration-dependent differences in the mechanisms by which caffeine potentiates etoposide cytotoxicity in HeLa cells. Cancer Res. 1994, 54, 4933–4939. [Google Scholar]
- Truţă, E.; Zamfirache, M.M.; Olteanu, Z. Caffeine induced genotoxic effects in Phaseolus vulgaris L. and Raphanus sativus L. Bot. Serbica 2011, 35, 49–54. [Google Scholar]
- Chung, W.H. Pleiotropic effects of caffeine leading to chromosome instability and cytotoxicity in eukaryotic microorganisms. J. Microbiol. Biotechnol. 2020, 31, 171. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Chen, Q.; Howes, N. Chromosome doubling of haploids of common wheat with caffeine. Genome 1997, 40, 552–558. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petti, C. Low Caffeine Concentrations Induce Callus and Direct Organogenesis in Tissue Cultures of Ornithogalum dubium. Plants 2025, 14, 1127. https://doi.org/10.3390/plants14071127
Petti C. Low Caffeine Concentrations Induce Callus and Direct Organogenesis in Tissue Cultures of Ornithogalum dubium. Plants. 2025; 14(7):1127. https://doi.org/10.3390/plants14071127
Chicago/Turabian StylePetti, Carloalberto. 2025. "Low Caffeine Concentrations Induce Callus and Direct Organogenesis in Tissue Cultures of Ornithogalum dubium" Plants 14, no. 7: 1127. https://doi.org/10.3390/plants14071127
APA StylePetti, C. (2025). Low Caffeine Concentrations Induce Callus and Direct Organogenesis in Tissue Cultures of Ornithogalum dubium. Plants, 14(7), 1127. https://doi.org/10.3390/plants14071127





