Heavy Metal Health Risk Assessment in Picea abies L. Forests Along an Altitudinal Gradient in Southern Romania
Abstract
1. Introduction
2. Results
2.1. Elemental Chemical Composition in Needles, Bark and Litter
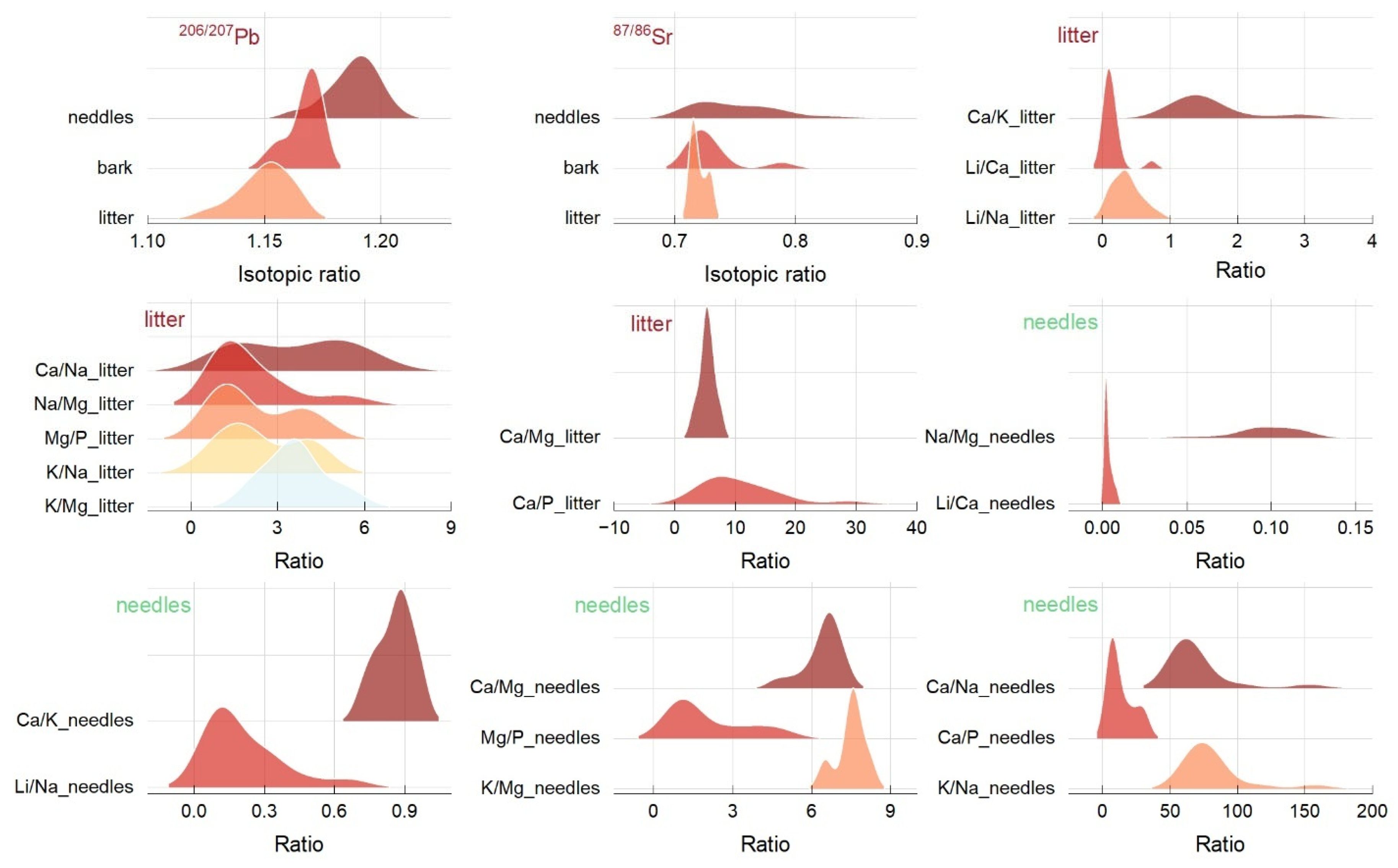
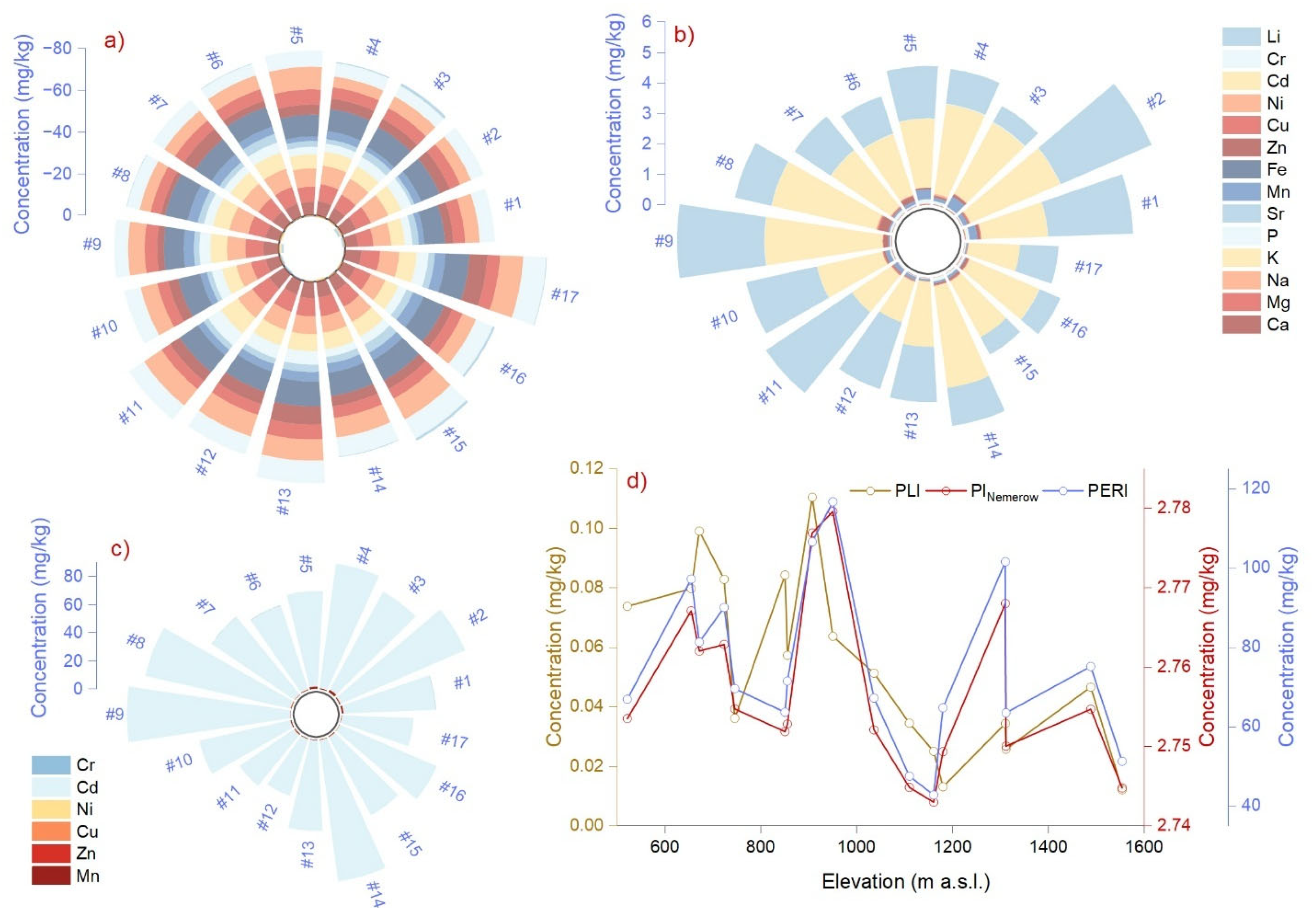
2.2. Isotopic Signature and Mole Ratios of Ca, K, Na, Mg, P, and Li
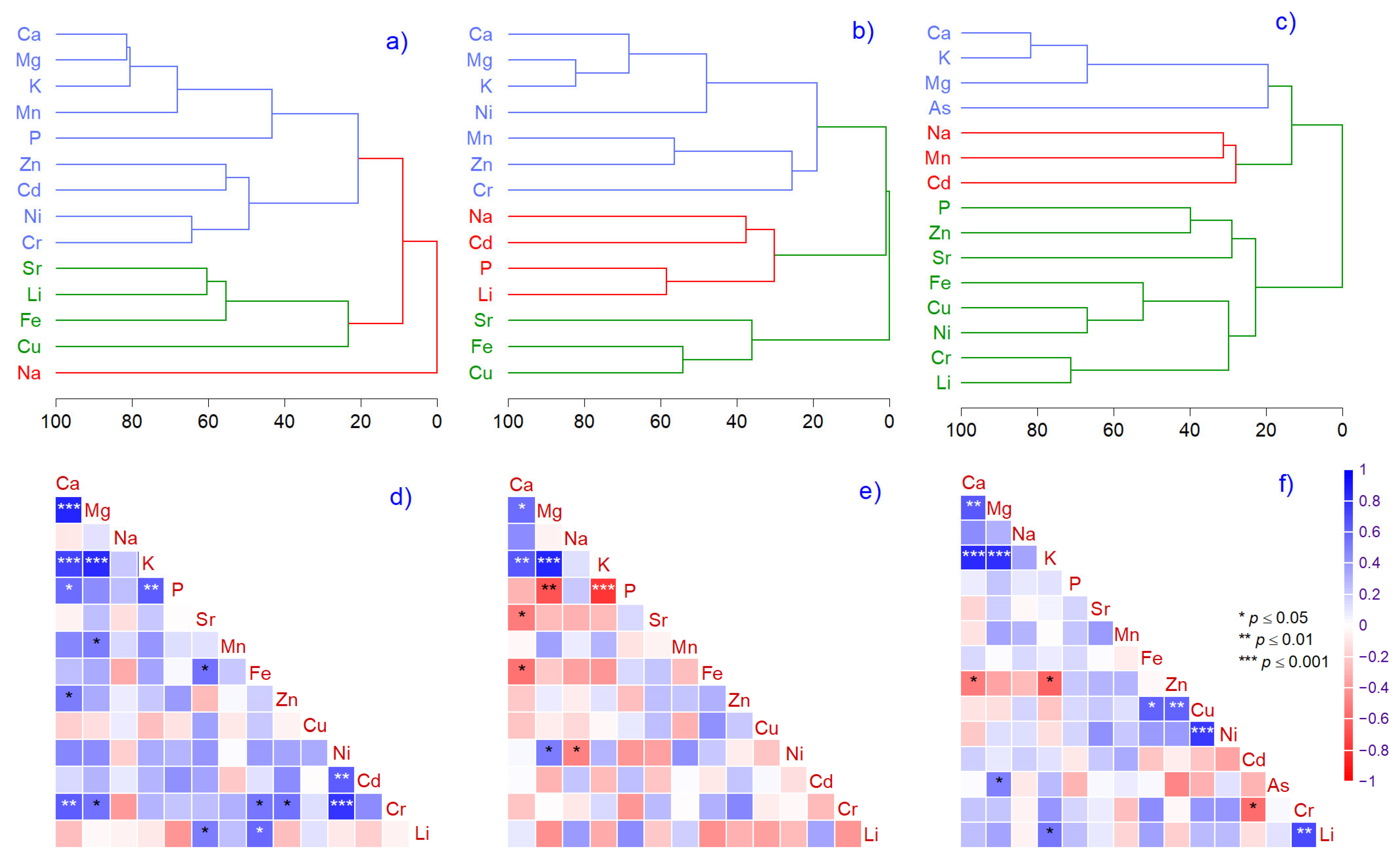
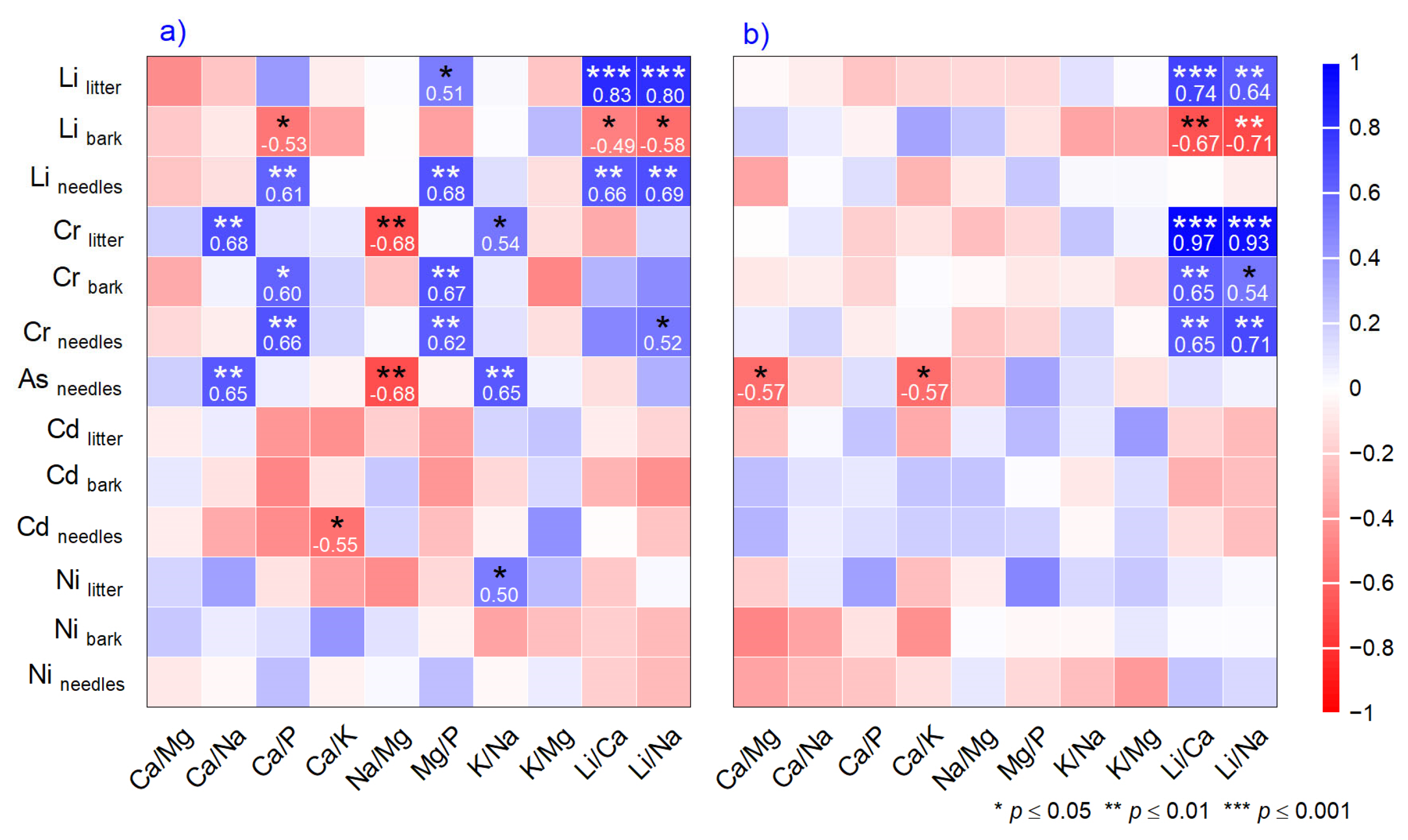
2.3. Correlation and Multivariate Analysis
2.4. Environmental Risk Assessment
3. Discussion
3.1. Elemental Distribution, Origins and Altitudinal Trends
3.2. Relationship Between Nutrients Mole Ratio and Toxic Metals
3.3. Environmental Health Risks
4. Materials and Methods
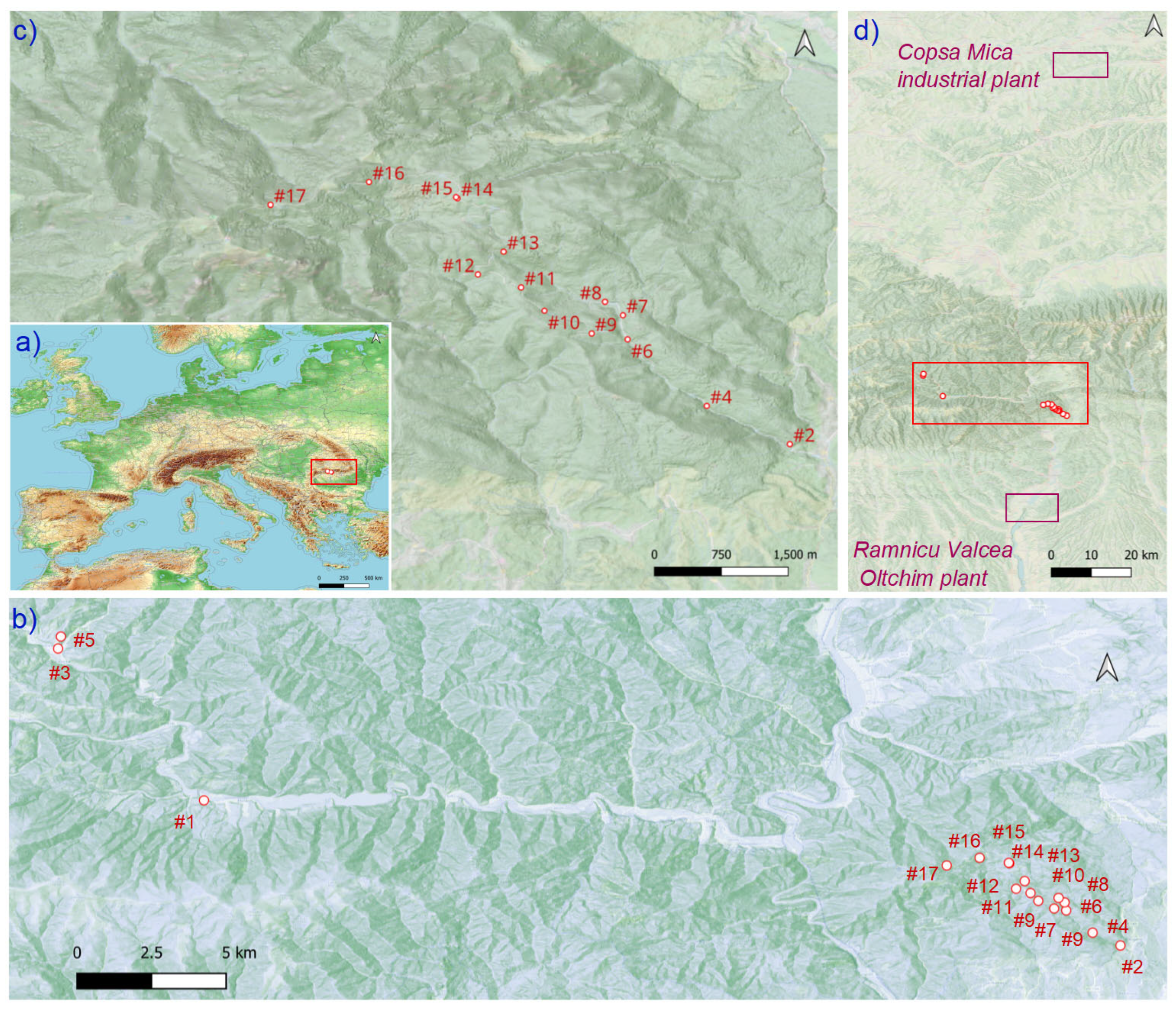
4.1. Study Area and Sample Collection
4.2. Sample Preparation and Analysis
4.2.1. Elemental Analysis
4.2.2. Analyses of 206/207Pb and 87Sr/86Sr Isotope Ratios
4.3. Statistical Analyses
4.4. Risk Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajkumar, M.; Prasad, M.N.V.; Swaminathan, S.; Freitas, H. Climate change driven plant–metal–microbe interactions. Environ. Int. 2013, 53, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Nechita, C.; Iordache, A.M.; Lemr, K.; Levanič, T.; Pluhacek, T. Evidence of declining trees resilience under long term heavy metal stress combined with climate change heating. J. Clean. Prod. 2021, 317, 128428. [Google Scholar] [CrossRef]
- Méndez-López, M.; Gómez-Armesto, A.; Eimil-Fraga, C.; Alonso-Vega, F.; Rodríguez-Soalleiro, R.; Álvarez-Rodríguez, E.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C. Needle age and precipitation as drivers of Hg accumulation and deposition in coniferous forests from a southwestern European Atlantic region. Environ. Res. 2022, 215, 114223. [Google Scholar] [CrossRef] [PubMed]
- Ershov, V.; Sukhareva, T.; Isaeva, L.; Ivanova, E.; Urbanavichus, G. Pollution-Induced Changes in the Composition of Atmospheric Deposition and Soil Waters in Coniferous Forests at the Northern Tree Line. Sustainability 2022, 14, 15580. [Google Scholar] [CrossRef]
- Iordache, A.M.; Nechita, C.; Voica, C.; Pluháček, T.; Schug, K.A. Climate change extreme and seasonal toxic metal occurrence in Romanian freshwaters in the last two decades—Case study and critical review. npj Clean Water 2022, 5, 2. [Google Scholar] [CrossRef]
- Lorenz, M.; Brunke, M. Trends of nutrients and metals in precipitation in northern Germany: The role of emissions and meteorology. Environ. Monit. Assess. 2021, 193, 325. [Google Scholar] [CrossRef]
- Urrutia-Pereira, M.; Varanda Rizzo, L.; Latour Staffeld, P.; Chong-Neto, H.J.; Viegi, G.; Solé, D. Dust from the Sahara to the American Continent: Health impacts: Dust from Sahara. Allergol. Et Immunopathol. 2021, 49, 187–194. [Google Scholar] [CrossRef]
- Abás, E.; Marina-Montes, C.; Laguna, M.; Lasheras, R.; Rivas, P.; Peribáñez, P.; del Valle, J.; Escudero, M.; Velásquez, A.; Cáceres, J.O.; et al. Evidence of human impact in Antarctic region by studying atmospheric aerosols. Chemosphere 2022, 307, 135706. [Google Scholar] [CrossRef]
- Byrne, A. Trouble in the air: Recent developments under the 1979 Convention on Long-Range Transboundary Air Pollution. Rev. Eur. Comp. Int. Environ. Law 2017, 26, 210–219. [Google Scholar] [CrossRef]
- Luo, H.; Wang, Q.; Guan, Q.; Ma, Y.; Ni, F.; Yang, E.; Zhang, J. Heavy metal pollution levels, source apportionment and risk assessment in dust storms in key cities in Northwest China. J. Hazard. Mater. 2022, 422, 126878. [Google Scholar] [CrossRef]
- Salazar-Rojas, T.; Cejudo-Ruiz, F.R.; Calvo-Brenes, G. Magnetic and Chemical Testing in Plants, Road Dust and Soil, as Indicators of Atmospheric Pollution. Water Air Soil Pollut. 2024, 235, 601. [Google Scholar] [CrossRef]
- Chen, L.-C.; Maciejczyk, P.; Thurston, G.D. Chapter 6—Metals and air pollution. In Handbook on the Toxicology of Metals, 5th ed.; Nordberg, G.F., Costa, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 137–182. [Google Scholar]
- Mirzaei Aminiyan, M.; Baalousha, M.; Mousavi, R.; Mirzaei Aminiyan, F.; Hosseini, H.; Heydariyan, A. The ecological risk, source identification, and pollution assessment of heavy metals in road dust: A case study in Rafsanjan, SE Iran. Environ. Sci. Pollut. Res. 2018, 25, 13382–13395. [Google Scholar] [CrossRef] [PubMed]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Cong, Y.; Yu, R.-l.; Yan, Y.; Weng, B.-S.; Hu, G.-R.; Sun, J.-W.; Cui, J.-Y.; Huang, Y.-Y. Source analysis of metals in the tea plant using linear correlation analysis combined with a lead-strontium isotope tracer. Catena 2023, 229, 107194. [Google Scholar] [CrossRef]
- Novak, M.; Holmden, C.; Farkaš, J.; Kram, P.; Hruska, J.; Curik, J.; Veselovsky, F.; Stepanova, M.; Kochergina, Y.V.; Erban, V.; et al. Calcium and strontium isotope dynamics in three polluted forest ecosystems of the Czech Republic, Central Europe. Chem. Geol. 2020, 536, 119472. [Google Scholar] [CrossRef]
- Cong, L.; Zhou, S.; Niyogi, D.; Wu, Y.; Yan, G.; Dai, L.; Liu, S.; Zhang, Z.; Hu, Y. Concentrations and isotopic analysis for the sources and transfer of lead in an urban atmosphere-plant-soil system. J. Environ. Manag. 2022, 311, 114771. [Google Scholar] [CrossRef]
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Khan, K.A.; Li, S. Health and environmental effects of heavy metals. J. King Saud Univ. Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- Remick, K.A.; Helmann, J.D. Chapter One—The elements of life: A biocentric tour of the periodic table. In Advances in Microbial Physiology; Poole, R.K., Kelly, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 82, pp. 1–127. [Google Scholar]
- Huang, D.; Gong, X.; Liu, Y.; Zeng, G.; Lai, C.; Bashir, H.; Zhou, L.; Wang, D.; Xu, P.; Cheng, M.; et al. Effects of calcium at toxic concentrations of cadmium in plants. Planta 2017, 245, 863–873. [Google Scholar] [CrossRef]
- Brown, P.H.; Zhao, F.-J.; Dobermann, A. What is a plant nutrient? Changing definitions to advance science and innovation in plant nutrition. Plant Soil 2022, 476, 11–23. [Google Scholar] [CrossRef]
- Marschner, H. Functions of Mineral Nutrients: Micronutrients, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Subbarao, G.V.; Ito, O.; Berry, W.L.; Wheeler, R.M. Sodium—A Functional Plant Nutrient. Crit. Rev. Plant Sci. 2003, 22, 391–416. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Afanaseva, L.V.; Kalugina, O.V.; Kharpukhaeva, T.M. Elemental Chemical Composition of the Larix Sibirica Needles in the Predbaikalia. Russ. J. Bioorganic Chem. 2024, 50, 2818–2825. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, X.; Dang, H.; Zhang, Q. Biomass:N:K:Ca:Mg:P ratios in forest stands world-wide: Biogeographical variations and environmental controls. Glob. Ecol. Biogeogr. 2020, 29, 2176–2189. [Google Scholar] [CrossRef]
- Gao, Y.; Yuan, Y.; Li, Q.; Kou, L.; Fu, X.; Dai, X.; Wang, H. Mycorrhizal type governs foliar and root multi-elemental stoichiometries of trees mainly via root traits. Plant Soil 2021, 460, 229–246. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Dong, Y.; Wei, S.; Zeng, M.; Jiao, R. Response strategies of slash pine (Pinus elliottii) to cadmium stress and the gain effects of inoculation with Herbaspirillum sp. YTG72 in alleviating phytotoxicity and enhancing accumulation of cadmium. Environ. Sci. Pollut. Res. 2024, 31, 31590–31604. [Google Scholar] [CrossRef]
- Kubov, M.; Schieber, B.; Janík, R. Seasonal dynamics of macronutrients in aboveground biomass of two herb-layer species in a beech forest. Biologia 2019, 74, 1415–1424. [Google Scholar] [CrossRef]
- Turkyilmaz, A.; Sevik, H.; Cetin, M. The use of perennial needles as biomonitors for recently accumulated heavy metals. Landsc. Ecol. Eng. 2018, 14, 115–120. [Google Scholar] [CrossRef]
- Cocozza, C.; Ravera, S.; Cherubini, P.; Lombardi, F.; Marchetti, M.; Tognetti, R. Integrated biomonitoring of airborne pollutants over space and time using tree rings, bark, leaves and epiphytic lichens. Urban For. Urban Green. 2016, 17, 177–191. [Google Scholar] [CrossRef]
- Alahabadi, A.; Ehrampoush, M.H.; Miri, M.; Ebrahimi Aval, H.; Yousefzadeh, S.; Ghaffari, H.R.; Ahmadi, E.; Talebi, P.; Abaszadeh Fathabadi, Z.; Babai, F.; et al. A comparative study on capability of different tree species in accumulating heavy metals from soil and ambient air. Chemosphere 2017, 172, 459–467. [Google Scholar] [CrossRef]
- Sevik, H.; Cetin, M.; Ozturk, A.; Ozel, H.; Pinar, B. Changes in Pb, Cr and Cu concentrations in some bioindicators depending on traffic density on the basis of species and organs. Appl. Ecol. Environ. Res. 2019, 17, 12843–12857. [Google Scholar] [CrossRef]
- Iordache, A.M.; Nechita, C.; Pluhacek, T.; Iordache, M.; Zgavarogea, R.; Ionete, R.E. Past and present anthropic environmental stress reflect high susceptibility of natural freshwater ecosystems in Romania. Environ. Pollut. 2020, 267, 115505. [Google Scholar] [CrossRef]
- Jeong, H.; Choi, J.Y.; Lee, J.; Lim, J.; Ra, K. Heavy metal pollution by road-deposited sediments and its contribution to total suspended solids in rainfall runoff from intensive industrial areas. Environ. Pollut. 2020, 265, 115028. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Davidson, C.I.; Grimm, T.C.; Nasta, M.A. Airborne Lead and Other Elements Derived from Local Fires in the Himalayas. Science 1981, 214, 1344–1346. [Google Scholar] [CrossRef]
- Cong, Z.; Kang, S.; Dong, S.; Liu, X.; Qin, D. Elemental and individual particle analysis of atmospheric aerosols from high Himalayas. Environ. Monit. Assess. 2010, 160, 323–335. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Miechówka, A. Identification of the factors determining the concentration and spatial distribution of Zn, Pb and Cd in the soils of the non-forest Tatra Mountains (southern Poland). Environ. Geochem. Health 2022, 44, 4323–4341. [Google Scholar] [CrossRef]
- He, X.; Liu, P.; Zhao, W.; Xu, H.; Zhang, R.; Shen, Z. Size distribution of water-soluble metals in atmospheric particles in Xi’an, China: Seasonal variations, bioavailability, and health risk assessment. Atmos. Pollut. Res. 2021, 12, 101090. [Google Scholar] [CrossRef]
- Morselli, L.; Olivieri, P.; Brusori, B.; Passarini, F. Soluble and insoluble fractions of heavy metals in wet and dry atmospheric depositions in Bologna, Italy. Environ. Pollut. 2003, 124, 457–469. [Google Scholar] [CrossRef]
- Pulatoglu, A.O.; Koç, İ.; Özel, H.B.; Şevik, H.; Yıldız, Y. Using trees to monitor airborne Cr pollution: Effects of compass direction and woody species on Cr uptake during phytoremediation. BioResources 2025, 20, 121–139. [Google Scholar] [CrossRef]
- Zeren Çetin, İ. Used in Urban Area for Landscape Planning and Design Spatial and Temporal Variations in Chromium (Cr) Concentrations in Picea orientalis L. Turk. J. Agric. Food Sci. Technol. 2024, 12, 1730–1738. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, W.; Li, F.; Liu, C.; Ma, J.; Yan, J.; Wang, Y.; Tian, R. Spatio-temporal distribution and source identification of heavy metals in particle size fractions of road dust from a typical industrial district. Sci. Total Environ. 2021, 780, 146357. [Google Scholar] [CrossRef]
- Popović, V.; Šešlija Jovanović, D.; Miletić, Z.; Milovanović, J.; Lučić, A.; Rakonjac, L.; Miljković, D. The evaluation of hazardous element content in the needles of the Norway spruce (Picea abies L.) that originated from anthropogenic activities in the vicinity of the native habitats. Environ. Monit. Assess. 2022, 195, 109. [Google Scholar] [CrossRef] [PubMed]
- Miranda, I.; Gominho, J.; Mirra, I.; Pereira, H. Chemical characterization of barks from Picea abies and Pinus sylvestris after fractioning into different particle sizes. Ind. Crops Prod. 2012, 36, 395–400. [Google Scholar] [CrossRef]
- Rothpfeffer, C.; Karltun, E. Inorganic elements in tree compartments of Picea abies—Concentrations versus stem diameter in wood and bark and concentrations in needles and branches. Biomass Bioenergy 2007, 31, 717–725. [Google Scholar] [CrossRef]
- Kuang, Y.W.; Wen, D.Z.; Zhou, G.; Liu, S.Z. Distribution of elements in needles of Pinus massoniana (Lamb.) was uneven and affected by needle age. Environ. Pollut. 2007, 145, 730–737. [Google Scholar] [CrossRef]
- Giertych, M.J.; de Temmerman, L.O.; Rachwal, L. Distribution of elements along the length of Scots pine needles in a heavily polluted and a control environment. Tree Physiol. 1997, 17, 697–703. [Google Scholar] [CrossRef]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef]
- Brække, F.H.; Salih, N. Reliability of foliar analyses of Norway spruce stands in a Nordic gradient. Silva Fenn. 2002, 36, 489–504. [Google Scholar] [CrossRef][Green Version]
- Wallander, H.; Thelin, G. The stimulating effect of apatite on ectomycorrhizal growth diminishes after PK fertilization. Soil Biol. Biochem. 2008, 40, 2517–2522. [Google Scholar] [CrossRef]
- Högberg, P.; Johannisson, C.; Yarwood, S.; Callesen, I.; Näsholm, T.; Myrold, D.D.; Högberg, M.N. Recovery of ectomycorrhiza after ‘nitrogen saturation’ of a conifer forest. New Phytol. 2011, 189, 515–525. [Google Scholar] [CrossRef]
- Novotný, R.; Fadrhonsová, V.; Šrámek, V. Long-Term Monitored Norway Spruce Plots in the Ore Mountains—30 Years of Changes in Forest Health, Soil Chemistry and Tree Nutrition After Air Pollution Calamity. Plants 2024, 13, 2379. [Google Scholar] [CrossRef]
- Zethof, J.H.T.; Julich, S.; Feger, K.-H.; Julich, D. Legacy effect of 25 years reduced atmospheric sulphur deposition on spruce tree nutrition. J. Plant Nutr. Soil Sci. 2024, 187, 834–843. [Google Scholar] [CrossRef]
- Jiao, C.; Zhang, J.; Yu, H.; He, N. Variation of magnesium drives plant adaption to heterogeneous environments by regulating efficiency in photosynthesis on a large scale. J. Ecol. 2024, 112, 2758–2770. [Google Scholar] [CrossRef]
- Erdem, R.; Koç, İ.; Çobanoglu, H.; Şevik, H. Variation of Magnesium, One of the Macronutrients, in Some Trees Based on Organs and Species. Forestist 2024, 74, 84–93. [Google Scholar] [CrossRef]
- Amirmohammadi, M.; Khademi, H.; Ayoubi, S.; Faz, A. Pine needles as bioindicator and biomagnetic indicator of selected metals in the street dust, a case study from southeastern Iran. Chemosphere 2024, 352, 141281. [Google Scholar] [CrossRef]
- Zsigmond, A.R.; Száraz, A.; Urák, I. Macro and trace elements in the black pine needles as inorganic indicators of urban traffic emissions. Environ. Pollut. 2021, 291, 118228. [Google Scholar] [CrossRef]
- Figas, A.; Siwik-Ziomek, A.; Kobierski, M. Heavy Metals and Sulphur in Needles of Pinus sylvestris L. and Soil in the Forests of City Agglomeration. Forests 2021, 12, 1310. [Google Scholar] [CrossRef]
- Ivanova, E.A.; Lukina, N.V.; Smirnov, V.E.; Isaeva, L.G. Effect of Industrial Airborne Pollution on the Chemical Composition of Pine Needle Litterfall at the Northern Distribution Limit of Pine Forests. Contemp. Probl. Ecol. 2022, 15, 851–862. [Google Scholar] [CrossRef]
- Parzych, A.; Mochnacky, S.; Sobisz, Z.; Polláková, N.; Šimanský, V. Needles and bark of Picea abies (L.) H. Karst and Picea omorika (Panciç) Purk. as bioindicators of environmental quality. Folia For. Pol. Ser. A For. 2018, 60, 230–240. [Google Scholar] [CrossRef]
- Konieczyński, P.; Moszczyński, J.; Wesołowski, M. Comparison of Fe, Zn, Mn, Cu, Cd and Pb concentration in spruce needles collected in the area of Gdansk and Gdynia in northern Poland. Environ. Prot. Nat. Resour 2018, 78, 1–9. [Google Scholar] [CrossRef]
- Schmidt, S.B.; Husted, S. The Biochemical Properties of Manganese in Plants. Plants 2019, 8, 381. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; Kabata-Pendias, A., Ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Jonczak, J.; Sut-Lohmann, M.; Polláková, N.; Parzych, A.; Šimanský, V.; Donovan, S. Bioaccumulation of Potentially Toxic Elements by the Needles of Eleven Pine Species in Low Polluted Area. Water Air Soil Pollut. 2021, 232, 28. [Google Scholar] [CrossRef]
- Świercz, A.; Świątek, B.; Pietrzykowski, M. Changes in the Concentrations of Trace Elements and Supply of Nutrients to Silver Fir (Abies alba Mill.) Needles as a Bioindicator of Industrial Pressure over the Past 30 Years in Świętokrzyski National Park (Southern Poland). Forests 2022, 13, 718. [Google Scholar] [CrossRef]
- Erdyneeva, S.A.; Shiretorova, V.G.; Radnaeva, L.D. Distinctive features of heavy metals’ accumulation in coniferous trees in Buryatia, Russia. IOP Conf. Ser. Earth Environ. Sci. 2019, 320, 012021. [Google Scholar] [CrossRef]
- Zeiner, M.; Juranović Cindrić, I. Accumulation of Major, Minor and Trace Elements in Pine Needles (Pinus nigra) in Vienna (Austria). Molecules 2021, 26, 3318. [Google Scholar] [CrossRef]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The Critical Role of Zinc in Plants Facing the Drought Stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Kaur, H.; Garg, N. Zinc toxicity in plants: A review. Planta 2021, 253, 129. [Google Scholar] [CrossRef]
- Tatuśko-Krygier, N.; Diatta, J.; Chudzińska, E.; Waraczewska, Z.; Gawroński, D.; Youssef, N. Bioactive levels of Zn, Pb, Cu, Cd and Mg, Fe in pollution sensitive and tolerant Scots pines needles—Is survival mineral-dependent? Ecol. Indic. 2023, 146, 109751. [Google Scholar] [CrossRef]
- Pongrac, P.; Vogel-Mikuš, K.; Regvar, M.; Kaligarič, M.; Vavpetič, P.; Kelemen, M.; Grlj, N.; Shelef, O.; Golan-Goldhirsh, A.; Rachmilevitch, S.; et al. On the distribution and evaluation of Na, Mg and Cl in leaves of selected halophytes. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 306, 144–149. [Google Scholar] [CrossRef]
- Sulhan, O.F.; Sevik, H.; Isinkaralar, K. Assessment of Cr and Zn deposition on Picea pungens Engelm. in urban air of Ankara, Türkiye. Environ. Dev. Sustain. 2023, 25, 4365–4384. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Han, H.; Du, R.; Wang, X. Physiological and Molecular Mechanisms of Plant Responses to Copper Stress. Int. J. Mol. Sci. 2022, 23, 12950. [Google Scholar] [CrossRef]
- Aznar, J.C.; Richer-Laflèche, M.; Bégin, C.; Bégin, Y. Lead Exclusion and Copper Translocation in Black Spruce Needles. Water Air Soil Pollut. 2009, 203, 139–145. [Google Scholar] [CrossRef]
- Solgi, E.; Keramaty, M.; Solgi, M. Biomonitoring of airborne Cu, Pb, and Zn in an urban area employing a broad leaved and a conifer tree species. J. Geochem. Explor. 2020, 208, 106400. [Google Scholar] [CrossRef]
- Vladimirovna Afanasyeva, L.; Ayushievna Ayushina, T. Accumulation of heavy metals and biochemical responses in Siberian larch needles in urban area. Ecotoxicology 2019, 28, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, H.; Schulz-Dobrick, B.; Wedepohl, K. Terrestrial geochemistry of Cd, Bi, Tl, Pb, Zn and Rb. Geochim. Cosmochim. Acta 1980, 44, 1519–1533. [Google Scholar] [CrossRef]
- Dytłow, S.; Górka-Kostrubiec, B. Concentration of heavy metals in street dust: An implication of using different geochemical background data in estimating the level of heavy metal pollution. Environ. Geochem. Health 2021, 43, 521–535. [Google Scholar] [CrossRef]
- Aral, H.; Vecchio-Sadus, A. Toxicity of lithium to humans and the environment—A literature review. Ecotoxicol. Environ. Saf. 2008, 70, 349–356. [Google Scholar] [CrossRef]
- Rodushkin, I.; Pallavicini, N.; Engström, E.; Sörlin, D.; Öhlander, B.; Ingri, J.; Baxter, D.C. Assessment of the natural variability of B, Cd, Cu, Fe, Pb, Sr, Tl and Zn concentrations and isotopic compositions in leaves, needles and mushrooms using single sample digestion and two-column matrix separation. J. Anal. At. Spectrom. 2016, 31, 220–233. [Google Scholar] [CrossRef]
- Wyttenbach, A.; Tobler, L. The seasonal variation of 20 elements in 1st and 2nd year needles of Norway spruce, Picea abies (L.) Karst. Trees 1988, 2, 52–64. [Google Scholar] [CrossRef]
- Belykh, O.A.; Chuparina, E.V.; Mokryy, A.V. Elemental Composition of Needles of the Family Pinaceae in the Territory with Accumulated Environmental Damage, Southern Baikal Region. Russ. J. Gen. Chem. 2020, 90, 2622–2626. [Google Scholar] [CrossRef]
- Burger, A.; Lichtscheidl, I. Strontium in the environment: Review about reactions of plants towards stable and radioactive strontium isotopes. Sci. Total Environ. 2019, 653, 1458–1512. [Google Scholar] [CrossRef]
- Pongrac, P.; Baltrenaite, E.; Vavpetič, P.; Kelemen, M.; Kladnik, A.; Budič, B.; Vogel-Mikuš, K.; Regvar, M.; Baltrenas, P.; Pelicon, P. Tissue-specific element profiles in Scots pine (Pinus sylvestris L.) needles. Trees 2019, 33, 91–101. [Google Scholar] [CrossRef]
- Srivastava, D.; Tiwari, M.; Dutta, P.; Singh, P.; Chawda, K.; Kumari, M.; Chakrabarty, D. Chromium Stress in Plants: Toxicity, Tolerance and Phytoremediation. Sustainability 2021, 13, 4629. [Google Scholar] [CrossRef]
- Ali, S.; Mir, R.A.; Tyagi, A.; Manzar, N.; Kashyap, A.S.; Mushtaq, M.; Raina, A.; Park, S.; Sharma, S.; Mir, Z.A.; et al. Chromium Toxicity in Plants: Signaling, Mitigation, and Future Perspectives. Plants 2023, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, U.; Haider, F.U.; Ahmad, M.; Hussain, S.; Maqsood, M.F.; Ishfaq, M.; Shahzad, B.; Waqas, M.M.; Ali, B.; Tayyab, M.N.; et al. Chromium toxicity, speciation, and remediation strategies in soil-plant interface: A critical review. Front. Plant Sci. 2023, 13, 1081624. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Hassan, W.; Shah, A.N.; Anjum, S.A.; Cheema, S.A.; Ali, I. Lithium toxicity in plants: Reasons, mechanisms and remediation possibilities—A review. Plant Physiol. Biochem. 2016, 107, 104–115. [Google Scholar] [CrossRef]
- Leung, D.W.M.; Prendergast, P.J. Physiological Responses of Plants to Environmental Lithium Pollution. In Lithium and Nickel Contamination in Plants and the Environment; World Scientific Series on Advances in Environmental Pollution Management; World Scientific: Singapore, 2023; Volume 1, pp. 49–62. [Google Scholar]
- Kuloğlu, S.S.; Yalçin, E.; Çavuşoğlu, K.; Acar, A. Dose-dependent toxicity profile and genotoxicity mechanism of lithium carbonate. Sci. Rep. 2022, 12, 13504. [Google Scholar] [CrossRef]
- Neves, O.; Moreno, F.; Pinheiro, D.; Pinto, M.C.; Inácio, M. Soil low-density geochemical mapping of technology-critical elements (TCEs) and its environmental implications: The case of lithium in Portugal. Sci. Total Environ. 2024, 934, 173207. [Google Scholar] [CrossRef]
- Cetin, M.; Sevik, H.; Cobanoglu, O. Ca, Cu, and Li in washed and unwashed specimens of needles, bark, and branches of the blue spruce (Picea pungens) in the city of Ankara. Environ. Sci. Pollut. Res. 2020, 27, 21816–21825. [Google Scholar] [CrossRef]
- Shin, J.H.; Yu, J.; Wang, L.; Kim, J.; Koh, S.-M. Investigation of Spectral Variation of Pine Needles as an Indicator of Arsenic Content in Soils. Minerals 2019, 9, 498. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Xu, Z.-M.; Li, Q.-S.; Yang, P.; Ye, H.-J.; Chen, Z.-S.; Guo, S.-H.; Wang, L.-L.; He, B.-Y.; Zeng, E.Y. Impact of osmoregulation on the differences in Cd accumulation between two contrasting edible amaranth cultivars grown on Cd-polluted saline soils. Environ. Pollut. 2017, 224, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Bing, H.; Luo, Z.; Wang, Y.; Jin, L. Impacts of atmospheric particulate matter pollution on environmental biogeochemistry of trace metals in soil-plant system: A review. Environ. Pollut. 2019, 255, 113138. [Google Scholar] [CrossRef] [PubMed]
- Pietrzykowski, M.; Woś, B.; Haus, N. Scots pine needles macronutrient (N, P, K, CA, MG, and S) supply at different reclaimed mine soil substrates--as an indicator of the stability of developed forest ecosystems. Environ. Monit. Assess. 2013, 185, 7445–7457. [Google Scholar] [CrossRef] [PubMed]
- Schiop, S.T.; Al Hassan, M.; Sestras, A.F.; Boscaiu, M.; Sestras, R.E.; Vicente, O. Identification of Salt Stress Biomarkers in Romanian Carpathian Populations of Picea abies (L.) Karst. PLoS ONE 2015, 10, e0135419. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W. A Review of the Use of the Basic Cation Saturation Ratio and the “Ideal” Soil. Soil Sci. Soc. Am. J. 2007, 71, 259–265. [Google Scholar] [CrossRef]
- Moore, P.A., Jr.; Miller, D.M. Decreasing Phosphorus Solubility in Poultry Litter with Aluminum, Calcium, and Iron Amendments. J. Environ. Qual. 1994, 23, 325–330. [Google Scholar] [CrossRef]
- Ribeiro, C.; Madeira, M.; Araújo, M.C. Decomposition and nutrient release from leaf litter of Eucalyptus globulus grown under different water and nutrient regimes. For. Ecol. Manag. 2002, 171, 31–41. [Google Scholar] [CrossRef]
- Benito, B.; Haro, R.; Amtmann, A.; Cuin, T.A.; Dreyer, I. The twins K+ and Na+ in plants. J. Plant Physiol. 2014, 171, 723–731. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef]
- Jacek, O.; Peter, B.R.; Roma, Z.; Piotr, K.; Mark, G.T. Needle nutrients in geographically diverse Pinus sylvestris L. populations. Ann. For. Sci. 2002, 59, 1–18. [Google Scholar]
- Rodríguez-Ruiz, M.; Aparicio-Chacón, M.V.; Palma, J.M.; Corpas, F.J. Arsenate disrupts ion balance, sulfur and nitric oxide metabolisms in roots and leaves of pea (Pisum sativum L.) plants. Environ. Exp. Bot. 2019, 161, 143–156. [Google Scholar] [CrossRef]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M.; Natasha, N. Arsenic Uptake, Toxicity, Detoxification, and Speciation in Plants: Physiological, Biochemical, and Molecular Aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Yang, W.; Tan, B.; Peng, Y.; Huang, C.; Xu, Z.; Ni, X.; Yang, Y.; Zhou, W.; Zhang, L.; et al. Immobilization of heavy metals during aquatic and terrestrial litter decomposition in an alpine forest. Chemosphere 2019, 216, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Pogge von Strandmann, P.A.E.; Burton, K.W.; Opfergelt, S.; Genson, B.; Guicharnaud, R.A.; Gislason, S.R. The lithium isotope response to the variable weathering of soils in Iceland. Geochim. Cosmochim. Acta 2021, 313, 55–73. [Google Scholar] [CrossRef]
- Iordache, A.M.; Voica, C.; Roba, C.; Nechita, C. Evaluation of potential human health risks associated with Li and their relationship with Na, K, Mg, and Ca in Romania’s nationwide drinking water. Front. Public Health 2024, 12, 1456640. [Google Scholar] [CrossRef]
- Penniston-Dorland, S.; Liu, X.-M.; Rudnick, R.L. Lithium Isotope Geochemistry. Rev. Mineral. Geochem. 2017, 82, 165–217. [Google Scholar] [CrossRef]
- Pogge von Strandmann, P.A.E.; Fraser, W.T.; Hammond, S.J.; Tarbuck, G.; Wood, I.G.; Oelkers, E.H.; Murphy, M.J. Experimental determination of Li isotope behaviour during basalt weathering. Chem. Geol. 2019, 517, 34–43. [Google Scholar] [CrossRef]
- von Strandmann, P.A.E.P.; Kasemann, S.A.; Wimpenny, J.B. Lithium and Lithium Isotopes in Earth’s Surface Cycles. Elements 2020, 16, 253–258. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.-M.; Chadwick, O.A. Lithium isotope behavior in Hawaiian regoliths: Soil-atmosphere-biosphere exchanges. Geochim. Cosmochim. Acta 2020, 285, 175–192. [Google Scholar] [CrossRef]
- Kang, H.; Liu, X.; Guo, J.; Wang, B.; Xu, G.; Wu, G.; Kang, S.; Huang, J. Characterization of mercury concentration from soils to needle and tree rings of Schrenk spruce (Picea schrenkiana) of the middle Tianshan Mountains, northwestern China. Ecol. Indic. 2019, 104, 24–31. [Google Scholar] [CrossRef]
- Erdem, R.; Aricak, B.; Cetin, M.; Sevik, H. Change in some heavy metal concentrations in forest trees by species, organ, and soil depth. Forestist 2023, 73, 257–263. [Google Scholar] [CrossRef]
- Çomaklı, E.; Bingöl, M.S. Heavy metal accumulation of urban Scots pine (Pinus sylvestris L.) plantation. Environ. Monit. Assess. 2021, 193, 192. [Google Scholar] [CrossRef]
- Isinkaralar, K.; Koç, İ.; Kuzmina, N.A.; Menshchikov, S.L.; Erdem, R.; Aricak, B. Determination of heavy metal levels using Betula pendula Roth. under various soil contamination in Southern Urals, Russia. Int. J. Environ. Sci. Technol. 2022, 19, 12593–12604. [Google Scholar] [CrossRef]
- Montoya, O.L.Q.; Niño-Ruiz, E.D.; Pinel, N. On the mathematical modelling and data assimilation for air pollution assessment in the Tropical Andes. Environ. Sci. Pollut. Res. 2020, 27, 35993–36012. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dong, W.; Zhao, Z.; Wang, H.; Li, W.; Chen, G.; Wang, F.; Zhao, Y.; Huang, J.; Zhou, T. Heavy metal pollution in urban river sediment of different urban functional areas and its influence on microbial community structure. Sci. Total Environ. 2021, 778, 146383. [Google Scholar] [CrossRef]
- Ma, S.; Qiao, L.; Liu, X.; Zhang, S.; Zhang, L.; Qiu, Z.; Yu, C. Microbial community succession in soils under long-term heavy metal stress from community diversity-structure to KEGG function pathways. Environ. Res. 2022, 214, 113822. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Liu, P.; Sun, Y.; Song, Z.; Hu, X. Characteristics of bacterial community structure and function associated with nutrients and heavy metals in coastal aquaculture area. Environ. Pollut. 2021, 275, 116639. [Google Scholar] [CrossRef]
- Iordache, A.M.; Nechita, C.; Zgavarogea, R.; Voica, C.; Varlam, M.; Ionete, R.E. Accumulation and ecotoxicological risk assessment of heavy metals in surface sediments of the Olt River, Romania. Sci. Rep. 2022, 12, 880. [Google Scholar] [CrossRef]
- Harja, M.; Ciocinta, R.C.; Ondrasek, G.; Bucur, D.; Dirja, M. Accumulation of Heavy Metal Ions from Urban Soil in Spontaneous Flora. Water 2023, 15, 768. [Google Scholar] [CrossRef]
- Hoaghia, M.-A.; Cadar, O.; Moisa, C.; Roman, C.; Kovacs, E. Heavy metals and health risk assessment in vegetables grown in the vicinity of a former non-metallic facility located in Romania. Environ. Sci. Pollut. Res. 2022, 29, 40079–40093. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST). Certificate of Analysis SRM 987 Strontium Isotopic Standard. Available online: https://www-s.nist.gov/srmors/certificates/987.pdf (accessed on 5 October 2024).
- National Institute of Standards and Technology (NIST). Certificate of Analysis SRM 981 Common Lead Isotopic Standard. Available online: https://www-s.nist.gov/srmors/certificates/981.pdf (accessed on 5 October 2024).
- Van Rossum, G.; Drake, F.L. Python 3 Reference Manual: (Python Documentation Manual Part 2). (CreateSpace Independent Publishing Platform, 2009); Centrum voor Wiskunde en Informatica: Amsterdam, The Netherlands, 1995; Volume 111. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. Geo J. 1969, 2, 108–118. [Google Scholar]
- Hans Wedepohl, K. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresunters 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
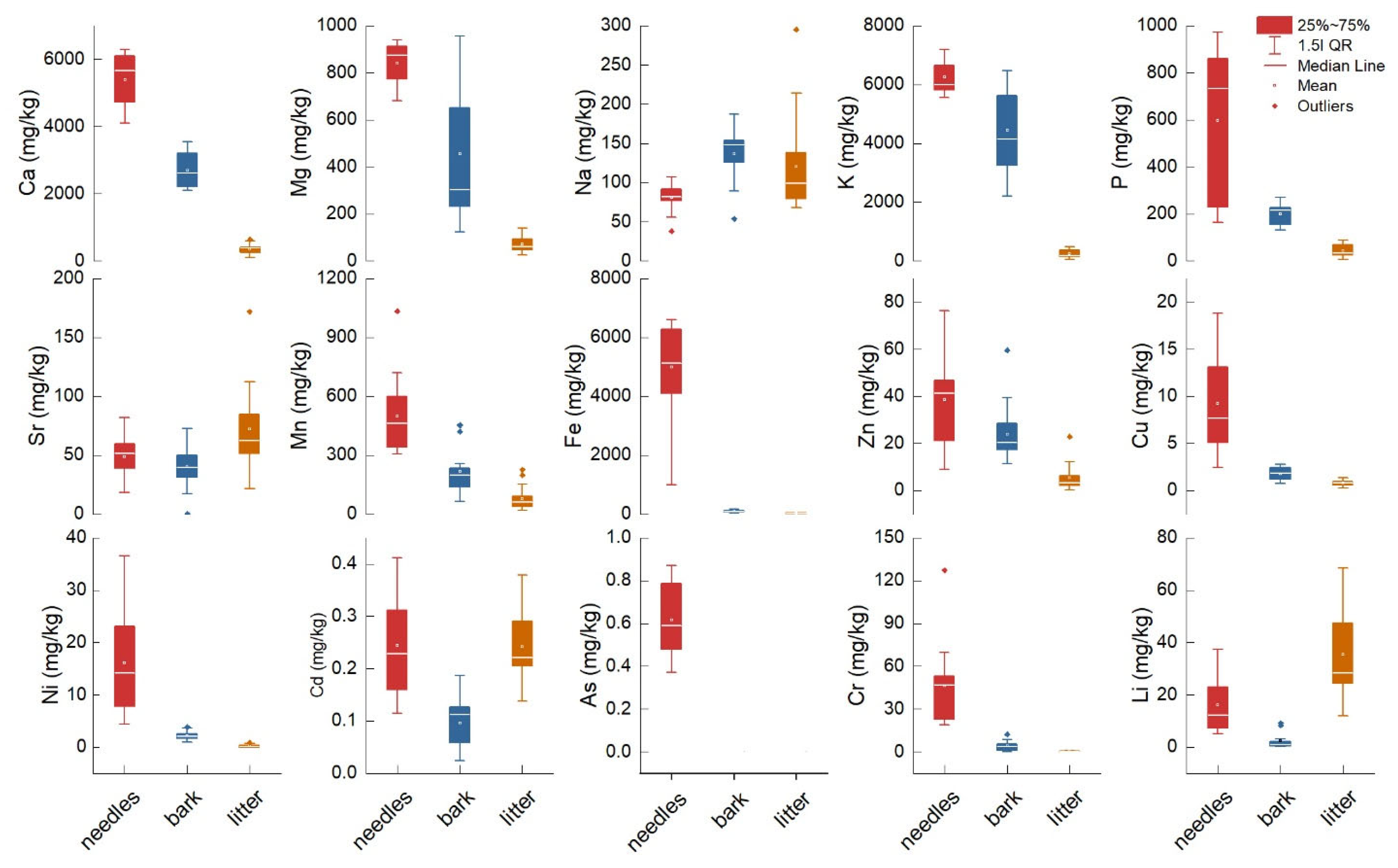
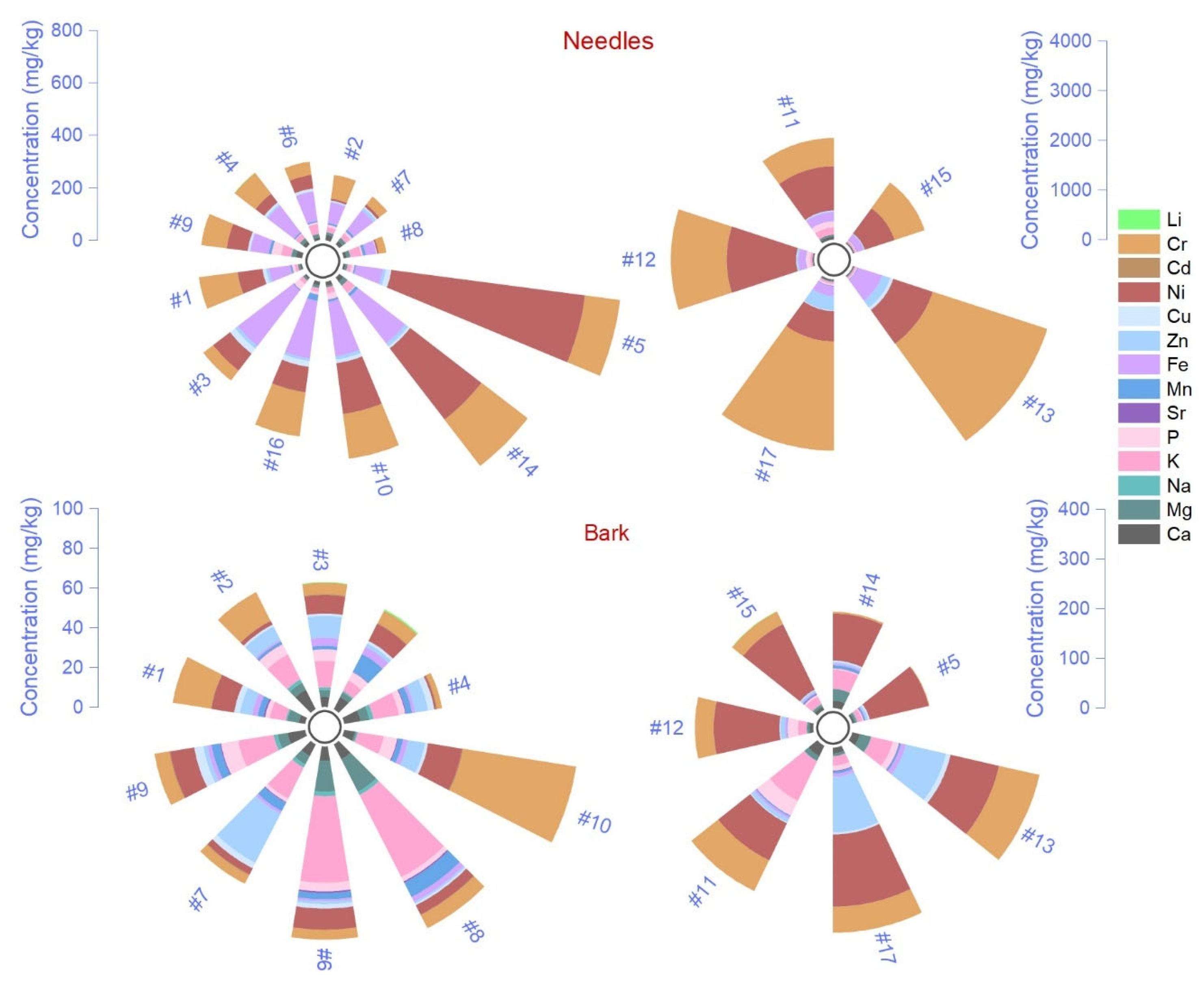
| Element | Tissue or Component | Bonferroni Test | Levene’s Test | RMSE | ||||
|---|---|---|---|---|---|---|---|---|
| Needles | Bark | Litter | Bark vs. Needles t-Value | Litter vs. Needles t-Value | Litter vs. Bark t-Value | Prob > F | ||
| K | 6263 ± 133 (A) | 4448 ± 339 (B) | 250 ± 30 (C) | −6.06 *** | −20.11 *** | −14.04 *** | <0.0001 | 871 |
| Ca | 5391 ± 182 (A) | 2692 ± 121 (B) | 359 ± 36 (C) | −14.87 *** | −27.73 *** | −12.86 *** | <0.0001 | 528 |
| Mg | 840 ± 21 (A) | 454 ± 71 (B) | 70 ± 8.5 (C) | −6.29 *** | −12.55 *** | −6.25 *** | <0.0001 | 178 |
| P | 597 ± 76 (A) | 199 ± 9.6 (B) | 44 ± 6.5 (B) | −6.34 *** | −8.79 *** | −2.45 | <0.0001 | 183 |
| Na | 81 ± 4.0 (B) | 136 ± 8.3 (A) | 120 ± 14 (A) | 3.91 ** | 2.74 * | −1.17 | 0.08 | 41 |
| Fe | 4991 ± 376 (A) | 94 ± 10 (B) | 37 ± 4.4 (B) | −15.90 *** | −16.09 *** | −0.18 | <0.0001 | 897 |
| Mn | 496 ± 48 (A) | 219 ± 28 (B) | 78 ± 14 (C) | −5.85 *** | −8.81 *** | −2.96 * | 0.001 | 138 |
| Zn | 38 ± 4.86 (A) | 23 ± 2.9 (B) | 5.2 ± 1.37 (C) | −3.09 ** | −6.98 *** | −3.88 *** | <0.0001 | 13 |
| Cu | 9.26 ± 1.1 (A) | 1.78 ± 0.16 (B) | 0.77 ± 0.07 (B) | −7.79 *** | −8.85 *** | −1.05 | <0.0001 | 2.7 |
| Sr | 48.79 ± 4.3 (B) | 39 ± 4.3 (B) | 72 ± 8.5 (A) | −1.03 | 2.74 * | 3.78 ** | 0.06 | 24 |
| Cr | 46 ± 6.4 (A) | 4.05 ± 0.82 (B) | 0.35 ± 0.07 (B) | −7.95 *** | −8.65 *** | −0.69 | <0.0001 | 15 |
| Ni | 16 ± 2.29 (A) | 2.22 ± 0.20 (B) | 0.23 ± 0.06 (B) | −7.40 *** | −8.45 *** | −1.05 | <0.0001 | 5.4 |
| Li | 16 ± 2.66 (B) | 2.3 ± 0.75 (C) | 35 ± 4.38 (A) | −3.24 ** | 4.58 *** | 7.82 *** | <0.0001 | 12 |
| As | 0.61 ± 0.04 (A) | — | — | — | — | — | — | — |
| Cd | 0.24 ± 0.02 (A) | 0.09 ± 0.01 (B) | 0.24 ± 0.01 (A) | −6.01 *** | −0.09 | 5.91 *** | 0.06 | 0.07 |
| Mole Ratio | Tissue or Component | Summary Statistics | One-Way ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | F-Value | Prob > F | ||
| Ca/Mg | litter | 5.34 | 1.26 | 2.78 | 7.68 | 9.57 | 0.004 |
| needles | 6.42 | 0.70 | 4.60 | 7.25 | |||
| Ca/Na | litter | 3.58 | 1.90 | 0.68 | 6.61 | 128.55 | <0.0001 |
| needles | 70.22 | 24.15 | 54.87 | 152.89 | |||
| Ca/P | litter | 10.82 | 6.40 | 2.45 | 28.67 | 0.80 | 0.37 |
| needles | 13.28 | 9.33 | 5.02 | 31.67 | |||
| Ca/K | litter | 1.58 | 0.58 | 0.89 | 3.03 | 25.83 | <0.0001 |
| needles | 0.85 | 0.07 | 0.71 | 0.96 | |||
| Na/Mg | litter | 0.09 | 0.01 | 0.04 | 0.12 | 33.73 | <0.0001 |
| needles | 2.07 | 1.40 | 0.81 | 5.72 | |||
| Mg/P | litter | 2.19 | 1.39 | 0.46 | 4.62 | 0.083 | 0.77 |
| needles | 2.05 | 1.43 | 0.87 | 4.83 | |||
| K/Na | litter | 2.42 | 1.37 | 0.31 | 4.55 | 189.96 | <0.0001 |
| needles | 81.40 | 23.58 | 60.44 | 157.68 | |||
| K/Mg | litter | 3.60 | 1.06 | 1.80 | 5.77 | 181.89 | <0.0001 |
| needles | 7.48 | 0.53 | 6.45 | 8.21 | |||
| Li/Ca | litter | 0.13 | 0.16 | 0.02 | 0.72 | 11.76 | 0.001 |
| needles | 0.002 | 0.002 | 0.00 | 0.008 | |||
| Li/Na | litter | 0.35 | 0.21 | 0.07 | 0.82 | 4.57 | 0.04 |
| needles | 0.21 | 0.16 | 0.05 | 0.66 | |||
| Indices | Interval of Values | Interpretation |
|---|---|---|
| BCF | ≤1 | plants only absorb metals |
| >1 | plants have the potential for accumulation | |
| Igeo | ≤1 | uncontaminated |
| 0 ≤ Igeo < 1 | uncontaminated to moderately contaminated | |
| 1 ≤ Igeo < 2 | moderately contaminated | |
| 2 ≤ Igeo < 3 | moderately to heavily contaminated | |
| 3 ≤ Igeo < 4 | heavily contaminated | |
| 4 ≤ Igeo < 5 | heavily to extremely contaminated | |
| Igeo ≥ 5 | extremely contaminated | |
| Cf | Cf < 1 | low contamination |
| 1 < Cf < 3 | moderate contamination | |
| 3 < Cf < 6 | considerable contamination | |
| Cf > 6 | very high contamination | |
| PLI | PLI < 1 | uncontaminated |
| 1 ≤ PLI < 2 | uncontaminated to moderately contaminated | |
| 2 ≤ PLI < 3 | moderately to strongly contaminated | |
| PLI ≥ 3 | strongly contaminated | |
| PINemerow | ≤0.7 | uncontaminated |
| 0.7–1 | danger range | |
| 1–2 | low contamination | |
| 2–3 | moderate contamination | |
| ≥3 | severe contamination | |
| Eri | Eri < 40 | low |
| 40 ≤ Eri < 80 | moderate | |
| 80 ≤ Eri < 160 | considerable | |
| 160 ≤ Eri < 320 | high | |
| 320 ≥ Eri | very high | |
| PERI | RI < 150 | moderate |
| 150 ≤ RI < 300 | considerable | |
| 300 ≤ RI < 600 | very high | |
| 600 ≥ RI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nechita, C.; Iordache, A.M.; Roba, C.; Sandru, C.; Zgavarogea, R.; Camarero, J.J. Heavy Metal Health Risk Assessment in Picea abies L. Forests Along an Altitudinal Gradient in Southern Romania. Plants 2025, 14, 968. https://doi.org/10.3390/plants14060968
Nechita C, Iordache AM, Roba C, Sandru C, Zgavarogea R, Camarero JJ. Heavy Metal Health Risk Assessment in Picea abies L. Forests Along an Altitudinal Gradient in Southern Romania. Plants. 2025; 14(6):968. https://doi.org/10.3390/plants14060968
Chicago/Turabian StyleNechita, Constantin, Andreea Maria Iordache, Carmen Roba, Claudia Sandru, Ramona Zgavarogea, and J. Julio Camarero. 2025. "Heavy Metal Health Risk Assessment in Picea abies L. Forests Along an Altitudinal Gradient in Southern Romania" Plants 14, no. 6: 968. https://doi.org/10.3390/plants14060968
APA StyleNechita, C., Iordache, A. M., Roba, C., Sandru, C., Zgavarogea, R., & Camarero, J. J. (2025). Heavy Metal Health Risk Assessment in Picea abies L. Forests Along an Altitudinal Gradient in Southern Romania. Plants, 14(6), 968. https://doi.org/10.3390/plants14060968








