Development of a Feasible and Efficient In Vitro Rescue Protocol for Immature Prunus spp. Embryos
Abstract
1. Introduction
2. Results
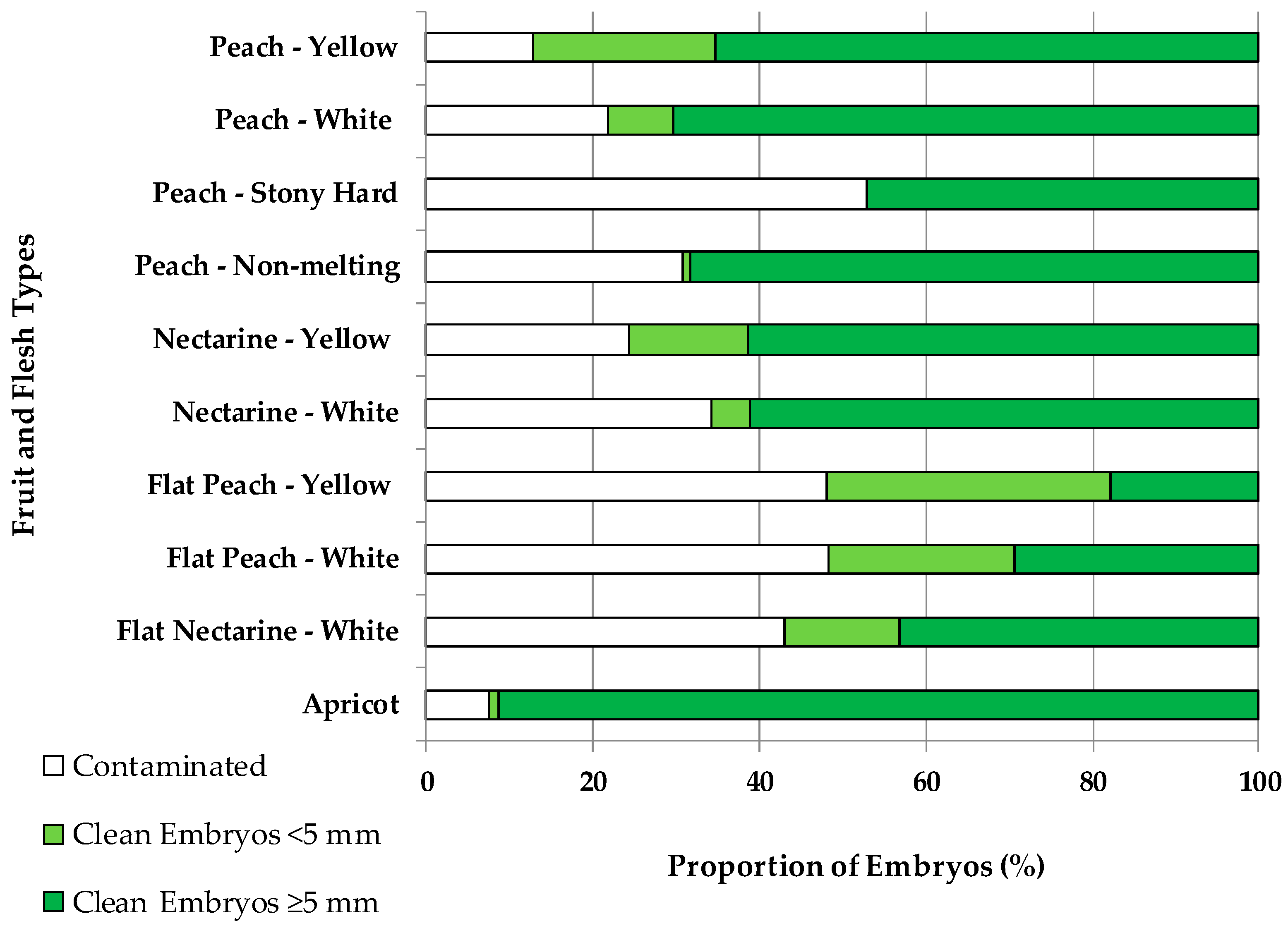
2.1. Contamination and Embryo Size Depending on the Fruit Type
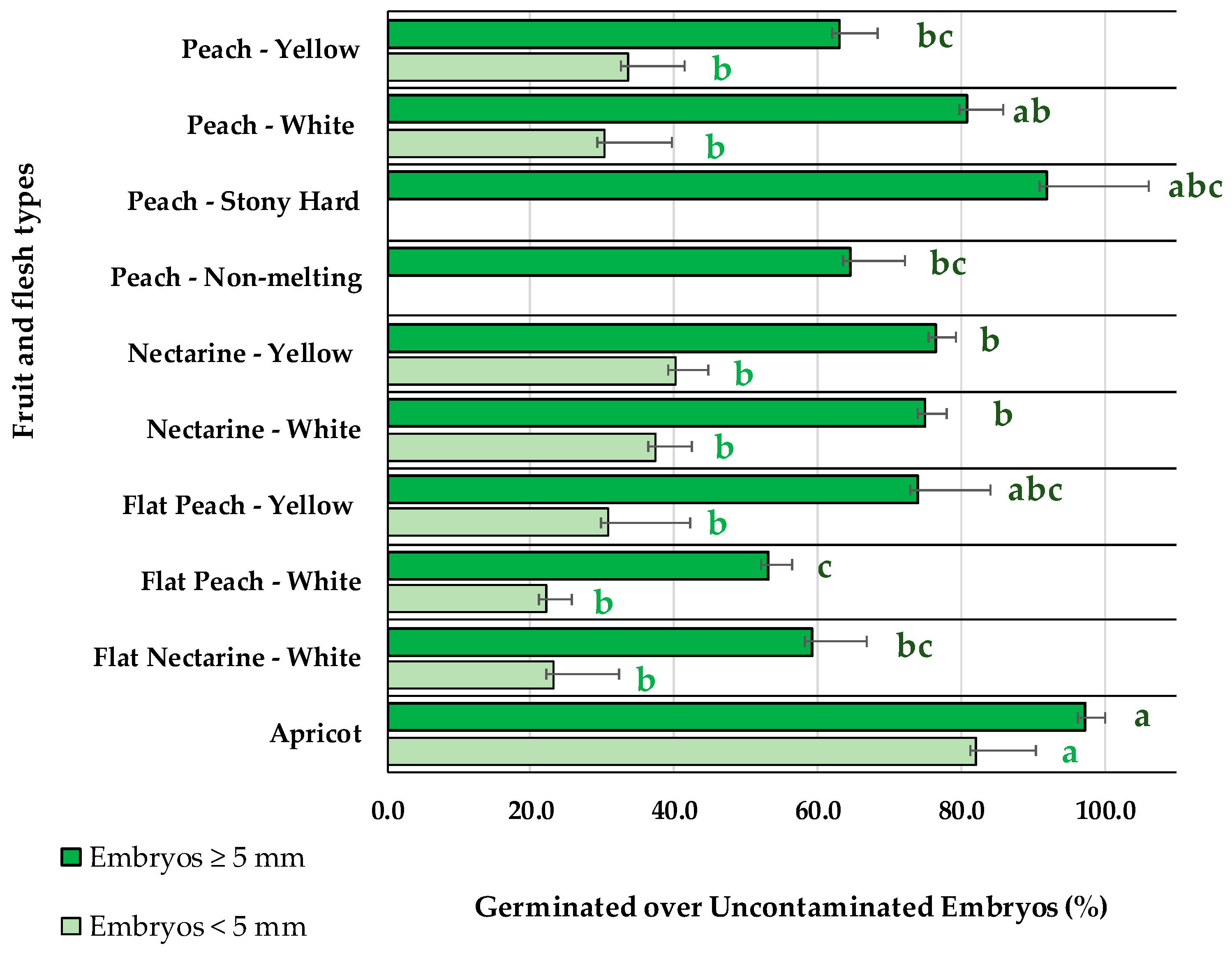
2.2. Embryo Germination Percentages Depending on the Fruit Type and Embryo Size
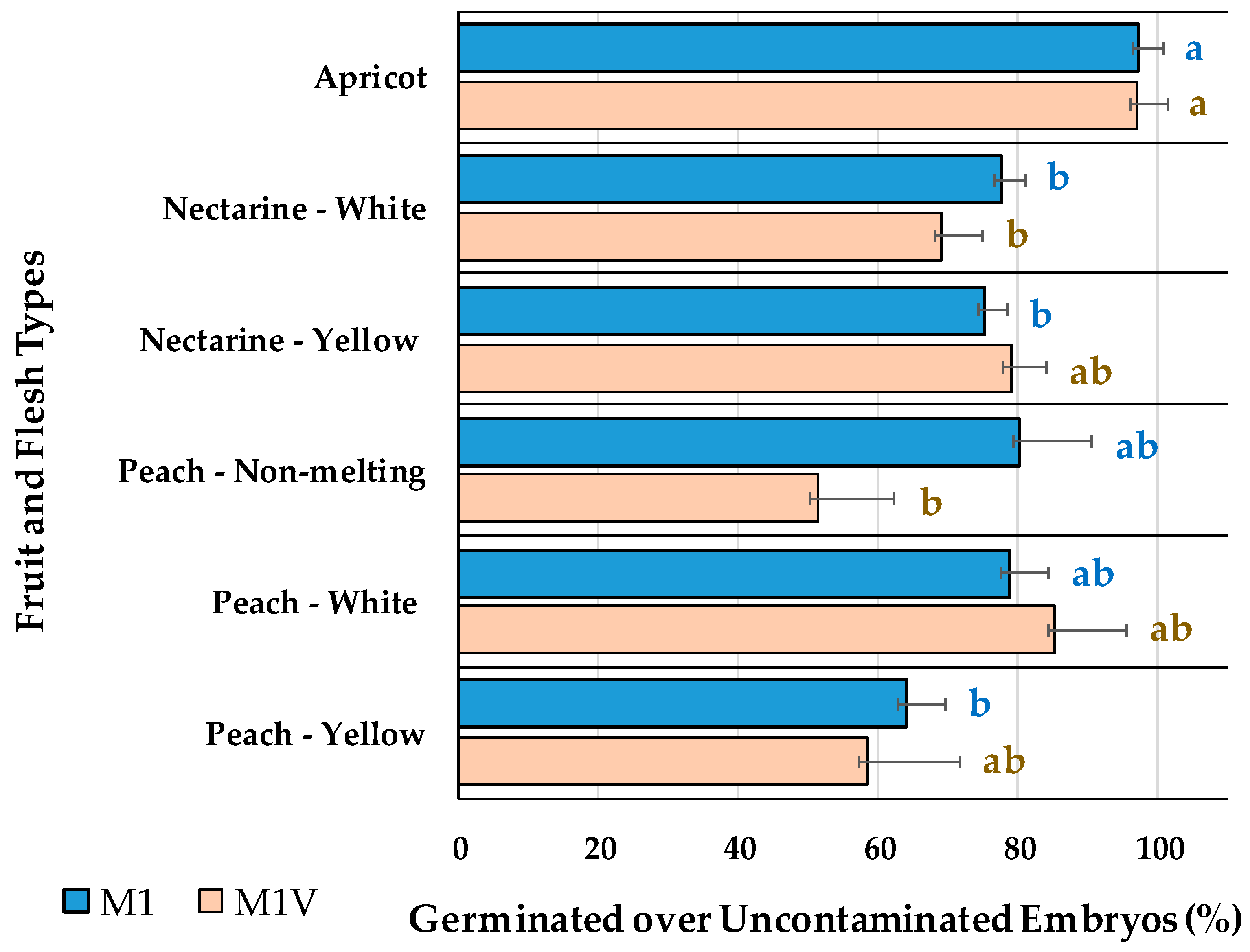
2.3. Embryo Germination Percentages Depending on the Use of Vermiculite
2.4. Effect of Vermiculite on In Vitro Plantlet Growth
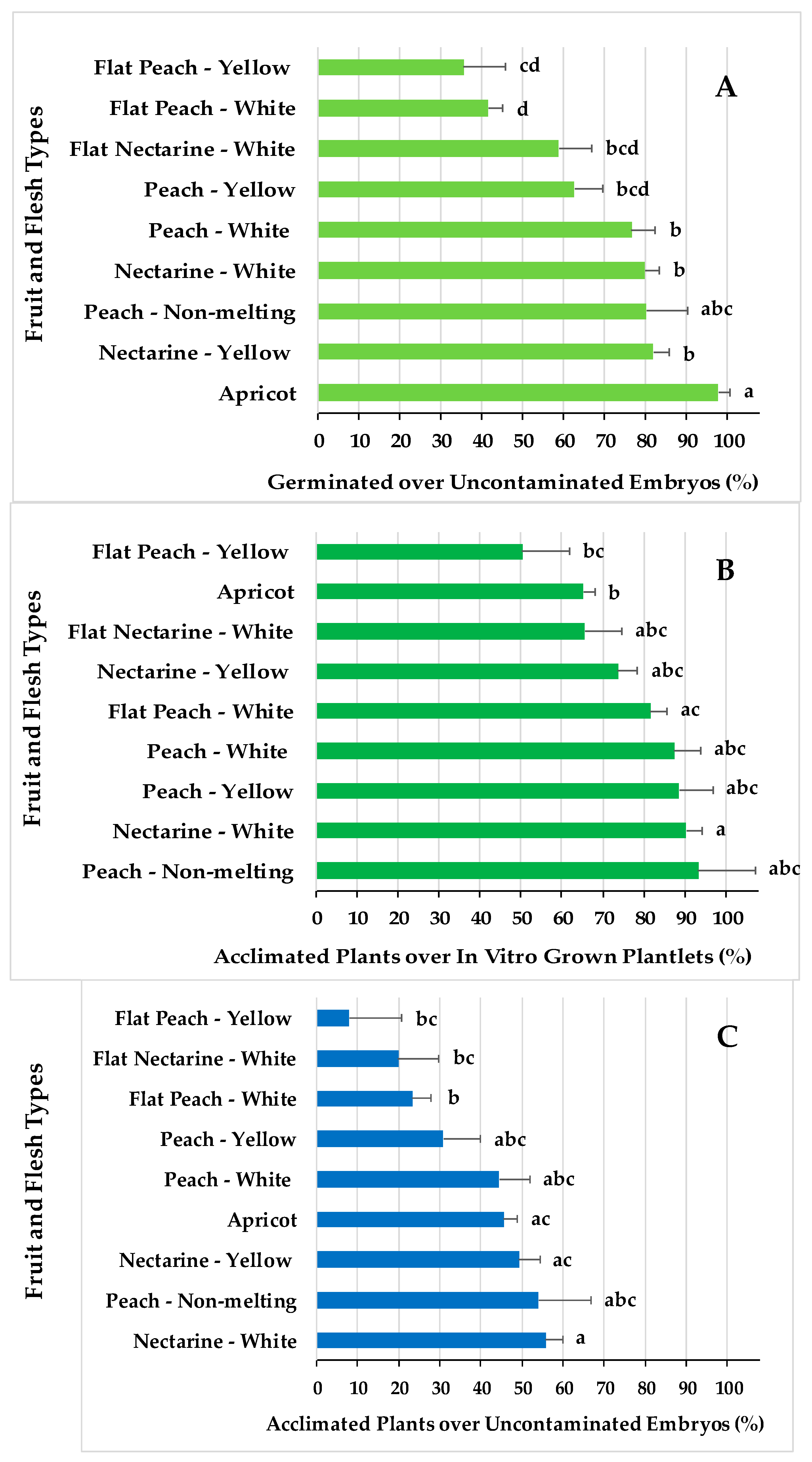
2.5. Embryo Rescue and Viable Plant Production Efficiencies for Different Fruit Types
3. Discussion
3.1. Contamination and Embryo Size Depending on the Fruit Type
3.2. Embryo Germination Percentages Depending on the Fruit Type and Embryo Size
3.3. Embryo Germination Percentage Depending on the Use of Vermiculite
3.4. Effect of Vermiculite on In Vitro Plantlet Growth
3.5. Embryo Rescue and Viable Plant Production Efficiencies for Different Fruit Types
4. Materials and Methods
4.1. Plant Material
4.2. Embryo Dissection and Culture
4.3. Culture Media
4.4. Containers Used for Embryo Rescue
4.5. Cold Stratification and Induction of Embryo Germination
4.6. Plantlet Growth till Acclimatization
4.7. Acclimatization to Greenhouse Conditions
4.8. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szymajda, M.; Żurawicz, E.; Maciorowski, R.; Pruski, K. Stratification Period Combined with Mechanical Treatments Increase Prunus Persica and Prunus Armeniaca Seed Germination. Dendrobiology 2019, 81, 47–57. [Google Scholar] [CrossRef]
- Rizzo, M.; Bassi, D.; Byrne, D.; Porter, K. Growth on Immature Peach (Prunus persica L. Batsch.) Embryos on Different Media. Acta Hort. 1998, 465, 141–144. [Google Scholar] [CrossRef]
- Sinclair, J.W.; Byrne, D.H. In Vitro Growth of Immature Peach Embryos as Related to Carbohydrate Source and PH. Acta Hort. 2002, 592, 157–159. [Google Scholar] [CrossRef]
- Hamill, S.D.; Beppu, K.; Topp, B.L.; Russell, D.M.; Defaveri, J. Effects of Media and Fruit Ripeness on Germination and Transplanting of In Vitro Cultured Embryos from Low-Chill Peach and Nectarine. Acta Hort. 2005, 694, 145–148. [Google Scholar] [CrossRef]
- Promchot, S.; Boonprakob, U. Replacing Agar with Vermiculite, Coconut Fiber and Charcoal Rice Husk in Culture Media for Embryo Rescue of Immature Nectarines Seeds. Thai J. Agric. Sci. 2007, 40, 167–173. [Google Scholar]
- Mansvelt, E.L.; Pieterse, W.-M.; Shange, S.B.D.; Mabiya, T.C.; Cronjé, C.; Balla, I.; Ham, H.; Rubio-Cabetas, M.-J. Embryo Rescue of Prunus Persica: Medium Composition Has Little Influence on Germination. Acta Hort. 2015, 1084, 207–210. [Google Scholar] [CrossRef]
- Singh, H.; Thakur, A.; Jawandha, S.K. Summer Stratification and Germination: A Viable Option for Recovery of Hybrid Seedlings in Low Chill Peach and Nectarines. Indian J. Hortic. 2017, 74, 151–155. [Google Scholar] [CrossRef]
- Devi, I.; Singh, H.; Thakur, A. Effect of Developmental Stage and Medium on Embryo Culture of Low Chill Peach Hybrids. Curr. Sci. 2017, 113, 1771–1775. [Google Scholar] [CrossRef]
- Sundouri, A.S.; Singh, H.; Gill, M.I.S.; Thakur, A.; Sangwan, A.K. In-Vitro Germination of Hybrid Embryo Rescued from Low Chill Peaches as affected by Stratification Period and Embryo Age. Indian J. Hort. 2014, 71, 151–155. [Google Scholar]
- Mancuso, M.L.; Caruso, T.; Germanà, M.A. Peach Breeding Programme for Early Ripening, Low Chilling Requirement Cultivars: Embryo Rescue and Somatic Embryogenesis. Acta Hort. 2002, 592, 125–129. [Google Scholar] [CrossRef]
- Sinclair, J.W.; Byrne, D.H. Improvement of Peach Embryo Culture Through Manipulation of Carbohydrate Source and PH. Hortscience 2003, 38, 582–585. [Google Scholar] [CrossRef]
- Burgos, L.; Ledbetter, C.A. Improved Efficiency in Apricot Breeding: Effects of Embryo Development and Nutrient Media on in Vitro Germination and Seedling Establishment. Plant Cell Tissue Organ Cult. 1993, 35, 217–222. [Google Scholar] [CrossRef]
- Dulić, J.; Ognjanov, V.; Ercisli, S.; Miodragović, M.; Barać, G.; Ljubojević, M.; Dorić, D. In Vitro Germination of Early Ripening Sweet Cherry Varieties (Prunus Avium L.) at Different Fruit Ripening Stages. Erwerbs-Obstbau 2016, 58, 113–118. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.; Liu, G.; Liang, Q.; He, T.; Feng, J. Interspecific Hybridization of Prunus Persica with P. Armeniaca and P. Salicina Using Embryo Rescue. Plant Cell Tissue Organ Cult. 2007, 88, 289–299. [Google Scholar] [CrossRef]
- Schulze, J.A.; Lattier, J.D.; Contreras, R.N. In Vitro Germination of Immature Prunus Lusitanica Seed. HortScience 2017, 52, 1122–1124. [Google Scholar] [CrossRef]
- Sallom, A.; Fatahi, R.; Zamani, Z.; Ebadi, A. Optimization in Vitro Conditions for Plum × Apricot Embryo Rescue and Modeling Some Critical Factors by Using Artificial Neural Networks Technology. Sci. Hortic. 2021, 289, 110487. [Google Scholar] [CrossRef]
- Romeu, J.F.; Sánchez, M.C.; García-Brunton, J. Potential Productivity Evolution of Flat Peach Cultivars (Prunus persica Var. platycarpa) Grown in Different Climatic Conditions of Southeast of Spain. Sci. Hortic. 2015, 197, 687–696. [Google Scholar] [CrossRef]
- Pinto, A.C.Q.; Dethier Rogers, S.M.; Byrne, D.H. Growth of Immature Peach Embryos in Response to Media, Ovule Support Method, and Ovule Perforation. Hortscience 1994, 29, 1081–1083. [Google Scholar] [CrossRef]
- Anderson, N.; Byrne, D.H.; Ramming, D.W. In Ovule Culture Success as Affected by Sugar Source and Fruit Storage Duration in Nectarine. Acta Hortic. 2006, 713, 89–92. [Google Scholar] [CrossRef]
- Ramming, D.W.; Emershad, R.L.; Foster, C. In Vitro Factors During Ovule Culture Affect Development and Conversion of Immature Peach and Nectarine Embryos. Hortscience 2003, 38, 424–428. [Google Scholar] [CrossRef]
- Yildirim, H.; Tilkat, E.; Onay, A.; Çetin Ozen, H. In Vitro Embryo Culture of Apricot, Prunus Armeniaca L. Cv. Hacıhaliloğlu. Int. J. Sci. Technol. 2007, 2, 99–104. [Google Scholar]
- Perez-Jimenez, M.; Guevara-Gazquez, A.; Carrillo-Navarro, A.; Cos-Terrer, J. How Carbon Source and Seedcoat Influence the in Vitro Culture of Peach (Prunus persica L. Batsch) Immature Seeds. HortScience 2021, 56, 136–137. [Google Scholar] [CrossRef]
- Navatel, J.; Bourrain, L. Influence of the Physical Structure of the Medium on in Vitro Rooting. Adv. Hort. Sci. 1994, 8, 57–59. [Google Scholar]
- Tuan, P.N.; Meier-Dinkel, A.; Höltken, A.M.; Wenzlitschke, I.; Winkelmann, T. Factors Affecting Shoot Multiplication and Rooting of Walnut (Juglans regia L.) in Vitro. Acta Hortic. 2017, 1155, 525–530. [Google Scholar] [CrossRef]
- Dolcet-Sanjuan, R.; Claveria, E.; Gruselle, R.; Meier-Dinkel, A.; Jay-Allemand, C.; Gaspar, T. Practical Factors Controlling in Vitro Adventitious Root Formation from Walnut Shoot Microcuttings. J. Am. Soc. Hortic. Sci. 2004, 129, 198–203. [Google Scholar] [CrossRef]
- Oakes, A.D.; Desmarais, T.; Powell, W.A.; Maynard, C.A. Improving Rooting and Shoot Tip Survival of Micropropagated Transgenic American Chestnut Shoots. HortScience 2016, 51, 171–176. [Google Scholar] [CrossRef]
- Kumari, S.; Singh, K.P.; Singh, S.K.; Kumar, S.; Sarkhel, S. Establishment of in Vitro Propagation Protocol for Hybrid Tea Rose Cv. Raktagandha. Indian J. Hortic. 2017, 74, 245–250. [Google Scholar] [CrossRef]
- Kalinina, A.; Brown, D.C.W. Micropropagation of Ornamental Prunus Spp. and GF305 Peach, a Prunus Viral Indicator. Plant Cell Rep. 2007, 26, 927–935. [Google Scholar] [CrossRef]
- Hamill, S.; Promchot, S.; Bignell, G.; Giles, J.; Topp, B. Vermiculite Improves Early Development and Survival of Low Chill Stone-Fruit Embryos Rescued In Vitro. Acta Hort. 2009, 829, 79–84. [Google Scholar] [CrossRef]
- Xu, L.; Li, S.; Shabala, S.; Jian, T.; Zhang, W. Plants Grown in Parafilm-Wrapped Petri Dishes Are Stressed and Possess Altered Gene Expression Profile. Front. Plant Sci. 2019, 10, 637. [Google Scholar] [CrossRef]
- Matuszkiewicz, M.; Koter, M.D.; Filipecki, M. Limited Ventilation Causes Stress and Changes in Arabidopsis Morphological, Physiological and Molecular Phenotype during in Vitro Growth. Plant Physiol. Biochem. 2019, 135, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Noferini, M. Effect of the Type of Closure for Culture Bottles on Micropropagation Efficiency of Apricot. Sci. Hortic. 2013, 161, 306–313. [Google Scholar] [CrossRef]
- Cati, M.; Gennari, F.; Marino, G. Effect of Culture Jar Seal on in Vitro Rooting and Subsequent Acclimatization of Three Italian Apricot Varieties. Sci. Hortic. 2014, 168, 120–123. [Google Scholar] [CrossRef]
- Puc, I.; Alegria, J.; Daniels, D.; Guerra, D.; Williams, S. Effects of Four Different Capping Systems in the Micropropagation of Sugarcane (Saccharum) Variety B79-474. Int. J. Sci. Eng. Res. 2018, 6, 13–16. [Google Scholar]
- Sáez, P.L.; Bravo, L.A.; Latsague, M.I.; Toneatti, M.J.; Coopman, R.E.; Álvarez, C.E.; Sánchez-Olate, M.; Ríos, D.G. Influence of in Vitro Growth Conditions on the Photosynthesis and Survival of Castanea Sativa Plantlets during Ex Vitro Transfer. Plant Growth Regul. 2015, 75, 625–639. [Google Scholar] [CrossRef]
- Anderson, N.; Byrne, D.H.; Sinclair, J.; Millie Burrell, A. Cooler Temperature During Germination Improves the Survival of Embryo Cultured Peach Seed. Resour. Hortscience 2002, 37, 402–403. [Google Scholar] [CrossRef]
- Cantabella, D.; Teixidó, N.; Solsona, C.; Casanovas, M.; Torres, R.; Dolcet-Sanjuan, R. Acidification of the Culture Medium as a Strategy to Control Endophytic Contaminations in Prunus Spp. Rootstocks Cultured in GreenTray TIS Bioreactor. Sci. Hortic. 2021, 290, 110521. [Google Scholar] [CrossRef]
- Cantabella, D.; Mendoza, C.R.; Teixidó, N.; Vilaró, F.; Torres, R.; Dolcet-Sanjuan, R. GreenTray® TIS Bioreactor as an Effective in Vitro Culture System for the Micropropagation of Prunus Spp. Rootstocks and Analysis of the Plant-PGPMs Interactions. Sci. Hortic. 2022, 291, 110622. [Google Scholar] [CrossRef]
- Cantabella, D.; Dolcet-Sanjuan, R.; Teixidó, N. Using Plant Growth-Promoting Microorganisms (PGPMs) to Improve Plant Development under in Vitro Culture Conditions. Planta 2022, 255, 117. [Google Scholar]
- Hesse, C.O.; Kester, D.E. Germination of Embryos of Prunus Related to Degree of Embryo Development and Method of Handling. Proc. Am. Soc. Hortic. Sci. 1955, 65, 251–264. [Google Scholar]
- Arbeloa, A.; Elena Daorden, M.; García, E.; Andreu, P.; Marín, J.A. In Vitro Culture of “Myrobalan” (Prunus Cerasifera Ehrh.) Embryos. Hortscience 2009, 44, 1672–1674. [Google Scholar] [CrossRef]
- Mehanna, H.T.; Martin, G.C.; Nishijima, C. Effects of Temperature, Chemical Treatments and Endogenous Hormone Content on Peach Seed Germination and Subsequent Seedling Growth. Sci. Hortic. 1985, 27, 63–73. [Google Scholar] [CrossRef]
- JiHong, D.; DanDan, W.; ZhengWen, Y.; MingShen, S.; HuiJuan, Z.; XiongWei, L.; XiaNan, Z. Study on the Development of Fruit and Embryo of Flat Peach. Acta Agric. Shanghai 2018, 34, 100–105. [Google Scholar]
- Guo, J.; Cao, K.; Yao, J.-L.; Deng, C.; Li, Y.; Zhu, G.; Fang, W.; Chen, C.; Wang, X.; Wu, J.; et al. Reduced Expression of a Subunit Gene of Sucrose Non-Fermenting 1 Related Kinase, PpSnRK1βγ, Confers Flat Fruit Abortion in Peach by Regulating Sugar and Starch Metabolism. BMC Plant Biol. 2021, 21, 88. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tornero, O.; Burgos, L. Different Media Requirements for Micropropagation of Apricot Cultivars. Plant Cell Tissue Organ Cult. 2000, 63, 133–141. [Google Scholar] [CrossRef]
- Nicolás, E.; Torrecillas, A.; Dell’Amico, J.; Alarcón, J.J. The Effect of Short-Term Flooding on the Sap Flow, Gas Exchange and Hydraulic Conductivity of Young Apricot Trees. Trees—Struct. Funct. 2005, 19, 51–57. [Google Scholar] [CrossRef]
- Rowe, R.N.; Catlin, P.B. Differential Sensitivity to Waterlogging and Cyanogenesis by Peach, Apricot, and Plum Roots. J. Amer. Soc. Hort. Sci. 1971, 96, 305–308. [Google Scholar] [CrossRef]
- Cid, M.; Pina, D.; Capote, I.; Escalona, M.; Daquinta Gradaile, M. Estrategias Para Disminuir La Necrosis Apical En La Multiplicación y El Enraizamiento in Vitro Del Pistacho. Rev. Colomb. Biotecnol. 2016, 18, 97–105. [Google Scholar] [CrossRef]
- Kataeva, N.V.; Alexandrova, I.G.; Butenko, R.G.; Dragavtceva, E.V. Effect of Applied and Internal Hormones on Vitrification and Apical Necrosis of Different Plants Cultured in Vitro. Plant Cell Tissue Organ Cult. 1991, 27, 149–154. [Google Scholar] [CrossRef]
- Carrasco-Cuello, F.; Jené, L.; Dolcet-Sanjuan, R.; Quiñones, A.; Rufat, J.; Torres, E. Differential Response to Calcium-Labelled (44Ca) Uptake and Allocation in Two Peach Rootstocks in Relation to Transpiration under in Vitro Conditions. Sci. Hortic. 2024, 326, 112718. [Google Scholar] [CrossRef]
- Kumar, R.; Kaul, M.K.; Saxena, S.N.; Kumar, P.; Singh, A.K. Micro Propagation Technique in Kinnow Mandarin (Citrus reticulata). Indian J. Agric. Sci. 2016, 86, 297–302. [Google Scholar] [CrossRef]
- Kumar, A.; Arora, R.L. Rapid in Vitro Multiplication of Early Maturing Peaches and Their in Vivo Acclimatization. Indian J. Hortic. 2007, 64, 258–262. [Google Scholar]
- Baskaran, P.; Kumari, A.; Naidoo, D.; Staden, J. Van In Vitro Propagation and Biochemical Changes in Aloe Pruinosa. Ind. Crops Prod. 2015, 77, 51–58. [Google Scholar] [CrossRef]
- Dev, R.; Singh, S.K.; Singh, A.K.; Verma, M.K. Comparative in Vitro Multiplication of Some Grape (Vitis vinifera) Genotypes. Indian J. Agric. Sci. 2015, 85, 1477–1483. [Google Scholar] [CrossRef]
- Hoang, N.N.; Kitaya, Y.; Shibuya, T.; Endo, R. Development of an in Vitro Hydroponic Culture System for Wasabi Nursery Plant Production—Effects of Nutrient Concentration and Supporting Material on Plantlet Growth. Sci. Hortic. 2019, 245, 237–243. [Google Scholar] [CrossRef]
- Cantabella, D.; Dolcet-Sanjuan, R.; Casanovas, M.; Solsona, C.; Torres, R.; Teixidó, N. Inoculation of in Vitro Cultures with Rhizosphere Microorganisms Improve Plant Development and Acclimatization during Immature Embryo Rescue in Nectarine and Pear Breeding Programs. Sci. Hortic. 2020, 273, 109643. [Google Scholar] [CrossRef]
- Alix, M.J.; Savvides, S.; Blake, J.; Herrmann, R.; Hornung, R. Effects of Illumination Source, Culture Ventilation and Sucrose on Potato (Solanum tuberosum) Microtuber Production under Short Days. Ann. Appl. Biol. 2001, 139, 175–187. [Google Scholar] [CrossRef]
- Mohamed, M.A.-H.; Alsadon, A.A. Effect of Vessel Type and Growth Regulators on Micropropagation of Capsicum Annuum. Biol. Plant 2011, 55, 370–374. [Google Scholar] [CrossRef]
- Kornova, K.M.; Popov, S.K. Effect of the In Vitro Container Type on Growth Characteristics of the Microplants in In Vitro Propagation of GF 677. In Proceedings of the I Balkan Symposium on Fruit Growing, Plovdiv, Bulgaria, 15–17 November 2007; Zhivondov, A., Gercheva, P., Koumanov, K., Eds.; Int Soc Horticultural Science: Leuven, Belgium, 2009; Volume 825, pp. 277–281. [Google Scholar]
- Cantabella, D.; Teixidó, N.; Segarra, G.; Torres, R.; Casanovas, M.; Dolcet-Sanjuan, R. Rhizosphere Microorganisms Enhance in Vitro Root and Plantlet Development of Pyrus and Prunus Rootstocks. Planta 2021, 253, 78. [Google Scholar] [CrossRef]
- McCown, B.H.; Lloyd, G. Woody Plant Medium (WPM)—A Mineral Nutrient Formulation for Microculture of Woody Plant-Species. HortScience 1981, 16, 453. [Google Scholar]




| Species | Fruit Type | Flesh Type | Crosses | Fruits | Embryos |
|---|---|---|---|---|---|
| Prunus persica (L.) Batsch | Peach | Yellow | 18 | 2778 | 2838 |
| Prunus persica (L.) Batsch | Peach | White | 16 | 3984 | 3469 |
| Prunus persica (L.) Batsch | Peach | Non-melting (Pavia) | 8 | 434 | 391 |
| Prunus persica (L.) Batsch | Peach | Stony Hard (Albino) | 3 | 717 | 660 |
| Prunus persica (L.) Batsch | Flat Peach | Yellow | 7 | 1350 | 1341 |
| Prunus persica (L.) Batsch | Flat Peach | White | 65 | 10,792 | 10,553 |
| Prunus persica (L.) Batsch | Nectarine | Yellow | 53 | 20,121 | 18,329 |
| Prunus persica (L.) Batsch | Nectarine | White | 47 | 14,684 | 12,555 |
| Prunus persica (L.) Batsch | Flat Nectarine | White | 14 | 4842 | 2009 |
| Prunus armeniaca | Apricot | - | 43 | 3847 | 3811 |
| Total | 274 | 63,549 | 55,956 |
| Species | Fruit Type | Flesh Type | Crosses | Fruits | Embryos |
|---|---|---|---|---|---|
| Prunus persica (L.) Batsch | Peach | Yellow | 6 | 1430 | 1289 |
| Prunus persica (L.) Batsch | Peach | White | 9 | 2234 | 1990 |
| Prunus persica (L.) Batsch | Peach | Non-melting (Pavia) | 3 | 93 | 89 |
| Prunus persica (L.) Batsch | Flat Peach | Yellow | 3 | 370 | 348 |
| Prunus persica (L.) Batsch | Flat Peach | White | 26 | 5089 | 4567 |
| Prunus persica (L.) Batsch | Nectarine | Yellow | 20 | 8053 | 7054 |
| Prunus persica (L.) Batsch | Nectarine | White | 27 | 7524 | 6517 |
| Prunus persica (L.) Batsch | Flat Nectarine | White | 5 | 989 | 799 |
| Prunus armeniaca | Apricot | - | 43 | 3847 | 3811 |
| Total | 142 | 29,629 | 26,464 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanovas, M.; Claveria, E.; Dolcet-Sanjuan, R. Development of a Feasible and Efficient In Vitro Rescue Protocol for Immature Prunus spp. Embryos. Plants 2024, 13, 2953. https://doi.org/10.3390/plants13212953
Casanovas M, Claveria E, Dolcet-Sanjuan R. Development of a Feasible and Efficient In Vitro Rescue Protocol for Immature Prunus spp. Embryos. Plants. 2024; 13(21):2953. https://doi.org/10.3390/plants13212953
Chicago/Turabian StyleCasanovas, Maria, Elisabet Claveria, and Ramon Dolcet-Sanjuan. 2024. "Development of a Feasible and Efficient In Vitro Rescue Protocol for Immature Prunus spp. Embryos" Plants 13, no. 21: 2953. https://doi.org/10.3390/plants13212953
APA StyleCasanovas, M., Claveria, E., & Dolcet-Sanjuan, R. (2024). Development of a Feasible and Efficient In Vitro Rescue Protocol for Immature Prunus spp. Embryos. Plants, 13(21), 2953. https://doi.org/10.3390/plants13212953






