Abstract
Plants have evolved complex terpene defenses. Terpenoids accumulate in plant tissues or release as volatile in response to ever-changing environment, playing essential roles in chemo-ecological functions as defense against pathogen and insect, improving pollination and seed dispersal, facilitation plant-to-plant communication. They are also gaining attention in pharmaceuticals, nutraceuticals, fragrance, and biofuels. Here, we highlight the recent progress in the fundamental pathways of terpenoid biosynthesis, key enzymes, and their corresponding genes involved in terpenoid synthesis. We identified the further exploration of biosynthetic networks and the development of novel terpenoid resources, proposed the need for further exploration of biosynthetic networks and the development of novel terpenoid resources. Based on that knowledge, future research should be directed towards the mechanisms governing terpenoid biosynthesis dependent environmental change and molecular breeding.
1. Introduction
Terpenoids constitute a significant proportion of plant secondary metabolites [1]. These compounds are derived from five-carbon precursors, commonly referred to as isoprene units, which have a propensity to decompose into isoprene (C5H8) at elevated temperatures [2,3]. Isoprene units assemble to yield a hierarchical series of terpenes, ranging from 5-carbon hemiterpenes to 40-carbon tetraterpenes, including monoterpenes (C10H16), sesquiterpenes (C15H24), diterpenes (C20H32), sesterterpenes (C25H40), and triterpenes (C30H48) [4].
For a long time, they were mistakenly regarded as mere metabolic waste or detoxification byproducts. Since the 1970s, scientists have revealed that many terpenoids exhibit bioactive properties, functioning as toxins, repellents, or attractants to other organisms [5]. In recent years, the biological significance of secondary metabolites, including terpenes and isoprenoids, is also gaining attention for their applications in pharmaceuticals, nutraceuticals, synthetic chemistry, the flavor and fragrance industries, and biofuels [6]. In addition, with the escalating demand for these natural products, synthetic biology has emerged as a transformative approach, offering innovative strategies to optimize the production of these bioactive compounds.
This review systematically summarizes the biochemical pathways of terpenoid biosynthesis in plant secondary metabolism, elucidating their synthetic principles from the perspective of metabolic network regulation. Through a comparative analysis of the molecular mechanisms underlying different terpenoid synthesis pathways, we reveal the foundational basis of structural diversity. Furthermore, we highlight recent advances in molecular regulation, including transcriptional control, miRNA-mediated modifications, and epigenetic regulation. These systematic insights not only provide a theoretical framework for deciphering unknown terpenoid biosynthesis mechanisms but also establish a critical foundation for the rational design and efficient production of terpenoids via synthetic biology approaches. The findings hold significant theoretical and practical value for further unraveling the regulatory logic of plant terpenoid metabolism and developing high-value terpenoid products.
2. Model of Terpenoid Biosynthesis
Terpene precursors, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), are synthesized through two distinct pathways: the mevalonate pathway (MVA pathway) and the 2C-methyl-D-erythritol-4-phosphate pathway (MEP pathway). These pathways are localized in different subcellular compartments and utilize different starting materials, providing a fundamental carbon skeleton for the synthesis of a wide variety of terpenoid compounds [7]. While the MEP pathway utilizes pyruvate and D-glyceraldehyde-3-phosphate (GAP), two central metabolites in glycolysis, the MVA pathway begins with acetyl-CoA, generated via the decarboxylation of pyruvate, another pivotal glycolytic product [8,9]. Isoprenyl diphosphate synthases (IDSs) subsequently catalyze the formation of geranyl diphosphate (GPP), farnesyl diphosphate (FPP) [10], and geranylgeranyl diphosphate (GGPP), which serve as the precursors for terpenoid biosynthesis. These intermediates are then processed by terpene synthase (TPS) enzymes, which generate a structurally diverse array of terpenes through stereospecific cyclization and rearrangement reactions [11]. Further structural elaboration occurs via oxidative modifications mediated by cytochrome P450 oxygenases (CYP450s), which modulate the functional diversity of terpenoids [12] (Figure 1).
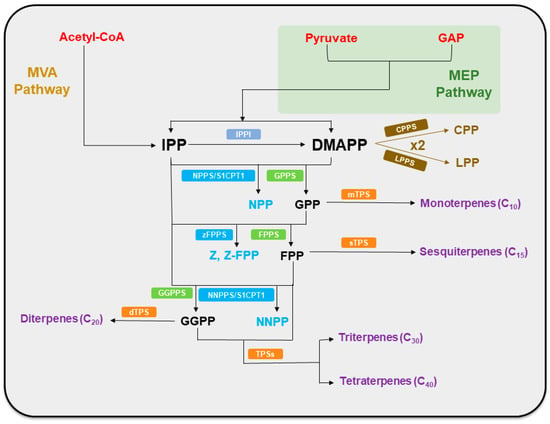
Figure 1.
Schematic representation of plant terpenoid and terpene biosynthesis. The green areas represent plastids. Abbreviations: GAP, D-glyceraldehyde-3-phosphate; MVA pathway, mevalonate pathway; MEP pathway, 2C-methyl-D-erythritol-4-phosphate pathway; IPP, isopentenyl diphosphate; IPPI, isopentenyl diphosphate isomerase; DMAPP, dimethylallyl diphosphate; CPPS, chrysanthemyl diphosphate synthase; CPP, chrysanthemyl diphosphate; LPPS, lavandulyl diphosphate synthase; LPP, lavandulyl diphosphate; NPPS/S1CPT1, nerolidol diphosphate synthase; GPPS, geranyl diphosphate synthase; NPP, nerolidol diphosphate; GPP, geranyl diphosphate; mTPS, monoterpene synthase; zFPPS, Z, Z-FPP synthases; FPPS, farnesyl diphosphate synthase; Z, Z-FPP, Z, Z-farnesyl diphosphate; FPP, farnesyl diphosphate; sTPS, sesquiterpene synthase; GGPPS, geranylgeranyl diphosphate synthase; NNPPS/S1CPT2, nerylneryl diphosphate synthase; dTPS, diterpene synthase; GGPP, geranylgeranyl diphosphate; NNPP, nerolidol diphosphate; TPSs, terpene synthases.
2.1. The MVA and MEP Pathways Generate Initial Precursors
The MVA pathway (Figure 2), a specialized metabolic network predominantly localized to the cytoplasm and endoplasmic reticulum (ER), with potential peroxisomal contributions [13], orchestrates the synthesis of IPP through a six-enzyme cascade. This process consumes three acetyl-CoA molecules, three ATP equivalents, and two NADPH molecules to yield a single IPP molecule [7]. A pivotal rate-limiting step occurs at the conversion of HMG-CoA to mevalonate, catalyzed by HMG-CoA reductase (HMGR) within the ER membrane. This reaction uniquely consumes two NADPH molecules, underscoring its regulatory significance [14]. The final step—conversion of mevalonate-5-diphosphate to IPP by mevalonate diphosphate decarboxylase (MVD)—is hypothesized to occur in peroxisomes [15,16], although cytoplasmic localization remains debated. It is notably that the IPP product undergoes reversible isomerization to DMAPP via IPPI, a critical reaction for downstream terpenoid biosynthesis [17].
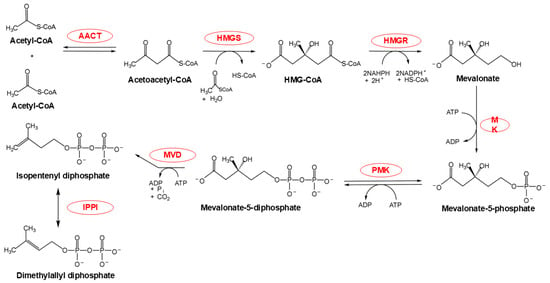
Figure 2.
MVA pathway chemical flow. The red portion highlights the enzymes participating in this reaction. Abbreviations: AACT, acetoacetyl-CoA thiolase; HMGS, 3-hydroxy-3-methylglutaryl-CoA synthase; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; HMGR, HMG-CoA reductase; MK, mevalonate kinase; PMK, phosphomevalonate kinase; MVD, mevalonate diphosphate decarboxylase; IPPI, isopentenyl diphosphate isomerase.
The metabolic sequence of the MEP pathway occurs exclusively within the plastid and involves a series of seven reactions that convert GAP and pyruvate into isopentenyl diphosphate (IDP) and DMADP (Figure 3). This conversion involves condensation and reduction processes, with the consumption of three ATP and three NADPH molecules [7]. Initially, 1-deoxy-D-xylulose-5-phosphate synthase (DXS) catalyzes the condensation of pyruvate and GAP to form 1-deoxy-D-xylulose-5-phosphate (DXP). This step is crucial in the MEP pathway, as DXS activity significantly influences the metabolic rate of the entire pathway [18]. Additionally, the MEP pathway provides essential precursors for the biosynthesis of carotenoids and chlorophyll alcohols in chloroplasts, which are critical components of photosynthesis and other primary metabolic processes [19].
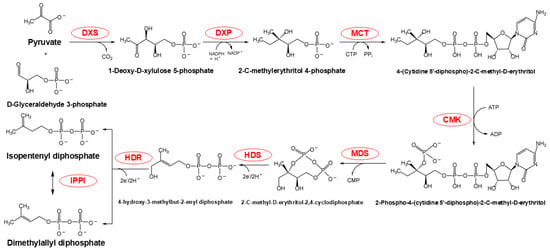
Figure 3.
MEP pathway chemical flow. The red portion highlights the enzymes participating in this reaction. Abbreviations: DXS, 1-deoxy-D-xylulose-5-phosphate synthase; DXR, DXP reductoisomerase; MCT, 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase; CMK, 4-(cytidine 5-diphospho)-2-C-methyl-D-erythritol kinase; MDS, 2-C-methyl-D-erythritol-2,4-cyclodiphosphate synthase; HDS, 4-hydroxy-3-methylbut-2-enyl diphosphate synthase; HDR, 4-hydroxy-3-methylbut-2-enyl diphosphate reductase; IPPI, isopentenyl diphosphate isomerase.
In plants, isoprene biosynthesis uniquely involves both the MVA pathway and the MEP pathway, contrasting with the single-pathway utilization observed in other organisms such as animals, fungi, and most bacteria [9]. This dual-pathway approach in plants is an evolutionary adaptation that provides significant survival advantages [20]. The compartmentalization of these pathways in distinct cellular locations (the MVA pathway predominantly in the cytoplasm/endoplasmic reticulum and the MEP pathway within the plastids) allows the efficient utilization of diverse carbon sources. This strategic localization facilitates the rapid and specific production of end products, reducing substrate competition and enhancing enzyme efficiency [21]. Furthermore, the presence of both pathways provides plants with the flexibility to adapt to various environmental conditions. It has been observed that, under light conditions, the MEP pathway becomes more active, supporting the synthesis of photosynthesis-related isoprenoids. In contrast, in the absence of light, the MVA pathway exhibits heightened activity, promoting the synthesis of phytosterols [9]. Despite these observations, the precise regulatory mechanisms governing the coordinated interaction between the MVA and MEP pathways remain unclear. Further research is needed to elucidate the complex regulatory networks that ensure the efficient coordination of these pathways in response to environmental change.
Subcellular localization studies of IPPI have revealed its presence in mitochondria, peroxisomes, and chloroplasts [22]. This indicated a potential transfer mechanism of IPPI across these distinct cellular compartments. Various experimental methodologies have been applied to confirm the existence of cross-pathway flow between the MVA and MEP pathways, including mutant analyses, chemical inhibitor interventions, and labeled substrate feeding experiments [23]. Skorupinska-Tudek et al. [24] utilized nuclear magnetic resonance (NMR) and mass spectrometry to observe frequent exchanges between plastids and the cytoplasm, leading to the formation of compounds with mixed MVA/MEP origins. Lipko et al. [25] employed a novel competitive labeling approach, providing additional evidence for the intricate interconnection and reciprocal regulation between the MEP and MVA pathways. Despite these findings, the extent of this metabolic flux remains limited and tightly regulated, the precise regulatory mechanisms governing this interaction continue to be an area of active investigation.
2.2. Direct Precursor Formation Stage
All terpenoids are derived from DMAPP and IPP through the enzymatic action of various IDSs. These enzymes catalyze the sequential condensation of isoprenoid units by ionizing the allylic diphosphate ester, followed by a rearrangement that results in the formation of prenyl diphosphate metabolites of varying chain lengths [26,27]. The initial condensation of one DMAPP and one IPP, catalyzed by GPPS, yields GPP, the precursor of monoterpenes. GPP synthesis primarily occurs in plastids and mitochondria [28]. Further elongation via FPPS incorporates an additional IPP with GPP to form FPP, serving as the backbone of sesquiterpenes [29]. GGPPS catalyzes the addition of three IPPs to one DMAPP, generating GGPP, a precursor for diterpenes that localizes to the cytoplasm, plastids, and mitochondria [28]. FPP and GGPP can then undergo further polymerization to form triterpenes and tetraterpenes, respectively [30] (Figure 1).
The structural diversity of terpenoids begins to diverge at the level of IDS enzymes, manifested through variations in enzyme architecture and alternative catalytic mechanisms that generate unconventional terpene scaffolds. Notably, direct precursors for terpenoid biosynthesis are initially in the trans (E) configuration and undergo “head-to-tail” condensation reactions to form terpenoids [31,32]. Cis-isoprenoid diphosphate synthases (cis-IDS) exhibit remarkable structural diversity in their active sites and broad functional capacities, unlike their trans-isoprenoid counterparts (trans-IDS). These enzymes are referred to as “butterfly-fold” proteins due to their characteristic structural resemblance to a spreading butterfly [33]. Research on the trichomes of cultivated tomato (Solanum lycopersicum) has identified three cis-configured intermediates: NPP, Z,Z-FPP, and NNPP. NPP is synthesized by NPPS1/S1CPT1; Z,Z-FPP is produced by zFPPS; and NNPP is generated by NNPPS/S1CPT2 [34]. The structural composition of the subunits of IDS determines whether they form homodimers or heterodimers. A cis-heterodimer IDS contains a non-catalytic subunit that facilitates the production of long-chain polyprenyl diphosphates. For instance, natural rubber (C10,000), the largest naturally occurring hydrocarbon molecule, is a long-chain Z-type isoprenoid produced through such mechanisms [26]. “Non-head-to-tail” condensation of two DMAPP molecules can produce branched structures. Rivera et al. [35] isolated a monoterpene with an irregular C1′-2-3 linkage between isoprenoid units, termed CPP, from Chrysanthemum cinerariaefolium, which was catalyzed by CPPS. Demissie et al. [36] also identified a novel cis-prenyl diphosphate synthase cDNA from Lavandula x intermedia, designated LiLPPS. This enzyme, LPPS, catalyzes the non-head-to-tail condensation of isoprene units to form LPP (Figure 1).
2.3. TPS Catalyzes the Subsequent Steps
The fundamental structural framework of terpenes is established by TPS [4]. The TPS gene family represents a moderate-sized group in plants, hypothesized to have originated from an ancestral bifunctional gene encoding an enzyme with both copalyl diphosphate synthase (CPS) and kaurene synthase (KS) activities. Over time, this gene diverged into two separate paralogs—one retaining CPS activity and the other KS activity—which became the basis for different TPS subfamilies [37]. The diversification of TPS subfamilies occurred after land plants split from Charophytic algae, driven by repeated gene duplications that led to the evolution of distinct subfamilies [38]. TPS enzymes exhibit remarkable regioselectivity in substrate binding, enabling them to utilize diverse precursors like GPP, FPP, and GGPP to generate a broad spectrum of terpenoid products [39]. Additionally, the conformation of a product can be dictated by TPS enzymes. For instance, in Mentha canadensis, a single TPS catalyzes the formation of both L-menthol and D-menthol [5].
The evolutionary relationships among these TPS classes and subfamilies are illustrated in Figure 4. TTS and PT, sharing a conserved α-domain with a DDx2D motif similar to ancestral IDS enzymes, evolved through gene fusion (forming αβγ domains) and subsequent domain loss/specialization, giving rise to modern plant Class I/II TPS and smaller TPS families. The TPS gene family is divided into seven subfamilies (TPS-a, b, c, d, e/f, g, and h) based on sequence and function [40]. TPS-a mainly synthesizes sesquiterpenes, while TPS-b produces monoterpenes. TPS-g, closely related to TPS-b, forms acyclic mono-, sesqui-, and diterpenes [38]. TPS-c is crucial for diterpenoid biosynthesis in plant secondary metabolism [41]. TPS-d acts as both mono- and diterpene synthase. TPS-e/f (evolutionarily linked) catalyzes diterpene synthesis, while TPS-h, unique to gymnosperms, generates mono- and sesquiterpenes [42]. TPS enzymes can be categorized into two primary groups based on their distinct catalytic mechanisms: Class I and Class II. Class I TPSs use an ionization-dependent mechanism. They contain two conserved domains—the C-terminal DDxxD motif and the (L, V) (V, L, A)—(N, D) D (L, I, V) × (S, T) xxxE domain (NSE/DTE domain)—which coordinate metal ions and stabilize carbocation intermediates. Class II TPSs employ a proton-dependent mechanism and feature the DxDD conserved domain [30,42].
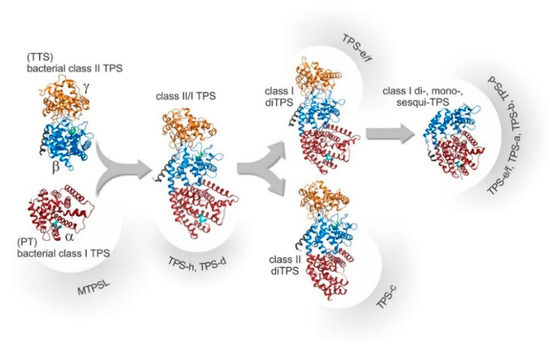
Figure 4.
Evolutionary relationships among TPS based on protein structures. The domain color marks the γ-domain (orange), the β-domain (blue), the α-domain (red) as well as the conserved DxDD (green) and DDx2D (cyan) motifs.
2.4. Modification of the Forming Terpenoids
After the synthesis of the terpenoid carbon skeleton, further structural diversification and functional modification are facilitated by CYP450 enzymes [4]. “P450” originates from the characteristic absorption band at 450 nm, observed when carbon monoxide binds to the reduced form of the heme iron in these enzymes [43]. Cytochrome P450 represents one of the largest and most ancient gene superfamilies, found across all living organisms. These enzymes catalyze a diverse range of chemical reactions [12]. In mint (Mentha spp.), the CYP450 enzyme limonene 3-hydroxylase specifically catalyzes the regiospecific hydroxylation of limonene at the C3 position, yielding trans-isopiperitenol [44]. In Artemisia annua, CYP71AV1 mediates the sequential oxidation of key intermediates in artemisinin biosynthesis, including amorpha-4,11-diene, artemisinol, and artemisinic aldehyde [45]. In Tripterygium wilfordii, members of the CYP71BE subfamily have been shown to catalyze the 18(4→3) methyl transfer reaction, a key step in the biosynthesis of diterpenoid triepoxides and the bicyclic sesquiterpene core structures found in numerous plant diterpenes [46].
Specific CYP450 proteins have been identified to catalyze the formation of homoterpenes—terpenoids with irregular carbon skeletons [4]. Two prominent examples are (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT) and (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene (TMTT), both widespread in plants. These compounds are typically found in the flowers or are released in response to herbivore feeding [47,48]. Stable isotope labeling studies show that DMNT biosynthesis starts from FDP, which is first converted to the intermediate (E)-nerolidol and then oxidatively degraded to form DMNT [49]. Similarly, GGPP is transformed into (E,E)-geranyllinalool before oxidative cleavage produces TMTT [50]. In Arabidopsis thaliana, the biosynthetic pathway of the volatile sesquiterpene TMTT has been fully characterized. This pathway involves the CYP82G1 enzyme, which specifically catalyzes the oxidative conversion of (E,E)-geranyllinalool to form TMTT [47,51].
3. Regulation of Terpene Biosynthesis
3.1. Transcription Factors in Terpene Biosynthesis
Transcription factors (TFs) are crucial regulatory proteins in plants that modulate gene expression by binding to cis-regulatory elements, either proximal or distal to their target genes. In total, 64 distinct transcription factor families have been identified in vascular plants. Among these, several families play pivotal roles in regulating plant secondary metabolism, including the following: WRKY, myeloblastosis-related (MYB), NAC, APETALA2/ethylene response factor (AP2/ERF), basic helix–loop–helix (bHLH), basic leucine zipper (bZIP), and others [52]. Kelimujiang et al. [53] identified 12 LaWRKY genes in Lavandula angustifolia that exhibit elevated expression levels in the buds and calyx, which are the primary organs of volatile terpenoid production. In tomato, the inhibition of SlMYB75 led to an increased accumulation of sesquiterpenes such as δ-elemene, β-caryophyllene, and α-humulene [54]. Li et al. [55] found that SaNAC30 in sandalwood (Santalum album) interacts with MKS during terpenoid biosynthesis in sandalwood heartwood. Meng et al. [56] observed a significant increase in SaAREB6 expression in sandalwood under drought conditions, accompanied by a concurrent increase in santalol content. Yi and colleagues [57] identified 12 TPS-related AarbHLH genes in Artemisia argyi that may be involved in the synthesis of 1,8-cineole or β-caryophyllene. Wang et al. [58] performed RNA-Seq analysis of the leaf, root, and stem tissues in Polygonatum kingianum, identifying 12 PkARF genes associated with metabolic pathways and secondary metabolite biosynthesis in roots.
3.2. MiRNAs in Terpene Biosynthesis
In addition to TFs, the involvement of microRNAs (miRNAs) in the regulation of terpenoid biosynthesis has been increasingly reported in recent years. MiRNAs regulate critical enzymes involved in both the MVA and MEP pathways, as well as downstream IDS and TPS. In Catharanthus roseus, miR-5021 has been identified as targeting HDS [59]. Tu et al. [60] conducted a multi-omics analysis to investigate miRNAs in Pinus elliottii. Their study revealed that pta-miR949, pta-miR946a-3p, and pab-miR3710 target PITA39236 (HDS), PITA24638 (GPPS), and PITA12789 (ACAT), respectively. MiRNAs also target non-conserved proteins specific to particular terpenoid biosynthesis pathways. These studies focus on species-specific terpenoid synthesis, investigating key factors that distinguish one terpenoid from another by modulating the mRNA expression of downstream non-conserved proteins. Pani et al. [61] found that miR-5021 targets UDP-glucose iridoid glucosyltransferase in C. roseus, which is responsible for the glucosylation step in the biosynthetic pathway of the secondary metabolite iridoid in higher plants. Additionally, miR-5021 targets strictosidine synthase, an enzyme that catalyzes the condensation of tryptamine and the iridoid secologanin to produce strictosidine, the universal precursor of terpenoid indole alkaloids (TIAs).
3.3. Epigenetic Regulatory Mechanisms in Terpene Biosynthesis
Epigenetic regulatory mechanisms play a crucial role in modulating terpenoid biosynthesis through processes such as DNA methylation, histone modification, and non-coding RNA regulation. MiRNA regulation is also recognized as a component of epigenetic control [62]. In Populus × canescens (gray poplar), diurnal variations in terpene emissions are closely linked to fluctuations in the expression of isoprene synthase (ISPS) genes. These fluctuations are regulated by circadian clock-associated elements, specifically CCA1/LHY, within the ISPS promoter region [63]. UV-B irradiation of indoor-grown spruce seedlings has been shown to induce epigenetic modifications, leading to selective terpene release [64]. Studies on Thymus kotschyanus have demonstrated that methyl jasmonate (MeJA) can induce DNA hypomethylation, leading to the upregulation of CYP450 gene expression [65]. These studies reveal the relationships between epigenetic regulation and plant circadian rhythms, growth environmental factors, and environmental signal transduction, indicating the critical role of epigenetic regulatory mechanisms in the biosynthesis of plant terpenoids.
3.4. Environmental Factors and Phytohormones in Modulating Terpene Biosynthetic Pathways
Various environmental stimuli, e.g., light, temperature, and biotic stress, significantly altered the expression of genes involved in terpenoid production [66,67]. Exposure to UV light and elevated temperatures often enhance the biosynthesis of protective terpenoids [68]. UV-B radiation can activate the plant defense system and promote the biosynthesis of secondary metabolites such as terpenes and phenolics [69]. Meanwhile, UV-A and visible light (400–700 nm) can act synergistically to facilitate the production of UV-B-absorbing compounds, thereby enhancing plant resistance to UV-B [70]. Phytohormones regulate terpenoid biosynthesis in response to environmental stress [71,72,73]. MeJA treatment increased terpene emissions in Picea abies, with linalool rising > 100-fold and sesquiterpenes > 30-fold, peaking during light periods [74]. Salicylic acid (SA) has been demonstrated to upregulate key enzymes in the terpenoid biosynthesis pathway, including FPPS in Euphorbia pekinensis [75] and SQS in licorice (Glycyrrhiza spp.) [76]. In Michelia chapensis, both JA and SA induced expression of the MichHMGR gene [71]. Under adverse conditions such as drought or salt stress, an imbalance occurs between the energy captured by plants and their capacity to process it. Plants employ various mechanisms to prevent and dissipate this excess excitation energy. Some of these mechanisms involving the upregulation of terpenoid levels occur concomitantly with increased SA levels [77,78].
Interestingly, environmental factors may modulate miRNA expression, thereby indirectly regulating downstream targets. For instance, in the leaves of Camellia sinensis (commonly used for tea production), terpenoids are a critical indicator for the tea’s aroma. Studies have shown that miRNAs in C. sinensis may be regulated by photoreceptors, in turn, which exert a negative regulatory effect on their target genes, ultimately modulating terpenoid biosynthesis [79]. This intricate regulatory network highlights the complexity and precision with which plants respond to environmental cues, optimizing the production of secondary metabolites such as terpenoids.
4. Novel Terpenoid Resources
Certain diterpenes possess semi-volatility and serve as phytoanticipins. They can either be synthesized as a response to pests, pathogens, or elicitors or they are constitutively produced during the normal growth process of plants. Many non-volatile terpenes are secreted by plant roots, acting as a primary defense mechanism and shaping rhizosphere communities [80,81]. In the contrast, plant-released volatile organic compounds (VOCs) facilitate plant-to-plant communication, and most of the VOCs are terpenes [82]. VOCs help plants lure pollinators, aiding in pollination [83].The formation of tree heartwood often involves the accumulation of secondary metabolites, which contribute to its distinctive darker color and provide protection against decay and insect infestation [84]. In the sandalwood heartwood, sandalwood oil (including α-santalol, β-santalol, epi-β-santalol, and α-exo-bergamotol) accumulates driven by the activity of SaSSY, a terpene synthase enzyme. These terpenoids provide resistance to decay and contribute to the long-lasting fragrance of sandalwood, enhancing its value as a high-quality timber [85]. Insects exposed to essential oil terpenes exhibited reduced respiratory rates, as well as avoidance or decreased movement on surfaces treated with these compounds. Terpenes such as eugenol, caryophyllene oxide, α-pinene, α-humulene, and α-phellandrene were found to be toxic to Sitophilus granarius and capable of deterring its predatory behavior [86].
Although terpenoids are primarily recognized for their roles in plant physiology and ecology, their economic and medicinal potential is increasingly being acknowledged [3,87]. The diversity of terpenoids arises from variations in enzymes, substrate specificities, and secondary modifications of terpene synthases. Terpenoid compounds exhibit diverse therapeutic applications. For instance, artemisinin, a potent antimalarial compound biosynthesized in Artemisia annua, has also demonstrated efficacy in cancer treatment [88,89]. Similarly, tanshinones, a class of abietane diterpenes derived from Salvia miltiorrhiza, are widely used in managing inflammatory conditions and show promising potential in treating neurological disorders [90,91,92,93]. Additionally, other terpenoids, such as oleanolic acid [94], myrcene [95], limonene [96], and linalool [97], have also been recognized for their significant pharmacological benefits.
Known for their aromatic properties and health benefits, terpenes are widely used in cosmetics and food products. Many essential oils (EOs) are rich in terpenoids, including sandalwood EO, as well as limonene (derived from citrus peels) [98], linalool (a key compound in producing household products, cosmetics, and fragrance chemicals such as geraniol, nerol, citral, and their derivatives) [99], and eucalyptus essential oils (EEOs) [100]. Due to their distinctive aromas, these terpenoid-rich EOs are widely used in personal care products such as hair tonics, soaps, and toothpaste. Limonene, linalool, and eucalyptol exhibit antioxidant properties, making them valuable additives in cosmetics to protect the skin against UV-induced damage [3]. As natural bioactive compounds, terpenoids can also be utilized to produce functional foods with health benefits. For instance, consuming terpenoid-enriched functional foods may help supplement type 2 diabetes treatment, potentially reducing the side effects associated with single-drug therapies [101]. Moreover, terpenoids serve as effective food preservatives. Terpenoids like erpenoids act as natural preservatives in the food industry due to their antimicrobial properties. Terpineol isomers (α-terpineol, terpinen-4-ol, and δ-terpineol) exhibit strong inhibitory effects against Gram-negative bacteria by disrupting membrane permeability and causing cell damage [102].
Interest in these compounds has grown due to the increasing demand for natural products and heightened regulatory scrutiny. Synthetic biology provides innovative solutions to enhance their production. For example, geraniol, an acyclic monoterpenoid alcohol and a major component of essential oils such as rose oil and citronella oil, has been produced at high levels in Escherichia coli through the recombinant overexpression of specific enzymes [33,103]. Similarly, antalene and santalol have been synthesized in vitro using engineered Saccharomyces cerevisiae with overexpressed enzymes [104]. Numerous high-performance engineered systems for plant terpene synthesis have been developed. Ignea et al. [105] engineered Saccharomyces cerevisiae by converting the yeast farnesyl diphosphate synthase (Erg20p) into a geranyl diphosphate synthase, establishing a dedicated platform for terpenoid biosynthesis. Basallo et al. [106] engineered rice lines with an MVA biosynthetic pathway, expressing three alternative versions of the MVA pathway in plastids.
5. Conclusions
Plant terpenoids are gaining prominence due to their broad application prospects. Recent advances have revealed their innovative uses in pharmaceuticals, food additives, and chemical industries, demonstrating significant market potential. Several critical knowledge gaps remain, particularly regarding the integration of different regulatory layers, including transcriptional, post-transcriptional, and epigenetic mechanisms. The precise roles of many newly identified TFs and miRNAs across various plant species, as well as their interactions with environmental factors, are still poorly understood. Moreover, the spatial and temporal dynamics of these regulatory networks during plant development and in response to biotic and abiotic stresses remain largely unexplored. These limitations underscore the need for more comprehensive systems biology approaches and functional validation studies to unravel the complex regulatory mechanisms governing terpenoid biosynthesis. Such investigations are crucial for deepening our understanding of plant metabolism and will provide valuable insights for metabolic engineering strategies aimed at optimizing terpenoid production for industrial and pharmaceutical applications. Based on that knowledge, future research should be directed toward the mechanisms governing terpenoid biosynthesis dependent on environmental change and molecular breeding.
Author Contributions
All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Key Research and Development Program of China (2022YFD2202000), the National Natural Science Foundation of China (31722012, 31901304 and 32401636), the Natural Science Foundation of Guangdong Province, China (2023A1515012709), and the Social Development Project of Guangzhou Municipal Science and Technology (202206010058).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Christianson, D.W. Structural and chemical biology of terpenoid cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.G.; Lathe, R. Terpenes, hormones and life: Isoprene rule revisited. J. Endocrinol. 2019, 242, R9–R22. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.; Perestrelo, R.; Ferreira, R.; Berenguer, C.V.; Pereira, J.A.; Castilho, P.C. Plant-derived terpenoids: A plethora of bioactive compounds with several health functions and industrial applications—A comprehensive overview. Molecules 2024, 29, 3861. [Google Scholar] [CrossRef] [PubMed]
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Tamogani, S.; Mitani, M.; Kodama, O.; Akatsuka, T. Oryzalexin S structure: A new stemarane-type rice plant phytoalexin and its biogenesis. Tetrahedron 1993, 49, 2025–2032. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically useful plant terpenoids: Biosynthesis, occurrence, and mechanism of action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Wang, K.C.; Ohnuma, S.-i. Isoprenyl diphosphate synthases. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2000, 1529, 33–48. [Google Scholar] [CrossRef]
- Jones, C.G.; Moniodis, J.; Zulak, K.G.; Scaffidi, A.; Plummer, J.A.; Ghisalberti, E.L.; Barbour, E.L.; Bohlmann, J. Sandalwood fragrance biosynthesis involves sesquiterpene synthases of both the terpene synthase (TPS)-a and TPS-b subfamilies, including santalene synthases. J. Biol. Chem. 2011, 286, 17445–17454. [Google Scholar] [CrossRef] [PubMed]
- Bathe, U.; Tissier, A. Cytochrome P450 enzymes: A driving force of plant diterpene diversity. Phytochemistry 2019, 161, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Sapir-Mir, M.; Mett, A.; Belausov, E.; Tal-Meshulam, S.; Frydman, A.; Gidoni, D.; Eyal, Y. Peroxisomal localization of Arabidopsis isopentenyl diphosphate isomerases suggests that part of the plant isoprenoid mevalonic acid pathway is compartmentalized to peroxisomes. Plant Physiol. 2008, 148, 1219–1228. [Google Scholar] [CrossRef]
- Soto, G.; Stritzler, M.; Lisi, C.; Alleva, K.; Pagano, M.E.; Ardila, F.; Mozzicafreddo, M.; Cuccioloni, M.; Angeletti, M.; Ayub, N.D. Acetoacetyl-CoA thiolase regulates the mevalonate pathway during abiotic stress adaptation. J. Exp. Bot. 2011, 62, 5699–5711. [Google Scholar] [CrossRef]
- Miziorko, H.M. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch. Biochem. Biophys. 2011, 505, 131–143. [Google Scholar] [CrossRef]
- Simkin, A.J.; Guirimand, G.; Papon, N.; Courdavault, V.; Thabet, I.; Ginis, O.; Bouzid, S.; Giglioli-Guivarc’h, N.; Clastre, M. Peroxisomal localisation of the final steps of the mevalonic acid pathway in planta. Planta 2011, 234, 903–914. [Google Scholar] [CrossRef]
- Berthelot, K.; Estevez, Y.; Deffieux, A.; Peruch, F. Isopentenyl diphosphate isomerase: A checkpoint to isoprenoid biosynthesis. Biochimie 2012, 94, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.P.; Rohwer, J.M.; Ghirardo, A.; Hammerbacher, A.; Ortiz-Alcaide, M.; Raguschke, B.; Schnitzler, J.-P.; Gershenzon, J.; Phillips, M.A. Deoxyxylulose 5-phosphate synthase controls flux through the methylerythritol 4-phosphate pathway in Arabidopsis. Plant Physiol. 2014, 165, 1488–1504. [Google Scholar] [CrossRef]
- Ruiz-Sola, M.Á.; Rodríguez-Concepción, M. Carotenoid biosynthesis in Arabidopsis: A colorful pathway. Arab. Book/Am. Soc. Plant Biol. 2012, 10, e0158. [Google Scholar] [CrossRef]
- Maeda, H.A.; Fernie, A.R. Evolutionary history of plant metabolism. Annu. Rev. Plant Biol. 2021, 72, 185–216. [Google Scholar] [CrossRef]
- Hemmerlin, A.; Harwood, J.L.; Bach, T.J. A raison d’être for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Prog. Lipid Res. 2012, 51, 95–148. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Baysal, C.; Gao, L.; Medina, V.; Drapal, M.; Ni, X.; Sheng, Y.; Shi, L.; Capell, T.; Fraser, P.D. The subcellular localization of two isopentenyl diphosphate isomerases in rice suggests a role for the endoplasmic reticulum in isoprenoid biosynthesis. Plant Cell Rep. 2020, 39, 119–133. [Google Scholar] [CrossRef]
- Mendoza-Poudereux, I.; Kutzner, E.; Huber, C.; Segura, J.; Eisenreich, W.; Arrillaga, I. Metabolic cross-talk between pathways of terpenoid backbone biosynthesis in spike lavender. Plant Physiol. Biochem. 2015, 95, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Skorupinska-Tudek, K.; Poznanski, J.; Wojcik, J.; Bienkowski, T.; Szostkiewicz, I.; Zelman-Femiak, M.; Bajda, A.; Chojnacki, T.; Olszowska, O.; Grunler, J. Contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthesis of dolichols in plants. J. Biol. Chem. 2008, 283, 21024–21035. [Google Scholar] [CrossRef] [PubMed]
- Lipko, A.; Pączkowski, C.; Perez-Fons, L.; Fraser, P.D.; Kania, M.; Hoffman-Sommer, M.; Danikiewicz, W.; Rohmer, M.; Poznanski, J.; Swiezewska, E. Divergent contribution of the MVA and MEP pathways to the formation of polyprenols and dolichols in Arabidopsis. Biochem. J. 2023, 480, 495–520. [Google Scholar] [CrossRef]
- Chen, C.-C.; Zhang, L.; Yu, X.; Ma, L.; Ko, T.-P.; Guo, R.-T. Versatile cis-isoprenyl diphosphate synthase superfamily members in catalyzing carbon–carbon bond formation. ACS Catal. 2020, 10, 4717–4725. [Google Scholar] [CrossRef]
- Nagel, R.; Schmidt, A.; Peters, R.J. Isoprenyl diphosphate synthases: The chain length determining step in terpene biosynthesis. Planta 2019, 249, 9–20. [Google Scholar] [CrossRef]
- Beck, G.; Coman, D.; Herren, E.; Ruiz-Sola, M.A.; Rodríguez-Concepción, M.; Gruissem, W.; Vranová, E. Characterization of the GGPP synthase gene family in Arabidopsis thaliana. Plant Mol. Biol. 2013, 82, 393–416. [Google Scholar] [CrossRef]
- Szkopińska, A.; Płochocka, D. Farnesyl diphosphate synthase; regulation of product specificity. Acta Biochim. Pol. 2005, 52, 45–55. [Google Scholar] [CrossRef]
- Li, C.; Zha, W.; Li, W.; Wang, J.; You, A. Advances in the biosynthesis of terpenoids and their ecological functions in plant resistance. Int. J. Mol. Sci. 2023, 24, 11561. [Google Scholar] [CrossRef]
- Chang, T.-H.; Hsieh, F.-L.; Ko, T.-P.; Teng, K.-H.; Liang, P.-H.; Wang, A.H.-J. Structure of a heterotetrameric geranyl pyrophosphate synthase from mint (Mentha piperita) reveals intersubunit regulation. Plant Cell 2010, 22, 454–467. [Google Scholar] [CrossRef]
- Song, X.; Qin, Y.G.; Zhang, Y.H.; Zhou, Y.B.; Li, Z.X. Farnesyl/geranylgeranyl diphosphate synthases regulate the biosynthesis of alarm pheromone in a unique manner in the vetch aphid Megoura viciae. Insect Mol. Biol. 2023, 32, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Fu, P.; Jun, X.; Cheng, P. Pharmacological properties of geraniol—A review. Planta Medica 2019, 85, 48–55. [Google Scholar] [CrossRef]
- Akhtar, T.A.; Matsuba, Y.; Schauvinhold, I.; Yu, G.; Lees, H.A.; Klein, S.E.; Pichersky, E. The tomato cis–prenyltransferase gene family. Plant J. 2013, 73, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Rivera, S.B.; Swedlund, B.D.; King, G.J.; Bell, R.N.; Hussey Jr, C.E.; Shattuck-Eidens, D.M.; Wrobel, W.M.; Peiser, G.D.; Poulter, C.D. Chrysanthemyl diphosphate synthase: Isolation of the gene and characterization of the recombinant non-head-to-tail monoterpene synthase from Chrysanthemum cinerariaefolium. Proc. Natl. Acad. Sci. USA 2001, 98, 4373–4378. [Google Scholar] [CrossRef]
- Demissie, Z.A.; Erland, L.E.; Rheault, M.R.; Mahmoud, S.S. The biosynthetic origin of irregular monoterpenes in Lavandula. J. Biol. Chem. 2013, 288, 6333–6341. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Jia, Q.; Brown, R.; Köllner, T.G.; Fu, J.; Chen, X.; Wong, G.K.-S.; Gershenzon, J.; Peters, R.J.; Chen, F. Origin and early evolution of the plant terpene synthase family. Proc. Natl. Acad. Sci. USA 2022, 119, e2100361119. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Li, M.; Wu, Z.; Liang, X.; Zheng, Q.; Li, D.; Wang, G.; An, T. The microbial biosynthesis of noncanonical terpenoids. Appl. Microbiol. Biotechnol. 2024, 108, 226. [Google Scholar] [CrossRef]
- Falara, V.; Akhtar, T.A.; Nguyen, T.T.; Spyropoulou, E.A.; Bleeker, P.M.; Schauvinhold, I.; Matsuba, Y.; Bonini, M.E.; Schilmiller, A.L.; Last, R.L. The tomato terpene synthase gene family. Plant Physiol. 2011, 157, 770–789. [Google Scholar] [CrossRef]
- Mao, L.; Jin, B.; Chen, L.; Tian, M.; Ma, R.; Yin, B.; Zhang, H.; Guo, J.; Tang, J.; Chen, T. Functional identification of the terpene synthase family involved in diterpenoid alkaloids biosynthesis in Aconitum carmichaelii. Acta Pharm. Sin. B 2021, 11, 3310–3321. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.-M.; Zhou, S.-S.; Liu, H.; Zhao, S.-W.; Tian, X.-C.; Shi, T.-L.; Bao, Y.-T.; Li, Z.-C.; Jia, K.-H.; Nie, S. Unraveling the evolutionary dynamics of the TPS gene family in land plants. Front. Plant Sci. 2023, 14, 1273648. [Google Scholar] [CrossRef]
- Omura, T.; Sato, R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J. Biol. Chem. 1964, 239, 2370–2378. [Google Scholar] [CrossRef]
- Mau, C.J.; Croteau, R. Cytochrome P450 oxygenases of monoterpene metabolism. Phytochem. Rev. 2006, 5, 373–383. [Google Scholar] [CrossRef]
- Teoh, K.H.; Polichuk, D.R.; Reed, D.W.; Nowak, G.; Covello, P.S. Artemisia annua L. (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett. 2006, 580, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.L.; Kjaerulff, L.; Heck, Q.K.; Forman, V.; Staerk, D.; Møller, B.L.; Andersen-Ranberg, J. Tripterygium wilfordii cytochrome P450s catalyze the methyl shift and epoxidations in the biosynthesis of triptonide. Nat. Commun. 2022, 13, 5011. [Google Scholar] [CrossRef]
- Lee, S.; Badieyan, S.; Bevan, D.R.; Herde, M.; Gatz, C.; Tholl, D. Herbivore-induced and floral homoterpene volatiles are biosynthesized by a single P450 enzyme (CYP82G1) in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 21205–21210. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, X.; Jing, W.; An, X.; Zhang, Q.; Zhang, H.; Zhou, J.; Zhang, Y.; Guo, Y. Identification and functional analysis of two P450 enzymes of Gossypium hirsutum involved in DMNT and TMTT biosynthesis. Plant Biotechnol. J. 2018, 16, 581–590. [Google Scholar] [CrossRef]
- Boland, W.; Gäbler, A.; Gilbert, M.; Feng, Z. Biosynthesis of C11 and C16 homoterpenes in higher plants; stereochemistry of the C-C-bond cleavage reaction. Tetrahedron 1998, 54, 14725–14736. [Google Scholar] [CrossRef]
- Richter, A.; Schaff, C.; Zhang, Z.; Lipka, A.E.; Tian, F.; Köllner, T.G.; Schnee, C.; Preiß, S.; Irmisch, S.; Jander, G. Characterization of biosynthetic pathways for the production of the volatile homoterpenes DMNT and TMTT in Zea mays. Plant Cell 2016, 28, 2651–2665. [Google Scholar] [CrossRef]
- Herde, M.; Gartner, K.; Kollner, T.G.; Fode, B.; Boland, W.; Gershenzon, J.; Gatz, C.; Tholl, D. Identification and regulation of TPS04/GES, an Arabidopsis geranyllinalool synthase catalyzing the first step in the formation of the insect-induced volatile C16-homoterpene TMTT. Plant Cell 2008, 20, 1152–1168. [Google Scholar] [CrossRef]
- Chowdhary, A.A.; Mishra, S.; Mehrotra, S.; Upadhyay, S.K.; Bagal, D.; Srivastava, V. Plant transcription factors: An overview of their role in plant life. In Plant Transcription Factors; Academic Press: Cambridge, MA, USA, 2022; pp. 3–20. [Google Scholar]
- Kelimujiang, K.; Zhang, W.; Zhang, X.; Waili, A.; Tang, X.; Chen, Y.; Chen, L. Genome-wide investigation of WRKY gene family in Lavandula angustifolia and potential role of LaWRKY57 and LaWRKY75 in the regulation of terpenoid biosynthesis. Front. Plant Sci. 2024, 15, 1449299. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Luo, Y.; Zhang, W.; Jian, W.; Zhang, L.; Gao, X.; Hu, X.; Yuan, Y.; Wu, M.; Xu, X. A SlMYB75-centred transcriptional cascade regulates trichome formation and sesquiterpene accumulation in tomato. J. Exp. Bot. 2021, 72, 3806–3820. [Google Scholar] [CrossRef]
- Li, X.; Meng, S.; Zhou, Y.; Wang, D.; Bian, Z.; Hu, L.; Lu, J. Genome-wide analysis of NAC gene family and function exploration of SaNAC30 in Santalum album L. Ind. Crop. Prod. 2025, 227, 120827. [Google Scholar] [CrossRef]
- Meng, S.; Lian, N.; Qin, F.; Yang, S.; Meng, D.; Bian, Z.; Xiang, L.; Lu, J. The AREB transcription factor SaAREB6 promotes drought stress-induced santalol biosynthesis in sandalwood. Hortic. Res. 2024, 12, uhae347. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Wang, X.; Wu, L.; Wang, M.; Yang, L.; Liu, X.; Chen, S.; Shi, Y. Integrated analysis of basic helix loop helix transcription factor family and targeted terpenoids reveals candidate AarbHLH genes involved in terpenoid biosynthesis in Artemisia argyi. Front. Plant Sci. 2022, 12, 811166. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-X.; Yang, C.; Xiong, W.; Chen, C.-Y.; Li, N. Transcriptome-wide identification of ARF gene family in medicinal plant Polygonatum kingianum and expression analysis of PkARF members in different tissues. Mol. Biol. Rep. 2024, 51, 648. [Google Scholar] [CrossRef]
- Oudin, A.; Mahroug, S.; Courdavault, V.; Hervouet, N.; Zelwer, C.; Rodríguez-Concepción, M.; St-Pierre, B.; Burlat, V. Spatial distribution and hormonal regulation of gene products from methyl erythritol phosphate and monoterpene-secoiridoid pathways in Catharanthus roseus. Plant Mol. Biol. 2007, 65, 13–30. [Google Scholar] [CrossRef]
- Tu, Z.; Hao, Z.; Liu, Q.; Gu, Z.; Zhang, W.; Yang, C. Multi-omics analyses reveal microRNAs’ role in terpene biosynthesis regulation in slash pine. Ind. Crop. Prod. 2024, 216, 118625. [Google Scholar] [CrossRef]
- Pani, A.; Mahapatra, R.K. Computational identification of microRNAs and their targets in Catharanthus roseus expressed sequence tags. Genom. Data 2013, 1, 2–6. [Google Scholar] [CrossRef]
- Morales, S.; Monzo, M.; Navarro, A. Epigenetic regulation mechanisms of microRNA expression. Biomol. Concepts 2017, 8, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Loivamäki, M.; Louis, S.; Cinege, G.n.; Zimmer, I.; Fischbach, R.J.; Schnitzler, J.r.-P. Circadian rhythms of isoprene biosynthesis in grey poplar leaves. Plant Physiol. 2007, 143, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.B.; Segerfeldt, P.; Lindström, A.; Borg-Karlson, A.-K.; Berglund, T. UV-B exposure of indoor-grown Picea abies seedlings causes an epigenetic effect and selective emission of terpenes. Z. Für Naturforschung C 2013, 68, 139–147. [Google Scholar]
- Kamali, S.; Iranbakhsh, A.; Ebadi, M.; Ardebili, Z.O.; Haghighat, S. Methyl jasmonate conferred Arsenic tolerance in Thymus kotschyanus by DNA hypomethylation, stimulating terpenoid metabolism, and upregulating two cytochrome P450 monooxygenases. J. Hazard. Mater. 2024, 465, 133163. [Google Scholar] [CrossRef]
- Shi, Z.; Deng, X.; Zeng, L.; Shi, S.; Lei, L.; Xiao, W. Acclimation strategy of Masson Pine (Pinus massoniana) by limiting flavonoid and terpenoid production under low light and drought. Int. J. Mol. Sci. 2022, 23, 8441. [Google Scholar] [CrossRef]
- Contreras-Avilés, W.; Heuvelink, E.; Marcelis, L.F.; Kappers, I.F. Ménage à trois: Light, terpenoids, and quality of plants. Trends Plant Sci. 2024, 29, 572–588. [Google Scholar] [CrossRef]
- Miao, W.; Luo, J.; Liu, J.; Howell, K.; Zhang, P. The Influence of UV on the Production of Free Terpenes in Vitis vinifera cv. Shiraz. Agronomy 2020, 10, 1431. [Google Scholar] [CrossRef]
- Gil, M.; Pontin, M.; Berli, F.; Bottini, R.; Piccoli, P. Metabolism of terpenes in the response of grape (Vitis vinifera L.) leaf tissues to UV-B radiation. Phytochemistry 2012, 77, 89–98. [Google Scholar] [CrossRef]
- Oliveira, A.d.; Nieddu, G. Vine growth and physiological performance of two red grape cultivars under natural and reduced UV solar radiation. Aust. J. Grape Wine Res. 2016, 22, 105–114. [Google Scholar] [CrossRef]
- Cao, X.-Y.; Li, C.-G.; Miao, Q.; Zheng, Z.-J.; Jiang, J.-H. Molecular cloning and expression analysis of a leaf-specific expressing 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase gene from Michelia chapensis Dandy. J. Med. Plants Res. 2011, 5, 3868–3875. [Google Scholar]
- Tounekti, T.; Hernández, I.; Munné-Bosch, S. Salicylic acid biosynthesis and role in modulating terpenoid and flavonoid metabolism in plant responses to abiotic stress. In Salicylic Acid: Plant Growth and Development; Springer: Berlin/Heidelberg, Germany, 2013; pp. 141–162. [Google Scholar]
- Lalel, H.; Singh, Z.; Tan, S. The role of ethylene in mango fruit aroma volatiles biosynthesis. J. Hortic. Sci. Biotechnol. 2003, 78, 485–496. [Google Scholar] [CrossRef]
- Martin, D.M.; Gershenzon, J.; Bohlmann, J.r. Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant Physiol. 2003, 132, 1586–1599. [Google Scholar] [CrossRef]
- Cao, X.; Yin, T.; Miao, Q.; Li, C.; Ju, X.; Sun, Y.; Jiang, J. Molecular characterization and expression analysis of a gene encoding for farnesyl diphosphate synthase from Euphorbia pekinensis Rupr. Mol. Biol. Rep. 2012, 39, 1487–1492. [Google Scholar] [CrossRef]
- Shabani, L.; Ehsanpour, A.A.; Esmaeili, A. Assessment of squalene synthase and beta-amyrin synthase gene expression in licorice roots treated with methyl jasmonate and salicylic acid using real-time qPCR. Russ. J. Plant Physiol. 2010, 57, 480–484. [Google Scholar] [CrossRef]
- Delfine, S.; Loreto, F.; Pinelli, P.; Tognetti, R.; Alvino, A. Isoprenoids content and photosynthetic limitations in rosemary and spearmint plants under water stress. Agric. Ecosyst. Environ. 2005, 106, 243–252. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Peñuelas, J. Photo- and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta 2003, 217, 758–766. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, X.; Yan, X.; Guo, L.; Mi, X.; Xu, Q.; Zhu, J.; Wu, A.; Liu, L.; Wei, C. Revealing of microRNA involved regulatory gene networks on terpenoid biosynthesis in Camellia sinensis in different growing time points. J. Agric. Food Chem. 2018, 66, 12604–12616. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Galhano, R.; Wiemann, P.; Bueno, E.; Tiernan, M.; Wu, W.; Chung, I.M.; Gershenzon, J.; Tudzynski, B.; Sesma, A. Genetic evidence for natural product-mediated plant–plant allelopathy in rice (Oryza sativa). New Phytol. 2012, 193, 570–575. [Google Scholar] [CrossRef]
- Carter, J.P.; Spink, J.; Cannon, P.F.; Daniels, M.J.; Osbourn, A.E. Isolation, characterization, and avenacin sensitivity of a diverse collection of cereal-root-colonizing fungi. Appl. Environ. Microbiol. 1999, 65, 3364–3372. [Google Scholar] [CrossRef]
- Rosenkranz, M.; Chen, Y.; Zhu, P.; Vlot, A.C. Volatile terpenes–mediators of plant-to-plant communication. Plant J. 2021, 108, 617–631. [Google Scholar] [CrossRef]
- Slavković, F.; Bendahmane, A. Floral phytochemistry: Impact of volatile organic compounds and nectar secondary metabolites on pollinator behavior and health. Chem. Biodivers. 2023, 20, e202201139. [Google Scholar] [CrossRef] [PubMed]
- Crang, R.; Lyons-Sobaski, S.; Wise, R. Plant Anatomy: A Concept-Based Approach to the Structure of Seed Plants; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Zha, W.; Zhang, F.; Shao, J.; Ma, X.; Zhu, J.; Sun, P.; Wu, R.; Zi, J. Rationally engineering santalene synthase to readjust the component ratio of sandalwood oil. Nat. Commun. 2022, 13, 2508. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Campos, J.M.; da Silva Rolim, G.; Martínez, L.C.; Dos Santos, M.H.; Fernandes, F.L.; Serrão, J.E.; Zanuncio, J.C. Terpenoid constituents of cinnamon and clove essential oils cause toxic effects and behavior repellency response on granary weevil, Sitophilus granarius. Ecotoxicol. Environ. Saf. 2018, 156, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Gutensohn, M.; Hartzell, E.; Dudareva, N. Another level of complex-ity: The role of metabolic channeling and metabolons in plant terpenoid metabolism. Front. Plant Sci. 2022, 13, 954083. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Yu, R. Artemisinin biosynthesis and its regulatory enzymes: Progress and perspective. Pharmacogn. Rev. 2011, 5, 189. [Google Scholar]
- Lai, H.C.; Singh, N.P.; Sasaki, T. Development of artemisinin compounds for cancer treatment. Investig. New Drugs 2013, 31, 230–246. [Google Scholar] [CrossRef]
- Tetali, S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef]
- Dong, Y.; Morris-Natschke, S.L.; Lee, K.-H. Biosynthesis, total syntheses, and antitumor activity of tanshinones and their analogs as potential therapeutic agents. Nat. Prod. Rep. 2011, 28, 529–542. [Google Scholar] [CrossRef]
- Javed, S.; Tariq, A.; Ahmed, T.; Budzyńska, B.; Tejada, S.; Daglia, M.; Nabavi, S.F.; Sobarzo-Sánchez, E.; Nabavi, S.M. Tanshinones and mental diseases: From chemistry to medicine. Rev. Neurosci. 2016, 27, 777–791. [Google Scholar] [CrossRef]
- Lai, Z.; He, J.; Zhou, C.; Zhao, H.; Cui, S. Tanshinones: An update in the medicinal chemistry in recent 5 years. Curr. Med. Chem. 2021, 28, 2807–2827. [Google Scholar] [CrossRef]
- Hu, W.; Meng, X.; Wu, Y.; Li, X.; Chen, H. Terpenoids, a Rising Star in Bioactive Constituents for Alleviating Food Allergy: A Review about the Potential Mechanism, Preparation, and Application. J. Agric. Food Chem. 2024, 72, 26599–26616. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Brock, P.L.; Vaughan, B.M.; Vollmer, D.L. Comparison of antimicrobial activities of natural essential oils and synthetic fragrances against selected environmental pathogens. Biochim. Open 2017, 5, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Pan, Z. Cannabidiol and terpenes from hemp–ingredients for future foods and processing technologies. J. Future Foods 2021, 1, 113–127. [Google Scholar] [CrossRef]
- Lowe, H.; Steele, B.; Bryant, J.; Toyang, N.; Ngwa, W. Non-cannabinoid metabolites of Cannabis sativa L. with therapeutic potential. Plants 2021, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- Aissou, M.; Chemat-Djenni, Z.; Yara-Varón, E.; Fabiano-Tixier, A.-S.; Chemat, F. Limonene as an agro-chemical building block for the synthesis and extraction of bioactive compounds. Comptes Rendus Chim. 2017, 20, 346–358. [Google Scholar] [CrossRef]
- Herman, A.; Tambor, K.; Herman, A. Linalool affects the antimicrobial efficacy of essential oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef]
- Shiekh, R.A.E.; Atwa, A.M.; Elgindy, A.M.; Mustafa, A.M.; Senna, M.M.; Alkabbani, M.A.; Ibrahim, K.M. Therapeutic applications of eucalyptus essential oils. Inflammopharmacology 2024, 33, 163–182. [Google Scholar] [CrossRef]
- Martirosyan, D.; Christopher, S. The benefits of terpenoids as functional foods for the managementof type 2 diabetes mellitus. Bioact. Compd. Health Dis.-Online 2024, 7, 345–347. [Google Scholar] [CrossRef]
- Huang, J.; Yang, L.; Zou, Y.; Luo, S.; Wang, X.; Liang, Y.; Du, Y.; Feng, R.; Wei, Q. Antibacterial activity and mechanism of three isomeric terpineols of Cinnamomum longepaniculatum leaf oil. Folia Microbiol. 2021, 66, 59–67. [Google Scholar] [CrossRef]
- Liu, W.; Xu, X.; Zhang, R.; Cheng, T.; Cao, Y.; Li, X.; Guo, J.; Liu, H.; Xian, M. Engineering Escherichia coli for high-yield geraniol production with biotransformation of geranyl acetate to geraniol under fed-batch culture. Biotechnol. Biofuels 2016, 9, 58. [Google Scholar]
- Wang, Y.; Gong, X.; Li, F.; Zuo, S.; Li, M.; Zhao, J.; Han, X.; Wen, M. Optimized biosynthesis of santalenes and santalols in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2021, 105, 8795–8804. [Google Scholar] [CrossRef] [PubMed]
- Ignea, C.; Pontini, M.; Maffei, M.E.; Makris, A.M.; Kampranis, S.C. Engineering monoterpene production in yeast using a synthetic dominant negative geranyl diphosphate synthase. ACS Synth. Biol. 2014, 3, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Basallo, O.; Perez, L.; Lucido, A.; Sorribas, A.; Marin-Saguino, A.; Vilaprinyo, E.; Perez-Fons, L.; Albacete, A.; Martínez-Andújar, C.; Fraser, P.D. Changing biosynthesis of terpenoid percursors in rice through synthetic biology. Front. Plant Sci. 2023, 14, 1133299. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).