Influence of Plant Part Selection and Drying Technique: Exploration and Optimization of Antioxidant and Antibacterial Activities of New Guinea Impatiens Extracts
Abstract
1. Introduction
2. Results
2.1. Quantitative Estimation of Bioactive Polyphenols by High-Performance Thin-Layer Chromatography (HPTLC)
2.2. Determination of TPC, TFC, and TMA Contents
2.3. Determination of In Vitro Antioxidant Activity Using Chemical Assays
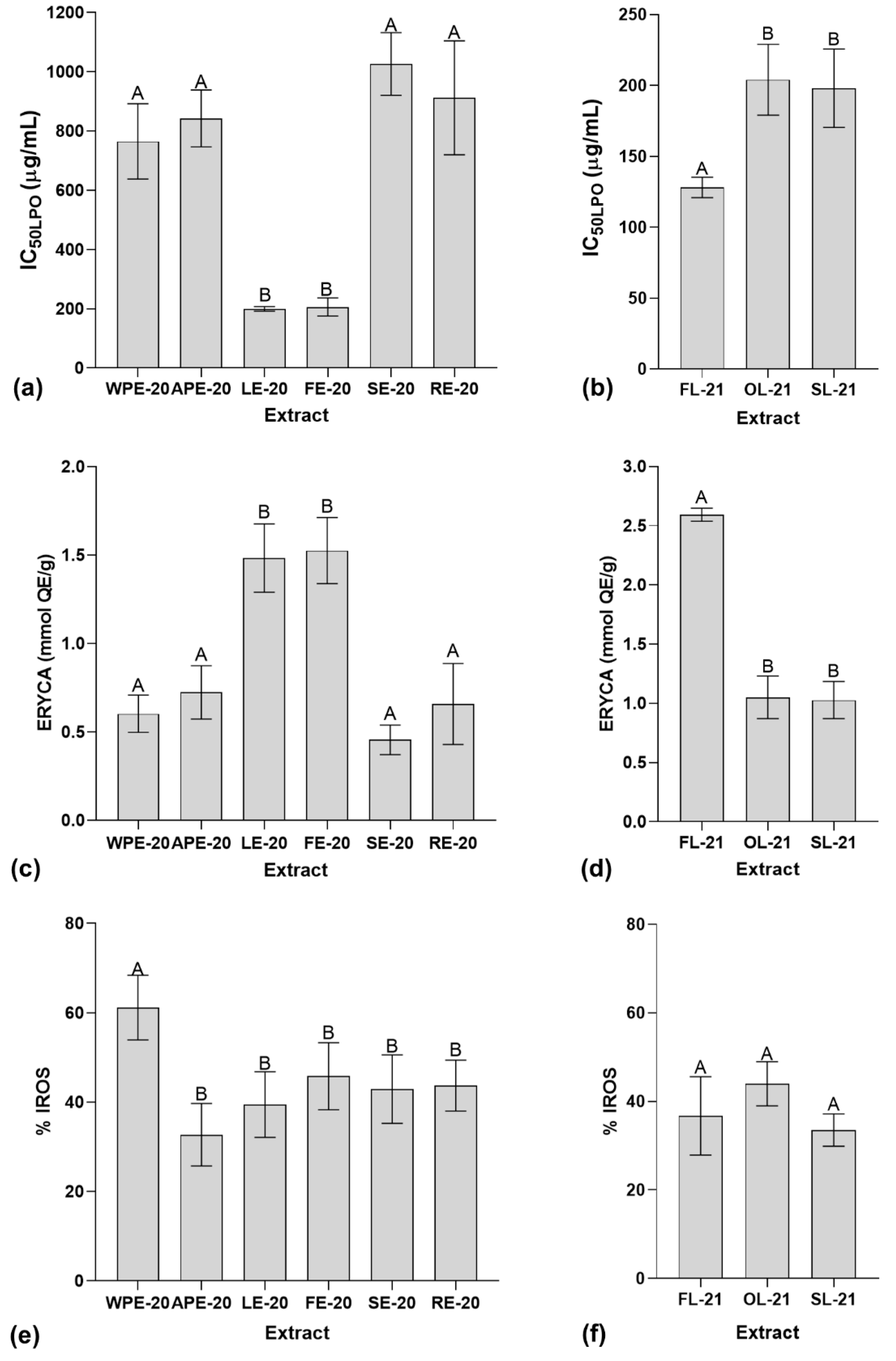
2.4. Determination of In Vitro Antioxidant Activity Using Biological Assays
2.5. In Vitro Antibacterial Microdilution Assay
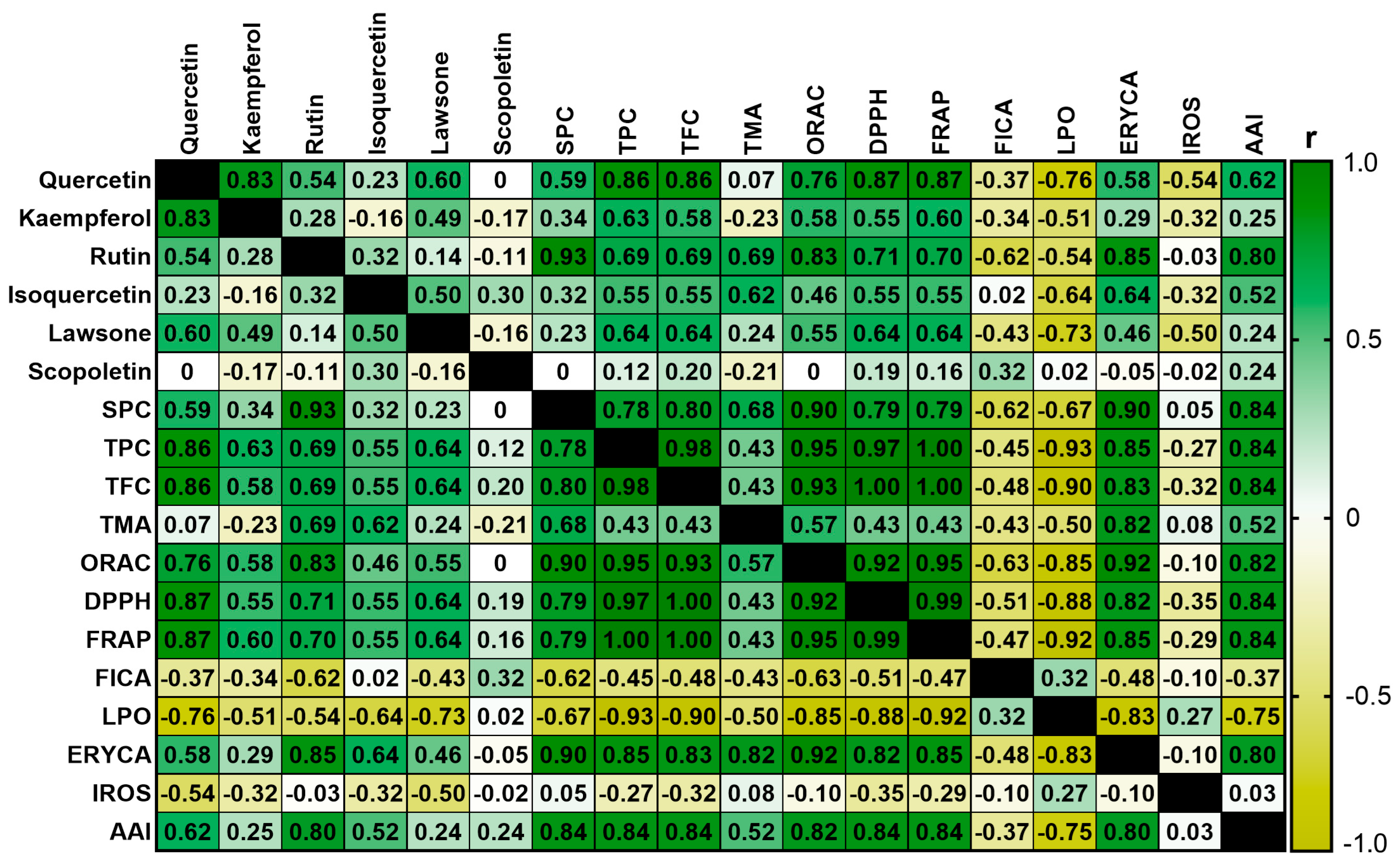
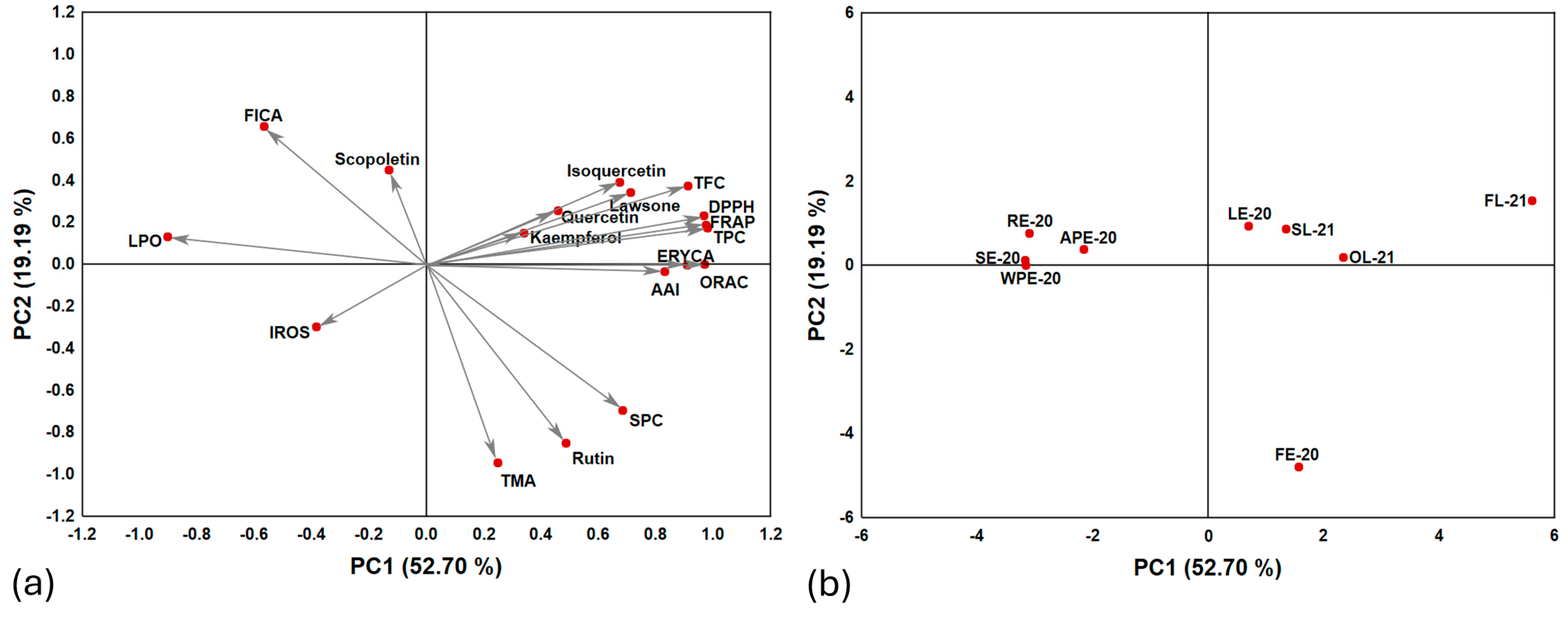
2.6. Correlation and Principal Component Analysis (PCA)
3. Discussion
3.1. Quantitative Estimation of Bioactive Polyphenols by High-Performance Thin-Layer Chromatography (HPTLC)
3.2. Determination of TPC, TFC, and TMA Contents
3.3. Determination of In Vitro Antioxidant Activity Using Chemical Assays
3.4. Determination of In Vitro Antioxidant Activity Using Biological Assays
3.5. In Vitro Antibacterial Microdilution Assay
3.6. Correlation and Principal Component Analysis
3.7. Further Directions Regarding Practical Applications
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material Collection
4.3. Extract Preparation
4.4. Quantitative Estimation of Bioactive Polyphenols by High-Performance Thin-Layer Chromatography (HPTLC)
4.4.1. General Chromatographic Conditions
4.4.2. Simultaneous Determination of Quercetin and Kaempferol
4.4.3. Simultaneous Determination of Quercetin 3-O-β-D-Rutinoside (Rutin) and Quercetin 3-O-β-D-Glucopyranoside (Isoquercetin)
4.4.4. Simultaneous Determination of 2-Hydroxy-1,4-Naphthoquinone (Lawsone) and 2-Methoxy-1,4-Naphtoquinone (2-MNQ)
4.4.5. Determination of 7-Hydroxy-6-Methoxycoumarin (Scopoletin)
4.5. Determination of Total Phenolic Content (TPC)
4.6. Determination of Total Flavonoid Content (TFC)
4.7. Determination of Total Monomeric Anthocyanins (TMA)
4.8. In Vitro Chemical Antioxidant Assays
4.8.1. Oxygen Radical Absorbance Capacity Assay (ORAC)
4.8.2. DPPH Radical Scavenging Activity Assay
4.8.3. Ferric Reducing Antioxidant Power Assay (FRAP)
4.8.4. Ferrous Iron Chelating Activity Assay (FICA)
4.9. In Vitro Biological Antioxidant Assays
4.9.1. Rat Liver Homogenate Lipid Peroxidation Inhibition Assay (LPO)
4.9.2. Erythrocyte Cellular Antioxidant Activity Assay (ERYCA)
4.9.3. Intracellular Reactive Oxygen Species Inhibition Assay (IROS)
4.10. In Vitro Antibacterial Microdilution Assay
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary Polyphenols in Chemoprevention and Synergistic Effect in Cancer: Clinical Evidences and Molecular Mechanisms of Action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef] [PubMed]
- Puttongsiri, T.; Manichart, N.; Teerarak, M.; Sikhao, P.; Somala, N.; Tongsri, P.; Pilasombut, K. Siamese Neem Tree as a Natural Preservative: Chemical Profile, Antioxidant Properties, and Antibacterial Efficacy against Foodborne Pathogens and Spoilage Bacteria. J. Agric. Food Res. 2025, 19, 101559. [Google Scholar] [CrossRef]
- Paiva, L.S.; Motta, M.H.; Baptista, J.A.B. Nutraceutical Value of Eleven Aromatic Medicinal Plants and Azorean Camellia Sinensis: Comparison of Antioxidant Properties and Phenolic and Flavonoid Contents. Processes 2024, 12, 1375. [Google Scholar] [CrossRef]
- Nakra, S.; Tripathy, S.; Srivastav, P.P. Drying as a Preservation Strategy for Medicinal Plants: Physicochemical and Functional Outcomes for Food and Human Health. Phytomedicine Plus 2025, 5, 100762. [Google Scholar] [CrossRef]
- Cör Andrejč, D.; Butinar, B.; Knez, Ž.; Tomažič, K.; Knez Marevci, M. The Effect of Drying Methods and Extraction Techniques on Oleuropein Content in Olive Leaves. Plants 2022, 11, 865. [Google Scholar] [CrossRef]
- Belwal, T.; Cravotto, C.; Prieto, M.A.; Venskutonis, P.R.; Daglia, M.; Devkota, H.P.; Baldi, A.; Ezzat, S.M.; Gómez-Gómez, L.; Salama, M.M.; et al. Effects of Different Drying Techniques on the Quality and Bioactive Compounds of Plant-Based Products: A Critical Review on Current Trends. Dry. Technol. 2022, 40, 1539–1561. [Google Scholar] [CrossRef]
- Abascal, K.; Ganora, L.; Yarnell, E. The Effect of Freeze-drying and Its Implications for Botanical Medicine: A Review. Phyther. Res. 2005, 19, 655–660. [Google Scholar] [CrossRef]
- Li, R.; Shang, H.; Wu, H.; Wang, M.; Duan, M.; Yang, J. Thermal Inactivation Kinetics and Effects of Drying Methods on the Phenolic Profile and Antioxidant Activities of Chicory (Cichorium intybus L.) Leaves. Sci. Rep. 2018, 8, 9529. [Google Scholar] [CrossRef]
- Zhang, W.; Zuo, Y.; Xu, F.; Wang, T.; Liu, J.; Wu, D. Study of the Mechanism of Change in Flavonoid Composition in the Processing of Chrysanthemum morifolium (Ramat.) Tzvel. ‘Boju’. BMC Chem. 2019, 13, 128. [Google Scholar] [CrossRef]
- Szewczyk, K. Phytochemistry of the Genus Impatiens (Balsaminaceae): A Review. Biochem. Syst. Ecol. 2018, 80, 94–121. [Google Scholar] [CrossRef]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.; El Borolossy, R.M.; Elsaid, T.; Sabri, N.A. Evaluation of the Combination Effect of Rutin and Vitamin C Supplementation on the Oxidative Stress and Inflammation in Hemodialysis Patients. Front. Pharmacol. 2022, 13, 961590. [Google Scholar] [CrossRef]
- Vida, R.G.; Fittler, A.; Somogyi-Végh, A.; Poór, M. Dietary Quercetin Supplements: Assessment of Online Product Informations and Quantitation of Quercetin in the Products by High-performance Liquid Chromatography. Phyther. Res. 2019, 33, 1912–1920. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.U. Quercetin and Its Role in Biological Functions: An Updated Review. EXCLI J. 2018, 17, 856–863. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.-M. Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef]
- Buathong, R.; Chamchumroon, V.; Schinnerl, J.; Bacher, M.; Santimaleeworagun, W.; Kraichak, E.; Vajrodaya, S. Chemovariation and Antibacterial Activity of Extracts and Isolated Compounds from Species of Ixora and Greenea (Ixoroideae, Rubiaceae). PeerJ 2019, 7, e6893. [Google Scholar] [CrossRef]
- Parama, D.; Girisa, S.; Khatoon, E.; Kumar, A.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakkara, A.B. An Overview of the Pharmacological Activities of Scopoletin against Different Chronic Diseases. Pharmacol. Res. 2022, 179, 106202. [Google Scholar] [CrossRef]
- Sakunphueak, A.; Panichayupakaranant, P. Comparison of Antimicrobial Activities of Naphthoquinones from Impatiens Balsamina. Nat. Prod. Res. 2012, 26, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.S.; Brighente, I.M.C.; Lund, R.G.; Llanes, L.C.; Nunes, R.J.; Bretanha, L.C.; Yunes, R.A.; Carvalho, P.H.A.; Ribeiro, J.S. Antioxidant and Antifungal Activity of Naphthoquinones Dimeric Derived from Lawsone. J. Biosci. Med. 2017, 05, 39–48. [Google Scholar] [CrossRef][Green Version]
- Chen, M.; Vial, M.L.; Gee, L.; Davis, R.A.; St John, J.A.; Ekberg, J.A.K. The Plant Natural Product 2-Methoxy-1,4-Naphthoquinone Stimulates Therapeutic Neural Repair Properties of Olfactory Ensheathing Cells. Sci. Rep. 2020, 10, 951. [Google Scholar] [CrossRef]
- Cui, H.; Xie, W.; Hua, Z.; Cao, L.; Xiong, Z.; Tang, Y.; Yuan, Z. Recent Advancements in Natural Plant Colorants Used for Hair Dye Applications: A Review. Molecules 2022, 27, 8062. [Google Scholar] [CrossRef]
- Szewczyk, K.; Zidorn, C.; Biernasiuk, A.; Komsta, Ł.; Granica, S. Polyphenols from Impatiens (Balsaminaceae) and Their Antioxidant and Antimicrobial Activities. Ind. Crops Prod. 2016, 86, 262–272. [Google Scholar] [CrossRef]
- Kang, S.-N.; Goo, Y.-M.; Yang, M.-R.; Ibrahim, R.; Cho, J.-H.; Kim, I.-S.; Lee, O.-H. Antioxidant and Antimicrobial Activities of Ethanol Extract from the Stem and Leaf of Impatiens balsamina L. (Balsaminaceae) at Different Harvest Times. Molecules 2013, 18, 6356–6365. [Google Scholar] [CrossRef]
- Pires, E.D.O.; Pereira, E.; Carocho, M.; Pereira, C.; Dias, M.I.; Calhelha, R.C.; Ćirić, A.; Soković, M.; Garcia, C.C.; Ferreira, I.C.F.R.; et al. Study on the Potential Application of Impatiens balsamina L. Flowers Extract as a Natural Colouring Ingredient in a Pastry Product. Int. J. Environ. Res. Public Health 2021, 18, 9062. [Google Scholar] [CrossRef]
- Chen, C.; Peng, X.; Zeng, R.; Wan, C.; Chen, M.; Chen, J. Physiological and Biochemical Responses in Cold-Stored Citrus Fruits to Carboxymethyl Cellulose Coating Containing Ethanol Extract of Impatiens balsamina L. Stems. J. Food Process. Preserv. 2017, 41, e12999. [Google Scholar] [CrossRef]
- Zeng, R.; Zhang, A.; Chen, J.; Fu, Y. Impact of Carboxymethyl Cellulose Coating Enriched with Extract of Impatiens balsamina Stems on Preservation of ‘Newhall’ Navel Orange. Sci. Hortic. 2013, 160, 44–48. [Google Scholar] [CrossRef]
- An, T.J.; Shin, Y.S.; Lee, S.E.; Ahn, Y.S.; Kim, Y.G.; Park, C.B.; Yu, S.H. Antifungal Activity of Impatiens balsamina against Ginseng Pathogen Alternaria Panax. Korean J. Med. Crop Sci. 2009, 17, 464–469. [Google Scholar]
- Sritrairat, N.; Nukul, N.; Inthasame, P.; Sansuk, A.; Prasirt, J.; Leewatthanakorn, T.; Piamsawad, U.; Dejrudee, A.; Panichayupakaranant, P.; Pangsomboon, K.; et al. Antifungal Activity of Lawsone Methyl Ether in Comparison with Chlorhexidine. J. Oral Pathol. Med. 2011, 40, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Singh, V.; Kant, V.; Ahuja, M. Current Status of 1,4-Naphthoquinones and Their Derivatives for Wound Healing. Eur. J. Med. Chem. Rep. 2024, 12, 100194. [Google Scholar] [CrossRef]
- United States Department of Agriculture 2019 Census of Horticultural Specialties. Available online: https://www.nass.usda.gov/Publications/AgCensus/2017/Online_Resources/Census_of_Horticulture_Specialties/hortic_1_0004_0004.pdf (accessed on 23 March 2025).
- Holdsworth, D.K. Medicinal Plants in Papua New Guinea; Technical Paper No. 175; South Pacific Commission: Noumea, New Caledonia, 1977. [Google Scholar]
- Telban, B. The role of medical ethnobotany in ethnomedicine: A New Guinea example. J. Ethnobiol. 1988, 8, 149–169. [Google Scholar]
- Holdsworth, D. High Altitude Medicinal Plants of Papua New Guinea. Int. J. Crude Drug Res. 1989, 27, 95–100. [Google Scholar] [CrossRef]
- Chua, L.S. Untargeted MS-Based Small Metabolite Identification from the Plant Leaves and Stems of Impatiens Balsamina. Plant Physiol. Biochem. 2016, 106, 16–22. [Google Scholar] [CrossRef]
- Szewczyk, K.; Olech, M. Optimization of Extraction Method for LC–MS Based Determination of Phenolic Acid Profiles in Different Impatiens Species. Phytochem. Lett. 2017, 20, 322–330. [Google Scholar] [CrossRef]
- Delgado-Rodriguez, F.; Hidalgo, O.; Loría-Gutiérrez, A.; Weng-Huang, N. In Vitro Antioxidant and Antimicrobial Activities of Ethanolic Extracts from Whole Plants of Three Impatiens Species (Balsaminaceae). Anc. Sci. Life 2017, 37, 16. [Google Scholar] [CrossRef]
- ElGamal, R.; Song, C.; Rayan, A.M.; Liu, C.; Al-Rejaie, S.; ElMasry, G. Thermal Degradation of Bioactive Compounds during Drying Process of Horticultural and Agronomic Products: A Comprehensive Overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Cao, X.; Cai, C.; Wang, Y.; Zheng, X. The Inactivation Kinetics of Polyphenol Oxidase and Peroxidase in Bayberry Juice during Thermal and Ultrasound Treatments. Innov. Food Sci. Emerg. Technol. 2018, 45, 169–178. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Rohn, S.; Buchner, N.; Driemel, G.; Rauser, M.; Kroh, L.W. Thermal Degradation of Onion Quercetin Glucosides under Roasting Conditions. J. Agric. Food Chem. 2007, 55, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Kovač Tomas, M.; Jurčević, I.; Šamec, D. Tissue-Specific Profiling of Biflavonoids in Ginkgo (Ginkgo biloba L.). Plants 2022, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, A.; Brenne, X.; Feist, E.; Metz, N.; Weniger, B.; Anton, R. Quantitative Determination of Naphthoquinones of Impatiens Species. Phytochem. Anal. 2001, 12, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Tusevski, O.; Krstikj, M.; Stanoeva, J.P.; Stefova, M.; Gadzovska Simic, S. Phenolic Profile and Biological Activity of Hypericum Perforatum L.: Can Roots Be Considered as a New Source of Natural Compounds? S. Afr. J. Bot. 2018, 117, 301–310. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Alrumaihi, F.; Almatroodi, S.A.; Alharbi, H.O.A.; Alwanian, W.M.; Alharbi, F.A.; Almatroudi, A.; Rahmani, A.H. Pharmacological Potential of Kaempferol, a Flavonoid in the Management of Pathogenesis via Modulation of Inflammation and Other Biological Activities. Molecules 2024, 29, 2007. [Google Scholar] [CrossRef]
- Mbikay, M.; Chrétien, M. Isoquercetin as an Anti-Covid-19 Medication: A Potential to Realize. Front. Pharmacol. 2022, 13, 830205. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Blunden, G.; Jacob, C. Antiviral Significance of Isoquercetin (Quercetin-3-O-Glucoside) with Special Reference to Its Anti-Coronaviral Potential. Nat. Prod. Commun. 2024, 19, 1934578X231219560. [Google Scholar] [CrossRef]
- Nair, A.; Sekar, M.; Gan, S.H.; Kumarasamy, V.; Subramaniyan, V.; Wu, Y.S.; Mat Rani, N.N.I.; Ravi, S.; Wong, L.S. Lawsone Unleashed: A Comprehensive Review on Chemistry, Biosynthesis, and Therapeutic Potentials. Drug Des. Dev. Ther. 2024, 18, 3295–3313. [Google Scholar] [CrossRef]
- Gao, X.-Y.; Li, X.-Y.; Zhang, C.-Y.; Bai, C.-Y. Scopoletin: A Review of Its Pharmacology, Pharmacokinetics, and Toxicity. Front. Pharmacol. 2024, 15, 1268464. [Google Scholar] [CrossRef]
- Oku, H.; Ishiguro, K. Screening Method for PAF Antagonist Substances: On the Phenolic Compounds from Impatients balsamina L. Phyther. Res. 1999, 13, 521–525. [Google Scholar] [CrossRef]
- Ishiguro, K.; Fukumoto, H. A Practical and Speedy Screening Method for Murine Anaphylaxis: On the Antianaphylactic Effect of Impatiens balsamina L. Phyther. Res. 1997, 11, 48–50. [Google Scholar] [CrossRef]
- Ishiguro, K.; Ohira, Y.; Oku, H. Preventive Effects of Impatiens balsamina on the Hen Egg-White Lysozyme (HEL)-Induced Decrease in Blood Flow. Biol. Pharm. Bull. 2002, 25, 505–508. [Google Scholar] [CrossRef]
- Fukumoto, H.; Yamaki, M.; Isoi, K.; Ishiguro, K. Antianaphylactic Effects of the Principal Compounds from the White Petals Of Impatiens balsamina L. Phyther. Res. 1996, 10, 202–206. [Google Scholar] [CrossRef]
- Oku, H.; Ishiguro, K. Antipruritic and Antidermatitic Effect of Extract and Compounds of Impatiens balsamina L. in Atopic Dermatitis Model NC Mice. Phyther. Res. 2001, 15, 506–510. [Google Scholar] [CrossRef]
- Ishiguro, K.; Oku, H. Antipruritic Effect of Flavonol and 1,4-Naphthoquinone Derivatives from Impatiens BalsaminaL. Phyther. Res. 1997, 11, 343–347. [Google Scholar] [CrossRef]
- Ishiguro, K.; Ohira, Y.; Oku, H. Antipruritic Dinaphthofuran-7,12-Dione Derivatives from the Pericarp of Impatiens balsamina. J. Nat. Prod. 1998, 61, 1126–1129. [Google Scholar] [CrossRef]
- Oku, H.; Kato, T.; Ishiguro, K. Antipruritic Effects of 1,4-Naphthoquinones and Related Compounds. Biol. Pharm. Bull. 2002, 25, 137–139. [Google Scholar] [CrossRef]
- Ak, G.; Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Picot-Allain, M.C.N.; Cakır, O.; Bensari, S.; Yılmaz, M.A.; Gallo, M.; Montesano, D. A Comparative Bio-Evaluation and Chemical Profiles of Calendula officinalis L. Extracts Prepared via Different Extraction Techniques. Appl. Sci. 2020, 10, 5920. [Google Scholar] [CrossRef]
- Buitrago, D.; Buitrago-Villanueva, I.; Barbosa-Cornelio, R.; Coy-Barrera, E. Comparative Examination of Antioxidant Capacity and Fingerprinting of Unfractionated Extracts from Different Plant Parts of Quinoa (Chenopodium quinoa) Grown under Greenhouse Conditions. Antioxidants 2019, 8, 238. [Google Scholar] [CrossRef]
- Oldenburg, K.; Henning, S.; Soendergaard, M. Antioxidant Activity and Phenolic Content of Chinese Balsam (Impatiens chinensis). FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Garcìa, L.M.; Ceccanti, C.; Negro, C.; De Bellis, L.; Incrocci, L.; Pardossi, A.; Guidi, L. Effect of Drying Methods on Phenolic Compounds and Antioxidant Activity of Urtica dioica L. Leaves. Horticulturae 2021, 7, 10. [Google Scholar] [CrossRef]
- Roslan, A.S.; Ismail, A.; Ando, Y.; Azlan, A. Effect of Drying Methods and Parameters on the Antioxidant Properties of Tea (Camellia sinensis) Leaves. Food Prod. Process. Nutr. 2020, 2, 8. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Effects of Different Drying Methods on Extractable Phenolic Compounds and Antioxidant Properties from Lemon Myrtle Dried Leaves. Heliyon 2019, 5, e03044. [Google Scholar] [CrossRef]
- Santos, J.S.; Alvarenga Brizola, V.R.; Granato, D. High-Throughput Assay Comparison and Standardization for Metal Chelating Capacity Screening: A Proposal and Application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Burillo, A.M.; La Penna, G.; Hegde, M.L.; Rao, K.S. Polyphenols as Potential Metal Chelation Compounds Against Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 82, S335–S357. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and Pitfalls in Measuring Antioxidant Efficacy: A Critical Evaluation of ABTS, DPPH, and ORAC Assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Snoussi, A.; Essaidi, I.; Ben Haj Koubaier, H.; Zrelli, H.; Alsafari, I.; Živoslav, T.; Mihailovic, J.; Khan, M.; El Omri, A.; Ćirković Veličković, T.; et al. Drying Methodology Effect on the Phenolic Content, Antioxidant Activity of Myrtus communis L. Leaves Ethanol Extracts and Soybean Oil Oxidative Stability. BMC Chem. 2021, 15, 31. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Gentile, F.; Arcaro, A.; Pizzimenti, S.; Daga, M.; Cetrangolo, G.P.; Dianzani, C.; Lepore, A.; Graf, M.; Ames, P.R.J.; Barrera, G. DNA Damage by Lipid Peroxidation Products: Implications in Cancer, Inflammation and Autoimmunity. AIMS Genet. 2017, 04, 103–137. [Google Scholar] [CrossRef]
- Avlasevich, S.; Pellegrin, T.; Godse, M.; Bryce, S.; Bemis, J.; Bajorski, P.; Dertinger, S. Biomarkers of DNA Damage Response Improve In Vitro Micronucleus Assays by Revealing Genotoxic Mode of Action and Reducing the Occurrence of Irrelevant Positive Results. Mutagenesis 2021, 36, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Memariani, T.; Hosseini, T.; Kamali, H.; Mohammadi, A.; Ghorbani, M.; Shakeri, A.; Spandidos, D.A.; Tsatsakis, A.M.; Shahsavand, S. Evaluation of the Cytotoxic Effects of Cyperus Longus Extract, Fractions and Its Essential Oil on the PC3 and MCF7 Cancer Cell Lines. Oncol. Lett. 2016, 11, 1353–1360. [Google Scholar] [CrossRef]
- García Fillería, S.; Tironi, V. Intracellular Antioxidant Activity and Intestinal Absorption of Amaranth Peptides Released Using Simulated Gastrointestinal Digestion with Caco-2 TC7 Cells. Food Biosci. 2021, 41, 101086. [Google Scholar] [CrossRef]
- Kubo, E.; Chhunchha, B.; Singh, P.; Sasaki, H.; Singh, D.P. Sulforaphane Reactivates Cellular Antioxidant Defense by Inducing Nrf2/ARE/Prdx6 Activity during Aging and Oxidative Stress. Sci. Rep. 2017, 7, 14130. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and Antagonism in Natural Product Extracts: When 1 + 1 Does Not Equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Chhetry, A.K.; Dhakal, S.; Chaudhary, L.; Karki, K.; Khadka, R.B.; Chaudhary, G.P.; Bastola, T.; Poudel, A.; Aryal, P.; Pandey, J. Study of Antibacterial Activity of Root Bark, Leaves, and Pericarp Extracts of Diploknema butyracea and Evaluation of Prospective Antioxidant Activity. J. Trop. Med. 2022, 2022, 6814901. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Wu, D.-C.; Liao, J.-J.; Wu, C.-H.; Li, W.-Y.; Weng, B.-C. In Vitro Activity of Impatiens balsamina L. Against Multiple Antibiotic-Resistant Helicobacter pylori. Am. J. Chin. Med. 2009, 37, 713–722. [Google Scholar] [CrossRef]
- Degu, S.; Abebe, A.; Gemeda, N.; Bitew, A. Evaluation of Antibacterial and Acute Oral Toxicity of Impatiens Tinctoria A. Rich Root Extracts. PLoS ONE 2021, 16, e0255932. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Malca-García, G.; Glenn, A.; Sharon, D.; Chait, G.; Díaz, D.; Pourmand, K.; Jonat, B.; Somogy, S.; Guardado, G.; et al. Minimum Inhibitory Concentrations of Medicinal Plants Used in Northern Peru as Antibacterial Remedies. J. Ethnopharmacol. 2010, 132, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Valle, D.L.; Andrade, J.I.; Puzon, J.J.M.; Cabrera, E.C.; Rivera, W.L. Antibacterial Activities of Ethanol Extracts of Philippine Medicinal Plants against Multidrug-Resistant Bacteria. Asian Pac. J. Trop. Biomed. 2015, 5, 532–540. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Li, H.; Qiu, Z.; Yu, Y. Comparative Assessment of the Antibacterial Efficacies and Mechanisms of Different Tea Extracts. Foods 2022, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- Oulahal, N.; Degraeve, P. Phenolic-Rich Plant Extracts with Antimicrobial Activity: An Alternative to Food Preservatives and Biocides? Front. Microbiol. 2022, 12, 753518. [Google Scholar] [CrossRef]
- De Rossi, L.; Rocchetti, G.; Lucini, L.; Rebecchi, A. Antimicrobial Potential of Polyphenols: Mechanisms of Action and Microbial Responses—A Narrative Review. Antioxidants 2025, 14, 200. [Google Scholar] [CrossRef]
- Golden, M.M.; Heppe, A.C.; Zaremba, C.L.; Wuest, W.M. Metal Chelation as an Antibacterial Strategy for Pseudomonas aeruginosa and Acinetobacter baumannii. RSC Chem. Biol. 2024, 5, 1083–1096. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, A.; Yang, Y.; Yang, P. Coumarin-Containing Hybrids and Their Antibacterial Activities. Arch. Pharm. 2020, 353, 1900380. [Google Scholar] [CrossRef]
- Song, R.; Yu, B.; Friedrich, D.; Li, J.; Shen, H.; Krautscheid, H.; Huang, S.D.; Kim, M.H. Naphthoquinone-Derivative as a Synthetic Compound to Overcome the Antibiotic Resistance of Methicillin-Resistant S. Aureus. Commun. Biol. 2020, 3, 529. [Google Scholar] [CrossRef]
- Jeyaraj, E.J.; Vidana Gamage, G.C.; Cintrat, J.C.; Choo, W.S. Acylated and Non-Acylated Anthocyanins as Antibacterial and Antibiofilm Agents. Discov. Food 2023, 3, 21. [Google Scholar] [CrossRef]
- Wiśniewski, P.; Trymers, M.; Chajęcka-Wierzchowska, W.; Tkacz, K.; Zadernowska, A.; Modzelewska-Kapituła, M. Antimicrobial Resistance in the Context of Animal Production and Meat Products in Poland—A Critical Review and Future Perspective. Pathogens 2024, 13, 1123. [Google Scholar] [CrossRef] [PubMed]
- Podkowik, M.; Seo, K.S.; Schubert, J.; Tolo, I.; Robinson, D.A.; Bania, J.; Bystroń, J. Genotype and Enterotoxigenicity of Staphylococcus Epidermidis Isolate from Ready to Eat Meat Products. Int. J. Food Microbiol. 2016, 229, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Espinosa-Gongora, C.; Guardabassi, L. Human Health Risks Associated with Antimicrobial-Resistant Enterococci and Staphylococcus Aureus on Poultry Meat. Clin. Microbiol. Infect. 2016, 22, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, A.G.; El-Zamkan, M.A.; Younis, W.; Saleh, S.O.; Abd-Elhafeez, H.H.; Yoseef, A.G. Phenotypic and Genotypic Characterization of Enterococcus faecalis and Enterococcus faecium Isolated from Fish, Vegetables, and Humans. Sci. Rep. 2024, 14, 21741. [Google Scholar] [CrossRef]
- Habib, I.; Lakshmi, G.B.; Mohamed, M.-Y.I.; Ghazawi, A.; Khan, M.; Al-Rifai, R.H.; Abdalla, A.; Anes, F.; Elbediwi, M.; Khalifa, H.O.; et al. Staphylococcus spp. in Salad Vegetables: Biodiversity, Antimicrobial Resistance, and First Identification of Methicillin-Resistant Strains in the United Arab Emirates Food Supply. Foods 2024, 13, 2439. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Patil, S.L.; Mallaiah, S.H.; Patil, R.K. Antioxidative and Radioprotective Potential of Rutin and Quercetin in Swiss Albino Mice Exposed to Gamma Radiation. J. Med. Phys. 2013, 38, 87–92. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, Z.; Li, Z.; Ding, Y.; Jiang, F.; Liu, J. Antioxidant and Antibacterial Study of 10 Flavonoids Revealed Rutin as a Potential Antibiofilm Agent in Klebsiella Pneumoniae Strains Isolated from Hospitalized Patients. Microb. Pathog. 2021, 159, 105121. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wąsik, T.J.; Zapletal-Pudełko, K.; Klim, M.; Wojtyczka, R.D. The Direction of the Antibacterial Effect of Rutin Hydrate and Amikacin. Antibiotics 2023, 12, 1469. [Google Scholar] [CrossRef]
- Koodkaew, I.; Sukonkhajorn, P. Anti-tyrosinase and antioxidant activities of Impatiens balsamina L. Songklanakarin J. Sci. Technol. 2019, 41, 686–692. [Google Scholar]
- Jang, J.Y.; Shin, H.; Lim, J.-W.; Ahn, J.H.; Jo, Y.H.; Lee, K.Y.; Hwang, B.Y.; Jung, S.-J.; Kang, S.Y.; Lee, M.K. Comparison of Antibacterial Activity and Phenolic Constituents of Bark, Lignum, Leaves and Fruit of Rhus verniciflua. PLoS ONE 2018, 13, e0200257. [Google Scholar] [CrossRef] [PubMed]
- Hmamou, A.; Eloutassi, N.; Alshawwa, S.Z.; Al kamaly, O.; Kara, M.; Bendaoud, A.; El-Assri, E.-M.; Tlemcani, S.; El Khomsi, M.; Lahkimi, A. Total Phenolic Content and Antioxidant and Antimicrobial Activities of Papaver rhoeas L. Organ Extracts Growing in Taounate Region, Morocco. Molecules 2022, 27, 854. [Google Scholar] [CrossRef] [PubMed]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-Laboratory Validation of Microplate Methods for Total Phenolic Content and Antioxidant Activity on Polyphenolic Extracts, and Comparison with Conventional Spectrophotometric Methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.; Almeida, M.I.G.S.; Barreiros, L.; Reis, S.; Segundo, M.A. Automatic Aluminum Chloride Method for Routine Estimation of Total Flavonoids in Red Wines and Teas. Food Anal. Methods 2012, 5, 530–539. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the PH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Kenny, O.; Brunton, N.P.; Smyth, T.J. In Vitro Protocols for Measuring the Antioxidant Capacity of Algal Extracts. In Methods in Molecular Biology 1308, Natural Products from Marine Algae; Stengel, D.B., Connan, S., Eds.; Springer Science & Business Media: New York, NY, USA, 2015; pp. 375–402. [Google Scholar]
- Chen, Z.; Bertin, R.; Froldi, G. EC50 Estimation of Antioxidant Activity in DPPH Assay Using Several Statistical Programs. Food Chem. 2013, 138, 414–420. [Google Scholar] [CrossRef]
- Shimamura, T.; Sumikura, Y.; Yamazaki, T.; Tada, A.; Kashiwagi, T.; Ishikawa, H.; Matsui, T.; Sugimoto, N.; Akiyama, H.; Ukeda, H. Applicability of the DPPH Assay for Evaluating the Antioxidant Capacity of Food Additives—Inter-Laboratory Evaluation Study. Anal. Sci. 2014, 30, 717–721. [Google Scholar] [CrossRef]
- Işıl Berker, K.; Güçlü, K.; Tor, İ.; Demirata, B.; Apak, R. Total Antioxidant Capacity Assay Using Optimized Ferricyanide/Prussian Blue Method. Food Anal. Methods 2010, 3, 154–168. [Google Scholar] [CrossRef]
- Azofeifa, G.; Quesada, S.; Pérez, A.M.; Vaillant, F.; Michel, A. Pasteurization of Blackberry Juice Preserves Polyphenol-Dependent Inhibition for Lipid Peroxidation and Intracellular Radicals. J. Food Compos. Anal. 2015, 42, 56–62. [Google Scholar] [CrossRef]
- González, E.; Vaillant, F.; Rojas, G.; Pérez, A. Novel Semiautomated Method for Assessing in Vitro Cellular Antioxidant Activity Using the Light-Scattering Properties of Human Erythrocytes. J. Agric. Food Chem. 2010, 58, 1455–1461. [Google Scholar] [CrossRef]
- Gómez-García, M.; Puente, H.; Argüello, H.; Mencía-Ares, Ó.; Rubio, P.; Carvajal, A. In Vitro Assessment of Antiviral Effect of Natural Compounds on Porcine Epidemic Diarrhea Coronavirus. Front. Vet. Sci. 2021, 8, 652000. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Determination of Minimum Inhibitory Concentrations (MICs) of Antibacterial Agents by Broth Dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [CrossRef]
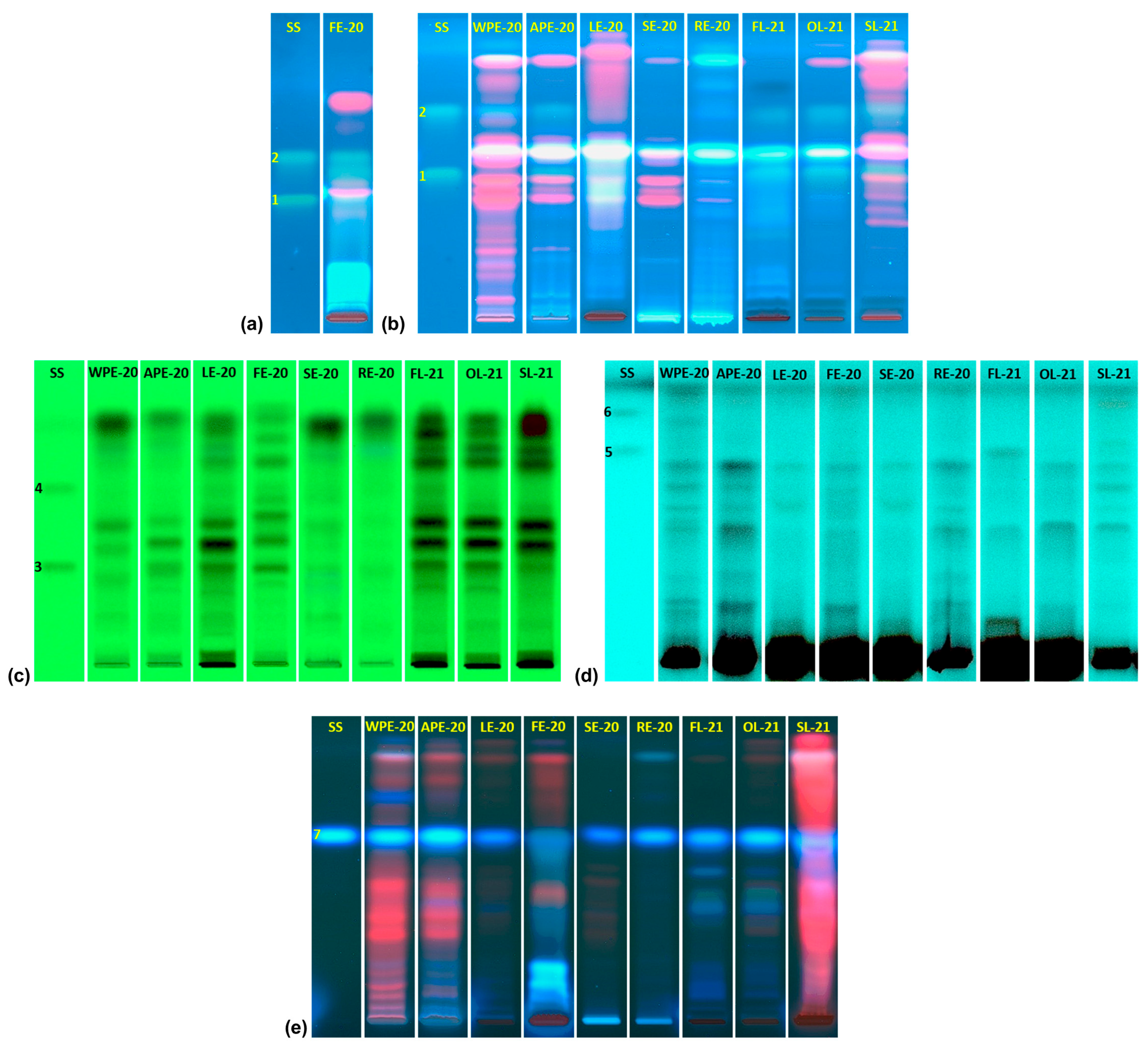
| Extract | Content (mg/g of Extract Dry Weight) | |||||
|---|---|---|---|---|---|---|
| Quercetin | Kaempferol | Rutin | Isoquercetin | Scopoletin | SPC | |
| WPE-20 | N.D | 0.19 ± 0.02 A | N.D | N.D | 0.47 ± 0.05 A | 0.66 |
| APE-20 | 0.42 ± 0.02 A | 0.30 ± 0.02 B | 2.68 ± 0.12 A | N.D | 0.78 ± 0.03 B | 4.18 |
| LE-20 | 0.17 ± 0.02 B | N.D | 4.58 ± 0.19 B | 1.65 ± 0.19 | 1.35 ± 0.07 C | 7.75 |
| FE-20 | 0.03 ± 0.02 C* | 0.17 ± 0.02 A | 23.80 ± 1.74 C | N.D | 0.05 ± 0.01 D | 24.05 |
| SE-20 | N.D | N.D | 0.24 ± 0.05 D | N.D | 0.41 ± 0.06 A | 0.65 |
| RE-20 | N.D | N.D | N.D | N.D | 2.24 ± 0.07 E | 2.24 |
| Extract | Content (mg/g of Extract Dry Weight) | ||||||
|---|---|---|---|---|---|---|---|
| Quercetin | Kaempferol | Rutin | Isoquercetin | Lawsone | Scopoletin | SPC | |
| FL-21 | 0.65 ± 0.05 A | 0.26 ± 0.02 A | 5.51 ± 0.28 A | 2.88 ± 0.24 | 0.99 ± 0.07 A | 0.81 ± 0.03 A | 11.10 |
| OL-21 | 2.03 ± 0.17 B | 0.76 ± 0.02 B | 9.74 ± 0.55 B | N.D | N.D | 1.42 ± 0.09 B | 13.95 |
| SL-21 | 2.72 ± 0.14 C | 1.70 ± 0.05 C | 2.53 ± 0.14 C | N.D | 0.13 ± 0.01 B | 0.16 ± 0.02 C | 7.24 |
| Extract | TPC (mg GAE/g DW) | TFC (mg QE/g DW) | TMA (mg C3GE/g DW) |
|---|---|---|---|
| WPE-20 | 15.59 ± 0.54 A | 23.40 ± 2.68 A | N.D |
| APE-20 | 37.79 ± 1.57 B | 57.97 ± 3.46 B | N.D |
| LE-20 | 156.49 ± 3.10 C | 333.66 ± 11.45 C | 0.14 ± 0.01 A |
| FE-20 | 124.75 ± 6.80 D | 100.48 ± 6.95 D | 4.96 ± 0.14 B |
| SE-20 | 7.50 ± 0.21 A | 11.33 ± 0.28 A | N.D |
| RE-20 | 14.83 ± 0.69 A | 28.88 ± 3.32 A,E | N.D |
| Extract | TPC (mg GAE/g DW) | TFC (mg QE/g DW) | TMA (mg C3GE/g DW) |
|---|---|---|---|
| FL-21 | 342.66 ± 4.34 A | 979.96 ± 43.34 A | 0.29 ± 0.02 |
| OL-21 | 232.10 ± 5.07 B | 534.03 ± 8.53 B | N.D |
| SL-21 | 165.93 ± 0.90 C | 344.70 ± 11.30 C | N.D |
| Extract | ORAC (mmol TE/g DW) | DPPH (mmol TE/g DW) | FRAP (mmol TE/g DW) | FICA (µmol EDTAE/g DW) |
|---|---|---|---|---|
| WPE-20 | 0.69 ± 0.07 A | 0.05 ± 0.00 A | 0.08 ± 0.01 A | 43.50 ± 2.51 A,B |
| APE-20 | 1.52 ± 0.03 B | 0.14 ± 0.00 A | 0.20 ± 0.02 B | 39.90 ± 1.47 A |
| LE-20 | 3.65 ± 0.02 C | 0.81 ± 0.06 B | 0.74 ± 0.02 C | 50.76 ± 3.04 B |
| FE-20 | 4.27 ± 0.07 D | 0.64 ± 0.06 C | 0.62 ± 0.02 D | 3.74 ± 0.12 C |
| SE-20 | 0.36 ± 0.02 E | 0.05 ± 0.00 A | 0.06 ± 0.00 A | 36.00 ± 4.45 A |
| RE-20 | 0.47 ± 0.01 A,E | 0.06 ± 0.00 A | 0.08 ± 0.00 A | 39.12 ± 3.14 A |
| Control (quercetin) | 23.11 ± 0.83 F | 5.87 ± 0.75 D | 12.53 ± 1.06 E | 23.32 ± 0.83 D |
| Extract | ORAC (mmol TE/g DW) | DPPH (mmol TE/g DW) | PFRAP (mmol TE/g DW) | FICA (µmol EDTAE/g DW) |
|---|---|---|---|---|
| FL-21 | 7.15 ± 0.02 A | 2.32 ± 0.13 A | 1.88 ± 0.05 A | 16.83 ± 0.24 A |
| OL-21 | 6.73 ± 0.10 A | 1.35 ± 0.11 B | 1.03 ± 0.03 B | 32.16 ± 1.12 B |
| SL-21 | 4.21 ± 0.36 B | 1.17 ± 0.07 B | 0.79 ± 0.03 C | 34.41 ± 0.43 C |
| Control (quercetin) | 23.11 ± 0.83 C | 5.87 ± 0.75 C | 12.53 ± 1.06 D | 23.32 ± 0.83 D |
| Extract | Minimal Inhibitory Concentration (MIC) (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| S.A | S.E | E.F | E.C | K.P | P.A | S.E.T | AAI | |
| WPE-20 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 0 |
| APE-20 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 0 |
| LE-20 | 10.00 ± 0.00 A | 8.33 ± 2.89 A | 10.00 ± 0.00 A | >10 | >10 | >10 | >10 | 0.32 |
| FE-20 | 8.33 ± 2.89 A | 10.00 ± 0.00 A | >10 | >10 | >10 | >10 | >10 | 0.22 |
| SE-20 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 0 |
| RE-20 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 0 |
| Control * | 2.75 ± 0.64 B | 2.00 ± 0.00 B | 0.50 ± 0.00 B | 0.03 ± 0.00 | 0.03 ± 0.00 | 24.00 ± 6.19 | 0.09 ± 0.02 | N.D |
| Extract | Minimal Inhibitory Concentration (MIC) (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| S.A | S.E | E.F | E.C | K.P | P.A | S.E.T | AAI | |
| FL-21 | >10 | 5.00 ± 0.00 A | 10.00 ± 0.00 A | >10 | >10 | >10 | >10 | 0.27 |
| OL-21 | 5.00 ± 0.00 A | 6.67 ± 2.89 A | 10.00 ± 0.00 A | >10 | >10 | >10 | >10 | 0.42 |
| SL-21 | >10 | 5.00 ± 0.00 A | >10 | >10 | >10 | >10 | >10 | 0.20 |
| Control * | 2.75 ± 0.64 B | 2.00 ± 0.00 B | 0.50 ± 0.00 B | 0.03 ± 0.00 | 0.03 ± 0.00 | 24.00 ± 6.19 | 0.09 ± 0.02 | N.D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado Rodríguez, F.; Azofeifa, G.; Quesada, S.; Weng Huang, N.T.; Loría Gutiérrez, A.; Morales Rojas, M.F. Influence of Plant Part Selection and Drying Technique: Exploration and Optimization of Antioxidant and Antibacterial Activities of New Guinea Impatiens Extracts. Plants 2025, 14, 1092. https://doi.org/10.3390/plants14071092
Delgado Rodríguez F, Azofeifa G, Quesada S, Weng Huang NT, Loría Gutiérrez A, Morales Rojas MF. Influence of Plant Part Selection and Drying Technique: Exploration and Optimization of Antioxidant and Antibacterial Activities of New Guinea Impatiens Extracts. Plants. 2025; 14(7):1092. https://doi.org/10.3390/plants14071092
Chicago/Turabian StyleDelgado Rodríguez, Fabián, Gabriela Azofeifa, Silvia Quesada, Nien Tzu Weng Huang, Arlene Loría Gutiérrez, and María Fernanda Morales Rojas. 2025. "Influence of Plant Part Selection and Drying Technique: Exploration and Optimization of Antioxidant and Antibacterial Activities of New Guinea Impatiens Extracts" Plants 14, no. 7: 1092. https://doi.org/10.3390/plants14071092
APA StyleDelgado Rodríguez, F., Azofeifa, G., Quesada, S., Weng Huang, N. T., Loría Gutiérrez, A., & Morales Rojas, M. F. (2025). Influence of Plant Part Selection and Drying Technique: Exploration and Optimization of Antioxidant and Antibacterial Activities of New Guinea Impatiens Extracts. Plants, 14(7), 1092. https://doi.org/10.3390/plants14071092





