Antioxidant Capacity and Accumulation of Caffeoylquinic Acids in Arnica montana L. In Vitro Shoots After Elicitation with Yeast Extract or Salicylic Acid
Abstract
1. Introduction
2. Results
2.1. Effect of YE on Shoot Organogenesis, Shoot Growth, and Development
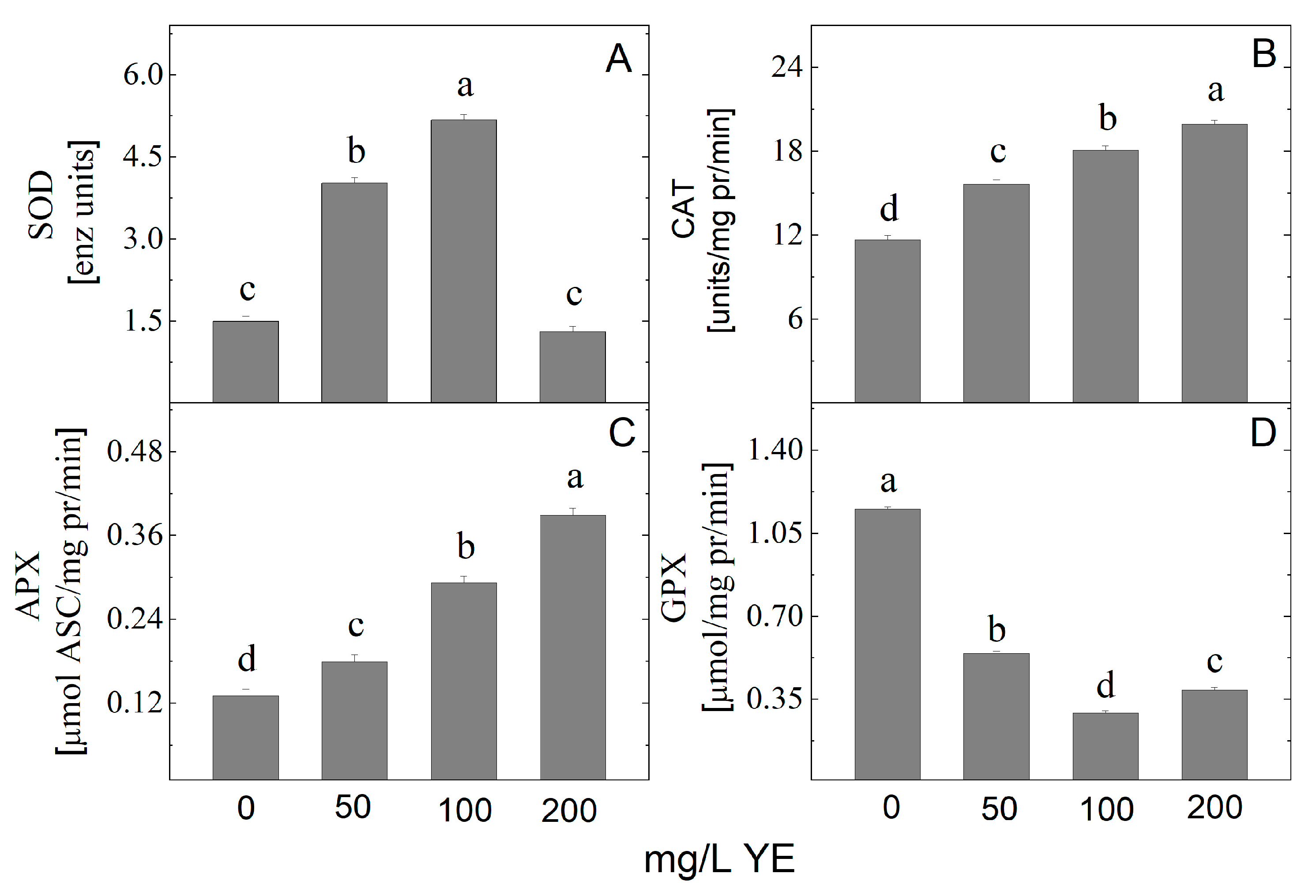
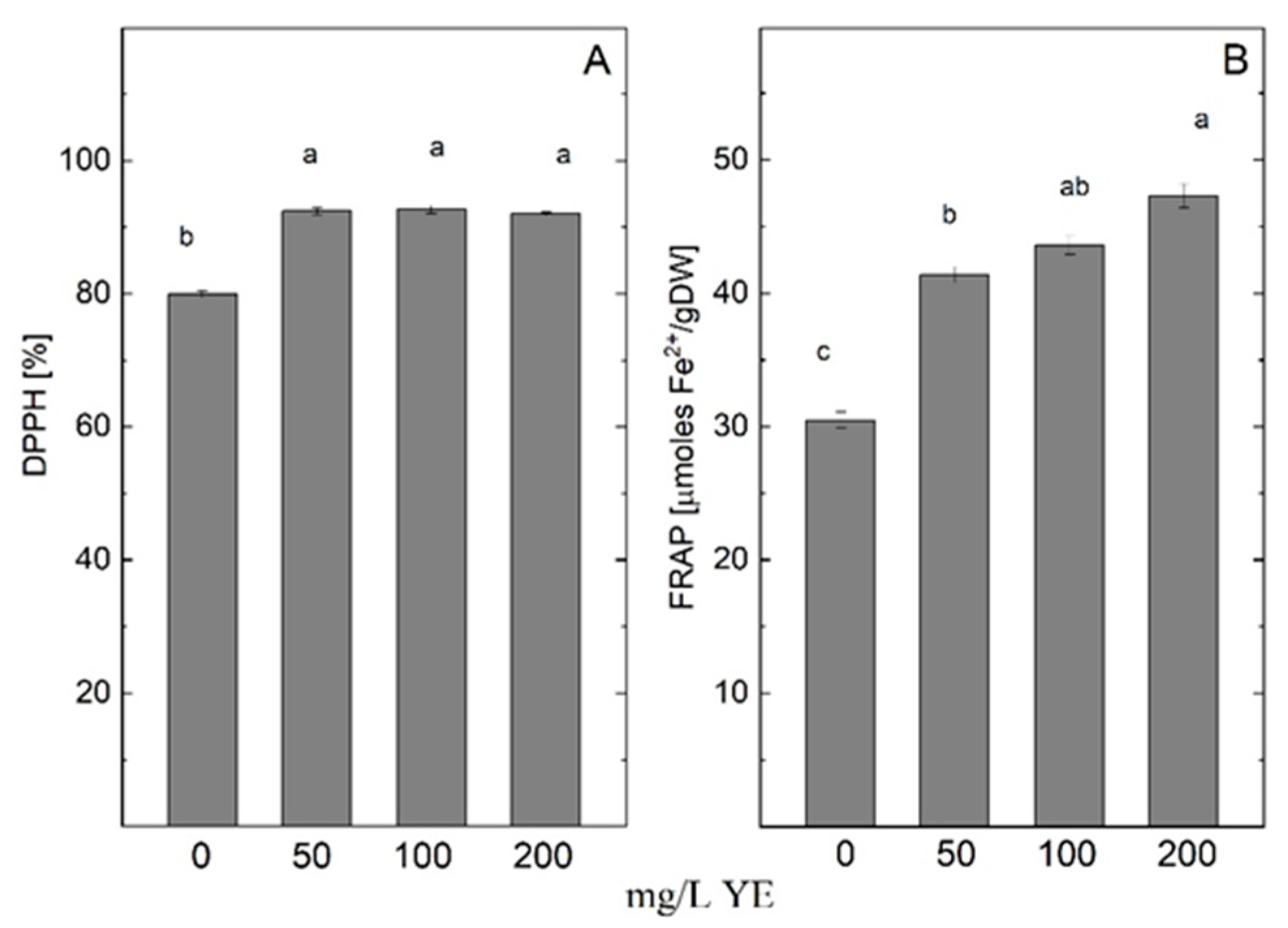
2.2. Effect of YE on Antioxidant Enzyme Activity
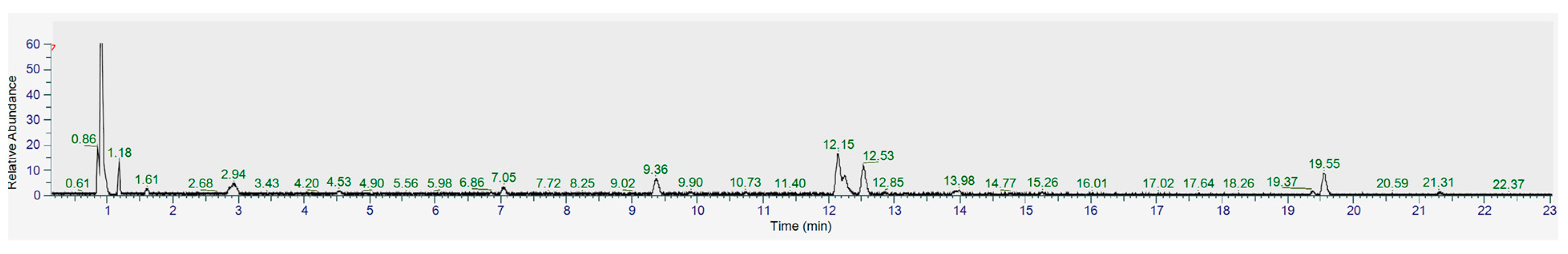
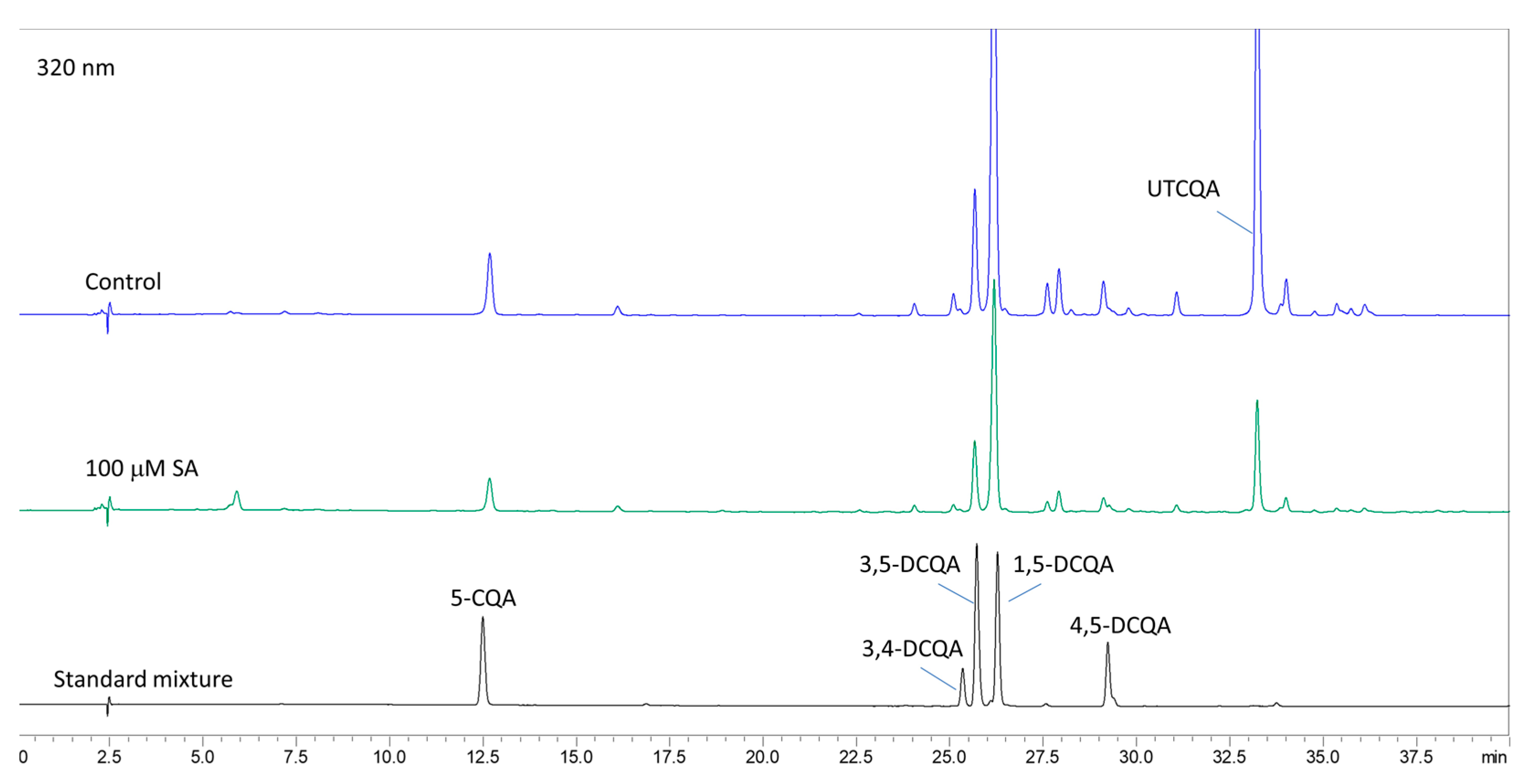
2.3. Identification of the Main Secondary Metabolites in A. montana Shoots
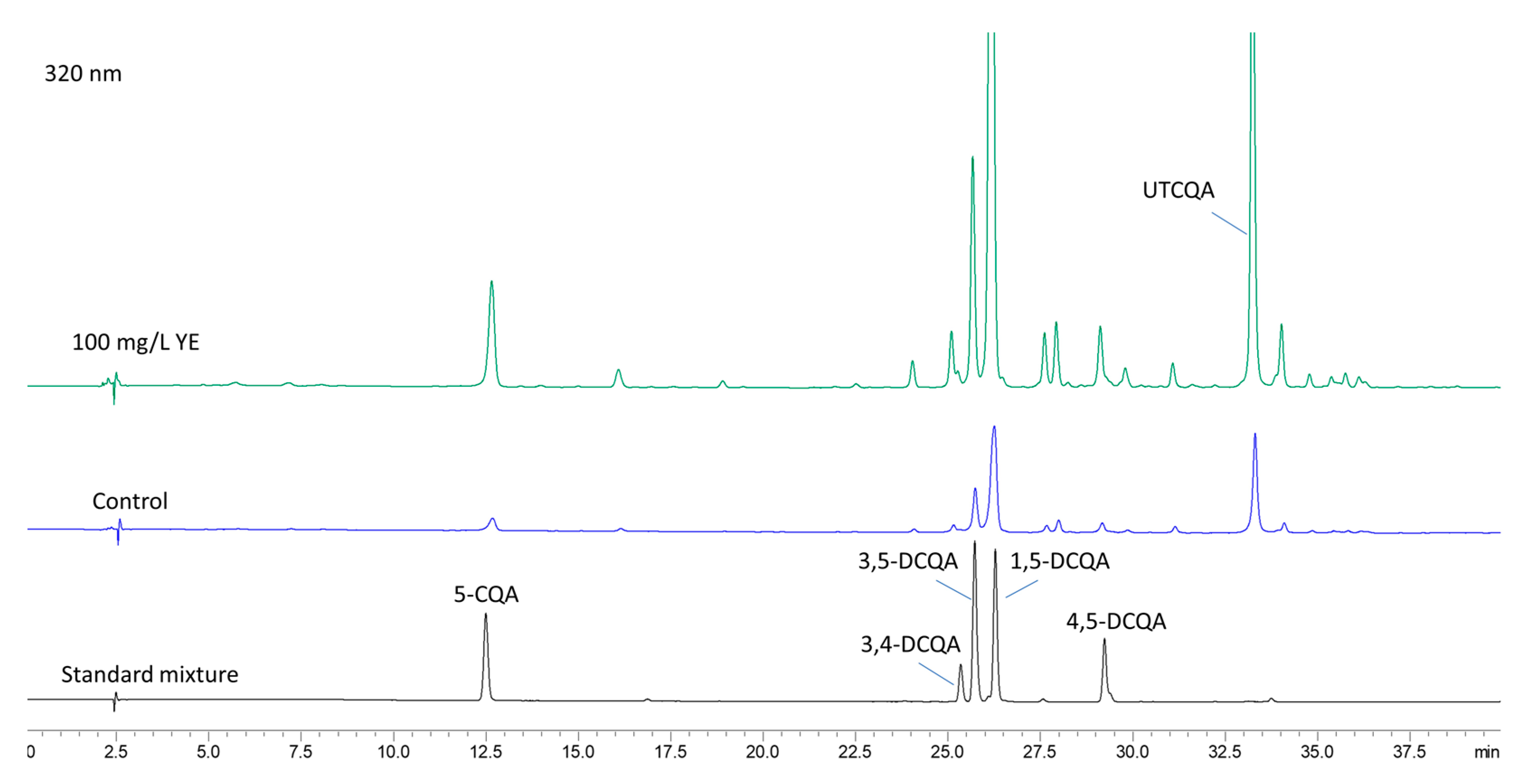
2.4. Content of Caffeoylquinic Acids After Elicitation with YE
2.5. Effect of SA on Shoot Organogenesis, Shoot Growth, and Development
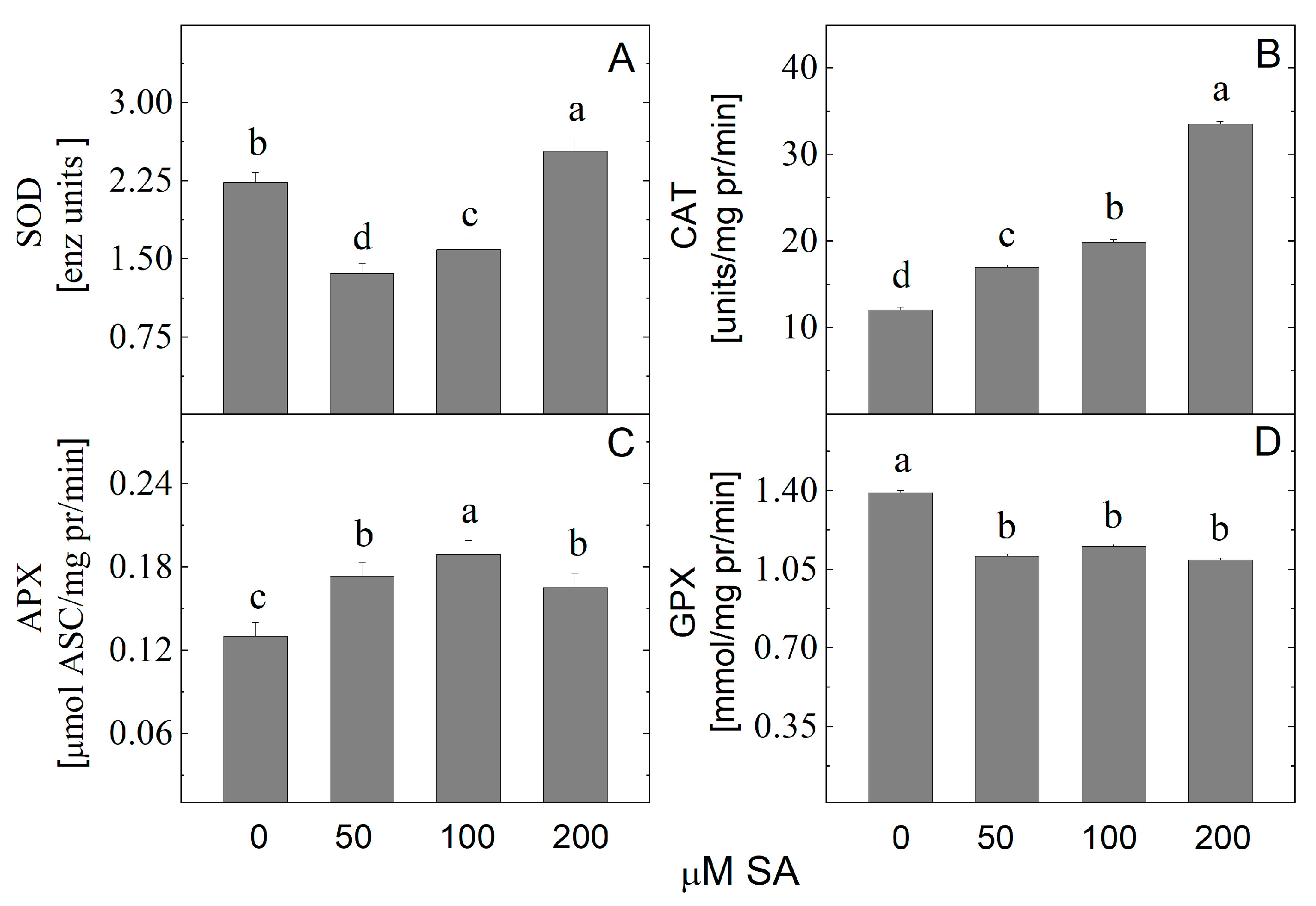
2.6. Effect of SA on Antioxidant Activity of Arnica montana In Vitro Shoots
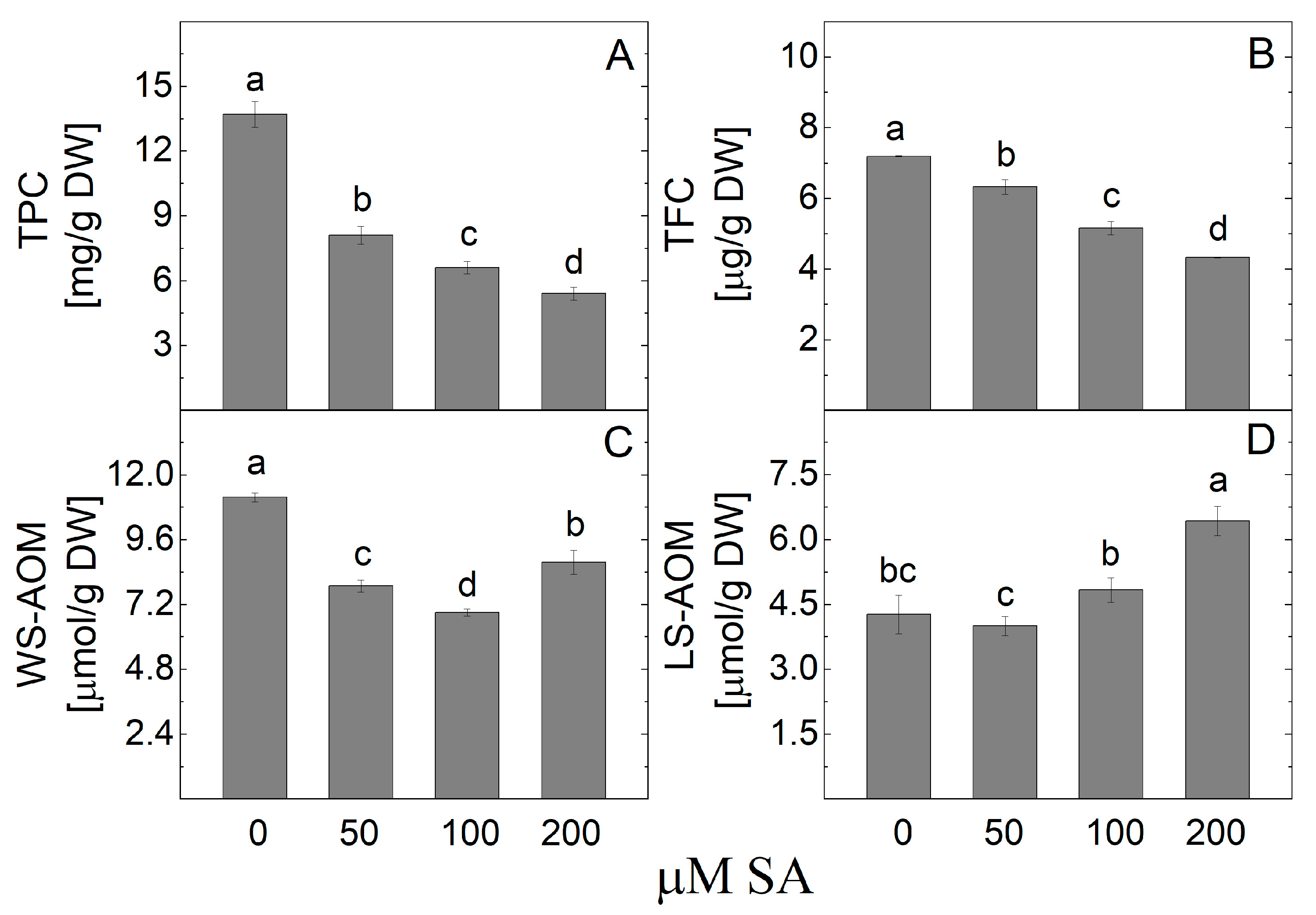
2.7. Content of Caffeoylquinic Acids After Treatment with SA
3. Discussion
3.1. Effect of Yeast Extract on Growth and Antioxidant Defense System
3.2. Effect of YE on Caffeoylquinic Acids Content
3.3. Effect of Salicylic Acid on Growth and Antioxidant Defense System
3.4. Effect of SA on Caffeoylquinic Acids Content
4. Materials and Methods
4.1. Plant Material
4.2. Elicitor Preparation and Culture Conditions
4.3. Antioxidant Capacity Assays
4.4. Preparation of the Samples for Qualitative and Quantitative Analysis of Caffeoylquinic Acids
4.5. UHPLC-MS/MS Analysis of the Methanol Extract of A. montana Shoots
4.6. HPLC-DAD Analysis of Caffeoylquinic Acids
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganzera, M.; Egger, C.; Zidorn, C.; Stuppner, H. Quantitative analysis of flavonoids and phenolic acids in Arnica montana L. by micellar electrokinetic capillary chromatography. Anal. Chim. Acta 2008, 614, 196–200. [Google Scholar] [CrossRef]
- Kriplani, P.; Guarve, K.; Baghael, U.S. Arnica montana L.—A plant of healing: Review. J. Pharm. Pharm. 2017, 69, 925–945. [Google Scholar] [CrossRef]
- Falniowski, A.; Bazos, I.; Hodálová, I.; Lansdown, R.; Petrova, A. “Arnica montana”. IUCN 2012. IUCN Red List of Threatened Species, version 2012.2; IUCN: Gland, Switzerland, 2012. [Google Scholar]
- Korneck, D.; Schnittler, M.; Vollmer, I. Red list of pteridophyta and spermatophyta in Germany. In Red List of Endangered Plants in Germany; Ludwig, G., Schnittler, M., Eds.; Bundesamt für Naturschutz: Bonn, Germany, 1996; Volume 28, pp. 21–187. [Google Scholar]
- Rather, G.A.; Verma, R.; Sharma, B.; Sharma, A.; Kumar, A. Tissue culture: A perpetual source for the conservation of medicinally important endangered plant species. In Advances in Plant Tissue Culture; Academic Press: Cambridge, MA, USA, 2022; pp. 373–393. [Google Scholar]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Bonfill Baldrich, M.M.; Cusidó Vidal, R.M.; Lalaleo, L.; Palazón Barandela, J. Plant Cell and Organ Cultures as a Source of Phytochemicals. In Recent Advances in Pharmaceutical Sciences V; Research Signpost: Ahmedabad, India, 2015; Chapter 3; pp. 33–49. ISBN 978-81-308-0561-0. [Google Scholar]
- Narayani, M.; Shrivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochemical. Rev. 2017, 16, 1227–1252. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Zhao, P.; Lu, G.H.; Yang, Y.H. Salicylic acid signaling and its role in responses to stresses in plants. Mech. Plant Horm. Signal. Under Stress 2017, 39, 413–441. [Google Scholar]
- Ali, B. Salicylic acid: An efficient elicitor of secondary metabolite production in plants. Biocatal Agric Biotechnol 2021, 31, 101884. [Google Scholar] [CrossRef]
- Gadzovska, S.; Maury, S.; Delaunay, A.; Spasenoski, M.; Hagège, D.; Courtois, D.; Joseph, C. The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tissue Organ Cult. 2012, 113, 25–39. [Google Scholar] [CrossRef]
- Ejtahed, R.; Radjabian, T.; Hoseini Tafreshi, S.A. Expression analysis of phenylalanine ammonia lyase gene and rosmarinic acid production in Salvia officinalis and Salvia virgata shoots under salicylic acid elicitation. Appl. Biochem. Biotechnol. 2015, 176, 1846–1858. [Google Scholar] [CrossRef]
- El-Serafy, R.S. Growth and productivity of roselle (Hibiscus sabdariffa L.) as affected by yeast and humic acid. Sci. J. Flowers Ornam. Plants. 2018, 5, 195–203. [Google Scholar] [CrossRef]
- Yan, Q.; Shi, M.; Ng, J.; Wu, J.Y. Elicitor-induced rosmarinic acid accumulation and secondary metabolism enzyme activities in Salvia miltiorrhiza hairy roots. Plant Sci. 2006, 170, 853–858. [Google Scholar] [CrossRef]
- Farjaminezhad, R.; Garoosi, G. Improvement and Prediction of Secondary Metabolites Production under Yeast Extract Elicitation of Azadirachta Indica Cell Suspension Culture Using Response Surface Methodology. AMB Express 2021, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Moharramnejad, S.; Sofalian, O.; Valizadeh, M.; Asgari, A.; Shiri, M. Response of antioxidant defense system to osmotic stress in maize seedlings. Fresenius Environ. Bull. 2016, 25, 805–811. [Google Scholar]
- Lin, L.Z.; Harnly, J.M. Identification of hydroxycinnamoylquinic acids of arnica flowers and burdock roots using a standardized LC-DAD-ESI/MS profiling method. J. Agricult. Food Chem. 2008, 56, 10105–10114. [Google Scholar] [CrossRef]
- Jaiswal, R.; Kuhnert, N. Identification and characterization of two new derivatives of chlorogenic acids in Arnica (Arnica montana L.) flowers by high-performance liquid chromatography/tandem mass spectrometry. J. Agricult. Food Chem. 2011, 59, 4033–4039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, P.; Qu, H.; Cheng, Y. Characterization of phenolic compounds in Erigeron breviscapus by liquid chromatography coupled to electrospray ionization mass spectrometry. Rapid Commun. Mass. Spectrom. 2007, 21, 2971–2984. [Google Scholar] [CrossRef]
- Heyman, H.M.; Senejoux, F.; Seibert, I.; Klimkait, T.; Maharaj, V.J.; Meyer, J.J.M. Identification of anti-HIV active dicaffeoylquinic-and tricaffeoylquinic acids in Helichrysum populifolium by NMR-based metabolomic guided fractionation. Fitoterapia 2015, 103, 155–164. [Google Scholar] [CrossRef]
- Ivanova, V.; Nedialkov, P.; Dimitrova, P.; Paunova-Krasteva, T.; Trendafilova, A. Inula salicina L.: Insights into its polyphenolic constituents and biological activity. Pharmaceuticals 2024, 17, 844. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Extensive characterisation of bioactive phenolic constituents from globe artichoke (Cynara scolymus L.) by HPLC–DAD-ESI-QTOF-MS. Food Chem. 2013, 141, 2269–2277. [Google Scholar] [CrossRef]
- Merghany, R.M.; Salem, M.A.; Ezzat, S.M.; Moustafa, S.F.; El-Sawi, S.A.; Meselhy, M.R. A comparative UPLC-orbitrap-MS-based metabolite profiling of three Pelargonium species cultivated in Egypt. Scient. Rep. 2024, 14, 22765. [Google Scholar] [CrossRef]
- MassBank of North America. Available online: http://mona.fiehnlab.ucdavis.edu (accessed on 1 January 2023).
- Naik, P.M.; Al-Khayri, J.M. Abiotic and biotic elicitors–role in secondary metabolites production through in vitro culture of medicinal plants. In Abiotic and Biotic Stress in Plants-Recent Advances and Future Perspectives; Shanker, A., Shanker, C., Eds.; IntechOpen: Rijeka, Croatia, 2016; pp. 247–277. [Google Scholar]
- Hidalgo, D.; Sanchez, R.; Lalaleo, L.; Bonfill, M.; Corchete, P.; Palazon, J. Biotechnological production of pharmaceuticals and biopharmaceuticals in plant cell and organ cultures. Curr. Med. Chem. 2018, 25, 3577–3596. [Google Scholar] [CrossRef]
- Danova, K.; Motyka, V.; Trendafilova, A.; Dobrev, P.I.; Ivanova, V.; Aneva, I. Evolutionary Aspects of Hypericin Productivity and Endogenous Phytohormone Pools Evidenced in Hypericum Species In Vitro Culture Model. Plants 2022, 11, 2753. [Google Scholar] [CrossRef]
- Chippy, S.L.; Vijay, V.T.A.; Hemanthakumar, A.S.; Pillai, P.P.; Preetha, T.S. Enhanced production of lupeol through elicitation in in vitro shoot cultures of snake grass (Clinacanthus nutans). Notul. Scient. Biol. 2022, 14, 11195. [Google Scholar] [CrossRef]
- Singh, A.; Dwivedi, P. Methyl-jasmonate and salicylic acid as potent elicitors for secondary metabolite production in medicinal plants: A review. J. Pharmacogn. Phytochem. 2018, 7, 750–757. [Google Scholar]
- Petrova, M.; Miladinova-Georgieva, K.; Geneva, M. Influence of Abiotic and Biotic Elicitors on Organogenesis, Biomass Accumulation, and Production of Key Secondary Metabolites in Asteraceae Plants. Int. J. Mol. Sci. 2024, 25, 4197. [Google Scholar] [CrossRef] [PubMed]
- George, E.F.; Hall, M.A.; Klerk, G.J.D. The components of plant tissue culture media II: Organic additions, osmotic and pH effects, and support systems. In Plant Propagation by Tissue Culture; Springer: Dordrecht, The Netherlands, 2008; pp. 115–173. [Google Scholar]
- Klotz, S.; Kuenz, A.; Prüße, U. Nutritional requirements and the impact of yeast extract on the D-lactic acid production by Sporolactobacillus inulinus. Green Chem. 2017, 19, 4633–4641. [Google Scholar] [CrossRef]
- Rasouli, D.; Werbrouck, S.; Maleki, B.; Jafary, H.; Schurdi-Levraud, V. Elicitor-induced in vitro shoot multiplication and steviol glycosides production in Stevia rebaudiana. S. Afr. J. Bot. 2021, 137, 265–271. [Google Scholar] [CrossRef]
- Jirakiattikul, Y.; Ruangnoo, S.; Sangmukdee, K.; Chamchusri, K.; Rithichai, P. Enhancement of Plumbagin Production through Elicitation in In Vitro-Regenerated Shoots of Plumbago indica L. Plants 2024, 13, 1450. [Google Scholar] [CrossRef]
- Kanthaliya, B.; Joshi, A.; Arora, J.; Alqahtani, M.D.; Abd_Allah, E.F. Effect of Biotic Elicitors on the Growth, Antioxidant Activity and Metabolites Accumulation in In Vitro Propagated Shoots of Pueraria tuberosa. Plants 2023, 12, 1300. [Google Scholar] [CrossRef]
- Abraham, F.; Bhatt, A.; Keng, C.L.; Indrayanto, G.; Sulaiman, S.F. Effect of yeast extract and chitosan on shoot proliferation, morphology and antioxidant activity of Curcuma mangga in vitro plantlets. Afr. J. Biotechnol. 2011, 10, 7787–7795. [Google Scholar]
- Gonçalves, S.; Mansinhos, I.; Rodríguez-Solana, R.; SantínE, P.; Coelho, N.; Romano, A. Elicitation improves rosmarinic acid content and antioxidant activity in Thymus lotocephalus shoot cultures. Ind. Crop. Prod. 2019, 137, 214–220. [Google Scholar] [CrossRef]
- Karalija, E.; Zeljković, S.Ć.; Parić, A. Harvest time-related changes in biomass, phenolics and antioxidant potential in Knautia sarajevensis shoot cultures after elicitation with salicylic acid and yeast. In Vitr. Cell. Dev. Biol. Plant. 2019, 56, 177–183. [Google Scholar] [CrossRef]
- Ishikawa, A.; Kitamura, Y.; Ozeki, Y.; Watanabe, M. Different responses of shoot and root cultures of Glehnia littoralis to yeast extract. J. Nat. Med. 2006, 61, 30–37. [Google Scholar] [CrossRef]
- Park, Y.J.; Kim, J.K.; Park, S.U. Yeast extract improved biosynthesis of astragalosides in hairy root cultures of Astragalus membranaceus. Prep. Biochem. Biotechnol. 2020, 51, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.M.S.; Oliveira, J.T.A.; Gondim, D.M.F.; Domingues, D.P.; Machado, O.L.T.; Jacinto, T. Assessment of stress–related enzymes in response to either exogenous salicylic acid or methyl jasmonate in Jatropha curcas L. leaves, an attractive plant to produce biofuel. S. Afr. J. Bot. 2016, 105, 163–168. [Google Scholar] [CrossRef]
- Gholami, F.; Amerian, M.R.; Asghari, H.R.; Ebrahimi, A. Assessing the effects of 24-epibrassinolide and yeast extract at various levels on cowpea’s morphophysiological and biochemical responses under water deficit stress. BMC Plant Biol. 2023, 23, 593. [Google Scholar] [CrossRef]
- Yousef, E.A.A.; Ali, M.A.M. Alleviation of Cold Stress on Tomato During Winter Season by Application of Yeast Extract and Glycinebetaine. Egypt. J. Hortic. 2019, 46, 117–131. [Google Scholar] [CrossRef]
- Abbas, S.M. The influence of biostimulants on the growth and on the biochemical composition of Vicia faba CV. Giza 3 beans. Rom. Biotechnol. Lett. 2013, 18, 8061–8068. [Google Scholar]
- Sarkate, A.; Banerjee, S.; Mir, J.I.; Roy, P.; Sircar, D. Antioxidant and cytotoxic activity of bioactive phenolic metabolites isolated from the yeast-extract treated cell culture of apple. Plant Cell Tiss. Organ. Cult. 2017, 130, 641–649. [Google Scholar] [CrossRef]
- Nadeem, M.; Abbasi, B.H.; Garros, L.; Drouet, S.; Zahir, A.; Ahmad, W.; Giglioli-Guivarc’h, N.; Hano, C. Yeast-extract improved biosynthesis of lignans and neolignans in cell suspension cultures of Linum usitatissimum L. Plant Cell Tiss. Organ. Cult. 2018, 135, 347–355. [Google Scholar] [CrossRef]
- Zaman, G.; Farooq, U.; Bajwa, M.N.; Jan, H.; Shah, M.; Ahmad, R.; Andleeb, A.; Drouet, S.; Hano, C.; Abbasi, B.H. Effects of yeast extract on the production of phenylpropanoid metabolites in callus culture of purple basil (Ocimum Basilicum L. var purpurascens) and their in-vitro evaluation for antioxidant potential. Plant Cell Tiss. Organ. Cult. 2022, 150, 543–553. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Kabera, J.N.; Semana, E.; Mussa, A.R.; He, X. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Nikolova, M.; Petrova, M.; Zayova, E.; Vitkova, A.; Evstatieva, L. Comparative study of in vitro, ex vitro and in vivo grown plants of Arnica montana-polyphenols and free radical scavenging activity. Acta Bot. Croat. 2013, 72, 13–22. [Google Scholar] [CrossRef]
- Nieto-Trujillo, A.; Cruz-Sosa, F.; Luria-Pérez, R.; Gutiérrez-Rebolledo, G.A.; Román-Guerrero, A.; Burrola-Aguilar, C.; Zepeda-Gómez, C.; Estrada-Zúñiga, M.E. Arnica montana Cell Culture Establishment, and Assessment of Its Cytotoxic, Antibacterial, α-Amylase Inhibitor, and Antioxidant In Vitro Bioactivities. Plants 2021, 10, 2300. [Google Scholar] [CrossRef] [PubMed]
- Sugier, D.; Sugier, P.; Jakubowicz-Gil, J.; Gawlik-Dziki, U.; Zając, A.; Król, B.; Chmiel, S.; Kończak, M.; Pięt, M.; Paduch, R. Nitrogen Fertilization and Solvents as Factors Modifying the Antioxidant and Anticancer Potential of Arnica montana L. Flower Head Extracts. Plants 2023, 12, 142. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Thiruvengadam, M.; Chung, I.M. Identification of elicitors enhances the polyphenolic compounds and pharmacological potential in hairy root cultures of Aster scaber. S. Afr. J. Bot. 2019, 125, 92–101. [Google Scholar] [CrossRef]
- Dowom, S.A.; Abrishamchi, P.; Radjabian, T.; Salami, S.A. Enhanced phenolic acids production in regenerated shoot cultures of Salvia virgata Jacq. after elicitation with Ag+ ions, methyl jasmonate and yeast extract. Ind. Crop. Prod. 2017, 103, 81–88. [Google Scholar] [CrossRef]
- Złotek, U. Effect of jasmonic acid and yeast extract elicitation on low-molecular antioxidants and antioxidant activity of marjoram (Origanum majorana L.). Acta Sci. Pol. Technol. Aliment. 2017, 16, 371–377. [Google Scholar]
- Eskandari-Samet, A.; Piri, K.; Kayhanfar, M.; Hasanloo, T. Enhancement of Tropane Alkaloid Production among Several Clones and Explants Types of Hairy Root of Atropa belladonna L. J. Med. Plants By-Product 2012, 1, 35–42. [Google Scholar]
- Fraisse, D.; Felgines, C.; Texier, O.; Lamaison, J.L. Caffeoyl derivatives: Major antioxidant compounds of some wild herbs of the Asteraceae family. Food Nut. Sci. 2011, 2, 181–192. [Google Scholar] [CrossRef]
- Uleberg, E.; Rohloff, J.; Jaakola, L.; Trôst, K.; Junttila, O.; Häggman, H.; Martinussen, I. Effects of temperature and photoperiod on yield and chemical composition of northern and southern clones of bilberry (Vaccinium myrtillus L.). J. Agr. Food Chem. 2012, 60, 10406–10414. [Google Scholar] [CrossRef]
- Hamed, Y.S.; Abdin, M.; Chen, G.; Akhtar, H.M.S.; Zeng, X. Effects of impregnate temperature on extraction of caffeoylquinic acid derivatives from Moringa oleifera leaves and evaluation of inhibitory activity on digestive enzyme, antioxidant, anti-proliferative and antibacterial activities of the extract. Int. J. Food Sci. Technol. 2020, 55, 3082–3090. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Vereshchagina, Y.V.; Veremeichik, G.N. Anticancer Polyphenols from Cultured Plant Cells: Production and New Bioengineering Strategies. Curr. Med. Chem. 2018, 25, 4671–4692. [Google Scholar] [CrossRef] [PubMed]
- Metwally, D.M.; Alajmi, R.A.; El-Khadragy, M.F.; Yehia, H.M.; Al-Megrin, W.A.; Akabawy, A.M.A.; Amin, H.K.; Abdel Moneim, A.E. Chlorogenic acid confers robust neuroprotection against arsenite toxicity in mice by reversing oxidative stress, inflammation, and apoptosis. J. Funct. Foods 2020, 75, 104202. [Google Scholar] [CrossRef]
- Matthews, D.G.; Caruso, M.; Alcazar Magana, A.; Wright, K.M.; Maier, C.S.; Stevens, J.F.; Gray, N.E.; Quinn, J.F.; Soumyanath, A. Caffeoylquinic Acids in Centella Asiatica Reverse Cognitive Deficits in Male 5XFAD Alzheimer’s Disease Model Mice. Nutrients 2020, 12, 3488. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Chamorro, F.; Donn, P.; Garcia-Perez, P.; Seyyedi-Mansour, S.; Silva, A.; Echave, J.; Simal-Gandara, J.; Cassani, L.; Prieto, M.A. Characterization of Phenolic Compounds of Arnica montana Conventional Extracts. Eng. Proc. 2023, 48, 61. [Google Scholar] [CrossRef]
- Kimel, K.; Krauze-Baranowska, M.; Godlewska, S.; Pobłocka-Olech, L. HPLC-DAD-ESI/MS comparison of the chemical composition of flowers from two Arnica species grown in Poland. Herba Pol. 2020, 66, 1–10. [Google Scholar] [CrossRef]
- Kimel, K.; Godlewska, S.; Krauze-Baranowska, M.; Pobłocka-Olech, L. HPLC-DAD-ESI/MS analysis of Arnica TM constituents. Acta Pol. Pharmaceut. 2019, 76, 1015–1027. [Google Scholar] [CrossRef]
- Clauser, M.; Aiello, N.; Scartezzini, F.; Innocenti, G.; Dall’Acqua, S. Differences in the chemical composition of Arnica montana flowers from wild populations of north Italy. Nat. Prod. Commun. 2014, 9, 3–6. [Google Scholar] [CrossRef]
- Perry, N.B.; Burgess, E.J.; Guitián, M.A.R.; Franco, R.R.; Mosquera, E.L.; Smallfield, B.M.; Joyce, N.I.; Littlejohn, R.P. Sesquiterpene lactones in Arnica montana: Helenalin and dihydrohelenalin chemotypes in Spain. Planta Med. 2009, 75, 660–666. [Google Scholar] [CrossRef]
- Custódio, L.; Cziáky, Z.; Castañeda-Loaiza, V.; Rodrigues, M.J. Establishment and elicitation of liquid adventitious root cultures of Inula crithmoides L. for increased caffeoylquinic acids production and hepatoprotective properties. Plant Cell Tissue Organ. Cult. (PCTOC) 2024, 156, 59. [Google Scholar] [CrossRef]
- Cai, Z.; Kastell, A.; Smetanska, I. Chitosan or yeast extract enhance the accumulation of eight phenolic acids in cell suspension cultures of Malus × domestica Borkh. J. Hort. Sci. Biotech. 2014, 89, 93–99. [Google Scholar] [CrossRef]
- Laezza, C.; Imbimbo, P.; D’Amelia, V.; Marzocchi, A.; Monti, D.M.; Di Loria, A.; Monti, S.M.; Novellino, E.; Tenore, G.C.; Rigano, M.M. Use of yeast extract to elicit a pulp-derived callus cultures from Annurca apple and potentiate its biological activity. J. Func. Foods 2024, 112, 105988. [Google Scholar] [CrossRef]
- Wang, Y.D.; Yuan, Y.J.; Wu, J.C. Induction studies of methyl jasmonate and salicylic acid on taxane production in suspension cultures of Taxus chinensis var. mairei. Biochem. Eng. J. 2004, 19, 259–265. [Google Scholar] [CrossRef]
- Li, A.; Sun, X.; Liu, L. Action of salicylic acid on plant growth. Front. Plant Sci. 2022, 13, 878076. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Grúz, J.; Bačkor, M.; Strnad, M.; Repčák, M. Salicylic acid-induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Rep. 2009, 28, 135–143. [Google Scholar] [CrossRef]
- Bayraktar, M.; Naziri, E.; Akgun, I.H.; Karabey, F.; Ilhan, E.; Akyol, B.; Gurel, A. Elicitor induced stevioside production, in vitro shoot growth, and biomass accumulation in micropropagated Stevia rebaudiana. Plant Cell Tissue Org. Cult. 2016, 127, 289–300. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Castroverde, C.D.M. Salicylic acid: A key regulator of redox signaling and plant immunity. Plant Physiol. Biochem. 2021, 168, 381–397. [Google Scholar] [CrossRef]
- Dong, J.; Wan, G.; Liang, Z. Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J. Biotech. 2010, 148, 99–104. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S. Arsenic toxicity and tolerance mechanisms in crop plants. In Handbook of Plant and Crop Physiology; CRC Press: Boca Raton, FL, USA, 2021; pp. 831–873. [Google Scholar]
- Moharramnejad, S.; Azam, A.T.; Panahandeh, J.; Dehghanian, Z.; Ashraf, M. Effect of methyl jasmonate and salicylic acid on in vitro growth, stevioside production, and oxidative defense system in Stevia rebaudiana. Sugar Tech. 2019, 21, 1031–1038. [Google Scholar] [CrossRef]
- Shaki, F.; Ebrahimzadeh Maboud, H.; Niknam, V. Central role of salicylic acid in resistance of safflower (Carthamus tinctorius L.) against salinity. J. Plant Interact. 2017, 12, 414–420. [Google Scholar] [CrossRef]
- Li, Q.; Wang, G.; Wang, Y.; Yang, D.; Guan, C.; Ji, J. Foliar application of salicylic acid alleviate the cadmium toxicity by modulation the reactive oxygen species in potato. Ecotox. Environ. Saf. 2019, 172, 317–325. [Google Scholar] [CrossRef]
- Lee, B.R.; Islam, M.T.; Park, S.H.; Jung, H.I.; Bae, D.W.; Kim, T.H. Characterization of salicylic acid-mediated modulation of the drought stress responses: Reactive oxygen species, proline, and redox state in Brassica napus. Environ. Exp. Bot. 2019, 157, 1–10. [Google Scholar]
- Gabr, A.M.M.; Ghareeb, H.; El Shabrawi, H.M.; Smetanska, I.; Bekheet, S.A. Enhancement of silymarin and phenolic compound accumulation in tissue culture of Milk thistle using elicitor feeding and hairy root cultures. J. Genet. Eng. Biotechnol. 2016, 14, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Salicylic acid elicitation improves antioxidant activity of spinach leaves by increasing phenolic content and enzyme levels. Food Chem. Adv. 2023, 2, 100156. [Google Scholar] [CrossRef]
- Pilbeam, D.J. Nitrogen. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 585–596. [Google Scholar]
- Elhanafi, L.; Houhou, M.; Rais, C.; Mansouri, I.; Elghadraoui, L.; Greche, H. Impact of excessive nitrogen fertilization on the biochemical quality, phenolic compounds, and antioxidant power of Sesamum indicum L. Seeds. J. Food Qual. 2019, 2019, 9428092. [Google Scholar] [CrossRef]
- Poshtdar, A.; Abdali Mashhadi, A.R.; Moradi, F.; Siadat, S.A.; Bakhshandeh, A. Effects of salicylic acid and nitrogen application on biochemistry, qualitative and quantitative yield of peppermint (Mentha piperita L.). Iran. J. Med. Arom. Plants Res. 2018, 34, 309–329. [Google Scholar]
- Perez-Llorca, M.; Pollmann, S.; Muller, M. Ethylene and jasmonates signaling network mediating secondary metabolites under abiotic stress. Int. J. Mol. Sci. 2023, 24, 5990. [Google Scholar] [CrossRef]
- Ncube, E.N.; Steenkamp, P.A.; Madala, N.E.; Dubery, I.A. Chlorogenic Acids Biosynthesis in Centella asiatica Cells Is not Stimulated by Salicylic Acid Manipulation. Appl. Biochem. Biotechnol. 2016, 179, 685–696. [Google Scholar] [CrossRef]
- Moglia, A.; Lanteri, S.; Comino, C.; Acquadro, A.; de Vos, R.; Beekwilder, J. Stress-induced biosynthesis of dicaffeoylquinic acids in globe artichoke. J. Agr. Food Chem. 2008, 56, 8641–8649. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Petrova, M.; Zayova, E.; Geneva, M.; Dimitrova, L.; Vitkova, A.; Stanilova, M. Multiplication and conservation of threatened medicinal plant Arnica montana L. by in vitro techniques. Agric. Consp. Sci. 2021, 86, 57–65. [Google Scholar]
- Hristozkova, M.; Geneva, M.; Stancheva, I.; Iliev, I.; Azcon-Aguilar, C. Symbiotic association between golden berry (Physalis peruviana) and arbuscular mycorrhizal fungi in heavy metal-contaminated soil. J. Plant Prot. Res. 2017, 57, 173–184. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts: Its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar]
- Urbanek, H.; Kuźniak-Gębarowska, E.; Herka, K. Elicitation of defence responses in bean leaves by Botrytis cinerea polygalacturonase. Acta Physiol. Plant. 1991, 13, 43–50. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pfeffer, H.; Dannel, F.; Römheld, V. Are there connections between phenol metabolism, ascorbate metabolism and membrane integrity in leaves of boron-deficient sunflower plants? Physiol Plant. 1998, 104, 479–485. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radical. Food Chem. J. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Tepe, B.; Sokmen, M.; Akpulat, H.A.; Sokmen, A. Screening of the antioxidant potentials of six Salvia species from Turkey. Food Chem. 2006, 95, 200–204. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Bioch. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Pineda, M.; Aguilar, M.A. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Bioch. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Angelova, P.; Hinkov, A.; Gerasimova, V.; Staleva, P.; Kamenova-Nacheva, M.; Alipieva, K.; Shivachev, D.; Shishkov, S. and Shishkova, K. Antiviral Activity of Water–Alcoholic Extract of Cistus incanus L. Int. J. Mol. Sci. 2025, 26, 947. [Google Scholar] [CrossRef]




| Nutrient Medium | Mean Number of Shoots Explant−1 | Mean Height cm Shoots−1 | Fresh Weight g Shoots−1 |
|---|---|---|---|
| Control | 3.20 ± 0.20 c | 1.73 ± 0.13 ab | 0.32 ± 0.03 c |
| 50 mg/L YE | 4.40 ± 0.41 ab | 2.09 ± 0.16 a | 0.44 ± 0.04 b |
| 100 mg/L YE | 5.20 ± 0.45 a | 2.10 ± 0.15 a | 0.58 ± 0.05 a |
| 200 mg/L YE | 4.10 ± 0.38 bc | 1.65 ± 0.15 b | 0.38 ± 0.03 bc |
| LSD | 1.05 | 0.42 | 0.11 |
| No | Rt, min | Compound | Molecular Formula | [M-H]−, m/z | Δ, ppm | MS/MS Fragments | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 0.93 | Quinic acid | C7H11O6 | 191.0555 | −3.22 | 191 *, 127, 85 | [22] |
| 2 | 1.00 | Dihydroxybenzoic acid O-hexoside | C13H15O9 | 315.0732 | 3.26 | 315, 153, 152, 109, 108 | [23] |
| 3 | 1.39 | Hydroxy-methoxybenzoic acid O-hexoside | C14H17O9 | 329.0883 | 1.65 | 167, 152, 123, 108 | [24] |
| 4 | 1.43 | Dihydroxybenzoic acid O- hexoside | C13H15O9 | 315.0728 | 2.09 | 315, 153, 152, 109, 108 | [23] |
| 5 | 1.61 | Syringic acid O-hexoside | C15H19O10 | 359.0992 | 2.26 | 359, 197, 167, 153, 123 | [23] |
| 6 | 1.83 | Neochlorogenic acid (3-O-caffeoylquinic acid) | C16H17O9 | 353.0880 | 0.89 | 353, 191, 179, 135 | St |
| 7 | 2.94 | Chlorogenic acid (5-O-caffeoylquinic acid) | C16H17O9 | 353.0884 | 1.3 | 353, 191, 179, 135 | St |
| 8 | 3.86 | Caffeic acid | C9H7O4 | 179.0341 | −4.65 | 179, 135 | St |
| 9 | 11.96 | 3,4-Dicaffeoylquinic acid | C25H23O12 | 515.1202 | 1.32 | 353, 191, 179, 173, 135 | St |
| 10 | 12.15 | 1,5-Dicaffeoylquinic acid | C25H23O12 | 515.1203 | 1.55 | 353, 191 | St |
| 11 | 12.19 | Kaempferol 3-O-glucoside | C21H19O11 | 447.0938 | 1.18 | 447, 284, 255, 227 | St |
| 12 | 12.53 | 3,5-Dicaffeoylquinic acid | C25H23O12 | 515.1202 | 1.32 | 353, 191, 179, 135 | St |
| 13 | 12.61 | Methoxyoxaloyl dicaffeoylquinic acid/Malonyl dicaffeoylquinic acid ** | C28H25O15 | 601.1210 | 1.9 | 395, 353,335, 233, 191, 179, 173, 162 | [18,19,20,21] |
| 14 | 12.86 | Isorhamnetin hexoside | C22H21O12 | 477.1044 | 1.22 | 477, 315, 299, 271, 243 | [25] |
| 15 | 13.98 | 4,5-Dicaffeoylquinic acid | C25H23O12 | 515.1201 | 1.08 | 353, 191, 179, 135 | St |
| 16 | 14.77 | Methoxyoxaloyl dicaffeoylquinic acid/Malonyl dicaffeoylquinic acid ** | C28H25O15 | 601.1213 | 2.4 | 395, 353, 233, 191, 179, 173, 162 | [18,19,20,21] |
| 17 | 15.26 | 1,3,5-Tricaffeoylquinic acid | C34H29O15 | 677.1526 | 2.15 | 515, 353, 191, 179, 161, 135 | [18] |
| 18 | 19.37 | 1,4,5-Tricaffeoylquinic acid | C34H29O15 | 677.1527 | 2.24 | 515, 353, 191, 179, 173, 161, 135 | [18] |
| 19 | 19.4 | 3,4,5-Tricaffeoylquinic acid | C34H29O15 | 677.1529 | 2.51 | 515, 353, 191, 179, 173, 161, 135 | [18] |
| 20 | 19.55 | Methoxyoxaloyl tricaffeoylquinic acid/Malonyl tricaffeoylquinic acid ** | C37H31O18 | 763.1532 | 2.11 | 557, 539, 515, 395, 233, 191, 179, 173, 161, 135 | [18,19,20,21] |
| 21 | 21.26 | Hispidulin | C16H11O6 | 299.0565 | 1.46 | 299, 284 | [25] |
| 22 | 21.31 | Methoxyoxaloyl tricaffeoylquinic acid/Malonyl tricaffeoylquinic acid ** | C37H31O18 | 763.1533 | 2.27 | 515, 395, 233, 191, 179, 173, 161, 135 | [18,19,20,21] |
| 23 | 22.37 | Dihydroxy-dimethoxyflavone | C17H13O6 | 313.0721 | 1.03 | 313, 298, 283, 255 | [25] |
| YE, mg/L | 5-CQA | 3,4-DCQA | 3,5-DCQA | 1,5-DCQA | 4,5-DCQA | UTCQA | Total |
|---|---|---|---|---|---|---|---|
| 0 | 0.23 ± 0.01 d | 0.08 ± 0.01 c | 0.35 ± 0.02 c | 1.34 ± 0.02 d | 0.09 ± 0.01 c | 0.94 ± 0.02 c | 3.02 ± 0.10 c |
| 50 | 0.28 ± 0.01 c | 0.08 ± 0.01 b,c | 0.28 ± 0.01 d | 1.44 ± 0.02 c | 0.09 ± 0.01 c | 0.96 ± 0.02 c | 3.13 ± 0.07 c |
| 100 | 0.68 ± 0.01 a | 0.17 ± 0.01 a | 0.61 ± 0.01 a | 3.02 ± 0.03 a | 0.21 ± 0.01 a | 1.77 ± 0.02 a | 6.45 ± 0.07 a |
| 200 | 0.37 ± 0.01 b | 0.11 ± 0.01 b | 0.42 ± 0.01 b | 1.79 ± 0.01 b | 0.15 ± 0.01 b | 1.32 ± 0.02 b | 4.16 ± 0.07 b |
| Nutrient Medium | Mean Number of Shoots Explant−1 | Mean Height cm Shoots−1 | Fresh Weight g Shoots−1 |
|---|---|---|---|
| Control | 3.00 ± 0.20 a | 1.46 ± 0.09 b | 0.32 ± 0.02 b |
| 50 µM SA | 2.95 ± 0.23 a | 1.56 ± 0.11 ab | 0.36 ± 0.03 ab |
| 100 µM SA | 2.60 ± 0.25 a | 1.96 ± 0.15 a | 0.47 ± 0.04 a |
| 200 µM SA | 1.70 ± 0.16 b | 1.90 ± 0.13 ab | 0.38 ± 0.03 ab |
| LSD | 0.61 | 0.35 | 0.09 |
| SA, µM | 5-CQA | 3,4-DCQA | 3,5-DCQA | 1,5-DCQA | 4,5-DCQA | UTCQA | Total |
|---|---|---|---|---|---|---|---|
| 0 | 0.39 ± 0.01 a | 0.07 ± 0 a | 0.38 ± 0.01 a | 2.07 ± 0.02 a | 0.14 ± 0.01 a | 1.79 ± 0.03 a | 4.85 ± 0.08 a |
| 50 | 0.28 ± 0.01 b | 0.04 ± 0.01 b | 0.28 ± 0.01 b | 1.30 ± 0.01 b | 0.06 ± 0 b | 0.66 ± 0.02 b | 2.62 ± 0.06 b |
| 100 | 0.22 ± 0.01 c | 0.03 ± 0 b,c | 0.23 ± 0.01 c | 1.00 ± 0.01 c | 0.04 ± 0.01 b,c | 0.42 ± 0.01 c | 1.94 ± 0.06 c |
| 200 | 0.19 ± 0 c | 0.02 ± 0 c | 0.14 ± 0.01 d | 0.66 ± 0.02 d | 0.03 ± 0 c | 0.20 ± 0 d | 1.25 ± 0.04 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrova, M.; Geneva, M.; Trendafilova, A.; Miladinova-Georgieva, K.; Dimitrova, L.; Sichanova, M.; Nikolova, M.; Ivanova, V.; Dimitrova, M.; Sozoniuk, M. Antioxidant Capacity and Accumulation of Caffeoylquinic Acids in Arnica montana L. In Vitro Shoots After Elicitation with Yeast Extract or Salicylic Acid. Plants 2025, 14, 967. https://doi.org/10.3390/plants14060967
Petrova M, Geneva M, Trendafilova A, Miladinova-Georgieva K, Dimitrova L, Sichanova M, Nikolova M, Ivanova V, Dimitrova M, Sozoniuk M. Antioxidant Capacity and Accumulation of Caffeoylquinic Acids in Arnica montana L. In Vitro Shoots After Elicitation with Yeast Extract or Salicylic Acid. Plants. 2025; 14(6):967. https://doi.org/10.3390/plants14060967
Chicago/Turabian StylePetrova, Maria, Maria Geneva, Antoaneta Trendafilova, Kamelia Miladinova-Georgieva, Lyudmila Dimitrova, Mariana Sichanova, Milena Nikolova, Viktoria Ivanova, Margarita Dimitrova, and Magdalena Sozoniuk. 2025. "Antioxidant Capacity and Accumulation of Caffeoylquinic Acids in Arnica montana L. In Vitro Shoots After Elicitation with Yeast Extract or Salicylic Acid" Plants 14, no. 6: 967. https://doi.org/10.3390/plants14060967
APA StylePetrova, M., Geneva, M., Trendafilova, A., Miladinova-Georgieva, K., Dimitrova, L., Sichanova, M., Nikolova, M., Ivanova, V., Dimitrova, M., & Sozoniuk, M. (2025). Antioxidant Capacity and Accumulation of Caffeoylquinic Acids in Arnica montana L. In Vitro Shoots After Elicitation with Yeast Extract or Salicylic Acid. Plants, 14(6), 967. https://doi.org/10.3390/plants14060967








