Abstract
Plant-based products play an increasingly vital role in the pharmaceutical industry, including Pogostemon cablin (Blanco) Benth. (patchouli), which is notable for its rich history and extensive use in traditional medicine. Patchouli has a longstanding historical use as a remedy for a wide range of health conditions, including colds, fevers, headaches, inflammation, digestive disorders, and insect and snake bites. Comprehensive phytochemical studies have revealed that patchouli leaves contain diverse valuable bioactive compounds, notably patchouli alcohol, β-patchoulene, pogostone, α-bulnesene, and β-caryophyllene. Recent studies have demonstrated that patchouli leaves exhibit various pharmacological properties, including anti-oxidant, anti-inflammatory, antimicrobial, antidepressant, and anticancer effects. Despite robust traditional knowledge, specific therapeutic applications of patchouli leaves require scientific validation and standardization of their bioactive compounds. This review provides a comprehensive overview of the existing literature on the phytochemical composition, pharmacological properties, and underlying mechanisms of action of patchouli essential oil (PEO) and plant extracts obtained from patchouli leaves. It offers detailed insights into potential therapeutic applications, aiming to inform and guide future research across multiple medical disciplines. Ultimately, this review underscores the need for further research to validate and develop the medicinal applications of patchouli leaves, providing a foundation for future healthcare advancements.
1. Introduction
Medicinal and aromatic plants represent a significant component of the world’s flora and serve as essential sources of raw materials for industries, such as fragrances, cosmetics, food flavoring, and pharmaceuticals [1,2]. These plants have been used in herbal medicine for centuries to address various human health issues. Despite advancements in synthetic pharmaceuticals, the global demand for herbal medicines continues to increase, underscoring their enduring significance and therapeutic potential [1,2,3]. Notably, many contemporary pharmaceutical drugs are derived from plant-based compounds, with traditional plant uses enhanced through modern technological applications [4].
Among the various medicinal species, Pogostemon cablin (Blanco) Benth. (patchouli), a member of the Lamiaceae family, stands out for its extensive use in traditional medicine across South and Southeast Asia [5,6]. Its therapeutic applications are well documented in Indian and Chinese medicine, where it addresses conditions such as exterior syndrome, relieving dampness, counteracting summer heat, and acting as an appetite stimulant and antiemetic [7,8,9]. In traditional Chinese medicine, patchouli is integral to formulations such as Pogostemon Herba and various pills, which are used for their anti-inflammatory properties [10,11]. The medicinal applications of patchouli are widespread in countries such as China, Japan, and Malaysia, where it is used to treat ailments such as diarrhea, colds, headaches, and snake and insect bites [12,13,14].
Patchouli leaf is distinguished by its rich profile of bioactive compounds, including terpenoids (monoterpenoids, sesquiterpenoids, and triterpenoids), flavonoids, lignins, phytosterols, organic acids, glycosides, aldehydes, alcohols, and pyrone [15,16,17]. Among these, the essential oil extracted from its leaves, commonly known as patchouli essential oil (PEO), represents the most prominent and extensively studied component, highly valued for its distinctive spicy aroma and extensively utilized in perfumery and aromatherapy [7,18,19]. Beyond its aromatic qualities, PEO exhibits a broad spectrum of pharmacological activities, including sedative, anti-inflammatory [20], anti-oxidant, astringent, anti-mutagenic, antidepressant, diuretic, and antiseptic [21,22]. These activities are primarily attributed to key constituents, such as patchouli alcohol (patchoulol), pogostone, α-, and β-patchoulene, α-, and δ-guaiene, β-caryophyllene, trans-caryophyllene, and β-elemene [23,24,25]. Additionally, the complex composition of PEO contributes to its diverse therapeutic potential, including antibacterial [26,27,28], anti-insecticidal, anti-allergic [29], immunomodulatory, antithrombotic [30], antiviral, anticancer, cytotoxic, and anti-mutagenic effects [22,31].
The unique properties of the patchouli plant make it a valuable commercial crop with diverse industrial applications globally [9,18,32]. This review analyzes the phytochemical composition, pharmacological activities, and mechanisms of action of patchouli plant extracts derived from patchouli leaves. The goal is to guide future research across various medical disciplines by offering detailed insights into potential therapeutic applications. This review also underscores the need for further studies to validate and expand the medicinal uses of patchouli leaves and establish a foundation for future healthcare innovations. Overall, it highlights the potential for developing innovative, effective, and sustainable therapeutic agents by bridging traditional medicine with modern pharmacology. To gather relevant information on P. cablin (Blanco) Benth. leaves, we conducted a literature search spanning the last 20 years using PubMed, Scopus, and Web of Science (WoS) with the keywords “Pogostemon cablin”, “Patchouli”, “phytochemistry”, and “pharmacological activity”. Additional articles were retrieved through Google Scholar and by screening references. Studies not directly related to P. cablin leaves or lacking sufficient methodological detail were excluded.
2. Botanical Description
2.1. Taxonomy
- Kingdom: Plantae
- Family: Lamiaceae
- Genus: Pogostemon
- Species: Pogostemon cablin (Patchouli)
P. cablin (Blanco) Benth., commonly referred to as patchouli, is a member of the Lamiaceae family, which is one of the most prominent families of flowering plants, comprising 12 subfamilies, 240 genera, and approximately 7200 recognized species [33,34]. The Pogostemon genus, which includes approximately 80 species, is primarily native to Southeast Asia, with 20 species also found in India [35].
2.2. General Habitat and Distribution
Patchouli is a tropical plant well suited to warm climates, originally native to the Philippines but now widely cultivated in several Asian countries, including China, Indonesia, Vietnam, India, and Malaysia, as well as in regions such as Brazil, Singapore, Seychelles, and West Africa [6,9,36]. It thrives under specific conditions: temperatures between 24 °C and 28 °C, relative humidity of approximately 75%, annual rainfall of 2000 to 3000 mm, and altitudes ranging from sea level to 1200 m [37].
2.3. Morphology
P. cablin is a dicotyledonous plant species [38]. Morphologically, it is a perennial, and bushy herb that is well-suited to hot and humid climates. The species typically attains a height of 1–1.2 m, exhibiting an upright stem and ovate stalked leaves measuring approximately 10 cm in length and 2 cm in width [39]. The leaf margins are slightly lobed, and lobs have crenate–serrate teeth [9]. The lobes and apex of the leaves are obtuse. On the undersurface of the leaves and along the ribs, numerous trichomes on the epidermis serve as the primary accumulation sites for PEO [40]. The roots of patchouli are extensive and branched. The roots can penetrate a considerable depth in mature plants that can grow without interference. The plant produces small flowers that are pale pinkish-white in color [41,42]. Leaves, flowers, and seeds freely leave their aromas [35].
3. Phytochemical Composition of Patchouli Leaves
In recent years, research and development have focused on the P. cablin plant as a whole [43,44]; however, studies specifically addressing P. cablin leaves remain relatively scarce. The leaves contain a variety of phytochemical constituents, including volatile and non-volatile compounds [45]. These include monoterpenes, sesquiterpenes, flavonoids, organic acids, glycosides, phenolic compounds, and other chemical constituents [42,45].
3.1. Volatile Chemical Composition
One of the main compounds extracted from patchouli leaves is PEO, which is well-known for its extensive range of pharmacological properties [46]. Several studies have investigated the various compounds present in this PEO [47], resulting in the identification of over 150 different compounds [48]. The chemical composition of PEO, including the volatile compounds, is detailed in Table 1, and the structures of several key volatile compounds are depicted in Figure 1.

Table 1.
The structures of some of the volatile chemical constituents in patchouli leaves.
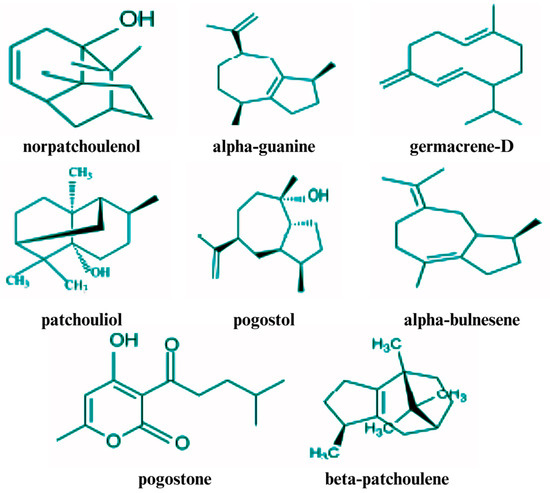
Figure 1.
Structures of some of the volatile chemical compounds in patchouli leaves.
PEO is composed of different phytochemicals, such as sesquiterpenes, ketones, alcohols, and other compounds [60]. However, sesquiterpenes are considered to be the main constituents of PEO [46]. Sesquiterpenes found in PEO include patchoulol (a tricyclic sesquiterpene), pogostone, pogostol, α-, β-, and δ-guaiene, α- and β-bulnesene, α-, β-, γ-, and δ-patchoulene, cis/trans-caryophyllene, and norpatchoulenol. Among these, patchoulol and patchoulene are present in the highest concentrations [61,62,63]. The biological activities of PEO are primarily determined by its constituent compounds, including pogostone, patchoulol, and α- and β-patchoulene [12,43]. Many researchers have identified patchoulol and α-patchoulene as key constituents that significantly influence the quality of PEO [12,64]. Additionally, the distinctive fragrance of PEO is primarily attributed to compounds such as pogostone and patchoulol, along with other sesquiterpene hydrocarbons, such as patchoulene, guaiene, and seychellene, which also play significant roles in defining its aromatic profile [43].
The chemical composition of PEO is highly variable and influenced by factors such as geographic location, environmental conditions, harvest timing, and processing techniques [46,65]. A study focusing on PEO from different regions in China identified nine primary components: patchoulol, pogostone, caryophyllene, α-, β-, and δ-guaiene, β-patchoulene, seychellene, and spathulenol [52]. Furthermore, analysis of leaves harvested from Hainan, China, between June and August revealed PEO contents of 0.8%, 0.7%, and 0.6%, respectively, with the highest concentration of patchoulol observed in June. Furthermore, gas chromatography-mass spectrometry (GC/MS) analysis of PEOs collected over a 24-h period revealed the presence of various compounds, including 1-octen-3-ol, α-guaiene, and β-patchoulene. However, it showed no significant compositional changes associated with harvest timing [59].
GC and GC/MS analyses revealed distinct profiles of PEO from different regions. In China, PEO from Gaoyao County in Guangdong Province was characterized by patchoulol (37.53%), patchouli ketone (pogostone) (21.31%), trans-caryophyllene (6.75%), α-guaiene (6.18%), and seychellene (1.99%) [66]. In Leizhou County, the PEO contain a variety of sesquiterpenes, including patchoulol, α-patchoulene, α-guaiene, aciphyllene, seychellene, and trans-caryophyllene [67]. Additional studies from Zhanxiang reported patchoulol (48.77%), α-guaiene (15.31%), δ-guaiacol (5.17%), α-guaiacol (4.52%), and patchoulone (0.18%), while research in Shipai noted patchoulol (60.59%), δ-guaiacol (6.14%), α-guaiacol (5.53%), and patchoulone (0.14%) [68].
In Vietnam, GC and nuclear magnetic resonance (NMR) analyses of patchouli leaves identified patchoulol as the major component at 32%, followed by α-guaiene at 14.9%, α-bulnesene at 14.6%, seychellene at 8.2%, and α-patchoulene at 5.5%, with another form of α-patchoulene at 5.2% [14,49]. Patchoulol was identified as a more odor-intensive component comprising 32–37% of the PEO [14,46]. In contrast, research in the Philippines indicates that the distinctive aroma of PEO is primarily attributed to germacrene-B, a major sesquiterpene component [69]. Additional volatile compounds identified in PEO include pogostol, δ-cardinene, daucosterol, germacrene-B, and three sesquiterpene hydroperoxides: 1α-hydroperoxyguaia-10(15),11-diene, 10α-hydroperoxyguaia-1,11-diene, and 15α-hydroperoxyguaia-1(10) [41].
In India, GC analysis identified patchoulol as the major component at 57.7%, followed by α-guaiene (13.2%), α-bulnesene (10.4%), seychellene (3.8%), (E)-caryophyllene (3.2%) α-humulene, (1.9%) norpatchoulenol, (1.8%) β-patchoulene, (1.7%), aciphyllene (1.6%), and γ-patchoulene (1.6%) [70]. In Indonesia, GC and GC/MS analyses of PEO have revealed a diverse array of compounds, including monoterpenes, such as α-, β-pinenes, and limonene. Among the sesquiterpenes identified were β-caryophyllene, α-humulene, α-copaene, δ-patchoulene, γ-gurjunene, germacrene D, α-, and δ-guaiene, β-, δ-elemene, seychellene, 7-epi-α-selinene, aciphyllene, and cycloseychellene. Additionally, several patchoulene isomers were identified, including α-, β-, γ-, and δ-patchoulene. Other terpenoids and related compounds identified include patchoulol, pogostol, isopatchoulenone, 9-oxopatchoulol, patchoulenone, caryophyllene oxide, norpatchoulenol, and nortetrapatchoulol [71]. In Brazil, GC/MS analysis identified 29 volatile compounds, including patchoulol (33.25%), pogostol (6.33%), seychellene (6.12%), norpatchoulenol (5.72%), α-bulnesene (4.11%), α-guaiene (2.99%), β-elemene (0.88%), and β-patchoulene (0.46%) [72].
The comparative analysis indicates that, while PEOs from China, Vietnam, India, Indonesia, and Brazil share several common compounds, such as patchoulol, α-guaiene, and seychellene, notable differences exist in their compositions and concentrations. Chinese PEOs exhibit significant regional variation, with some areas, such as Shipai County, producing oils with high levels of patchoulol (up to 60.59%) and unique compounds, like patchouli ketone. Vietnamese PEO is characterized by a balanced composition, with substantial amounts of patchoulol (32%), α-guaiene (14.9%), and α-bulnesene (14.6%). Indian PEO stands out for its exceptionally high patchoulol content (57.7%). Indonesian PEO contains a diverse range of compounds, including α-pinene, limonene, and δ-guaiene, which contribute to its distinct profile. Brazilian PEO, in contrast, features significant amounts of patchoulol (33.25%), pogostol (6.33%), and α-bulnesene (4.11%), along with a unique set of volatile compounds. These variations underscore the influence of geographical and environmental factors on the chemical composition of PEOs across different regions.
3.2. Non-Volatile Chemical Composition
Patchouli leaves are abundant in non-volatile compounds, with over 50 such compounds identified and characterized through various analytical methods [14]. These compounds encompass a range of chemical classes, including flavonoids, lignans, non-volatile terpenoids (e.g., triterpenoids), glycosides, organic acids, and higher molecular weight aldehydes [48,70]. The non-volatile compounds of PEO are detailed in Table 2, and the structures of several key non-volatile compounds are depicted in Figure 2.

Table 2.
The structures of some of the non-volatile chemical constituents in patchouli leaves.
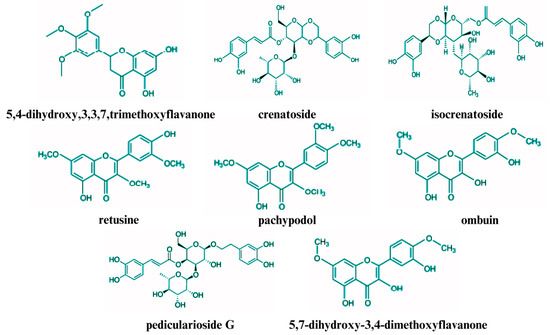
Figure 2.
Structures of some non-volatile chemical compounds in patchouli leaves.
Studies have identified a variety of flavones and other non-volatile compounds in the aerial parts of the patchouli plant, particularly in the leaves [79]. These compounds include ombuin, licochalcone A, 3,3′,4′,5,7-pentahydroxyflavone, 4′,5,7-trihydroxyflavone (a derivative of apigenin), 5,7-dihydroxy-3′,4′-dimethoxyflavone, 5-hydroxy-3′,4′,7-trimethoxyflavone, 5-hydroxy-3′,4′,7-trimethoxyflavanone, 5-hydroxy-4′,7-dimethoxyflavone, 3,5-dihydroxy-4′,7-dimethoxyflavone, and 4′,5-dihydroxy-7-methoxyflavone [22].
In addition to these flavones, numerous studies have identified other significant non-volatile compounds in patchouli leaves, including pachypodol, retusine, stigmasterol, tilianin, dibutyl phthalate, and tschimganical A [80]. Pachypodol has garnered attention owing to its diverse biological activities [81]. Furthermore, patchouli leaves are rich in various glycosides, including phenylethanoid glycosides, such as verbascoside and pedicularioside G, and other glycosides, such as actinosides, campeoside, and isocrenatoside [14]. Recent research has also identified two novel glycosidic epimers, cablinosides A and B [82].
Methanol, ethanol, and hexane extracts of patchouli leaves have been extensively studied and have revealed a wide array of non-volatile chemical compounds. Through column chromatography and spectral analysis, researchers have successfully isolated nine distinct compounds: apigenin-7-O-β-D-(-6″-p-coumaroyl)-glucoside, 3″-O-methylcrenatoside, isocrenatoside, crenatoside, epifriedelinol, 5-hydroxymethyl-2-furfural, succinic acid, β-sitosterol, and daucosterol [83]. Additionally, high-speed countercurrent chromatography (HSCCC) and preparative high-performance liquid chromatography (HPLC) facilitated the isolation of five more compounds, four of which were identified as flavonoids: 5-hydroxy-7,3′,4′-trimethoxyflavanone, 5-hydroxy-3,7,3′,4′-tetramethoxyflavanone, 4′,5-dihydroxy-3′,7-dimethoxyflavanone, and 5,4′-dihydroxy-3,7,3′-trimethoxyflavanone [84]. Advanced analytical methods, such as HPLC-Q-TOF-MS, HPLC, and Thin-layer chromatography (TLC), have been employed to explore non-volatile components further, leading to the identification of compounds such as 7R-campeoside II, 7S-campeoside II, osmanthuside B, 2″,3″-O-acetylmartynoside, and 3′-methoxyisocrenatoside [62,75,76]. Zhou et al. (2013) [85] isolated 13 compounds from the aerial parts of patchouli using column chromatography and spectroscopic techniques. Notable compounds include methyl oleanolate, (−)-guaiacylglycerol, stigmast-4-ene-3-one, oleanolic acid, and 5α-stigmast-3,6-dione [85]. Furthermore, an HPLC-Diode Array Detection (HPLC-DAD) method was developed for the precise quantification of non-volatile components, including eight distinct flavonoids: ombuine, rhamnetin, 5-hydroxy-7,3′,4′-trimethoxyflavanone, 5-hydroxy-3,4′,7-trimethoxyflavone, 3,5-dihydroxy-7,4′-dimethoxyflavone, 4′,5-dihydroxy-3,3′,7-trimethoxyflavone, and 5-hydroxy-3,3′,4′,7-tetramethoxyflavone [74]. These studies collectively highlight the diverse chemical compositions of patchouli leaves and the advanced analytical techniques used to identify and quantify these compounds.
4. Pharmacological Activities
Patchouli leaves display various pharmacological activities attributed to their diverse bioactive compounds. Extensive experimental studies have investigated the pharmacological effects of these compounds, broadly summarized in Figure 3 and detailed separately as volatile and non-volatile in Table 3 and Table 4. These activities include antimicrobial properties, where patchouli leaves demonstrate significant antibacterial, antifungal, and antiviral effects, particularly against influenza virus and HIV/AIDS [39,86,87]. In anticancer and tumor-related activities, patchouli leaf compounds inhibit cancer cell proliferation, induce apoptosis, and modulate the immune system, enhancing its ability to combat tumors and cancer cells [88,89]. Gastrointestinal protective activities are also notable, with patchouli leaves providing protection against gastrointestinal issues, antiemetic effects, regulation of defecation and constipation, anti-peptic ulcer properties, and anti-diarrheal benefits [90,91]. Additionally, patchouli leaves influence blood-related activities by promoting blood coagulation and fibrinolysis and exerting antithrombotic and antihypertensive effects [43,92]. The general protective and therapeutic activities of patchouli leaves include anti-oxidant, analgesic, anti-inflammatory, anti-mutagenic, and insecticidal properties, as well as benefits in treating skin diseases, aromatherapy, and aphrodisiac applications [93,94]. The metabolic and obesity-related activities of patchouli leaves include effects on obesity, intestinal microecology, and diabetes, further highlighting their comprehensive therapeutic potential [95,96].
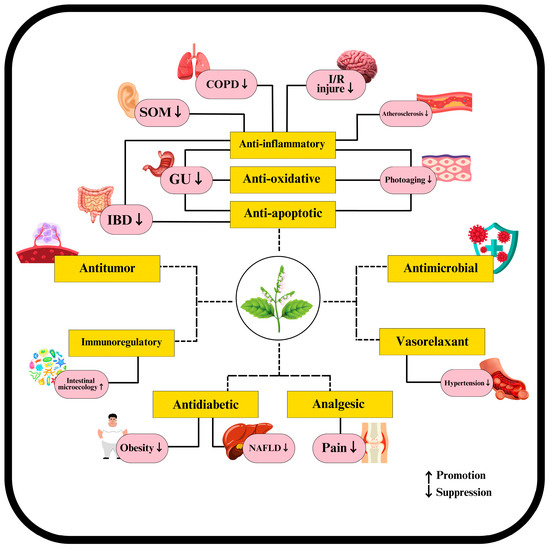
Figure 3.
The pharmacological activities of chemical constituents in patchouli leaves.

Table 3.
The pharmacological activities of the volatile chemical constituents in patchouli leaves.

Table 4.
The pharmacological activities of the non-volatile chemical constituents in patchouli leaves.
4.1. Antimicrobial Activities
4.1.1. Antibacterial Activities
Studies have demonstrated the antimicrobial properties of patchouli leaf extracts, effective against both Gram-negative and Gram-positive bacteria [39,86]. Punithavathy (2012) and Chuwen et al. (2013) showed that ethyl acetate (PCLE) and hexane (PCLH) extracts inhibit the growth of Klebsiella pneumoniae and Staphylococcus aureus in a concentration-dependent manner, with PCLE being more potent, particularly effective against K. pneumoniae at 0.03 mg/mL [127,128]. TLC of ethanol extracts identified terpenes, phenolic acids, and flavonoids, which inhibited the growth of Escherichia coli, Erwinia sp., S. aureus, and Xanthomonas campestris [129]. Aqueous patchouli extracts have also demonstrated enhanced resistance against Moraxella catarrhalis, S. aureus, and Vibrio harveyi, inhibiting biofilm formation by multidrug-resistant V. harveyi at a minimum inhibitory concentration (MIC) of 31.25 mg/mL through modulation of biofilm-associated genes [80,130].
PEO exhibits broad-spectrum antibacterial activity, including against Acinetobacter baumannii, Aeromonas veronii, Candida albicans, Enterobacter aerogenes, Enterococcus faecalis, E. coli, K. pneumoniae, Pseudomonas aeruginosa, Salmonella enterica, and S. aureus [28,103]. It is particularly effective against methicillin-resistant S. aureus (MRSA), with patchoulol and hexane extracts from dried patchouli leaves (Pogostemonis Herba) showing superior antibacterial efficacy [13,46]. PEO also demonstrates vigorous bactericidal activity against Campylobacter jejuni, Listeria monocytogenes, and Shigella flexneri [131]. One of the main constituents, pogostone, demonstrated antibacterial activity with a MIC of 4 µg/mL against S. aureus, disrupting bacterial cell membranes by interacting with membrane proteins [80,132]. Additionally, PEO resolves middle ear infections caused by M. catarrhalis, reducing inflammatory cell infiltration with MICs of 0.21 mg/mL and 0.026 mg/mL [80].
Patchoulol, a key compound in PEO, has potent antimicrobial effects, especially against Helicobacter pylori, which is linked to gastritis, peptic ulcers, and gastric cancer [104,133]. It targets H. pylori while preserving normal gut microbiota, reducing the risk of bacterial resistance [134]. Patchoulol inhibits H. pylori adhesion, motility, and urease activity by downregulating acid resistance genes, thus decreasing urease production [135]. It also protects gastric epithelial cells by preserving mitochondrial function, reducing oxidative stress, and activating anti-oxidant enzymes. Patchoulol’s anti-inflammatory effects modulate NLRP3 and NF-κB inflammasome pathways, and reduce neutrophil recruitment, cytokine production, and H. pylori survival within intracellular lysosomes [42,136].
4.1.2. Antifungal Activities
PEO has been evaluated for its antifungal properties against the pathogenic fungi Aspergillus niger and C. albicans, both in vitro and in vivo, showing significant antifungal activity [28,86,103]. Patchoulol, a major constituent of PEO, has also demonstrated strong efficacy against [103]. Additionally, pogostone, another compound in PEO, and its synthetic analogs exhibit potent antifungal activity against C. albicans [131,137]. Li et al. provided compelling evidence for the therapeutic potential of pogostone, particularly in treating vulvovaginal candidiasis caused by Candida infections [138].
Furthermore, TLC analysis of the ethanol extract from the patchouli plant using solvents, such as ethanol, chloroform, and ether, identified the presence of terpenes, phenolic acids, and flavonoids. This extract exhibited significant antifungal activity, effectively inhibiting the growth of C. albicans and Fusarium oxysporum [129].
4.1.3. Antiviral Activities
Anti-IFV Treatment of HIV/AIDS and Opportunistic Infections
Herbal medicines, such as patchouli, have been studied for their antiviral properties, particularly against influenza. Methanol extracts of patchouli leaves have shown potent antiviral activity, inhibiting the influenza A/PR/8/34 (H1N1) virus by up to 99.8% at 10 μg/mL with an IC50 of 2.6 μM [139]. Patchoulol, a compound in patchouli, also demonstrates significant anti-influenza effects in vivo. In mouse models, patchoulol administration (20–80 mg/kg for 7 days) improved survival rates, reduced viral loads, and modulated immune responses, including T cell levels, antibodies, and cytokine concentrations [35]. Patchoulol increased serum titers of anti-influenza IgA, IgM, and IgG antibodies, as well as CD3+, CD4+, and CD8+ T cells, while also reducing lung inflammation through the regulation of cytokines, such as TNF-α, IL-10, and IFN-γ [140,141].
Wu et al. further confirmed the antiviral efficacy of patchoulol, demonstrating its activity against influenza A (H2N2) with an IC50 of 4.0 μM, supporting its potential as an anti-influenza agent [140]. Additionally, a study found that patchoulol enhanced the innate immune response while suppressing the inflammatory factor IFN-α, reducing inflammation [14]. PEO has also been used in managing HIV/AIDS, particularly for treating opportunistic infections [142,143].
4.2. Anticancer and Tumor-Related Activities
4.2.1. Anticancer Activity
Studies on patchouli have identified several bioactive compounds, including betulinic acid, moronic acid, and pachypodol, which show notable anticancer properties across various cancers, such as oral, lung, breast, colorectal, and pancreatic cancers. These compounds primarily exert their effects through antiproliferative and pro-apoptotic mechanisms [87,89].
Pachypodol, a key bioflavonoid from patchouli, has shown mild cytotoxicity in colon cancer cells, with an LD50 of 185.6 µM for cytotoxicity and 435.8 µM for general toxicity, indicating its potential to inhibit colon cancer cell growth [144]. Other phytochemicals from patchouli also demonstrate antitumor properties, further supporting its therapeutic potential in cancer treatment [145,146].
Patchoulol, another major compound in patchouli, exhibits significant anticancer activity, particularly against colorectal cancer. It inhibits cell proliferation, induces G1 phase arrest in CaCo-2 cells, and reduces tumor growth in animal models following dextran sodium sulfate (DSS)-induced tumorigenesis [147]. Patchoulol targets histone deacetylase 2 (HDAC2) and c-Myc oncogenes and activates NF-kB transcription factors to promote apoptosis, further suppressing tumor growth [148]. It also modulates key cell cycle regulators, including p21, cyclin D1, and CDK4, contributing to its anticancer effects.
Patchoulol’s anticancer effects extend to multiple mechanisms, including autophagy induction and modulation of the Akt/mTOR and EGFR-MAPK pathways, which are crucial for cancer cell proliferation [149]. In non-small cell lung cancer (NSCLC) models, patchoulol reduces cell viability by inducing cell cycle arrest and promoting oxidative DNA damage, as evidenced by increased levels of reactive oxygen species (ROS) and the DNA damage marker 8-OHdG [107].
Patchoulol also activates key DNA damage response pathways, including the phosphorylation of CHK1, CHK2, and H2A.X, while upregulating p21 and p53 levels. It inhibits NSCLC cell proliferation via the ATM/CHK2 and ATR/CHK1 pathways, promoting apoptosis with increased levels of Bax, cleaved caspase-3 and caspase-9, and decreased BCL-2 expression [107].
Furthermore, patchoulol addresses drug resistance in NSCLC by inhibiting the P-glycoprotein (P-gp) efflux pump, which typically reduces the efficacy of chemotherapy. It also impacts cancer stem cell markers, such as CD133 and CD44, which are associated with treatment resistance [150]. By targeting these mechanisms, patchoulol enhances chemotherapy effectiveness, particularly in drug-resistant NSCLC cells, highlighting its potential to improve treatment outcomes [151].
4.2.2. Apoptosis Induction
Research using cell cultures and animal models has suggested that the cytotoxic and anticancer effects of pachypodol may involve several mechanisms. These include the induction of apoptosis and necrosis, activation of both specific and general immune responses, inhibition of cell cycle progression, and the release of β-endorphins into the bloodstream [81]. Based on these findings, research has explored the role of natural compounds in enhancing apoptosis as a part of cancer treatment strategies. For example, mistletoe extract has been used as complementary oncotherapy, particularly for its potential to induce apoptosis in cancer cells. Friedel et al. (2009) conducted a cohort analysis to assess the efficacy of mistletoe extract (Iscador) in patients with non-metastatic colorectal cancer [152]. The study found that the treatment decreased survival rates and induced apoptosis in several cell lines, including human leukemia K562, human plasmacytoma RPMI-8226, and murine lymphocytic leukemia L1210 cells [152]. This apoptotic process is facilitated by the activation of the intrinsic apoptosis pathway, as evidenced by the activation of caspase-9, JNK-1/2, and p38-MAPK, along with the inhibition of ERK-1/2 and PKB phosphorylation and the downregulation of Mcl-1 [81]. Moreover, anti-mutagenic protection is crucial for cancer prevention, with pachypodol showing considerable effectiveness against mutagenic agents [153].
4.2.3. Effects on Tumors/Cancer Cells and the Immune System
Immunoregulatory Effect
The immune system plays a vital role in protecting an organism from pathogens and cancer. Patchoulol, a key compound in patchouli, has been shown to enhance immune functions by activating the mononuclear phagocytic system and suppressing excessive cellular immune responses [154]. PEO also exhibits immunomodulatory effects, notably through the stimulation of secretory immunoglobulin A (SIgA) production, which is crucial in mucosal immunity, defending against toxins, viruses, bacteria, and food antigens [155].
Further research on patchoulol’s immunomodulatory effects in Kunming mice found that it enhances the humoral immune response while suppressing cellular immunity [156]. Additionally, Xinxiang granule (XXG), which includes patchouli along with other herbs, has shown therapeutic benefits for allergic rhinitis and skin irritability [132,157]. PEO’s anti-allergic and anti-nociceptive properties have also been validated in mouse models [158].
Patchouli plants also contain non-volatile compounds, such as ombuin, licochalcone A, and 5,7-dihydroxy-3′,4′-dimethoxy flavanone, which exhibit cytotoxic effects. Licochalcone A has been shown to inhibit PI-PLC gamma 1, inducing terminal differentiation and monocyte production in promyelocytic leukemia cells (HL-60) [42]. Patchoulol inhibits HeLa cell proliferation, reduces cell differentiation, and induces apoptosis in colorectal cancer cell lines by inhibiting histone deacetylase 2, downregulating c-Myc, and activating the NF-κB signaling pathway [159,160]. Furthermore, α-bulnesene, a sesquiterpenoid in patchouli, demonstrates antiplatelet effects by inhibiting cyclooxygenase activity, thus reducing thromboxane production and preventing platelet aggregation [161,162].
4.3. Gastrointestinal Protective Activities
4.3.1. Gastrointestinal Protective Activity
The aqueous extract of the patchouli plant has been shown to protect and preserve the membrane fluidity of intestinal epithelial cells by regulating the serum levels of tumor necrosis factor and nitric oxide (NO). This study establishes an experimental basis for utilizing patchouli for gastrointestinal protection following trauma or surgical procedures [163]. Li et al. investigated the metabolic profile of pogostone both in vitro and in vivo using LC–MS, revealing the significant gastroprotective effects of PEO against gastric ulceration [164]. The mechanisms underlying these anti-ulcerogenic effects are likely related to the stimulation of COX-mediated prostaglandin E2 (PGE2) production along with enhanced anti-inflammatory and anti-oxidant activities [90]. Additionally, PEO administration has been demonstrated to promote the repair of the intestinal epithelial ultrastructure, reduce intestinal permeability, and safeguard the intestinal mucosal mechanical barrier in a rat model [91].
4.3.2. Antiemetic Activity
Patchouli has long been valued for its antiemetic effects and is traditionally used to address conditions such as dyspepsia, vomiting, diarrhea, and poor appetite. Research supports these uses, with studies showing that the n-hexane extract of patchouli leaves significantly reduces vomiting in young chicks induced by copper sulfate (CuSO4), outperforming other extracts, such as water, methanol, and chloroform, by 58.6%. This effect is primarily attributed to patchoulol, which is present in significant concentrations in patchouli and contributes to its antiemetic properties [165]. Additionally, patchoulol has demonstrated calcium ion (Ca2+) antagonist activity in vitro, suggesting that its ability to inhibit Ca2+ influx might alleviate excessive smooth muscle contractions in the digestive system, thereby reducing symptoms, such as diarrhea, nausea, and vomiting [166]. Other compounds from the n-hexane extract, including pogostol, stigmast-4-en-3-one, retusin, and pachypodol, also exhibit antiemetic effects [35].
Additionally, pogostone exhibits notable antiemetic properties and has proven effective in managing conditions such as diarrhea, anorexia, and dyspepsia, as well as in preventing vomiting and stimulating appetite. This therapeutic efficacy is partly linked to its high concentrations of elements, such as aluminum, vanadium, and manganese, which are believed to contribute to its beneficial effects [167].
4.3.3. Defecation and Constipation
Anti-Peptic Ulcer Effect
Studies have shown that PEO influences bowel movements and constipation in two mouse models: one with flaccid bowel movements and another induced by a low-fiber diet [168]. Exposure to the aroma of PEO increased both fecal quantity and dry weight, likely due to the activation of olfactory neurotransmission pathways, which help relieve constipation [169].
Gastrointestinal ulcers, particularly in the stomach and duodenum, are complex disorders with widespread impact, often exacerbated by the chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs) [166,170]. Pro-inflammatory cytokines and apoptotic factors are key in ulcer development [171]. Pogostone, a major component of PEO, has been shown to reduce oxidative stress and protect the gastrointestinal mucosa from ulcers induced by indomethacin. It upregulates anti-oxidant enzymes and reduces malondialdehyde (MDA) levels in rats while increasing prostaglandin E2 levels and modulating apoptosis-related proteins, which helps prevent cellular apoptosis [90,172].
Patchoulol, another component of PEO, similarly protects against gastric ulcers in rat models. Pretreatment with patchoulol reduces the severity of ethanol-induced gastric ulcers by enhancing anti-oxidant enzyme activity and decreasing MDA levels in gastric tissues [173]. Additionally, β-patchoulene, a compound found in patchouli, has gastroprotective effects, with its primary metabolite proving more effective than the parent compound in reducing ulcer size in rat models. This action is mediated through the modulation of the ERK1/2 and NF-κB signaling pathways [174,175].
Inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn’s disease, is a chronic gastrointestinal disorder marked by severe symptoms and mucosal ulceration [176]. Research indicates that PEO alleviates colonic damage and reduces disease activity markers, such as colonic myeloperoxidase (MPO), in rat models of UC induced by 2,4,6-trinitrobenzenesulfonic acid [177]. Patchoulol also reduces MPO levels and pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) while increasing anti-inflammatory cytokines (IL-4, IL-10) in UC. It modulates mucin gene expression and tight junction proteins, which are vital for maintaining intestinal barrier integrity. Patchoulol also influences apoptosis-related proteins and reduces necrosis by downregulating receptor-interacting protein kinase 3 in acute colitis models [178]. Furthermore, patchoulol’s role as a pregnane X receptor (PXR) agonist and its involvement in PXR–NF-κB signaling pathways suggest new therapeutic options for treating colitis.
4.3.4. Anti-Diarrheal Effect
Irritable bowel syndrome (IBS), especially the diarrhea-predominant subtype (IBS-D), is a prevalent functional gastrointestinal disorder [179]. Research shows that patchoulol inhibits spontaneous contractions of colonic longitudinal smooth muscle in a concentration-dependent manner, with an EC50 of 41.9 µM [180]. Additionally, patchoulol has proven effective in alleviating IBS-D symptoms in rat colon models [181]. These findings indicate that patchoulol plays a key role in the anti-diarrheal effects of patchouli, although the exact pharmacological mechanisms remain to be elucidated.
4.4. Blood-Related Activities
4.4.1. Blood Coagulation and Fibrinolytic Activities
In vitro enzymatic assays have assessed various essential oils’ coagulation and fibrinolytic properties, including patchouli oil. These assays evaluate fibrin formation from fibrinogen through thrombin and the subsequent breakdown of fibrin by urokinase. The findings indicated that essential oils, such as chamomile, eucalyptus, and neroli, demonstrated coagulation and fibrinolytic activities. Conversely, PEO and citrus, pine, and frankincense oils exhibited pronounced hyper fibrinolytic activity, highlighting their potential efficacy in fibrin degradation [43].
4.4.2. Antithrombotic Activities
Antihypertensive Activity
Hypertension is a major contributor to disability and early mortality, with over one billion people affected globally and approximately 9.4 million deaths annually [92]. Effective antihypertensive agents can significantly reduce the risk of cardiovascular events, such as stroke and coronary heart disease [182].
Patchoulol has been shown to exert antihypertensive effects through an endothelium-independent mechanism. It inhibits receptor-operated Ca2+ channels (ROCCs) and enhances α-receptor activity, both of which are essential for vasoconstriction [183]. Patchoulol modulates Ca2+ ion influx, reducing potassium chloride-induced contractions in the aorta [7,184]. By interfering with voltage-dependent Ca2+ channels (VDCCs) and membrane depolarization, patchoulol reduces the contraction response typically triggered by phenylephrine (PHE) and potassium chloride [185].
In addition, patchoulol decreases Ca2+-induced muscle contraction in endothelium-aortic rings and counteracts potassium ion depolarization. Combined with ruthenium red and heparin, patchoulol blocks Ca2+ ion channels, such as inositol triphosphate receptors (IP3R) and ryanodine receptors (RYR), which further reduces the effects of PHE [186]. This indicates that patchoulol inactivates ROCCs, limits Ca2+ influx, and enhances α1 receptor sensitivity, contributing to vasoconstriction [187,188].
Patchoulol also demonstrates vasodilatory effects by acting as a Ca2+ ion antagonist. It relaxes smooth muscle and dilates blood vessels by inhibiting Ca2+ influx across vascular smooth muscle cell membranes. This effect is mediated through the disruption of Ca2+ ion influx via the IP3R and RYR pathways [189]. Additionally, pocahemiketal B, a compound derived from PEO, exhibits significant vasorelaxant properties, effectively counteracting PHE-induced contractions in rat aortic rings with an EC50 value of 16.32 µM [188].
4.5. General Protective and Therapeutic Activities
4.5.1. Anti-Oxidant Activity
Oxidative stress, characterized by elevated ROS levels, causes damage to lipids, proteins, and DNA due to free radical activity [93,94]. PEO, known for its free radical-scavenging properties, mitigates oxidative damage by preventing the oxidation of hexanal to hexanoic acid [14]. It protects brain cells from ROS-induced injury and cell death, suggesting potential benefits in neurodegenerative diseases [190]. Additionally, PEO combats photoaging by preserving skin integrity and preventing cellular ATP depletion and cytochrome C release caused by H2O2 [35]. It also neutralizes superoxide anions and hydroxyl free radicals and inhibits lipid peroxidation [191].
Pachypodol, a methoxy flavonoid, has demonstrated protective effects against oxidative damage in various tissues. In a study by Ijaz et al. (2022), pachypodol mitigated perfluorooctane sulfonate (PFOS)-induced testicular injury by scavenging ROS, improving spermatogenesis [124]. In the liver, pachypodol protects hepatocytes from oxidative stress by activating the Nrf2 via ERK1/2 signaling pathway [81].
Patchoulol, another compound from patchouli, protects intestinal epithelial cells (IEC-6) from heat shock-induced oxidative damage. Treatment with patchoulol increased HO1, and Nrf2 expression, enhancing cellular defense mechanisms and reducing markers of oxidative stress such as MDA [32]. However, high doses of patchoulol (above 80 ng) increase ROS production, suggesting a dose-dependent relationship with oxidative stress through the Nrf2-Keap1 pathway [192]. These findings underscore the potential of patchouli-derived compounds in reducing oxidative stress when administered in controlled amounts.
4.5.2. Photoaging Activity
Skin aging, influenced by intrinsic and extrinsic factors, progressively disrupts the structural integrity and physiological function [193]. Photoaging is linked to increased activity of ROS-induced matrix metalloproteinases (MMPs) [194]. These MMP-mediated changes in the extracellular matrix commonly result in skin wrinkling, which is a key sign of premature aging. Pogostone administration has been demonstrated to alleviate both macroscopic and histopathological damage in UV-exposed mouse skin. This protective effect is achieved by boosting the activity of anti-oxidant enzymes, such as catalase (CAT), glutathione peroxidase (GSH-PX), and superoxide dismutase (SOD), while reducing MDA levels [195].
Furthermore, PEO demonstrated significant therapeutic effects on photoaged rat skin by modulating the p38-MAPK/ERK signaling pathway and associated apoptotic pathways. It inhibits the aberrant expression of MMP-1 and MMP-3 and prevents abnormal increases in markers such as Bax, Caspase-9, c-Fos, c-Jun, ERK1/2, MEK, MDA, p38-MAPK, Raf, and Ras, while maintaining levels of Bcl2, CAT, GSH-PX, and SOD [80,196].
4.5.3. Analgesic and Anti-Inflammatory Activities
Analgesic Activity
Pain, a common symptom of many diseases, significantly disrupts normal physiological processes. It is triggered by the activation of nociceptors through various stimuli. Research has shown that patchouli can effectively reduce pain in mice subjected to acetic acid-induced writhing, indicating their analgesic potential in vivo [197]. Cyclooxygenase-2 (COX-2), an enzyme associated with inflammation, is often upregulated in response to inflammatory stimuli and certain cancers [198]. Notably, patchoulol increases the expression of COX-2 mRNA and protein both in vivo and in vitro [197].
Furthermore, the analgesic effects of patchoulol are linked to the mu-opioid receptor (MOR), a key player in pain modulation and relief through the opioid pathways [199]. Upregulation of MOR by patchoulol also affects intracellular Ca2+ levels, indicating its interaction with MOR [77,200,201]. These findings suggest that patchoulol’s dual modulation of COX-2 and MOR is a promising avenue for developing novel analgesic agents.
Anti-Inflammatory Activity
Inflammation, characterized by redness, swelling, heat, and pain, is a key defense mechanism against pathogens, driven by pro-inflammatory mediators, such as IL-1β, IL-6, NO, PGE2, and TNF-α [202]. Lipopolysaccharide (LPS), a component of Gram-negative bacteria, triggers inflammation by activating macrophages to produce these mediators [203].
β-patchoulene exhibits strong anti-inflammatory effects, particularly in LPS-stimulated RAW 264.7 macrophages, where it regulates the balance of pro- and anti-inflammatory cytokines. It reduces IL-1β, IL-6, and TNF-α levels while increasing IL-10 expression. β-patchoulene also inhibits COX-2 and iNOS activity, leading to decreased NO and PGE2 production, and suppresses NF-κB activity by increasing IκBα levels, preventing NF-κB translocation to the nucleus [100,204].
In vivo, β-patchoulene demonstrates dose-dependent anti-inflammatory effects in acute inflammation models, such as carrageenan-induced paw edema, xylene-induced ear edema, and acetic acid-induced vascular permeability. Histopathological analysis shows reduced cell infiltration, MDA levels, and MPO activity in inflamed tissues. Additionally, β-patchoulene lowers pro-inflammatory cytokines (IL-1β, IL-6, TNF-α), NO, and PGE2 and reduces vascular permeability by inhibiting mediators, such as serotonin, prostaglandins, and histamines [205,206].
Patchoulol oxide, an oxidative derivative of β-patchoulene, exhibits superior anti-inflammatory effects by reducing IL-1β, IL-12, MCP-1, and TNF-α expression at both mRNA and protein levels. It is more effective than β-patchoulene in decreasing NO and PGE2 production by inhibiting COX-2 and iNOS pathways [173]. PEO also limits leukocyte recruitment and modulates the NF-κB pathway to balance pro- and anti-inflammatory cytokines [207].
Methanol extracts of patchouli, containing phenolic compounds such as ombuine and pachypodol, show significant anti-inflammatory effects in various mouse models. Pachypodol inhibits NO production, a key player in organ edema pathogenesis, and pogostone reduces pro-inflammatory signaling by blocking p38-MAPK and JNK phosphorylation [80,81].
4.5.4. Anti-Mutagenic Activity
The anti-mutagenic properties of pachypodol have been demonstrated in various assays. The umu test, using S. typhimurium TA1535/pSK1002, assessed its effectiveness against mutagenic chemicals, such as 2-(2-furyl)-3-(5-nitro-2-furyl) acrylamide (furylfuramide), and 3-amino-1,4-dimethyl-5H-pyrido [4,3-b] indole (Trp-P-1). The observed suppression effects in the umu test are likely attributed to the presence of a single methoxy group (–OCH3) at the 4′-position of the aromatic ring [35].
Additionally, pachypodol exhibited significant inhibition of SOS induction, showing more substantial effects at lower doses than furylfuramide. Specifically, at a 0.06 mol/mL concentration, pachypodol reduced over 80% of Trp-SOS-inducing P-1 activity. Additionally, pachypodol demonstrated more potent inhibition of the Trp-P-1-induced SOS response than furylfuramide. The Ames test with S. typhimurium TA100 further confirmed the anti-mutagenic properties of pachypodol against furylfuramide, Trp-P-1, and activated Trp-P-1 [87,208].
4.5.5. Dermato-Protective Activity
PEO, at a concentration of 12%, has proven effective in managing skin infections and odors in patients with ulcers, abrasions, pressure sores, and torn skin. Its application significantly reduced healing time [46]. Furthermore, PEO helps preserve the structural integrity of UV-irradiated skin, prevents photoaging, and enhances the recovery of skin lesions [209,210].
4.5.6. Insecticidal Activity
Controlling vectors and pests remains a significant challenge, especially in developing countries [211]. Pogostone, a major compound, exhibits acute toxicity to cockroaches (LC50 = 8.51 µg/adult) and inhibits dust mites (Dermatophagoides farinae) when extracted with petroleum ether [212]. Additionally, mosquito coils containing a 75:25 patchouli-to-valamus ratio significantly knocked down Aedes aegypti at a 7.5% concentration [213]. Pogostone also demonstrates larvicidal, antifeedant, pupicidal, and growth-inhibitory effects on pests such as Spodoptera spp., indicating its potential for crop protection [214].
Various studies confirm PEO’s larvicidal activity, with lethal concentration 50 (LC50) values ranging from 47.88 ppm against A. albopictus [215] to 254.79 ppm in different assessments [216]. Discrepancies in LC50 values highlight the need for standardized testing. Gokulakrishnan et al. (2013) found patchoulol to be a highly effective repellent, offering 100% protection against insects at 2 mg/cm2 for up to 280 min [217]. This underscores patchoulol’s potential as a superior natural repellent. In addition, PEO extract at 7% concentration provided over 90% protection for up to 6 h against mosquito bites [218], and undiluted oil provided complete repellency for 2 h [219]. These findings demonstrate the efficacy of essential oil-based repellents as alternatives to synthetic insecticides. However, further research is needed to identify the specific constituents of PEO responsible for its insecticidal activity and to understand its bio-insecticidal mechanisms better.
4.5.7. Aromatherapeutic Activity
PEO is widely used in aromatherapy to relieve tension, insomnia, and anxiety [220]. Its distinct wine-like aroma is believed to act as an aphrodisiac, improve concentration, and enhance mental clarity [23]. Spiritually, patchouli incense creates a serene atmosphere [43]. Studies on healthy adults have shown that the fragrance of PEO reduces sympathetic activity, as evidenced by lower serum catecholamine levels and blood pressure [220].
In care settings, applying PEO in an aqueous cream five times daily has been shown to decrease dementia-related behaviors and improve mental alertness [43,221]. Additionally, PEO’s anti-inflammatory and cooling properties help alleviate menopausal symptoms, such as hot flashes and sweating [12,222]. A blend of patchouli with rose, ylang-ylang, jasmine, sandalwood, and vetiver is thought to balance thyroid function and promote harmonious energy flow [223].
PEO also has sedative and uplifting effects on cerebral function [224]. Inhalation of the oil has been shown to reduce physiological stress markers, such as blood pressure, pulse rate, and brain wave activity, suggesting both mood-enhancing and therapeutic properties [46,221]. These effects are primarily attributed to its primary compound, patchoulol [225]. Aromatherapy, including the use of PEO, is increasingly integrated into nursing care to manage stress, reduce chronic pain during cancer treatment, maintain skin integrity, and treat various psychiatric disorders [226].
4.5.8. Aphrodisiac Activities
Patchoulol significantly increased aphrodisiac activity by increasing the production of norepinephrine by 23% when 40 mg/kg patchoulol was administered; however, there was also an increase in dopamine by 15% [227]. Administration of patchoulol increased NO [228]. Upregulation of NO from the endothelium and specific tissues induces the relaxation of blood vessels and smooth muscle in the penile; the relaxation of trabecular smooth muscle also lowers blood flow resistance accompanied by a decrease in vascular resistance, which influences blood flow in the penile, and decreased blood outflow triggers penile erection and engorgement with blood [22].
4.5.9. Antidepressant Activity
A study investigating the antidepressant properties of patchouli plant extracts revealed significant findings with a 70% ethanol extract. When administered at doses of 500 mg/kg and 750 mg/kg, the extract notably reduced the immobility time in rats during both the despair swimming and tail suspension tests, compared to the vehicle control. Moreover, Dunnett’s test indicated that the 750 mg/kg dose significantly decreased spontaneous locomotor activity relative to the vehicle-treated group [35].
4.6. Metabolic and Obesity-Related Activities
4.6.1. Gut Microbiota Modulatory Activity
Human gut microbiota plays a crucial role in maintaining homeostasis, influencing immune responses, nutrient absorption, and defense against pathogens [96,229]. An imbalance in the gut microbiota (dysbiosis) can lead to diseases such as IBD, obesity, and cancer [230,231].
Recent studies highlight that PEO, including key compounds such as pogostone, patchoulol, and β-patchoulene, positively influences gut health. These compounds strengthen the gut epithelial barrier, modulate macrophage activity, and improve gut microbiota composition. In mouse models, PEO has been shown to enhance gut microbiota diversity, reduce pro-inflammatory cytokines, and promote the transition of macrophages from the M1 to the M2 phenotype, supporting immune regulation and reducing inflammation [232].
PEO also aids in the production of antimicrobial proteins by upregulating goblet and Paneth cells, promoting mucin synthesis, and enhancing the secretion of lysozymes and defensins, which inhibit harmful bacteria [233,234]. Moreover, patchouli compounds improve gut barrier function by enhancing tight junction protein activity, which is crucial for maintaining epithelial integrity and preventing cancer development [235,236].
Additionally, PEO and its components inhibit adhesion molecules, such as intracellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), which are involved in tumor progression, suggesting their potential role in cancer suppression [237,238]. Patchouli compounds also modulate macrophage polarization by reducing pro-inflammatory M1 markers (e.g., CXCL10 and iNOS) and promoting M2 markers (e.g., arginase-1, mannose receptor), further supporting cancer suppression and reducing inflammation [134].
Beyond their direct effects on immune modulation, patchouli compounds enhance gut microbiota health by promoting beneficial bacteria, such as Akkermansia muciniphila, and increasing short-chain fatty acid (SCFA) production, which plays a role in immune regulation and anti-inflammatory responses [239]. The modulation of the gut microbiota, combined with epithelial nourishment, suggests that PEO and its active compounds exhibit significant potential in preventing and managing inflammatory diseases and cancer.
4.6.2. Antidiabetic Effect
Obesity is strongly associated with the development of type 2 diabetes and is a major risk factor for several metabolic disorders [240]. Recent studies have highlighted the impact of patchoulol on obesity management. Administration of patchoulol results in a significant reduction in body weight in high-fat diet (HFD)-induced obese mice, primarily by inhibiting adipogenesis and fat accumulation in adipocytes through enhanced β-catenin expression and activation [241]. Furthermore, inhalation of PEO at concentrations ranging from 0.3% to 1% markedly improves metabolic parameters in obesity-induced rats [242]. Chronic intake of high-fat diets is linked to various health issues, including non-alcoholic fatty liver disease (NAFLD), which affects approximately 38% of the population and is the most prevalent chronic liver disorder globally [243,244]. Recent studies have demonstrated that patchoulol exerts protective effects against HFD-induced hepatic steatosis in rats by alleviating endoplasmic reticulum stress and modulating the metabolism of very low-density lipoproteins (VLDL). Patchoulol also influences the expression of apolipoprotein B100, VLDL receptors, and microsomal triglyceride transfer protein, thereby improving liver health [245].
4.7. Other Activities
Secretory otitis media, marked by middle ear inflammation, tympanic effusion, and hearing loss, often arises from bacterial infections causing auditory tube dysfunction [246]. In guinea pig models, pogostone alleviates secretory otitis media-related hearing loss by reducing mucosal thickening and neutrophil infiltration, primarily inhibiting ICAM-1 and TNF-α expression in the ear mucosa [80]. Similarly, patchoulol has demonstrated anti-inflammatory effects in lung diseases, such as acute respiratory distress syndrome and chronic obstructive pulmonary disease (COPD) [247]. In mouse models of LPS-induced acute lung injury, patchoulol effectively suppressed pro-inflammatory cytokines IL-1β, IL-6, and TNF-α and inhibited the phosphorylation of IκB-α and p65 NF-κB [102,248]. Pogostone also offers protection against lung injury in COPD by decreasing inflammatory proteins p-IκBα and p-NF-κBp65 while increasing HO-1 and Nrf-2 expression, indicating a central role of the NF-κB signaling pathway in lung tissue preservation [80]. Additionally, patchoulol has shown cardiovascular benefits in atherosclerosis models, reducing atherosclerotic plaques, macrophage infiltration, and inflammatory cytokines in ApoE knockout mice [249].
5. Mechanisms of Action
Recent experimental studies have expanded our knowledge of patchouli’s pharmacological properties, revealing a broad spectrum of therapeutic effects. These include anti-peptic ulcer [172], antimicrobial [62,122,250], anticancer [111], antioxidant [81,251], anti-inflammatory [7,248], ischemia-reperfusion injury protection [252], analgesic [253], antitumor [107,254], antidiabetic [255], antihypertensive [200], immunoregulatory [256], intestinal microecology [134], and anti-diarrheal activities [20]. Patchouli leaves-derived compounds, such as β-patchoulene, pachypodol, patchoulene epoxide, patchoulol, and pogostone, have shown protective effects in various organs, including the intestines, middle ear, and liver [36,257]. Advances in research methodology have allowed for an in-depth investigation of these monomeric components, with patchoulol and pachypodol being particularly well-studied. Their therapeutic efficacy is linked to the suppression of inflammation, oxidative stress reduction, apoptosis regulation, endoplasmic reticulum stress mitigation, and VLDL metabolism enhancement, as illustrated in Figure 4 [248].
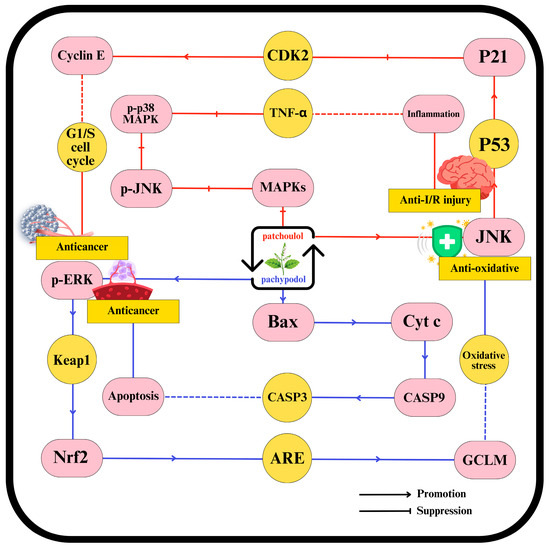
Figure 4.
The molecular pathways are activated by patchoulol and pachypodol from patchouli leaves.
Recent studies have identified multiple signaling pathways through which patchoulol exerts its therapeutic effects. For peptic ulcer treatment, patchoulol activates the PXR pathway while inhibiting the nuclear factor NF-κB pathway. These actions help maintain intestinal barrier integrity, reduce tryptophan catabolism, and inhibit cell death pathways. Key molecular changes include reduced levels of pro-inflammatory cytokines, downregulation of necroptosis-related proteins (MLKL and RIP3), decreased expression of indoleamine 2,3-dioxygenase-1 and tryptophan hydroxylase 1 (TPH-1), lowered anti-apoptotic Bcl-2, and pro-apoptotic Bax [248].
In diabetes treatment, patchoulol inhibits crucial pathways, such as activating transcription factor 6 (ATF6), inositol-requiring enzyme 1 (IRE1), protein kinase RNA-like ER kinase (PERK), and Wnt/β-catenin. This inhibition results in downregulation of ATF6, IRE1, and PERK; inhibition of very low-density lipoprotein receptor (VLDLR); increased apolipoprotein B 100 (apoB 100); enhanced microsomal triglyceride transfer protein (MTP) expression; increased Smad7 expression; and stabilization of β-catenin [258].
Patchoulol also mitigates ischemia-reperfusion injury by activating the ERK pathway and inhibiting the mitogen-activated protein kinase (MAPK) pathway [259]. Moreover, emerging research indicates patchoulol’s potential to prevent obesity and provide analgesia, though the underlying mechanisms are still being explored [258]. Pachypodol has been shown to protect hepatocytes from oxidative damage, likely through ERK1/2-dependent activation of Nrf2, which enhances endogenous antioxidant defenses [81]. Additionally, pachypodol exhibits cytotoxic and anticancer properties by inducing apoptosis and necrosis through mechanisms involving the activation of Bax, caspase-3, caspase-9, JNK-1/2, and p38 MAPK while inhibiting ERK-1/2 and PKB phosphorylation, and downregulating Mcl-1. It also offers significant anti-mutagenic protection, suggesting its potential for cancer prevention [81]. Despite these promising findings, further research is needed to elucidate other patchouli compounds’ molecular mechanisms and protein targets and address safety concerns through comprehensive preclinical studies. As existing research primarily relies on in vitro and small animal models, there is an urgent need for human clinical trials to validate these pharmacological effects and facilitate the development of patchouli-based traditional medicine formulations for broader applications.
6. Commercial Applications
6.1. Applications in Pharmaceuticals: Role in Medicine
Patchouli leaves have gained attention in the pharmaceutical industry due to their diverse pharmacological properties. The essential oil extracted from patchouli contains bioactive compounds, such as patchoulol, α-bulnesene, and pogostone, which exhibit anti-inflammatory, anti-mutagenic, and antioxidant effects [87,260]. In traditional Chinese medicine, patchouli is used in formulations, such as the Baoji and Houdan Pills, to treat inflammatory diseases [10,11]. Hopitan pills derived from patchouli address stomach discomfort, chest pain, diarrhea, and vomiting [167]. Globally, patchouli is used in various cultures for therapeutic purposes. In Uruguay, leaf infusions treat nervous disorders, while crushed leaves are applied topically to relieve rheumatism and arthritis [35]. Patchouli is also central to Ayurvedic formulations, such as Rasa, Guna, and Virya, and is recognized in several Asian countries for its antidepressant, antiseptic, and aphrodisiac properties [12,22,91]. These diverse applications suggest its potential for developing new therapeutic agents [42,261].
Recent research has focused on patchouli’s potential in treating skin infections and inflammatory conditions, as well as an adjunct therapy in cancer. PEO has demonstrated effectiveness against bacterial and fungal pathogens, such as Cryptococcus neoformans, C. albicans, herpes simplex, and methicillin-resistant S. aureus in AIDS patients, indicating its promise for developing new antibiotics and antifungal agents [103,262]. Additionally, PEO inhibits dermatophytes and fungi such as Scopulariopsis brevicaulis and Chaetomium globosum, suggesting its potential for treating pneumonia and chronic meningitis in 8–30% of AIDS patients [57].
6.2. Applications in Cosmetics: Role in Beauty and Skincare
PEO is employed extensively in the cosmetic industry due to its fragrant properties and ability to fixate aroma, particularly in the production of perfumes, aromatic incense, and other scented items [172,263]. It is a common ingredient in soaps, cosmetics, after-shave lotions, detergents, and various fancy products [2,264,265]. In addition to its aromatic appeal, PEO has numerous skin benefits [266]. Its anti-inflammatory and antiseptic properties effectively treat acne, eczema, and other skin conditions [36].
The regenerative properties of PEO contribute to wound healing and scar reduction, making it a valuable ingredient in anti-aging products. It enhances muscle, skin, and nerve contractions, which helps improve gum adherence to teeth, reduces hair loss, decreases muscle looseness, and prevents skin sagging [267]. Additionally, its antimicrobial properties promote skin health and hygiene, further supporting its use in cosmetic formulations [268]. The versatility of PEO was also demonstrated by its effectiveness in spray gels and microemulsions, offering both aesthetic and therapeutic benefits in beauty and skincare applications [269].
6.3. Other Industries: Additional Commercial Applications
In addition to pharmaceuticals and cosmetics, patchouli has applications in several industries. PEO is sometimes used as a flavoring agent in the food and beverage industry because of its distinctive taste and aroma [45]. PEOs are recognized by the Food and Drug Administration (FDA) as a safe, natural flavoring component for human consumption. They are commonly used as flavoring agents in various food products, including meat and meat products, gelatin, frozen dairy desserts, and non-alcoholic and alcoholic beverages [270,271]. The agricultural sector utilizes patchouli as a natural pesticide and insect repellent because of its potent insecticidal properties [272]. The bioactive compounds in patchouli exhibit selective toxicity, minimizing adverse effects on higher organisms and the environment [272,273]. Moreover, PEO is employed in aromatherapy for its calming and grounding effects, which help alleviate stress and anxiety. The fragrance industry also uses patchouli leaves to form incense and scented candles, capitalizing on their long-lasting aroma [274].
Additionally, manufacturers have integrated the aroma of patchouli into air fresheners, paper towels, laundry detergents, and other similar products to enhance their fragrance [275]. Overall, the multifaceted applications of patchouli leaves in pharmaceutical, cosmetic, and other industries highlight their significant commercial potential and contribution to various sectors. Advancements in extraction methods, chemical analyses, and economic studies have enhanced the commercial value of patchouli leaves, positioning them as versatile resources in the commercial sector [276].
7. Challenges and Limitations
7.1. Phytochemical and Genetic Variabilities
Despite significant advancements in the study of the patchouli plant, and specifically its leaves, several challenges and limitations have affected our understanding and utilization of this valuable plant resource. The phytochemical composition of patchouli leaves can vary significantly owing to geographical and environmental factors, including geographical location, climate, soil conditions, and cultivation practices, all of which influence the consistency and efficacy of therapeutic products derived from leaves [16]. Genetic variability among different strains or cultivars of patchouli contributes to diverse profiles of its active compounds, further leading to inconsistent therapeutic effects. Mechanistic studies are needed to elucidate the molecular mechanisms underlying the pharmacological effects of patchouli, which are not well understood [48]. Furthermore, rigorous, large-scale clinical trials are scarce to substantiate the therapeutic claims associated with patchouli extracts [277].
7.2. Methodology and Quality Control
Methodological challenges in current research on patchouli leaves include a lack of standardized extraction methods [278]. The techniques for extracting active compounds from patchouli leaves can influence the quality and concentration of phytochemicals, highlighting the need for standardized extraction protocols to ensure consistency. The distillation process for extracting PEO is time-consuming, primarily because of the high sesquiterpene content of the leaves. This extended distillation time can affect the efficiency of oil extraction processes and may require innovative approaches to streamline production while maintaining the oil quality [45].
Additionally, some studies suffer from inadequate experimental design, including insufficient control groups and small sample sizes, which can compromise the validity and reproducibility of the findings. Another methodological challenge is the reliance on conventional analytical techniques that may not detect all bioactive compounds, highlighting the need for more advanced methods [21]. Quality assurance is also crucial, as contamination with pesticides, heavy metals, or adulterants can pose significant health risks, emphasizing the importance of ensuring the quality and purity of patchouli extract. Addressing these methodological limitations is crucial for enhancing the scalability and sustainability of PEO production [45].
7.3. Regulatory, Standardization and Safety Issues
Regulatory, standardization, and safety challenges further complicate the commercialization of patchouli products. Obtaining regulatory approval for therapeutic use involves rigorous testing and compliance with safety standards, making the process time-consuming and expensive. A significant issue is the lack of standardized guidelines for the quality control of patchouli leaf products, leading to variable product quality and efficacy [279]. Regulatory challenges include inconsistencies in legislative frameworks governing the use and distribution of herbal products, which can affect their marketability and acceptance. These regulatory and standardization challenges hinder the consistent therapeutic use of patchouli products [280]. Finally, comprehensive toxicological studies are necessary to ensure the safety of patchouli-based products, as potential side effects, interactions with other drugs, and long-term safety profiles must be thoroughly investigated. Ensuring consistency in product quality and adherence to regulatory standards are essential for market acceptance and consumer trust. Harmonizing regulations and establishing industry standards for patchouli products can help overcome these challenges and promote market growth [281].
7.4. Bioavailability and Efficacy
Another challenge is the low bioavailability of certain phytochemicals in patchouli leaves, which means that they are not easily absorbed or metabolized by the human body, and enhancing their bioavailability is essential for the effective therapeutic use of these compounds [282]. Variations in the composition of bioactive compounds, such as patchoulol, across different plant parts and growth stages can affect the overall efficacy of patchouli-based products [283]. Additionally, the efficacy of these extracts can be influenced by factors such as extraction method, formulation, and individual patient differences [19]. Finally, determining the optimal dosages that are both effective and safe is complex and requires extensive clinical testing and validation. Achieving consistent bioavailability and therapeutic effects requires a deeper understanding of the factors influencing plant compound accumulation and activity. Developing strategies to enhance bioavailability and ensure consistent efficacy is crucial for maximizing patchouli leaves’ medicinal and commercial value [284].
Although patchouli leaves offer various commercial applications, challenges and limitations impede their full utilization. Addressing research gaps, overcoming methodological issues, navigating regulatory complexities, and optimizing bioavailability are essential steps in advancing the understanding and commercialization of patchouli products [285]. By addressing these challenges through interdisciplinary research and collaborative efforts, the potential of patchouli leaves as a valuable herbal resource can be fully realized.
8. Future Perspectives
8.1. Emerging Trends: New Directions in Research
Emerging trends in research on patchouli leaves indicate a growing interest in understanding their comprehensive phytochemical profiles and their health implications. Advances in analytical techniques, such as MS and HPLC, have facilitated more detailed studies of the chemical constituents of patchouli [46]. There is a growing emphasis on the genetic engineering of patchouli plants to optimize the production of specific bioactive compounds. The use of omics technologies, including genomics, proteomics, and metabolomics, has become increasingly prevalent and provides deeper insights into the metabolic pathways involved in the biosynthesis of these compounds [286]. By leveraging biotechnological approaches, researchers aim to enhance the sustainable production of PEO components, opening new avenues for industrial applications and therapeutic uses [287].
8.2. Potential for New Applications: Opportunities for Novel Therapeutic Uses
The potential for new applications of patchouli extends beyond their traditional use. Research currently explores its efficacy in treating various conditions, including inflammatory and neurodegenerative diseases. The antioxidant and anti-inflammatory properties of patchouli extracts suggest their potential use in managing conditions such as arthritis and Alzheimer’s disease [288]. Additionally, the antimicrobial properties of patchouli are being investigated to develop novel antibiotics and antifungal agents. Understanding the metabolic dynamics of patchouli leaves can lead to the development of targeted interventions to modulate bioactive compound production for specific applications in medicine, cosmetics, and other fields [39].
8.3. Directions for Future Studies: Suggestions for Further Research
Future studies on patchouli should address several key areas to advance this field. First, more comprehensive phytochemical analyses are needed to identify and quantify bioactive compounds in different parts of the plant [70]. Second, in-depth mechanistic studies are essential to elucidate the pathways through which these compounds exert their pharmacological effects [154]. Third, well-designed clinical trials are crucial to validate the therapeutic potential of patchouli extracts in humans [132]. Additionally, research should focus on optimizing extraction and formulation techniques to improve the consistency and efficacy of patchouli-based products [14]. Finally, interdisciplinary collaborations integrating pharmacology, molecular biology, and bioinformatics could accelerate the discovery of new applications and enhance our understanding of the therapeutic potential of patchouli [132]. In conclusion, patchouli research has immense potential for uncovering new trends, applications, and directions for further studies. By leveraging advances in metabolic engineering, metabolomics, genetic analysis, and pharmacological research, researchers can unlock patchouli leaves’ total therapeutic and commercial value, paving the way for innovative products and interventions in diverse industries [286,289].
9. Conclusions
This review comprehensively summarizes patchouli leaves’ phytochemical diversity and pharmacological potential, highlighting their rich profile of volatile and non-volatile bioactive compounds. Our evaluation showed that patchouli leaves contain various bioactive compounds, including flavonoids, sesquiterpenes, glycosides, phytosterols, alcohols, organic acids, and phenolic acids, all contributing to their therapeutic properties. These compounds exhibit significant anti-oxidant, anti-inflammatory, anticancer, antidepressant, diuretic, antithrombotic, cytotoxic, antimicrobial, and insect-repellent properties, indicating a broad spectrum of potential medical applications. These findings highlight the potential of patchouli leaves as a versatile medicinal resource with broad-spectrum applications. However, despite their traditional use and promising pharmacological properties, there is a critical need for the scientific validation and standardization of these bioactive compounds. Future studies should prioritize elucidating precise mechanisms of action, optimizing extraction methodologies, and conducting robust clinical trials to establish the efficacy and safety of patchouli-derived therapeutics. Many fundamental and applied aspects of the bioactive compounds in patchouli remain underexplored, highlighting the urgent need for further research on patchouli leaves to understand their potential fully. By bridging traditional medicine with modern pharmacology, this review highlights the opportunities for innovative healthcare solutions and the development of novel, effective, and sustainable therapeutic agents.
Author Contributions
J.H. and E.C. conceptualized the manuscript; I.I.M. and E.M. prepared the initial draft; I.I.M., E.M., H.C., H.Z., J.X., X.L., J.H. and E.C. reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Major Science and Technology Projects in Jilin Province (project No. 20240303059NC) and Jilin Provincial Drug Administration Project (project No. JLPZGF-2020-026).
Acknowledgments
We thank the editors and reviewers for their critical and helpful comments on this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BCL-2 | B-cell lymphoma 2 |
| cAMP | cyclic adenosine monophosphate pathway |
| CAT | catalase |
| CDK 1 | cyclin-dependent kinase 1 |
| CDK4 | cyclin-dependent kinase 4 |
| COPD | chronic obstructive pulmonary disease |
| COX-1 | cyclooxygenase 1 |
| DNA | deoxyribonucleic acid |
| DSS | dextran sodium sulfate |
| FDA | food and drug administration |
| GC/MS | gas chromatography mass spectrum |
| GCLC | glutamate cysteine ligase comprising of catalytic subunit |
| GCLM | glutamate-cysteine ligase, modifier subunit |
| GES-1 | gastric epithelial cells |
| GSH | glutathione |
| GUs | gastric ulcers |
| HCT116 | human carcinoma cells and large intestine cells |
| HDAC | histone deacetylase |
| HFD | high-fat diet |
| HIV/AIDS | human immunodeficiency virus/acquired immunodeficiency syndrome |
| HO-1 | heme oxygenase-1 |
| HPLC-DAD | high-performance liquid chromatography-diode array detection |
| IBD | inflammatory bowel disease |
| ICAM-1 | intercellular adhesion molecule 1 |
| IFN-γ | interferon gamma |
| IkB-α | inhibitor of kappa B alpha |
| IL-1β | interleukin-1 beta |
| IP3R | inositol triphosphate receptors |
| KEAP1 | kelch-like ECH-associated protein 1 |
| LC50 | lethal concentration 50 |
| M1 | macrophages markers-1 |
| MCP-1 | monocyte chemotactic protein-1 |
| MDA | malondialdehyde |
| MIC | minimum inhibitory concentration |
| MMPs | matrix metalloproteinases |
| MOR | mu-opioid receptor |
| MR | mannose receptor |
| NAD(P)H | nicotinamide adenine dinucleotide (phosphate) |
| NAFLD | non-alcoholic fatty liver disease |
| NF-κB | nuclear factor-kappa B |
| NMR | nuclear magnetic resonance |
| NO | nitrous oxide |
| NQO1 | quinone oxidoreductase 1 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| NSCLC | non-small cell lung cancer |
| PCLE | patchouli ethyl acetate |
| PCLH | patchouli hexane |
| PFOS | perfluoro octane sulfonate |
| PGE2 | prostaglandin E2 |
| P-gp | P-glycoprotein |
| PHE | phenylephrine |
| PXR | pregnane X receptor |
| ROCCS | rapid overlay of chemical conformations and structures |
| ROCCS | receptor-operated Ca2+ channels |
| ROS | reactive oxygen species |
| RYR | ryanodine receptors |
| SCFA-GPR | short-chain fatty acid-G protein-coupled receptor |
| SCLC | small cell lung cancer |
| SOD | superoxide dismutase |
| SOM | secretory otitis media |
| TLC | thin-layer chromatography |
| TNF-α | tumor necrosis factor-α |
| TOF | time-of-flight |
| UC | ulcerative colitis |
| VCAM-1 | vascular cell adhesion molecule 1 |
| VCR | vincristine |
| VDCCS | voltage-dependent calcium channel subunit |
| VLDL | very low-density lipoproteins |
| β-PAE | β-patchoulene |
References
- Yeşilyurt, S.; Gürgan, M.; Sertkahya, M. Biologically Active Compounds from Medicinal and Aromatic Plants for Industrial Applications. In Medicinal and Aromatic Plants: Current Research Status, Value-Addition to Their Waste, and Agro-Industrial Potential (Vol I); Springer: Cham, Switzerland, 2024; pp. 1–11. [Google Scholar] [CrossRef]
- Abate, L.; Bachheti, A.; Bachheti, R.K.; Husen, A.; Getachew, M.; Pandey, D.P. Potential Role of Forest-Based Plants in Essential Oil Production: An Approach to Cosmetic and Personal Health Care Applications. In Non-Timber Forest Products; Springer: Cham, Switzerland, 2021; pp. 1–18. [Google Scholar] [CrossRef]
- Ahad, B.; Shahri, W.; Rasool, H.; Reshi, Z.A.; Rasool, S.; Hussain, T. Medicinal Plants and Herbal Drugs: An Overview. In Medicinal and Aromatic Plants; Springer: Cham, Switzerland, 2021; pp. 1–40. [Google Scholar] [CrossRef]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Liu, X.; Cao, S.; Yu, J.; Zhang, J.; Yao, G.; Yang, H.; Yang, D.; Wu, Y. Molecular Basis of Pogostemon cablin Responding to Continuous Cropping Obstacles Revealed by Integrated Transcriptomic, MiRNA and Metabolomic Analyses. Ind. Crops Prod. 2023, 200, 116862. [Google Scholar] [CrossRef]
- Bano, H.; Khan, J.A. Identification of Viruses Naturally Infecting Patchouli (Pogostemon cablin (Blanco) Benth.) in India. Biol. Forum Int. J. 2023, 15, 549–558. [Google Scholar]
- Ijaz, M.U.; Azmat, R.; Yeni, D.K.; Ashraf, A.; Mariappan, V. Pachypodol, a Plant Flavonoid, Mitigates Cisplatin-Induced Hepatotoxicity Through Anti-Oxidant, Anti-Inflammatory and Anti-Apoptotic Mechanisms. J. Food Nutr. Res. 2023, 11, 513–518. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Liu, Y.; Wu, D.; Huang, H.; Zhan, R.; Chen, W.; Chen, L. PatSWC4, a Methyl Jasmonate-Responsive MYB (v-Myb Avian Myeloblastosis Viral Oncogene Homolog)-Related Transcription Factor, Positively Regulates Patchoulol Biosynthesis in Pogostemon cablin. Ind. Crops Prod. 2020, 154, 112672. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, N.; Chanotiya, C.S.; Lal, R.K. The Pharmacological Potential and the Agricultural Significance of the Aromatic Crop Patchouli (Pogostemon cablin Benth.): A Review. Ecol. Front. 2024, 44, 1109–1118. [Google Scholar] [CrossRef]
- Yeong, K.Y.; Chin, K.W. Neuroprotection with the Functional Herbs from the Lamiaceae Family. Front. Nat. Prod. Chem. 2021, 8, 173–212. [Google Scholar] [CrossRef]
- Zhou, Q.-M.; Zhu, H.; Ma, C.; Guo, L.; Peng, C.; Xiong, L. Pogocablanols A–G, a New Class of Sesquiterpenoids with an Unprecedented Bicyclic Sesquiterpenoid Skeleton from Pogostemon cablin (Patchouli) and Their Activities. Arab. J. Chem. 2023, 16, 105245. [Google Scholar] [CrossRef]
- Zahra, S.S.; Nayik, G.A.; Khalida, T. Patchouli Essential Oil. In Essential Oils; Elsevier: Amsterdam, The Netherlands, 2023; pp. 429–457. [Google Scholar] [CrossRef]
- Wan, F.; Peng, F.; Xiong, L.; Chen, J.; Peng, C.; Dai, M. In Vitro and in Vivo Antibacterial Activity of Patchouli Alcohol from Pogostemon cablin. Chin. J. Integr. Med. 2021, 27, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Isnaini, N.; Khairan, K.; Faradhilla, M.; Sufriadi, E.; Prajaputra, V.; Ginting, B.; Muhammad, S.; Lufika, R.D. A Study of Essential Oils from Patchouli (Pogostemon cablin Benth.) and Its Potential as an Antivirus Agent to Relieve Symptoms of COVID-19. J. Patchouli Essent. Oil Prod. 2022, 1, 27–35. [Google Scholar] [CrossRef]
- Yunus, R.; Supiati, S.; Suwarni, S.; Afrini, I.M.; Mubarak, M. Larvicidal and Repellent Potential of Patchouli Extract (Pogostemon cablin) Varieties of Southeast Sulawesi for Aedes aegypti Vector. Egypt. J. Chem. 2023, 66, 89–98. [Google Scholar] [CrossRef]
- Lallo, S.; Hasmiranti, A.; Hardianti, B. Effect of the Growth Environment on Patchouli (Pogostemon cablin Benth.) Oil Character at Southeast Sulawesi, Indonesia. Med. Plants-Int. J. Phytomed. Relat. Ind. 2020, 12, 77–81. [Google Scholar] [CrossRef]
- Jadid, N.; Soleha, I.D.; Rahayu, A.E.; Puspaningtyas, I.; Sari, S.A.; Rahmawati, M.; Hidayati, D. Exogenous Methyl Jasmonate (MeJA) Altered Phytochemical Composition and Enhanced the Expression of PatAACT Gene of in Vitro Culture-Derived Patchouli Var. Sidikalang (Pogostemon cablin Benth.). J. King Saud Univ. 2024, 36, 103301. [Google Scholar] [CrossRef]
- Ermaya, D.; Widodo, Y.R.; Teguh, D. Isolation and Utilization of Patchouli Oil (Pogostemon cablin Benth) as an Anti-Bacterial Alternative. IOP Conf. Ser. Earth Environ. Sci. 2022, 1012, 12015. [Google Scholar] [CrossRef]
- Muhammad, S.; HPS, A.K.; Abd Hamid, S.; Danish, M.; Marwan, M.; Yunardi, Y.; Abdullah, C.K.; Faisal, M.; Yahya, E.B. Characterization of Bioactive Compounds from Patchouli Extracted via Supercritical Carbon Dioxide (SC-CO2) Extraction. Molecules 2022, 27, 6025. [Google Scholar] [CrossRef]
- Wu, Z.; Zeng, H.; Zhang, L.; Pu, Y.; Li, S.; Yuan, Y.; Zhang, T.; Wang, B. Patchouli Alcohol: A Natural Sesquiterpene against Both Inflammation and Intestinal Barrier Damage of Ulcerative Colitis. Inflammation 2020, 43, 1423–1435. [Google Scholar] [CrossRef]
- Srivastava, S.; Lal, R.K.; Singh, V.R.; Rout, P.K.; Padalia, R.C.; Yadav, A.K.; Bawitlung, L.; Bhatt, D.; Maurya, A.K.; Pal, A. Chemical Investigation and Biological Activities of Patchouli (Pogostemon cablin (Blanco) Benth) Essential Oil. Ind. Crops Prod. 2022, 188, 115504. [Google Scholar] [CrossRef]
- Khairan, K.; Amanda, R.; Hasbi, S.Y.; Diah, M.; Hasballah, K. A Perspective Study of Pogostemon cablin Benth as an Aphrodisiac. Malacca Pharm. 2023, 1, 62–70. [Google Scholar] [CrossRef]
- Lima Santos, L.; Barreto Brandão, L.; da Costa, P.; Luiz, A.; Lopes Martins, R.; Lobato Rodrigues, A.B.; Alves Lobato, A.; Moreira da Silva de Almeida, S.S. Bioinsecticidal and Pharmacological Activities of the Essential Oil of Pogostemon cablin Benth Leaves: A Review. Pharmacogn. Rev. 2022, 16, 139–145. [Google Scholar] [CrossRef]
- de Sousa, D.P.; de Assis Oliveira, F.; Arcanjo, D.D.R.; da Fonsêca, D.V.; Duarte, A.B.S.; de Oliveira Barbosa, C.; Ong, T.P.; Brocksom, T.J. Essential Oils: Chemistry and Pharmacological Activities—Part II. Biomedicines 2024, 12, 1185. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, L.; Fan, X.; Zhang, H.; Dong, Z.; Tao, T. β-Patchoulene Alleviates Cognitive Dysfunction in a Mouse Model of Sepsis Associated Encephalopathy by Inhibition of Microglia Activation through Sirt1/Nrf2 Signaling Pathway. PLoS ONE 2023, 18, e0279964. [Google Scholar] [CrossRef]
- Muhammad, S.; Isnaini, N.; Indra, I.; Prajaputra, V.; Sufriadi, E.; Ernawati, E. Patchouli Essential Oil: Unlocking Antimicrobial Potential for Dandruff Control. IOP Conf. Ser. Earth Environ. Sci. 2024, 1356, 12095. [Google Scholar] [CrossRef]
- Handayani, W.; Yunilawati, R.; Imawan, C. The Antibacterial Effect from Combining Cinnamon, Patchouli and Coriander Essential Oils. In Proceedings of the 2nd International Conference of Essential Oils, Banda Aceh, Indonesia, 29–30 October 2020; pp. 153–158. [Google Scholar] [CrossRef]
- Da Cunha, S.M.D.; Alves, C.A.; Ribeiro, L.S.M.; Macedo, M.A.; Da Silva, A.L.S.; Cunha, F.N.; De Lima, B.; Silva, D.F.; Lima, E.O. Bioprospecting of the Antifungal Activity of Patchouli Essential Oil (Pogostemon cablin Benth) against Strains of the Genus Candida. J. Med. Plants Res. 2023, 17, 1–7. [Google Scholar] [CrossRef]
- Green, M. Aromatherapy in the Treatment of Sexual Dysfunction and Relationship Problems. In Integrative Sexual Health; Oxford University Press: Oxford, UK, 2018; pp. 471–497. [Google Scholar]
- Arya, R.K.K.; Sethiya, N.K.; Bisht, D.; Rashid, M.; Kumar, D.; Singh, A.; Gupta, R.; Rana, V.S. An Overview on Chemical Features and Metabolism of Synthetic and Natural Product-Based Medicine for Combating COVID-19. Res. J. Pharm. Technol. 2023, 16, 908–916. [Google Scholar] [CrossRef]
- Kemala, P.; Idroes, R.; Khairan, K.; Ramli, M.; Tallei, T.E.; Helwani, Z.; Abd Rahman, S. The Potent Antimicrobial Spectrum of Patchouli: Systematic Review of Its Antifungal, Antibacterial, and Antiviral Properties. Malacca Pharm. 2024, 2, 10–17. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Y.; Huang, H.; Wu, D.; Chen, X.; Li, J.; Zheng, H.; Zhan, R.; Chen, L. Functional Analysis of Pogostemon cablin Farnesyl Pyrophosphate Synthase Gene and Its Binding Transcription Factor PcWRKY44 in Regulating Biosynthesis of Patchouli Alcohol. Front. Plant Sci. 2022, 13, 946629. [Google Scholar] [CrossRef]
- Pérez-González, C.; Pérez-Ramos, J.; Méndez-Cuesta, C.A.; Serrano-Vega, R.; Martell-Mendoza, M.; Pérez-Gutiérrez, S. Cytotoxic Activity of Essential Oils of Some Species from Lamiaceae Family. In Cytotoxicity—Definition, Identification, and Cytotoxic Compounds; IntechOpen: London, UK, 2019; pp. 29–43. [Google Scholar] [CrossRef]
- Ferreira, D.L.; de Mendonça, M.S.; de Araujo3 Branca, M.G.P.; Lescano, F.M.; Simão, M.O. de A.R. Leaf Anatomy and Histochemistry of Oriza (Pogostemon cablin Beth., LAMIACEAE): Medicinal Plant Used in Community Arari Region, Itacoatiara, Amazonas. Int. J. Adv. Eng. Res. Sci. 2020, 7, 2349–6495. [Google Scholar] [CrossRef]
- Dongare, P.; Dhande, S.; Kadam, V. A Review on Pogostemon Patchouli. Res. J. Pharmacogn. Phytochem. 2014, 6, 41–47. [Google Scholar]
- Deliana, F.; Illian, D.N.; Nadirah, S.; Sari, F.; Khairan, K. Therapeutic Effects of Patchouli (Pogostemon cablin) Essential Oil in Relieving Eczema Symptoms in Infants and Toddlers: A Literature Review. J. Patchouli Essent. Oil Prod. 2023, 2, 1–8. [Google Scholar] [CrossRef]
- Yao, G.; Drew, B.T.; Yi, T.-S.; Yan, H.-F.; Yuan, Y.-M.; Ge, X.-J. Phylogenetic Relationships, Character Evolution and Biogeographic Diversification of Pogostemon s.l. (Lamiaceae). Mol. Phylogenet. Evol. 2016, 98, 184–200. [Google Scholar] [CrossRef]
- Lubis, A.; Mandang, T.; Hermawan, W. Study of the Physical and Mechanical Characteristics of Patchouli Plants. AIMS Agric. Food 2021, 6, 525–537. [Google Scholar] [CrossRef]
- Adhayani, L.; Suhartono, S.; Amalia, A. Aceh Patchouli Oil (Pogostemon cablin Benth) as Antibacterial and Antibiofilm: Mechanism, Challenges, and Opportunities. AIP Conf. Proc. 2023, 2480, 050005. [Google Scholar] [CrossRef]
- Qur’ania, A.; Herdiyeni, Y.; Kusuma, W.A.; Wigena, A.H.; Suhesti, S. Detection and Calculation of Stomatal Density Using YOLOv5: A Study of High-Yielding Patchouli Varieties. J. Image Graph. 2024, 12, 228–238. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R. A Comprehensive Review on the Phytochemical Constituents and Pharmacological Activities of Pogostemon cablin Benth.: An Aromatic Medicinal Plant of Industrial Importance. Molecules 2015, 20, 8521–8547. [Google Scholar] [CrossRef]
- Singh, S.; Agrawal, N. Exploring the Pharmacological Potential and Bioactive Components of Pogostemon cablin (Blanco) Benth, Traditional Chinese Medicine. Pharmacol. Res.—Mod. Chin. Med. 2024, 10, 100382. [Google Scholar] [CrossRef]
- Ijaz, M.U.; Akbar, A.; Anwar, H.; Inam, S.; Ashraf, A.; Riaz, M. Patchouli. In Essentials of Medicinal and Aromatic Crops; Springer: Cham, Switzerland, 2023; pp. 249–279. [Google Scholar]
- Tang, Y.; Zhong, L.; Wang, X.; Zheng, H.; Chen, L. Molecular Identification and Expression of Sesquiterpene Pathway Genes Responsible for Patchoulol Biosynthesis and Regulation in Pogostemon cablin. Bot. Stud. 2019, 60, 11. [Google Scholar] [CrossRef]
- Jain, P.L.B.; Patel, S.R.; Desai, M.A. Patchouli Oil: An Overview on Extraction Method, Composition and Biological Activities. J. Essent. Oil Res. 2022, 34, 1–11. [Google Scholar] [CrossRef]
- Pandey, S.K.; Bhandari, S.; Sarma, N.; Begum, T.; Munda, S.; Baruah, J.; Gogoi, R.; Haldar, S.; Lal, M. Essential Oil Compositions, Pharmacological Importance and Agro Technological Practices of Patchouli (Pogostemon cablin Benth.): A Review. J. Essent. Oil Bear. Plants 2021, 24, 1212–1226. [Google Scholar] [CrossRef]
- Sundarajan, S.; Abdurahman, N.H. Chemical Compound Characterizations of Patchouli Leaf Extract via GC-MS, LC-QTOF-MS, FTIR, and 1H NMR. Int. J. Eng. Res. 2019, 8, 1054–1059. [Google Scholar] [CrossRef]
- van Beek, T.A.; Joulain, D. The Essential Oil of Patchouli, Pogostemon cablin: A Review. Flavour Fragr. J. 2018, 33, 6–51. [Google Scholar] [CrossRef]
- Hanh, N.P.; Binh, N.Q.; Thuy, D.T.T.; Ogunwande, I.A. Chemical Constituents and Antimicrobial Activity of Essential Oils from Pogostemon cablin (Blanco) Benth and Coriandrum sativum L. from Vietnam. Am. J. Essent. Oil Nat. Prod 2022, 10, 10–15. [Google Scholar]
- Keerthiraj, M. Bioprospecting of Pogostemon cablin Benth. for Insecticidal Activity. Ph.D. Thesis, Division of Agricultural Chemicals ICAR-Indian Agricultural Research Institute, New Delhi, India, 2020. [Google Scholar]
- Reyon, D.; Tsai, S.Q.; Khayter, C.; Foden, J.A.; Sander, J.D.; Joung, J.K. FLASH Assembly of TALENs for High-Throughput Genome Editing. Nat. Biotechnol. 2012, 30, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.F.; Li, S.P.; Cao, H.; Liu, J.J.; Gao, J.L.; Yang, F.Q.; Wang, Y.T. GC–MS Fingerprint of Pogostemon cablin in China. J. Pharm. Biomed. Anal. 2006, 42, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Hsu, H.-C.; Yang, W.-C.; Tsai, W.-J.; Chen, C.-C.; Watanabe, T. α-Bulnesene, a PAF Inhibitor Isolated from the Essential Oil of Pogostemon cablin. Fitoterapia 2007, 78, 7–11. [Google Scholar] [CrossRef]
- Bunrathep, S.; Lockwood, G.B.; Songsak, T.; Ruangrungsi, N. Chemical Constituents from Leaves and Cell Cultures of Pogostemon cablin and Use of Precursor Feeding to Improve Patchouli Alcohol Level. Sci. Asia 2006, 32, 293–296. [Google Scholar] [CrossRef]
- Akhila, A.; Sharma, P.K.; Thakur, R.S. Biosynthetic Relationships of Patchouli Alcohol, Seychellene and Cycloseychellene in Pogostemon cablin. Phytochemistry 1988, 27, 2105–2108. [Google Scholar] [CrossRef]
- Rakotonirainy, O.; Gaydou, E.M.; Faure, R.; Bombarda, I. Sesquiterpenes from Patchouli (Pogostemon cablin) Essential Oil. Assignment of the Proton and Carbon-13 NMR Spectra. J. Essent. Oil Res. 1997, 9, 321–327. [Google Scholar] [CrossRef]
- Karimi, A. Characterization and Antimicrobial Activity of Patchouli Essential Oil Extracted from Pogostemon cablin [Blanco] Benth. [Lamiaceae]. Adv. Environ. Biol. 2014, 8, 2301–2310. [Google Scholar]
- Buré, C.M.; Sellier, N.M. Analysis of the Essential Oil of Indonesian Patchouli (Pogostemon cabin Benth.) Using GC/MS (EI/CI). J. Essent. Oil Res. 2004, 16, 17–19. [Google Scholar] [CrossRef]
- Silva, M.A.S.; Ehlert, P.A.D.; Ming, L.C.; Marques, M.O.M. Composition and Chemical Variation during Daytime of Constituents of the Essential Oil of Pogostemon Pachouli Pellet Leaves. Acta Hortic. 2004, 629, 145–147. [Google Scholar] [CrossRef]
- Souhoka, F.A.; Al Aziz, A.Z.; Nazudin, N. Patchouli Oil Isolation and Identification of Chemical Components Using GC-MS. Indones. J. Chem. Res. 2020, 8, 108–113. [Google Scholar] [CrossRef]
- Kusumaningrum, H.P.; Ni’mah, R.S.; Nurlindasari, Y.; Imrani, A.; Arrois, S.; Purnomo, E.; Arfiansyah, F.R.; Setyowati, E.; Loka, B.D. Patchouli Oil Analysis of Chemical Components Using FT-IR and GCMS Method. AIP Conf. Proc. 2023, 2738, 040026. [Google Scholar] [CrossRef]
- Xie, S.; Li, L.; Zhan, B.; Shen, X.; Deng, X.; Tan, W.; Fang, T. Pogostone Enhances the Antibacterial Activity of Colistin against MCR-1-Positive Bacteria by Inhibiting the Biological Function of MCR-1. Molecules 2022, 27, 2819. [Google Scholar] [CrossRef]
- Cano-Reinoso, D.M.; Purwanto, Y.A.; Budiastra, I.W.; Kuroki, S.; Widodo, S.; Kamanga, B.M. Determination of α-Guaiene and Azulene Chemical Content in Patchouli Aromatic Oil (Pogostemon cablin Benth.) from Indonesia by Near-Infrared Spectroscopy. Indian J. Nat. Prod. Resour. (IJNPR) 2021, 12, 256–262. [Google Scholar]
- Singh, S.; Pathak, S.; Sharma, N.; Agrawal, N. Patchouli (Pogostemon cablin Benth.): A Comprehensive Review on Medicinal Aromatic Plant with Industrial Utilization. In Advances in Medicinal and Aromatic Plants; Apple Academic Press: Palm Bay, FL, USA, 2025. [Google Scholar] [CrossRef]
- Sufriadi, E.; Meilina, H.; Munawar, A.A.; Idroes, R. Fourier Transformed Infrared (FTIR) Spectroscopy Analysis of Patchouli Essential Oils Based on Different Geographical Area in Aceh. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1087, 12067. [Google Scholar] [CrossRef]
- Luo, J.; Feng, Y.; Guo, X.; Li, X. GC-MS analysis of volatile oil of herba Pogostemonis collected from Gaoyao county. J. Chin. Med. Mater. 1999, 22, 25–28. [Google Scholar]
- Feng, Y.; Guo, X.; Luo, J. GC-MS Analysis of Volatile Oil of Herba Pogostemonis Collected from Leizhou County. J. Chin. Med. Mater. 1999, 22, 241–243. [Google Scholar]
- Zhi, Z.; Lixian, T.; Shaojin, M.; Han, Z. Study on the Chemical Constituents and Fingerprint of Pogostemon cablin from Three Culture Varieties. Chin. J. Anal. Chem. 2006, 34, 1249–1254. [Google Scholar]
- Hasegawa, Y.; Tajima, K.; Toi, N.; Sugimura, Y. An Additional Constituent Occurring in the Oil from a Patchouli Cultivar. Flavour Fragr. J. 1992, 7, 333–335. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, V.R. Chemical Composition of Leaves, Inflorescence, Whole Aerial-Parts and Root Essential Oils of Patchouli {Pogostemon cablin (Blanco) Benth.}. J. Essent. Oil Res. 2019, 31, 319–325. [Google Scholar] [CrossRef]
- Fahmi, Z.; Mudasir, M.; Rohman, A. Attenuated Total Reflectance-FTIR Spectra Combined with Multivariate Calibration and Discrimination Analysis for Analysis of Patchouli Oil Adulteration. Indones. J. Chem. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Lima Santos, L.; Barreto Brandão, L.; Lopes Martins, R.; de Menezes Rabelo, E.; Lobato Rodrigues, A.B.; da Conceição Vieira Araújo, C.M.; Fernandes Sobral, T.; Ribeiro Galardo, A.K.; Moreira da Silva de Ameida, S.S. Evaluation of the Larvicidal Potential of the Essential Oil Pogostemon cablin (Blanco) Benth in the Control of Aedes aegypti. Pharmaceuticals 2019, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- IToKAWA, H.; SUTO, K.; TAKEYA, K. Studies on a Novel P-Coumaroyl Glucoside of Apigenin and on Other Flavonoids Isolated from Patchouli (Labiatae). Chem. Pharm. Bull. 1981, 29, 254–256. [Google Scholar] [CrossRef]
- Li, P.; Yin, Z.-Q.; Li, S.L.; Huang, X.; Ye, W.; Zhang, Q.-W. Simultaneous Determination of Eight Flavonoids and Pogostone in Pogostemon cablin by High Performance Liquid Chromatography. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 1771–1784. [Google Scholar] [CrossRef]
- Amakura, Y.; Yoshimura, M.; Mouri, C.; Mikage, M.; Kawahara, N.; Goda, Y.; Yoshida, T. Convenient TLC-Based Identification Test for the Crude Drug “Pogostemoni Herba”. Yakugaku Zasshi 2008, 128, 1833–1837. [Google Scholar] [CrossRef]
- Chen, J.; Han, L.; Li, G.; Sun, L.; Qian, F.; Chen, K.; Zhang, L.; Li, Y. Lignans and Phenylethanoid Glycosides from the Aerial Parts of Pogostemon cablin. Chem. Biodivers. 2022, 19, e202200889. [Google Scholar] [CrossRef]
- Kim, K.H.; Beemelmanns, C.; Clardy, J.; Cao, S. A New Antibacterial Octaketide and Cytotoxic Phenylethanoid Glycosides from Pogostemon cablin (Blanco) Benth. Bioorg. Med. Chem. Lett. 2015, 25, 2834–2836. [Google Scholar] [CrossRef]
- Xie, B.; Wu, X.-F.; Luo, H.-T.; Huang, X.-L.; Huang, F.; Zhang, Q.-Y.; Zhou, X.; Wu, H.-Q. Chemical Profiling and Quality Evaluation of Pogostemon cablin Benth by Liquid Chromatography Tandem Mass Spectrometry Combined with Multivariate Statistical Analysis. J. Pharm. Biomed. Anal. 2022, 209, 114526. [Google Scholar] [CrossRef]
- Wenguang, J.; Xiaoyu, L.; Chu, L.I.; Xiaoliang, Z.; Xianlong, C.; Penglong, W.; Feng, W.; Shuangcheng, M.A. Anti-Inflammatory Mechanism of the Non-Volatile Ingredients Originated from Guanghuoxiang Based on High Performance Liquid Chromatography-Heated Electron Spray Ionization-High Resolution Mass Spectroscope and Cell Metabolomics. J. Tradit. Chin. Med. 2024, 44, 260–267. [Google Scholar] [CrossRef]
- Junren, C.; Xiaofang, X.; Mengting, L.; Qiuyun, X.; Gangmin, L.; Huiqiong, Z.; Guanru, C.; Xin, X.; Yanpeng, Y.; Fu, P.; et al. Pharmacological Activities and Mechanisms of Action of Pogostemon cablin Benth: A Review. Chin. Med. 2021, 16, 5. [Google Scholar] [CrossRef]
- Kim, E.K.; Kim, J.H.; Jeong, S.; Choi, Y.W.; Choi, H.J.; Kim, C.Y.; Kim, Y.-M. Pachypodol, a Methoxyflavonoid Isolated from Pogostemon cablin Bentham Exerts Antioxidant and Cytoprotective Effects in HepG2 Cells: Possible Role of ERK-Dependent Nrf2 Activation. Int. J. Mol. Sci. 2019, 20, 4082. [Google Scholar] [CrossRef]
- Huang, X.; Li, P.; Yin, Z.; Lu, J.; Lin, L.; Wang, Y.; Ye, W.; Zhang, Q. Cablinosides A and B, Two Glycosidic Phenylacetic Acid Derivatives from the Leaves of Pogostemon cablin. Chem. Biodivers. 2019, 16, e1900137. [Google Scholar] [CrossRef]
- Huang, L.; Mu, S.; Zhang, J.; Deng, B.; Song, Z.; Hao, X. Chemical constituents from involatile moiety of Pogostemon cablin. China J. Chin. Mater. Medica 2009, 34, 410–413. [Google Scholar]
- Li, K.; Zhang, H.; Xie, H.; Liang, Y.; Wang, X.; Ito, Y. Preparative Isolation and Purification of Five Flavonoids from Pogostemon cablin Benth by High-Speed Countercurrent Chromatography and Preparative High-Performance Liquid Chromatography. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1617–1629. [Google Scholar] [CrossRef]
- Zhou, Q.-M.; Peng, C.; Li, X.-H.; Guo, L.; Xiong, L.; Lin, D.-S. Study on constituents of the aerial parts of Pogostemon cablin. J. Chin. Med. Mater. 2013, 36, 915–918. [Google Scholar]
- Beşirik, N.Ş.; Göger, G. Antimicrobial Evaluation of the Patchouli (Pogostemon cablin Benth.) Leaf Essential Oil Combination with Standard Antimicrobial Compounds. Int. J. Second. Metab. 2023, 10, 385–393. [Google Scholar] [CrossRef]
- Fatima, S.; Farzeen, I.; Ashraf, A.; Aslam, B.; Ijaz, M.U.; Hayat, S.; Sarfraz, M.H.; Zafar, S.; Zafar, N.; Unuofin, J.O. A Comprehensive Review on Pharmacological Activities of Pachypodol: A Bioactive Compound of an Aromatic Medicinal Plant Pogostemon cablin Benth. Molecules 2023, 28, 3469. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Romane, A.; Efferth, T.; Salgueiro, L. North African Medicinal Plants Traditionally Used in Cancer Therapy. Front. Pharmacol. 2017, 8, 383. [Google Scholar] [CrossRef]
- Chien, J.-H.; Lee, S.-C.; Chang, K.-F.; Huang, X.-F.; Chen, Y.-T.; Tsai, N.-M. Extract of Pogostemon cablin Possesses Potent Anticancer Activity against Colorectal Cancer Cells In Vitro and In Vivo. Evid. Based Complement. Altern. Med. 2020, 2020, 9758156. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Chen, H.-M.; Liu, Y.-H.; Zhang, Z.-B.; Zheng, Y.-F.; Su, Z.-Q.; Zhang, X.; Xie, J.-H.; Liang, Y.-Z.; Fu, L.-D.; et al. The Gastroprotective Effect of Pogostone from Pogostemonis herba against Indomethacin-Induced Gastric Ulcer in Rats. Exp. Biol. Med. 2016, 241, 193–204. [Google Scholar] [CrossRef]
- Han, H.; Gao, M.; Wang, F.; Luo, Z.; Jiang, X.; Qiu, Y.; Su, J.; Duan, X.; Luo, S.; Tang, S. Protective Effects of Patchouli Alcohol against DSS-Induced Ulcerative Colitis. Sci. Rep. 2024, 14, 16745. [Google Scholar] [CrossRef]
- Ojangba, T.; Boamah, S.; Miao, Y.; Guo, X.; Fen, Y.; Agboyibor, C.; Yuan, J.; Dong, W. Comprehensive Effects of Lifestyle Reform, Adherence, and Related Factors on Hypertension Control: A Review. J. Clin. Hypertens. 2023, 25, 509–520. [Google Scholar] [CrossRef]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef]
- Kıran, T.R.; Otlu, O.; Karabulut, A.B. Oxidative Stress and Antioxidants in Health and Disease. J. Lab. Med. 2023, 47, 1–11. [Google Scholar] [CrossRef]
- Clark, J.A.; Coopersmith, C.M. Intestinal Crosstalk: A New Paradigm for Understanding the Gut as the “Motor” of Critical Illness. Shock 2007, 28, 384–393. [Google Scholar] [CrossRef]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; de Vos, W.M. Intestinal Microbiota in Human Health and Disease: The Impact of Probiotics. Genes Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef] [PubMed]
- de Barros, D.B.; e Lima, L.d.O.; da Silva, L.A.; Fonseca, M.C.; Ferreira, R.C.; Neto, H.D.; da Nóbrega Alves, D.; da Silva Rocha, W.P.; Scotti, L.; de Oliveira Lima, E. α-Pinene: Docking Study, Cytotoxicity, Mechanism of Action, and Anti-Biofilm Effect against Candida albicans. Antibiotics 2023, 12, 480. [Google Scholar] [CrossRef]
- Nóbrega, J.R.; Silva, D.d.F.; de Andrade, F.P., Jr.; Sousa, P.M.S.; de Figueiredo, P.T.R.; Cordeiro, L.V.; Lima, E.d.O. Antifungal Action of α-Pinene against Candida Spp. Isolated from Patients with Otomycosis and Effects of Its Association with Boric Acid. Nat. Prod. Res. 2021, 35, 6190–6193. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A Never-Ending Story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Chen, H.; Wang, L.; Liang, J.; Luo, D.; Liu, Y.; Yang, H.; Li, Y.; Xie, J. Anti-Inflammatory Activity of β-Patchoulene Isolated from Patchouli Oil in Mice. Eur. J. Pharmacol. 2016, 781, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Peng, X.; Wu, R.; Jin, J.; Ang, S.; Li, D. Four New Sesquiterpenoids from the Aerial Parts of Pogostemon cablin (Blanco.) Benth. and Their Hypoglycemic Activity. Fitoterapia 2024, 177, 106054. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhao, J.; Gao, P.; Xia, Y. Patchouli Alcohol Suppresses Castration-Resistant Prostate Cancer Progression by Inhibiting NF-ΚB Signal Pathways. Transl. Androl. Urol. 2022, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, J.; Zhang, Y.; Sui, Y.; Du, Y.; Yang, L.; Yin, Y. Antifungal and Anti-Biofilm Activities of Patchouli Alcohol against Candida albicans. Int. J. Med. Microbiol. 2024, 314, 151596. [Google Scholar] [CrossRef]
- Zhong, Y.; Tang, L.; Deng, Q.; Jing, L.; Zhang, J.; Zhang, Y.; Yu, F.; Ou, Y.; Guo, S.; Huang, B. Unraveling the Novel Effect of Patchouli Alcohol against the Antibiotic Resistance of Helicobacter pylori. Front. Microbiol. 2021, 12, 674560. [Google Scholar] [CrossRef]
- Chen, M.; Wen, H.; Zhou, S.; Yan, X.; Li, H. Patchouli Alcohol Inhibits D-gal Induced Oxidative Stress and Ameliorates the Quality of Aging Cartilage via Activating the Nrf2/HO-1 Pathway in Mice. Oxid. Med. Cell. Longev. 2022, 2022, 6821170. [Google Scholar] [CrossRef]
- Xu, L.; Huang, Q.; Tan, X.; Zhao, Q.; Wu, J.; Liao, H.; Ai, W.; Liu, Y.; Lai, Z.; Fu, L. Patchouli Alcohol Ameliorates Acute Liver Injury via Inhibiting Oxidative Stress and Gut-Origin LPS Leakage in Rats. Int. Immunopharmacol. 2021, 98, 107897. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, H.; Li, R.; Li, H.; Rui, X.; Zhou, L.; Liu, N.; Ji, Q.; Li, Q. Mufangji Decoction and Its Active Ingredient Patchouli Alcohol Inhibit Tumor Growth through Regulating Akt/MTOR-mediated Autophagy in Nonsmall-cell Lung Cancer. Evid.-Based Complement. Altern. Med. 2021, 2021, 2373865. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Ma, J.; Wu, J.; Li, H.; Li, R.; Chen, X. Anti-Influenza Patchoulol-Type Sesquiterpenoids from Pogostemon cablin. Phytochem. Lett. 2022, 48, 34–39. [Google Scholar] [CrossRef]
- Li, D.; Xing, Z.; Yu, T.; Dong, W.; Wang, Z.; Peng, C.; Yang, C. Pogostone Attenuates Adipose Tissue Inflammation by Regulating the Adipocyte–Macrophage Crosstalk via Activating SIRT1. Food Funct. 2022, 13, 11853–11864. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, Y.; Zhao, X.; Qi, M.; Liu, X. Pogostone Alleviates Angiotensin II-Induced Cardiomyocyte Hypertrophy in H9c2 Cells through MAPK and Nrf2 Pathway. Trop. J. Pharm. Res. 2023, 22, 1373–1378. [Google Scholar] [CrossRef]
- Zheng, T.; Lin, Z.; Jiang, G.; Chen, H.; Yang, Y.; Zeng, X. Pogostone Attenuates Osteolysis in Breast Cancer by Inhibiting the NF-KB and JNK Signaling Pathways of Osteoclast. Life Sci. 2023, 328, 121611. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Ma, X.; Yang, X.; Cai, Y.; Yin, W. Pogostone Inhibits the Activity of CYP3A4, 2C9, and 2E1 In Vitro. Pharm. Biol. 2021, 59, 530–534. [Google Scholar] [CrossRef]
- Rezazadeh, D.; Asadi, S.; Nemati, H.; Jalili, C. The Effect of Pogostone on Viability, Membrane Integrity, and Apoptosis of Liver Cancer Cells. World Cancer Res. J. 2022, 9, e2436. [Google Scholar] [CrossRef]
- Wang, D.; Yuan, C.; Li, J.; Fang, Y.; Zhang, Z. Synthesis of Pogostone-Inspired Dehydroacetic Acid Derivatives and Their Antifungal Activity against Sclerotinia sclerotiorum (Lib.) de Bary. Chem. Biodivers. 2023, 20, e202300128. [Google Scholar] [CrossRef]
- Wang, D.; Yuan, C.; Li, Y.; Bai, S.; Feng, J.; Wang, Y.; Fang, Y.; Zhang, Z. Chelation of the Optimal Antifungal Pogostone Analogue with Copper (II) to Explore the Dual Antifungal and Antibacterial Agent. J. Agric. Food Chem. 2024, 72, 3894–3903. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Wu, J.-X.; Yang, S.-F.; Yang, C.-K.; Chen, T.-H.; Hsiao, Y.-H. Anticancer Effects and Molecular Mechanisms of Apigenin in Cervical Cancer Cells. Cancers 2022, 14, 1824. [Google Scholar] [CrossRef]
- Imran, M.; Aslam Gondal, T.; Atif, M.; Shahbaz, M.; Batool Qaisarani, T.; Hanif Mughal, M.; Salehi, B.; Martorell, M.; Sharifi-Rad, J. Apigenin as an Anticancer Agent. Phyther. Res. 2020, 34, 1812–1828. [Google Scholar] [CrossRef]
- Syahraini, A.; Harnelly, E.; Hermanto, F.E. Pro-Apoptosis Activity of Pogostemon cablin Benth. Against Nasopharyngeal Carcinoma through the BCL-2 Inhibition Signaling Pathway: A Computational Investigation. Makara J. Sci. 2023, 27, 6. [Google Scholar] [CrossRef]
- Nisari, M.; Bozkurt, Ö.; Ertekin, T.; Ceylan, D.; İnanç, N.; Al, Ö.; Güler, H.; Unur, E. Rhamnetin Improves Antioxidant Status in the Liver of Ehrlich Solid Tumor Bearing Mice. Med. Sci. Discov. 2020, 7, 494–500. [Google Scholar] [CrossRef]
- Li, B.; Feng, F.; Jia, H.; Jiang, Q.; Cao, S.; Wei, L.; Zhang, Y.; Lu, J. Rhamnetin Decelerates the Elimination and Enhances the Antitumor Effect of the Molecular-Targeting Agent Sorafenib in Hepatocellular Carcinoma Cells via the MiR-148a/PXR Axis. Food Funct. 2021, 12, 2404–2417. [Google Scholar] [CrossRef]
- Peng, X.; Ang, S.; Zhang, Y.; Fan, F.; Wu, M.; Liang, P.; Wen, Y.; Gan, L.; Zhang, K.; Li, D. Chemical Constituents with Antiproliferative Activity from Pogostemon cablin (Blanco) Benth. Front. Chem. 2022, 10, 938851. [Google Scholar] [CrossRef] [PubMed]
- Krithika, S.; Sadiq, M.; Ramanujam, G.M.; Maruthapillai, A. Trimethoxy Flavone, Pachypodol Containing Pogostemon cablin Leaf Extract Shows Broad Spectrum Antimicrobial Activity. Mater. Today Proc. 2022, 50, 297–300. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, M.; Zhang, J. Pachypodol Protects Newborn Rats from Anaesthesia-induced Apoptosis in the Developing Brain by Regulating the JNK/ERK Pathway. Int. J. Dev. Neurosci. 2021, 81, 633–642. [Google Scholar] [CrossRef]
- Ijaz, M.U.; Rauf, A.; Mustafa, S.; Ahmed, H.; Ashraf, A.; Al-Ghanim, K.; Mruthinti, S.S.; Mahboob, S. Pachypodol Attenuates Perfluorooctane Sulphonate-Induced Testicular Damage by Reducing Oxidative Stress. Saudi J. Biol. Sci. 2022, 29, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Ghaani, M.R.; Bayazeid, O. Phenylethanoid Glycosides as a Possible COVID-19 Protease Inhibitor: A Virtual Screening Approach. J. Mol. Model. 2021, 27, 341. [Google Scholar] [CrossRef]
- Park, E.J.; Park, H.R.; Lee, J.S.; Kim, J. Licochalcone A: An Inducer of Cell Differentiation and Cytotoxic Agent from Pogostemon cabling. Planta Med. 1998, 64, 464–466. [Google Scholar] [CrossRef]
- Punithavathy, P.M.; Nalina, K.; Menon, T. Antifungal Susceptibility Testing of Candida Tropicalis Biofilms against Fluconazole Using Calorimetric Indicator Resazurin. Indian J. Pathol. Microbiol. 2012, 55, 72–74. [Google Scholar] [CrossRef]
- Chuwen, L.; Wu, X.-L.; Zhao, X.-N.; Su, Z.-Q.; Chen, H.-M.; Wang, X.-F.; Zhang, X.-J.; Zeng, H.-F.; Chen, J.-N.; Li, Y.-C.; et al. Anti-Inflammatory Property of the Ethanol Extract of the Root and Rhizome of Pogostemon cablin (Blanco) Benth. Sci. World J. 2013, 2013, 434151. [Google Scholar] [CrossRef]
- Lavanya, G.; Brahmaprakash, G.P. Phytochemical Screening and Antimicrobial Activity of Compounds from Selected Medicinal and Aromatic Plants. Int. J. Sci. Nat. 2010, 2, 287–291. [Google Scholar]
- Mansuri, A.; Lokhande, K.; Kore, S.; Gaikwad, S.; Nawani, N.; Swamy, K.V.; Junnarkar, M.; Pawar, S. Antioxidant, Anti-Quorum Sensing, Biofilm Inhibitory Activities and Chemical Composition of Patchouli Essential Oil: In Vitro and in Silico Approach. J. Biomol. Struct. Dyn. 2022, 40, 154–165. [Google Scholar] [CrossRef]
- Tang, Z.; Peng, C.; Dai, M.; Han, B. Cytotoxic and Antibacterial Activities of the Analogues of Pogostone. Fitoterapia 2015, 106, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A. Therapeutic Potential of Pogostemon cablin Herb: A Comprehensive Review. Pharm. Pat. Anal. 2022, 11, 213–224. [Google Scholar] [CrossRef]
- Kim, J.; Wang, T.C. Helicobacter pylori and Gastric Cancer. Gastrointest. Endosc. Clin. 2021, 31, 451–465. [Google Scholar] [CrossRef]
- Leong, W.; Huang, G.; Liao, W.; Xia, W.; Li, X.; Su, Z.; Liu, L.; Wu, Q.; Wong, V.K.W.; Law, B.Y.K. Traditional Patchouli Essential Oil Modulates the Host’s Immune Responses and Gut Microbiota and Exhibits Potent Anti-Cancer Effects in ApcMin/+ Mice. Pharmacol. Res. 2022, 176, 106082. [Google Scholar] [CrossRef]
- Tran, T.H.; Luong, T.M.N.; Le Bui, V.; Tran, T.H. Effects of Plant Essential Oils and Their Constituents on Helicobacter pylori: A Review. Plant Sci. Today 2023, 10, 334–344. [Google Scholar] [CrossRef]
- Ren, W.-K.; Xu, Y.-F.; Wei, W.-H.; Huang, P.; Lian, D.-W.; Fu, L.-J.; Yang, X.-F.; Chen, F.-J.; Wang, J.; Cao, H.-Y. Effect of Patchouli Alcohol on Helicobacter pylori-Induced Neutrophil Recruitment and Activation. Int. Immunopharmacol. 2019, 68, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.-Y.; He, J.-J.; Su, J.-Q.; Kong, S.-Z.; Su, J.-Y.; Li, Y.-C.; Li, C.-W.; Lai, X.-P.; Su, Z.-R. Synthesis and Antimicrobial Evaluation of Pogostone and Its Analogues. Fitoterapia 2013, 84, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Liang, H.-C.; Chen, H.-M.; Tan, L.-R.; Yi, Y.-Y.; Qin, Z.; Zhang, W.-M.; Wu, D.-W.; Li, C.-W.; Lin, R.-F. Anti-Candida albicans Activity and Pharmacokinetics of Pogostone Isolated from Pogostemonis herba. Phytomedicine 2012, 20, 77–83. [Google Scholar] [CrossRef]
- Kiyohara, H.; Ichino, C.; Kawamura, Y.; Nagai, T.; Sato, N.; Yamada, H. Patchouli Alcohol: In Vitro Direct Anti-Influenza Virus Sesquiterpene in Pogostemon cablin Benth. J. Nat. Med. 2012, 66, 55–61. [Google Scholar] [CrossRef]
- Wu, H.; Li, B.; Wang, X.; Jin, M.; Wang, G. Inhibitory Effect and Possible Mechanism of Action of Patchouli Alcohol against Influenza A (H2N2) Virus. Molecules 2011, 16, 6489–6501. [Google Scholar] [CrossRef]
- Li, Y.C.; Peng, S.Z.; Chen, H.M.; Zhang, F.X.; Xu, P.P.; Xie, J.H.; He, J.J.; Chen, J.N.; Lai, X.P.; Su, Z.R. Oral Administration of Patchouli Alcohol Isolated from Pogostemonis herba Augments Protection against Influenza Viral Infection in Mice. Int. Immunopharmacol. 2012, 12, 294–301. [Google Scholar] [CrossRef]
- Mandal, A.; Biswas, D.; Hazra, B. Natural Products from Plants with Prospective Anti-HIV Activity and Relevant Mechanisms of Action. Stud. Nat. Prod. Chem. 2020, 66, 225–271. [Google Scholar] [CrossRef]
- Buckle, J. Clinical Aromatherapy and AIDS. J. Assoc. Nurses AIDS Care 2002, 13, 81–99. [Google Scholar] [CrossRef]
- Ali, H.-A.; Chowdhury, A.K.A.; Rahman, A.K.M.; Borkowski, T.; Nahar, L.; Sarker, S.D. Pachypodol, a Flavonol from the Leaves of Calycopteris floribunda, Inhibits the Growth of CaCo 2 Colon Cancer Cell Line In Vitro. Phytother. Res. 2008, 22, 1684–1687. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, P.; Narayana, K.; Shridhar, N.B.; Bhat, A. Toxicity Studies of Calycopteris floribunda Lam. in Calf, Rabbit and Rat. J. Ethnopharmacol. 2006, 107, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.J.; Nahar, L.; Shilpi, J.A.; Shoeb, M.; Borkowski, T.; Gibbons, S.; Middleton, M.; Byres, M.; Sarker, S.D. Gedunin, a Limonoid from Xylocarpus granatum, Inhibits the Growth of CaCo-2 Colon Cancer Cell Line In Vitro. Phytother. Res. 2007, 21, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, H.-S.; Lee, S.-H. Preventive Activity of Patchouli Alcohol against Colorectal Cancer and Diabetes. J. Med. Food 2023, 26, 255–261. [Google Scholar] [CrossRef]
- Jeong, J.B.; Choi, J.; Lou, Z.; Jiang, X.; Lee, S.H. Patchouli alcohol, an essential oil of Pogostemon cablin, exhibits antitumorigenic activity in human colorectal cancer cells. Int. Immunopharmacol. 2013, 16, 184–190. [Google Scholar] [CrossRef]
- Gao, F.; Niu, Y.; Sun, L.; Li, W.; Xia, H.; Zhang, Y.; Geng, S.; Guo, Z.; Lin, H.; Du, G. Integrating Network Pharmacology and Transcriptomic Validation to Investigate the Efficacy and Mechanism of Mufangji Decoction Preventing Lung Cancer. J. Ethnopharmacol. 2022, 298, 115573. [Google Scholar] [CrossRef]
- Škubník, J.; Pavlíčková, V.S.; Ruml, T.; Rimpelová, S. Vincristine in Combination Therapy of Cancer: Emerging Trends in Clinics. Biology 2021, 10, 849. [Google Scholar] [CrossRef]
- Liang, C.; Chang, K.; Huang, Y.; Huang, X.; Sheu, G.; Kuo, C.; Hsiao, C.; Tsai, N. Patchouli Alcohol Induces G0/G1 Cell Cycle Arrest and Apoptosis in Vincristine-resistant Non-small Cell Lung Cancer through ROS-mediated DNA Damage. Thorac. Cancer 2023, 14, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Friedel, W.E.; Matthes, H.; Bock, P.R.; Zänker, K.S. Systematic Evaluation of the Clinical Effects of Supportive Mistletoe Treatment within Chemo- and/or Radiotherapy Protocols and Long-Term Mistletoe Application in Nonmetastatic Colorectal Carcinoma: Multicenter, Controlled, Observational Cohort Study. J. Soc. Integr. Oncol. 2009, 7, 137–145. [Google Scholar] [PubMed]
- Burkhart, J.; Wälchli, C.; Heusser, P.; Weissenstein, U.; Baumgartner, S.; Andres, A.-C. In Vitro Investigation into the Potential of a Mistletoe Extract to Alleviate Adverse Effects of Cyclophosphamide. Altern. Ther. Health Med. 2010, 16, 40–48. [Google Scholar]
- Hu, G.; Peng, C.; Xie, X.; Zhang, S.; Cao, X. Availability, Pharmaceutics, Security, Pharmacokinetics, and Pharmacological Activities of Patchouli Alcohol. Evid.-Based Complement. Altern. Med. 2017, 2017, 4850612. [Google Scholar] [CrossRef]
- Sheng, X.; Yan, J.; Meng, Y.; Kang, Y.; Han, Z.; Tai, G.; Zhou, Y.; Cheng, H. Immunomodulatory Effects of Hericium erinaceus Derived Polysaccharides Are Mediated by Intestinal Immunology. Food Funct. 2017, 8, 1020–1027. [Google Scholar] [CrossRef]
- Liao, J.B.; Wu, D.W.; Peng, S.Z.; Xie, J.H.; Li, Y.C.; Su, J.Y.; Chen, J.N.; Su, Z.R. Immunomodulatory Potential of Patchouli Alcohol Isolated from Pogostemon cablin (Blanco) Benth (Lamiaceae) in Mice. Trop. J. Pharm. Res. 2013, 12, 559–565. [Google Scholar] [CrossRef]
- Tanaka, C.; Okada, A.; Nedachi, M.; Noda, S.; Takahashi-Ando, N. The Effects of Essential Oils on Skin Homeostasis and Inflammation Using Human Keratinocyte HaCaT Cells. Cell Biol. Res. 2023, 4, 10–25. [Google Scholar]
- Sim, L.Y.; Abd Rani, N.Z.; Husain, K. Lamiaceae: An Insight on Their Anti-Allergic Potential and Its Mechanisms of Action. Front. Pharmacol. 2019, 10, 677. [Google Scholar] [CrossRef]
- Chang, K.; Lai, H.; Lee, S.; Huang, X.; Huang, Y.; Chou, T.; Hsiao, C.; Tsai, N. The Effects of Patchouli Alcohol and Combination with Cisplatin on Proliferation, Apoptosis and Migration in B16F10 Melanoma Cells. J. Cell. Mol. Med. 2023, 27, 1423–1435. [Google Scholar] [CrossRef]
- Song, Y.; Chang, L.; Wang, X.; Tan, B.; Li, J.; Zhang, J.; Zhang, F.; Zhao, L.; Liu, G.; Huo, B. Regulatory Mechanism and Experimental Verification of Patchouli Alcohol on Gastric Cancer Cell Based on Network Pharmacology. Front. Oncol. 2021, 11, 711984. [Google Scholar] [CrossRef]
- Tsoupras, A.; Gkika, D.A.; Siadimas, I.; Christodoulopoulos, I.; Efthymiopoulos, P.; Kyzas, G.Z. The Multifaceted Effects of Non-Steroidal and Non-Opioid Anti-Inflammatory and Analgesic Drugs on Platelets: Current Knowledge, Limitations, and Future Perspectives. Pharmaceuticals 2024, 17, 627. [Google Scholar] [CrossRef] [PubMed]
- Orf, M.; Kurian, M.; Nelson, C.; Simmons, D.; Espinosa, L.; Chickos, J. Correlation Gas Chromatographic Study of the Vaporization Enthalpies and Vapor Pressures of the Major Sesquiterpene Hydrocarbons in Patchouli Oil. J. Chem. Eng. Data 2021, 66, 2538–2549. [Google Scholar] [CrossRef]
- Xie, Y.-C.; Tang, F. Protective effect of Pogostemon cablin on membrane fluidity of intestinal epithelia cell in ischemia/reperfusion rats after ischemia/reperfusion. Chin. J. Integr. Tradit. West. Med. 2009, 29, 639–641. [Google Scholar]
- Li, Y.; Su, Z.; Lin, S.; Li, C.; Zhao, Y.; Gao, X.; Lai, Y.; Wu, X.; Wu, H.; Cai, Z. Characterisation of the Metabolism of Pogostone In Vitro and In Vivo Using Liquid Chromatography with Mass Spectrometry. Phytochem. Anal. 2014, 25, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kinoshita, K.; Koyama, K.; Takahashi, K.; Tai, T.; Nunoura, Y.; Watanabe, K. Anti-Emetic Principles of Pogostemon cablin (Blanco) Benth. Phytomedicine 1999, 6, 89–93. [Google Scholar] [CrossRef]
- Khan, A.H.; Dar, M.A.; Mir, M.A. Gastric Ulcer: An Overview. Int. J. Curr. Res. Physiol. Pharmacol. 2023, 1–7. [Google Scholar]
- He, P. Study on the Fingerprint of Patchouli Leaves and Its Medicinal Chemistry in Serum. Master’s Thesis, Liaoning University of Traditional Chinese Medicine, Shenyang, China, 2010. [Google Scholar]
- Lu, Q.; Tan, D.; Luo, J.; Ye, Y.; Zuo, M.; Wang, S.; Li, C. Potential of Natural Products in the Treatment of Irritable Bowel Syndrome. Phytomedicine 2022, 106, 154419. [Google Scholar] [CrossRef]
- Almikhlafi, M.A. A Review of the Gastrointestinal, Olfactory, and Skin Abnormalities in Patients with Parkinson’s Disease. Neurosci. J. 2024, 29, 4–9. [Google Scholar] [CrossRef]
- Pujari, N.M.; Khushtar, M.; Mishra, A.; Gupta, D. Comprehensive Insights into Gastric Ulcer Therapies: A Review of Current Approaches. J. Appl. Pharm. Sci. Res. 2024, 7, 4–12. [Google Scholar] [CrossRef]
- Saad, R.A.; Qutob, H.M.H. Alterations in Hemostatic and Hematological Parameters after Gastric Ulcer Induction in Rats. Possible Role of IL-6 and TNF-α. J. Evol. Biochem. Physiol. 2023, 59, 82–93. [Google Scholar] [CrossRef]
- Chen, G.; Xie, X.; Peng, F.; Wang, T.; Chen, J.; Li, G.; Liu, J.; Peng, C. Protective Effect of the Combination of Essential Oil from Patchouli and Tangerine Peel against Gastric Ulcer in Rats. J. Ethnopharmacol. 2022, 282, 114645. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liang, J.-L.; Liu, Y.-H.; Wu, J.-Z.; Huang, Q.-H.; Li, Y.-C.; Xie, Q.-F. Comparison of Anti-Inflammatory Effect between β-Patchoulene Epoxide and β-Patchoulene in LPS-Stimulated RAW264.7 Macrophages. Eur. J. Inflamm. 2018, 16, 205873921878507. [Google Scholar] [CrossRef]
- Hao, D.-C.; Wang, F.; Xiao, P.-G. Impact of Drug Metabolism/Pharmacokinetics and Its Relevance Considering Traditional Medicine-Based Anti-COVID-19 Drug Research. Curr. Drug Metab. 2022, 23, 374–393. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, J.; Wu, J.; Chen, H.; Zhang, Z.; Yang, H.; Chen, L.; Chen, H.; Su, Z.; Li, Y. Transformation of Patchouli Alcohol to β-Patchoulene by Gastric Juice: β-Patchoulene Is More Effective in Preventing Ethanol-Induced Gastric Injury. Sci. Rep. 2017, 7, 5591. [Google Scholar] [CrossRef]
- Ashton, J.J.; Beattie, R.M. Inflammatory Bowel Disease: Recent Developments. Arch. Dis. Child. 2024, 109, 370–376. [Google Scholar] [CrossRef]
- Yu, X.; Yang, G.; Jiang, H.; Lin, S.; Liu, Y.; Zhang, X.; Zeng, H.; Su, Z.; Huang, S.; Shen, L.; et al. Patchouli Oil Ameliorates Acute Colitis: A Targeted Metabolite Analysis of 2,4,6-Trinitrobenzenesulfonic Acid-Induced Rats. Exp. Ther. Med. 2017, 14, 1184–1192. [Google Scholar] [CrossRef]
- Qu, C.; Yuan, Z.-W.; Yu, X.-T.; Huang, Y.-F.; Yang, G.-H.; Chen, J.-N.; Lai, X.-P.; Su, Z.-R.; Zeng, H.-F.; Xie, Y.; et al. Patchouli Alcohol Ameliorates Dextran Sodium Sulfate-Induced Experimental Colitis and Suppresses Tryptophan Catabolism. Pharmacol. Res. 2017, 121, 70–82. [Google Scholar] [CrossRef]
- Di Rosa, C.; Altomare, A.; Terrigno, V.; Carbone, F.; Tack, J.; Cicala, M.; Guarino, M.P.L. Constipation-Predominant Irritable Bowel Syndrome (IBS-C): Effects of Different Nutritional Patterns on Intestinal Dysbiosis and Symptoms. Nutrients 2023, 15, 1647. [Google Scholar] [CrossRef]
- Zhou, T.-R.; Huang, J.-J.; Huang, Z.-T.; Cao, H.-Y.; Tan, B. Inhibitory Effects of Patchouli Alcohol on Stress-Induced Diarrhea-Predominant Irritable Bowel Syndrome. World J. Gastroenterol. 2018, 24, 693–705. [Google Scholar] [CrossRef]
- Pei, Y.; Huang, C.; Chen, W.; Zhang, Y.; Bao, R.; Yi, S.; Li, T.; Xu, Y.; Cao, H.; Tan, B. Patchouli Alcohol Improves Intestinal Motility in Ibs-D Rats Through Muscularis Macrophages Modulation of Enteric Neurons; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood Pressure Lowering for Prevention of Cardiovascular Disease and Death: A Systematic Review and Meta-Analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- Liu, X.; Luo, D.; Zhang, J.; Du, L. Distribution and Relative Expression of Vasoactive Receptors on Arteries. Sci. Rep. 2020, 10, 15383. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Pérez, J.; López-Canales, Ó.A.; Castillo-Hernández, M.C.; Padilla-Keymole, J.; López-Canales, J.S.; Zambrano-Padilla, R. Fast Kinetics of Fundamental Aortic Contraction to Phenylephrine Compared to KCl Only in Male Prepubertal and Adult Rats. Rev. Méd. Hosp. Gen. Méx. 2023, 86, 93–98. [Google Scholar] [CrossRef]
- Moser, J.C.; da Silva, R.d.C.V.; Costa, P.; da Silva, L.M.; Cassemiro, N.S.; Gasparotto Junior, A.; Silva, D.B.; de Souza, P. Role of K+ and Ca2+ Channels in the Vasodilator Effects of Plectranthus barbatus (Brazilian Boldo) in Hypertensive Rats. Cardiovasc. Ther. 2023, 2023, 9948707. [Google Scholar] [CrossRef] [PubMed]
- Muraki, K.; Bolton, T.B.; Imaizumi, Y.; Watanabe, M. Effect of Isoprenaline on Ca2+ Channel Current in Single Smooth Muscle Cells Isolated from Taenia of the Guinea-pig Caecum. J. Physiol. 1993, 471, 563–582. [Google Scholar] [CrossRef]
- Tugrul, I.; Dost, T.; Demir, O.; Gokalp, F.; Oz, O.; Girit, N.; Birincioglu, M. Effects of a PPAR-Gamma Receptor Agonist and an Angiotensin Receptor Antagonist on Aortic Contractile Responses to Alpha Receptor Agonists in Diabetic and/or Hypertensive Rats: Cardiovascular Topics. Cardiovasc. J. Afr. 2016, 27, 164–169. [Google Scholar] [CrossRef][Green Version]
- Zhu, H.; Zhou, Q.-M.; Peng, C.; Chen, M.-H.; Li, X.-N.; Lin, D.-S.; Xiong, L. Pocahemiketals A and B, Two New Hemiketals with Unprecedented Sesquiterpenoid Skeletons from Pogostemon cablin. Fitoterapia 2017, 120, 67–71. [Google Scholar] [CrossRef]
- Hu, G.-Y.; Peng, C.; Xie, X.-F.; Xiong, L.; Zhang, S.-Y.; Cao, X.-Y. Patchouli Alcohol Isolated from Pogostemon cablin Mediates Endothelium-Independent Vasorelaxation by Blockade of Ca2+ Channels in Rat Isolated Thoracic Aorta. J. Ethnopharmacol. 2018, 220, 188–196. [Google Scholar] [CrossRef]
- Lin, R.-F.; Feng, X.-X.; Li, C.-W.; Zhang, X.-J.; Yu, X.-T.; Zhou, J.-Y.; Zhang, X.; Xie, Y.-L.; Su, Z.-R.; Zhan, J.Y.-X. Prevention of UV Radiation-Induced Cutaneous Photoaging in Mice by Topical Administration of Patchouli Oil. J. Ethnopharmacol. 2014, 154, 408–418. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Merecz-Sadowska, A.; Ghorbanpour, M.; Szemraj, J.; Piekarski, J.; Bijak, M.; Śliwiński, T.; Zajdel, R.; Sitarek, P. Enhanced Natural Strength: Lamiaceae Essential Oils and Nanotechnology in in Vitro and in Vivo Medical Research. Int. J. Mol. Sci. 2023, 24, 15279. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, L.; Liu, F.; Chen, Y.; Xu, L.; Li, D.; Ma, Y.; Li, H.; Xu, J. Effect of Patchouli Alcohol on the Regulation of Heat Shock-Induced Oxidative Stress in IEC-6 Cells. Int. J. Hyperth. 2016, 32, 474–482. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Kim, M. Structural and Functional Changes and Possible Molecular Mechanisms in Aged Skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhi, J.; Zhao, R.; Sun, Y.; Wen, H.; Cai, H.; Chen, W.; Jiang, X.; Bai, R. Discovery of Matrix Metalloproteinase Inhibitors as Anti-Skin Photoaging Agents. Eur. J. Med. Chem. 2024, 267, 116152. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Huang, Y.-F.; Wang, L.; Xu, L.-Q.; Yu, X.-T.; Liu, Y.-H.; Li, C.-L.; Zhan, J.Y.-X.; Su, Z.-R.; Chen, J.-N.; et al. Photo-Protective Activity of Pogostone against UV-Induced Skin Premature Aging in Mice. Exp. Gerontol. 2016, 77, 76–86. [Google Scholar] [CrossRef]
- Gupta, A.K.; Parasar, D.; Sagar, A.; Choudhary, V.; Chopra, B.S.; Garg, R.; Ashish; Khatri, N. Analgesic and Anti-Inflammatory Properties of Gelsolin in Acetic Acid Induced Writhing, Tail Immersion and Carrageenan Induced Paw Edema in Mice. PLoS ONE 2015, 10, e0135558. [Google Scholar] [CrossRef]
- Desai, S.J.; Prickril, B.; Rasooly, A. Mechanisms of Phytonutrient Modulation of Cyclooxygenase-2 (COX-2) and Inflammation Related to Cancer. Nutr. Cancer 2018, 70, 350–375. [Google Scholar] [CrossRef]
- Thirkettle-Watts, D. Impedance-Based Analysis of Mu Opioid Receptor Signaling and Underlying Mechanisms. Biochem. Biophys. Rep. 2016, 6, 32–38. [Google Scholar] [CrossRef]
- Li, J.; Hu, G.; Liu, W.; Cao, X.; Chen, G.; Peng, F.; Xiaofang, X.; Peng, C. Patchouli Alcohol against Renal Fibrosis of Spontaneously Hypertensive Rats via Ras/Raf-1/ERK1/2 Signalling Pathway. J. Pharm. Pharmacol. 2023, 75, 995–1010. [Google Scholar] [CrossRef]
- Yu, X.; Wang, X.; Yan, X.; Jiang, J.; Lei, F.; Xing, D.; Guo, Y.; Du, L. Anti-Nociceptive Effect of Patchouli Alcohol: Involving Attenuation of Cyclooxygenase 2 and Modulation of Mu-Opioid Receptor. Chin. J. Integr. Med. 2019, 25, 454–461. [Google Scholar] [CrossRef]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 Suppresses Macrophage Inflammatory Response by Blocking Proinflammatory Cytokine Transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-H.; Liu, Y.-H.; Liang, J.-L.; Lin, Z.; Kong, Q.-L.; Xian, Y.; Guo, D.-Q.; Lai, Z.-Q.; Su, Z.-R.; Xiaoqi, H. β-Patchoulene, Isolated from Patchouli Oil, Suppresses Inflammatory Mediators in LPS-Stimulated RAW264.7 Macrophages. Eur. J. Inflamm. 2017, 15, 1721727X1771469. [Google Scholar] [CrossRef]
- Hijazy, H.H.A.; Dahran, N.; Althagafi, H.A.; Alharthi, F.; Habotta, O.A.; Oyouni, A.A.A.; Algahtani, M.; Theyab, A.; Al-Amer, O.; Lokman, M.S. Thymoquinone Counteracts Oxidative and Inflammatory Machinery in Carrageenan-Induced Murine Paw Edema Model. Environ. Sci. Pollut. Res. 2023, 30, 16597–16611. [Google Scholar] [CrossRef]
- Stojanović, N.M.; Ranđelović, P.J.; Simonović, M.; Radić, M.; Todorović, S.; Corrigan, M.; Harkin, A.; Boylan, F. Essential Oil Constituents as Anti-Inflammatory and Neuroprotective Agents: An Insight through Microglia Modulation. Int. J. Mol. Sci. 2024, 25, 5168. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Dou, Y.; Wu, X.; Li, H.; Wu, J.; Huang, Q.; Luo, D.; Yi, T.; Liu, Y.; Su, Z. Prophylactic Efficacy of Patchoulene Epoxide against Ethanol-Induced Gastric Ulcer in Rats: Influence on Oxidative Stress, Inflammation and Apoptosis. Chem. Biol. Interact. 2018, 283, 30–37. [Google Scholar] [CrossRef]
- Miyazawa, M.; Okuno, Y.; Nakamura, S.; Kosaka, H. Antimutagenic Activity of Flavonoids from Pogostemon cablin. J. Agric. Food Chem. 2000, 48, 642–647. [Google Scholar] [CrossRef]
- Lu, T.-C.; Liao, J.-C.; Huang, T.-H.; Lin, Y.-C.; Liu, C.-Y.; Chiu, Y.; Peng, W.-H. Analgesic and Anti-Inflammatory Activities of the Methanol Extract from Pogostemon cablin. Evidence-Based Complement. Altern. Med. 2011, 2011, 671741. [Google Scholar] [CrossRef]
- Xian, Y.-F.; Li, Y.-C.; Ip, S.-P.; Lin, Z.-X.; Lai, X.-P.; Su, Z.-R. Anti-Inflammatory Effect of Patchouli Alcohol Isolated from Pogostemonis herba in LPS-Stimulated RAW264.7 Macrophages. Exp. Ther. Med. 2011, 2, 545–550. [Google Scholar] [CrossRef]
- van Den Berg, H.; Velayudhan, R.; Yadav, R.S. Management of Insecticides for Use in Disease Vector Control: Lessons from Six Countries in Asia and the Middle East. PLoS Negl. Trop. Dis. 2021, 15, e0009358. [Google Scholar] [CrossRef]
- Fu, Q.; Zeng, B.; Xiao, Q.; He, B.; Huang, C.; Bao, M. Essential Oils for the Treatment of Dust Mites. E3S Web Conf. 2021, 271, 4032. [Google Scholar] [CrossRef]
- Phal, D.; Naik, R.K.; Deobhankar, K.P.; Vitonde, S.; Ghatpande, N. Laboratory Evaluation of Herbal Mosquito Coils against Aedes aegypti Mosquito. Bull. Environ. Pharmacol. Life Sci. 2012, 1, 16–20. [Google Scholar]
- Huang, S.-H.; Xian, J.-D.; Kong, S.-Z.; Li, Y.-C.; Xie, J.-H.; Lin, J.; Chen, J.-N.; Wang, H.-F.; Su, Z.-R. Insecticidal Activity of Pogostone against Spodoptera litura and Spodoptera exigua (Lepidoptera: Noctuidae). Pest Manag. Sci. 2014, 70, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Ga’al, H.; Fouad, H.; Mao, G.; Tian, J.; Jianchu, M. Larvicidal and Pupicidal Evaluation of Silver Nanoparticles Synthesized Using Aquilaria sinensis and Pogostemon cablin Essential Oils against Dengue and Zika Viruses Vector Aedes albopictus Mosquito and Its Histopathological Analysis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1171–1179. [Google Scholar] [CrossRef]
- Tennyson, S.; Samraj, A.D.; Jeyasundar, D.; Chalieu, K. Larvicidal Efficacy of Plant Oils against the Dengue Vector Aedes aegypti (L.) (Diptera: Culicidae). World J. Pharm. Res. 2013, 13, 64–68. [Google Scholar] [CrossRef]
- Gokulakrishnan, J.; Kuppusamy, E.; Shanmugam, D.; Appavu, A.; Kaliyamoorthi, K. Pupicidal and Repellent Activities of Pogostemon Cablin Essential Oil Chemical Compounds against Medically Important Human Vector Mosquitoes. Asian Pacific J. Trop. Dis. 2013, 3, 26–31. [Google Scholar] [CrossRef]
- Widawati, M.; Riandi, M.U. Study of Herbal Topical Repellent Made of Betel Leaves (Piper Betle) and Patchouli Oil (Pogostemon cablin) Mixture Against Yellow Fever Mosquito (Aedes aegypti). Biotropia 2015, 22, 45–51. [Google Scholar] [CrossRef]
- Mardin, S.; Ramadhan, A.; Afiat, N.; Istadewi, I. Repellent and Larvacidal Activity of Patchouli Oil and Curcuma Aeruginosarhizome Extract against Aedesaegypti. L. (Diptera: Culicidae). Egypt. J. Chem. 2024, 67, 117–125. [Google Scholar] [CrossRef]
- Shin, Y.K.; Lee, S.-Y.; Lee, J.-M.; Kang, P.; Seol, G.H. Effects of Short-Term Inhalation of Patchouli Oil on Professional Quality of Life and Stress Levels in Emergency Nurses: A Randomized Controlled Trial. J. Altern. Complement. Med. 2020, 26, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Bharathi, M.; Kunaviktikul, W.; Klunklin, A.; Chanthapoon, C.; Chaiyasut, C. Essential Oils, Phytoncides, Aromachology, and Aromatherapy—A Review. Appl. Sci. 2022, 12, 4495. [Google Scholar] [CrossRef]
- Vishali, S.; Kavitha, E.; Selvalakshmi, S. Therapeutic Role of Essential Oils. In Essential Oils: Extraction Methods and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 953–976. [Google Scholar] [CrossRef]
- Le Callaway, P. Aromatherapy and Its Applications for Body, Mind and Spirit; Lulu Press: Morrisville, NC, USA, 2019; ISBN 0359964354. [Google Scholar]
- Lizarraga-Valderrama, L.R. Effects of Essential Oils on Central Nervous System: Focus on Mental Health. Phyther. Res. 2021, 35, 657–679. [Google Scholar] [CrossRef]
- Ermaya, D.; Sari, S.P.; Patria, A.; Hidayat, F.; Razi, F. Identification of Patchouli Oil Chemical Components as the Results on Distillation Using GC-MS. IOP Conf. Ser. Earth Environ. Sci. 2019, 365, 12039. [Google Scholar] [CrossRef]
- Triana, K.Y.; Allenidekania, A.; Hayati, H. The Effect of Aromatherapy Inhalation on Reducing Chronic Pain for Children with Cancer: A Pilot Study. Trends Sci. 2022, 19, 2669. [Google Scholar] [CrossRef]
- Sah, S.P.; Mathela, C.S.; Chopra, K. Antidepressant Effect of Valeriana wallichii Patchouli Alcohol Chemotype in Mice: Behavioural and Biochemical Evidence. J. Ethnopharmacol. 2011, 135, 197–200. [Google Scholar] [CrossRef]
- Astuti, P.; Khairan, K.; Marthoenis, M.; Hasballah, K. Antidepressant-like Activity of Patchouli Oil Var. Tapak Tuan (Pogostemon cablin Benth) via Elevated Dopamine Level: A Study Using Rat Model. Pharmaceuticals 2022, 15, 608. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M. The Gut Microbiota and Host Health: A New Clinical Frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Ekstedt, N.; Jamioł-Milc, D.; Pieczyńska, J. Importance of Gut Microbiota in Patients with Inflammatory Bowel Disease. Nutrients 2024, 16, 2092. [Google Scholar] [CrossRef]
- Cho, K.Y. Association of Gut Microbiota with Obesity in Children and Adolescents. Clin. Exp. Pediatr. 2023, 66, 148. [Google Scholar] [CrossRef]
- Leong, W.; Huang, G.; Khan, I.; Xia, W.; Li, Y.; Liu, Y.; Li, X.; Han, R.; Su, Z.; Hsiao, W.L.W. Patchouli Essential Oil and Its Derived Compounds Revealed Prebiotic-Like Effects in C57BL/6J Mice. Front. Pharmacol. 2019, 10, 1229. [Google Scholar] [CrossRef]
- Sninsky, J.A.; Shore, B.M.; Lupu, G.V.; Crockett, S.D. Risk Factors for Colorectal Polyps and Cancer. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 195–213. [Google Scholar] [CrossRef]
- Bel, S.; Pendse, M.; Wang, Y.; Li, Y.; Ruhn, K.A.; Hassell, B.; Leal, T.; Winter, S.E.; Xavier, R.J.; Hooper, L. V Paneth Cells Secrete Lysozyme via Secretory Autophagy during Bacterial Infection of the Intestine. Science 2017, 357, 1047–1052. [Google Scholar] [CrossRef]
- Förster, C. Tight Junctions and the Modulation of Barrier Function in Disease. Histochem. Cell Biol. 2008, 130, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Orbán, E.; Szabó, E.; Lotz, G.; Kupcsulik, P.; Páska, C.; Schaff, Z.; Kiss, A. Different Expression of Occludin and ZO-1 in Primary and Metastatic Liver Tumors. Pathol. Oncol. Res. 2008, 14, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Wagener, F.A.; Feldman, E.; De Witte, T.; Abraham, N.G. Heme Induces the Expression of Adhesion Molecules ICAM-1, VCAM-1, and E Selectin in Vascular Endothelial Cells. Proc. Soc. Exp. Biol. Med. 1997, 216, 456–463. [Google Scholar] [CrossRef]
- Maurer, C.A.; Friess, H.; Kretschmann, B.; Wildi, S.; Müller, C.; Graber, H.; Schilling, M.; Büchler, M.W. Over-expression of ICAM-1, VCAM-1 and ELAM-1 Might Influence Tumor Progression in Colorectal Cancer. Int. J. Cancer 1998, 79, 76–81. [Google Scholar] [CrossRef]
- West, A.C.; Johnstone, R.W. New and Emerging HDAC Inhibitors for Cancer Treatment. J. Clin. Investig. 2014, 124, 30–39. [Google Scholar] [CrossRef]
- Sampath, S.J.P.; Venkatesan, V.; Ghosh, S.; Kotikalapudi, N. Obesity, Metabolic Syndrome, and Osteoarthritis—An Updated Review. Curr. Obes. Rep. 2023, 12, 308–331. [Google Scholar] [CrossRef]
- Kim, T.; Al Mijan, M.; Lee, J.; Yun, J.; Chung, J.H.; Son, S.M.; Kwon, R.J. Essential Oils for the Treatment and Management of Nonalcoholic Fatty Liver Disease (NAFLD). Nat. Prod. Commun. 2024, 19, 4. [Google Scholar] [CrossRef]
- Hong, S.J.; Cho, J.; Boo, C.G.; Youn, M.Y.; Pan, J.H.; Kim, J.K.; Shin, E.-C. Inhalation of Patchouli (Pogostemon cablin Benth.) Essential Oil Improved Metabolic Parameters in Obesity-Induced Sprague Dawley Rats. Nutrients 2020, 12, 2077. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Ekstedt, M.; Wong, G.L.-H.; Hagström, H. Changing Epidemiology, Global Trends and Implications for Outcomes of NAFLD. J. Hepatol. 2023, 79, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.L.P.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.H.; Lim, W.H.; Yang, J.D.; Tan, E.; Muthiah, M.D. Global Incidence and Prevalence of Nonalcoholic Fatty Liver Disease. Clin. Mol. Hepatol. 2023, 29, S32. [Google Scholar] [CrossRef]
- Wu, X.; Xu, N.; Li, M.; Huang, Q.; Wu, J.; Gan, Y.; Chen, L.; Luo, H.; Li, Y.; Huang, X.; et al. Protective Effect of Patchouli Alcohol Against High-Fat Diet Induced Hepatic Steatosis by Alleviating Endoplasmic Reticulum Stress and Regulating VLDL Metabolism in Rats. Front. Pharmacol. 2019, 10, 1134. [Google Scholar] [CrossRef]
- Cole, L.K.; Rajala-Schultz, P.J.; Lorch, G.; Daniels, J.B. Bacteriology and Cytology of Otic Exudates in 41 Cavalier King Charles Spaniels with Primary Secretory Otitis Media. Vet. Dermatol. 2019, 30, 151-e44. [Google Scholar] [CrossRef] [PubMed]
- Borek, I.; Birnhuber, A.; Voelkel, N.F.; Marsh, L.M.; Kwapiszewska, G. The Vascular Perspective on Acute and Chronic Lung Disease. J. Clin. Investig. 2023, 133, e170502. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, M.; Song, M.; Wang, J.; Cai, J.; Lin, C.; Li, Y.; Jin, X.; Shen, C.; Chen, Z. Patchouli Alcohol Activates PXR and Suppresses the NF-ΚB-Mediated Intestinal Inflammatory. J. Ethnopharmacol. 2020, 248, 112302. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-T.; Wang, Z.-Z.; Wang, Z.-C.; Wang, S.-M.; Cai, X.-J.; Su, G.-H.; Yuan, Z.-Y. Patchouli Alcohol Attenuates Experimental Atherosclerosis via Inhibiting Macrophage Infiltration and Its Inflammatory Responses. Biomed. Pharmacother. 2016, 83, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Aisyah, Y.; Yunita, D.; Amanda, A. Antimicrobial Activity of Patchouli (Pogostemon cablin Benth) Citronella (Cymbopogon nardus), and Nutmeg (Myristica fragrans) Essential Oil and Their Mixtures against Pathogenic and Food Spoilage Microbes. IOP Conf. Ser. Earth Environ. Sci. 2021, 667, 12020. [Google Scholar] [CrossRef]
- Xie, L.; Guo, Y.; Chen, Y.; Zhang, L.; Wang, Z.; Zhang, T.; Wang, B. A Potential Drug Combination of Omeprazole and Patchouli Alcohol Significantly Normalizes Oxidative Stress and Inflammatory Responses against Gastric Ulcer in Ethanol-Induced Rat Model. Int. Immunopharmacol. 2020, 85, 106660. [Google Scholar] [CrossRef]
- Lu, Y.; Li, S.; Lou, H. Patchouli Alcohol Protects against Myocardial Ischaemia–Reperfusion Injury by Regulating the Notch1/Hes1 Pathway. Pharm. Biol. 2022, 60, 949–957. [Google Scholar] [CrossRef]
- Lee, H.-S.; Lee, J.; Smolensky, D.; Lee, S.-H. Potential Benefits of Patchouli Alcohol in Prevention of Human Diseases: A Mechanistic Review. Int. Immunopharmacol. 2020, 89, 107056. [Google Scholar] [CrossRef]
- Homayoun, M.; Sajedi, N.; Soleimani, M. In Vitro Evaluation of the Pogostone Effects on the Expression of PTEN and DACT1 Tumor Suppressor Genes, Cell Cycle, and Apoptosis in Ovarian Cancer Cell Line. Res. Pharm. Sci. 2022, 17, 164–175. [Google Scholar] [CrossRef]
- Ji, L.; Lou, S.; Fang, Y.; Wang, X.; Zhu, W.; Liang, G.; Lee, K.; Luo, W.; Zhuang, Z. Patchouli Alcohol Protects the Heart against Diabetes-Related Cardiomyopathy through the JAK2/STAT3 Signaling Pathway. Pharmaceuticals 2024, 17, 631. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-X.; Yao, Y.-Y.; Ai, C.-H.; Yang, G.; Liang, X.-Y.; Liu, T.-D.; He, M.-L.; Xia, J.-H. Dietary Supplementation of Patchouli Oil Increases the Resistance against Streptococcus Agalactiae Infection in GIFT Tilapia as Revealed by Transcriptome and 16s Amplicon Profiling. Aquac. Rep. 2023, 33, 101754. [Google Scholar] [CrossRef]
- Tang, S.; Huang, S.; Huang, J.; Lai, X.; Guo, J.; Huang, J.; Zhong, Y. Pogostone Attenuated High-Fat Diet–Induced Nonalcoholic Fatty Liver Disease in Mice through Inhibition of NLRP3 Inflammasome Signaling. Eur. J. Pharmacol. 2024, 970, 176463. [Google Scholar] [CrossRef]
- Lee, J.; Kong, B.; Lee, S.-H. Patchouli Alcohol, a Compound from Pogostemon cablin, Inhibits Obesity. J. Med. Food 2020, 23, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.-L.; Chen, Y.; Yu, Q.-Y.; Wang, Y.; Liu, G. Patchouli Alcohol Protects against Ischemia/Reperfusion-Induced Brain Injury via Inhibiting Neuroinflammation in Normal and Obese Mice. Brain Res. 2018, 1682, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Fei, F.; Zhang, C. Patchouli Alcohol Suppresses Inflammation and Autophagy, and Reduces Osteoclast Formation in Periodontitis via Regulation of ERK and NF ΚB Signaling Pathways. Trop. J. Pharm. Res. 2022, 21, 1607–1613. [Google Scholar] [CrossRef]
- Lalthafamkimi, L.; Kumar, A.; Wann, S.B.; Kumar, D.; Bhattacharyya, P.; Kumar, S. Metabolite Bioprospection and Expression Analysis of Patchoulol Synthase Gene in Different in Vitro Systems of Pogostemon cablin: An Important Medicinal Aromatic Plant. Ind. Crops Prod. 2023, 205, 116689. [Google Scholar] [CrossRef]
- Kusumaningrum, H.P.; Subagio, A.; Zainuri, M.; Herida, A.P.; Wiryawan, A.Z.T.P.; Nuha, I.; Syaifudin, A.M.P.; Ghozi, A.F.; Suhariyanto, B.G.; Samudra, J.A. Antibacterial Activity Test of Patchouli Essential Oil (Pogostemon cablin Benth.), Clove (Syzygium aromaticum), and Lemongrass (Cymbopogon citratus) against Bacteria Escherichia coli and Bacillus subtilis. AIP Conf. Proc. 2024, 3165, 050009. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L.; Moreno-Toral, E. Pharmacy and Fragrances: Traditional and Current Use of Plants and Their Extracts. Cosmetics 2023, 10, 157. [Google Scholar] [CrossRef]
- Devi, M.; Thalkari, A.B.; Thorat, V.M. Overview of Herbal Cosmetics. Res. J. Top. Cosmet. Sci. 2022, 13, 27–34. [Google Scholar] [CrossRef]
- Sharma, A.; Gumber, K.; Gohain, A.; Bhatia, T.; Sohal, H.S.; Mutreja, V.; Bhardwaj, G. Importance of Essential Oils and Current Trends in Use of Essential Oils (Aroma Therapy, Agrofood, and Medicinal Usage). In Essential Oils; Elsevier: Amsterdam, The Netherlands, 2023; pp. 53–83. [Google Scholar] [CrossRef]
- Taufik, M.; Rizal, S.; Harnelly, E.; Muhammad, S.; Syahrizal, D.; Prajaputra, V.; Isnaini, N. The Therapeutic Potential of Aceh Patchouli Oil (Pogostemon cablin Benth.) in Enhancing Full-Thickness Wound Healing in Mice. Trop. J. Nat. Prod. Res. 2024, 8. [Google Scholar] [CrossRef]
- Rahmi, D.; Yunilawati, R.; Jati, B.N.; Setiawati, I.; Riyanto, A.; Batubara, I.; Astuti, R.I. Antiaging and Skin Irritation Potential of Four Main Indonesian Essential Oils. Cosmetics 2021, 8, 94. [Google Scholar] [CrossRef]
- Kumar, K.J.S.; Vani, M.G.; Chinnasamy, M.; Lin, W.-T.; Wang, S.-Y. Patchouli Alcohol: A Potent Tyrosinase Inhibitor Derived from Patchouli Essential Oil with Potential in the Development of a Skin-Lightening Agent. Cosmetics 2024, 11, 38. [Google Scholar] [CrossRef]
- Arahman, N.; Deski, D.; Akbar, A.; Surya, A.; Pitra, M.N.; Pratama, R.N. Effect of Addition of Patchouli Leaf Essential Oil (Pogostemon cablin Benth) on Eyecream Products. J. Patchouli Essent. Oil Prod. 2023, 2, 16–20. [Google Scholar] [CrossRef]
- Khare, N.; Khokhar, D.; Patel, S.; Mishra, N.K. Study on Physico-Chemical Properties of Patchouli Oil. Int. J. Chem. Stud. 2020, 8, 1206–1211. [Google Scholar] [CrossRef]
- Hassid, A.; Salla, M.; Krayem, M.; Khaled, S.; Hassan, H.; El Khatib, S. A Review on the Versatile Applications of Plant-Based Essential Oils in Food Flavoring, Culinary Uses and Health Benefits. Preprint 2024. [Google Scholar] [CrossRef]
- Hikal, W.M.; Baz, M.M.; Alshehri, M.A.; Bahattab, O.; Baeshen, R.S.; Selim, A.M.; Alhwity, L.; Bousbih, R.; Alshourbaji, M.S.; Ahl, H.A.H.S.-A. Sustainable Pest Management Using Novel Nanoemulsions of Honeysuckle and Patchouli Essential Oils against the West Nile Virus Vector, Culex Pipiens, under Laboratory and Field Conditions. Plants 2023, 12, 3682. [Google Scholar] [CrossRef]
- Silvério, M.R.S.; Espindola, L.S.; Lopes, N.P.; Vieira, P.C. Plant Natural Products for the Control of Aedes aegypti: The Main Vector of Important Arboviruses. Molecules 2020, 25, 3484. [Google Scholar] [CrossRef]
- Ahmad, I.; Ayu, N.A.; Sushanti, G.; Muhtar, I. Aromatherapy Candle Formulation Using Grapefruit Essential Oil with Patchouli Essential Oil Fixative. J. Agric. 2022, 1, 159–166. [Google Scholar] [CrossRef]
- Ramya, H.G.; Palanimuthu, V.; Rachna, S. An Introduction to Patchouli (Pogostemon cablin Benth.)–A Medicinal and Aromatic Plant: It’s Importance to Mankind. Agric. Eng. Int. CIGR J. 2013, 15, 243–250. [Google Scholar]
- Soh, S.H.; Jain, A.; Lee, L.Y.; Chin, S.K.; Yin, C.-Y.; Jayaraman, S. Techno-Economic and Profitability Analysis of Extraction of Patchouli Oil Using Supercritical Carbon Dioxide. J. Clean. Prod. 2021, 297, 126661. [Google Scholar] [CrossRef]
- Zaizen, K.; Kusano, Y.; Kabayahi, T. Study about a Novel Odor-active Compound in Patchouli Oil. In Proceedings of the 58th Symposium Chemistry Terpenes, Essential Oils, and Aromatics (TEAC), Wakayama, Japan, 20–22 September 2014. [Google Scholar]
- Peter, K.V.; Sharangi, A.B. Medicinal Plants: Future Thrust Areas and Research Directions. In Medicinal Plants; Apple Academic Press: Palm Bay, FL, USA, 2022; pp. 521–536. [Google Scholar]
- Istiningrum, R.B.; Saepuloh, A.; Jannah, W.; Aji, D.W. Measurement Uncertainty of Ester Number, Acid Number and Patchouli Alcohol of Patchouli Oil Produced in Yogyakarta. AIP Conf. Proc. 2017, 1823, 020080. [Google Scholar] [CrossRef]
- Mortimer, S.; Reeder, M. Botanicals in Dermatology: Essential Oils, Botanical Allergens, and Current Regulatory Practices. Dermatitis 2016, 27, 317–324. [Google Scholar] [CrossRef]
- Barbieri, C.; Borsotto, P. Essential Oils: Market and Legislation. In Potential of Essential Oils; IntechOpen: London, UK, 2018; pp. 107–127. [Google Scholar] [CrossRef]
- Zhang, R.; Yan, P.; Li, Y.; Xiong, L.; Gong, X.; Peng, C. A Pharmacokinetic Study of Patchouli Alcohol after a Single Oral Administration of Patchouli Alcohol or Patchouli Oil in Rats. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 441–448. [Google Scholar] [CrossRef]
- Nour, A.H.; Modather, R.H.; Yunus, R.M.; Elnour, A.A.M.; Ismail, N.A. Characterization of Bioactive Compounds in Patchouli Oil Using Microwave-Assisted and Traditional Hydrodistillation Methods. Ind. Crops Prod. 2024, 208, 117901. [Google Scholar] [CrossRef]
- de Matos, S.P.; Lucca, L.G.; Koester, L.S. Essential Oils in Nanostructured Systems: Challenges in Preparation and Analytical Methods. Talanta 2019, 195, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Achagar, R.; Ait-Touchente, Z.; El Ati, R.; Boujdi, K.; Thoume, A.; Abdou, A.; Touzani, R. A Comprehensive Review of Essential Oil–Nanotechnology Synergy for Advanced Dermocosmetic Delivery. Cosmetics 2024, 11, 48. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Liu, Y.; Yu, J.; Yao, G.; Yang, H.; Yang, D.; Wu, Y. An Integrated Transcriptome and Metabolome Analysis Reveals the Gene Network Regulating Flower Development in Pogostemon cablin. Front. Plant Sci. 2023, 14, 1201486. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Mahfud, M. The Extraction of Essential Oils from Patchouli Leaves (Pogostemon cablin Benth) Using a Microwave Air-Hydrodistillation Method as a New Green Technique. RSC Adv. 2017, 7, 1336–1347. [Google Scholar] [CrossRef]
- Lin, L.-T.; Zhang, S.-T.; Shang, B.-L.; Dai, Y.-Q.; Cheng, X.-Q.; Wu, Q.-G.; Zhan, R.-T.; Liu, S.-J. The Effect and Mechanism of Patchouli Alcohol on Cognitive Dysfunction in AD Mice Induced by Aβ1-42 Oligomers through AMPK/MTOR Pathway. Brain Res. Bull. 2024, 215, 111030. [Google Scholar] [CrossRef]
- Yan, W.; Ye, Z.; Cao, S.; Yao, G.; Yu, J.; Yang, D.; Chen, P.; Zhang, J.; Wu, Y. Transcriptome Analysis of Two Pogostemon cablin Chemotypes Reveals Genes Related to Patchouli Alcohol Biosynthesis. PeerJ 2021, 9, e12025. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).