Novel PCR-Based Detection Methods for the Lettuce Bacterial Leaf Spot Pathogen, Xanthomonas hortorum pv. vitians Morinière et al., 2020
Abstract
1. Introduction
2. Results
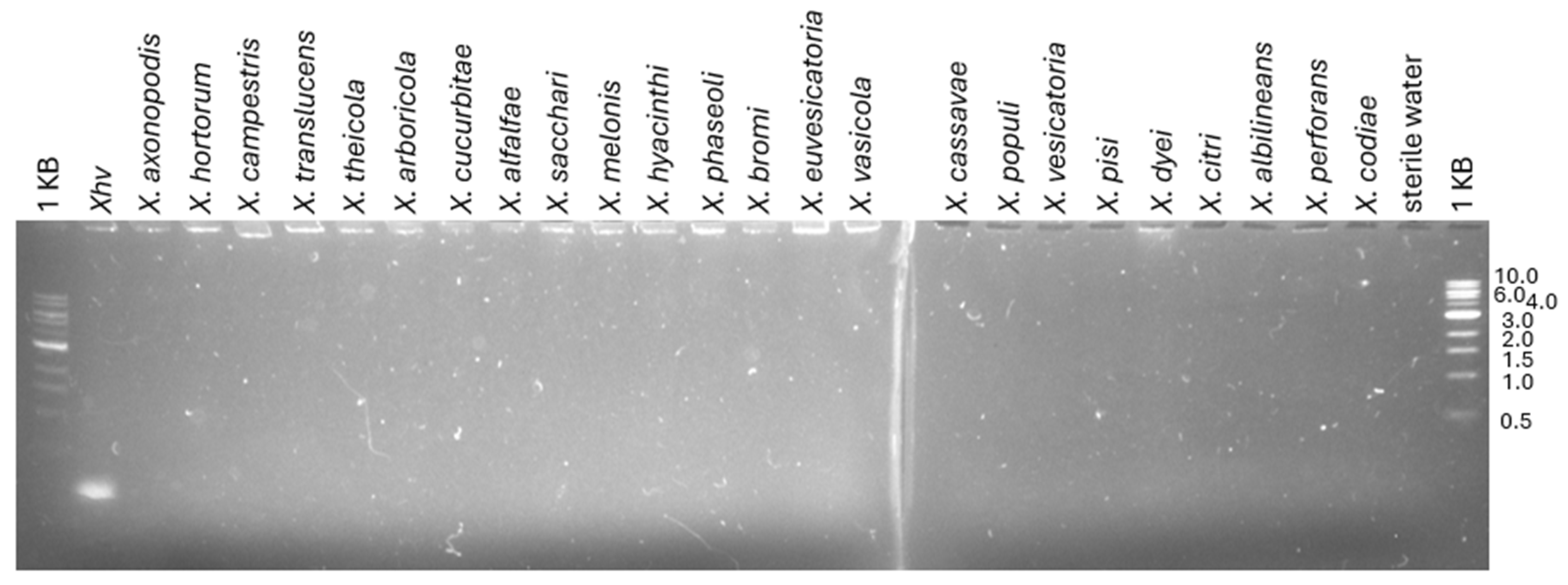
2.1. The Specificity of the Previously Published Detection Methods
2.2. A Pangenome Analysis Revealed Xhv Pathovar- and Race-Specific Gene Clusters
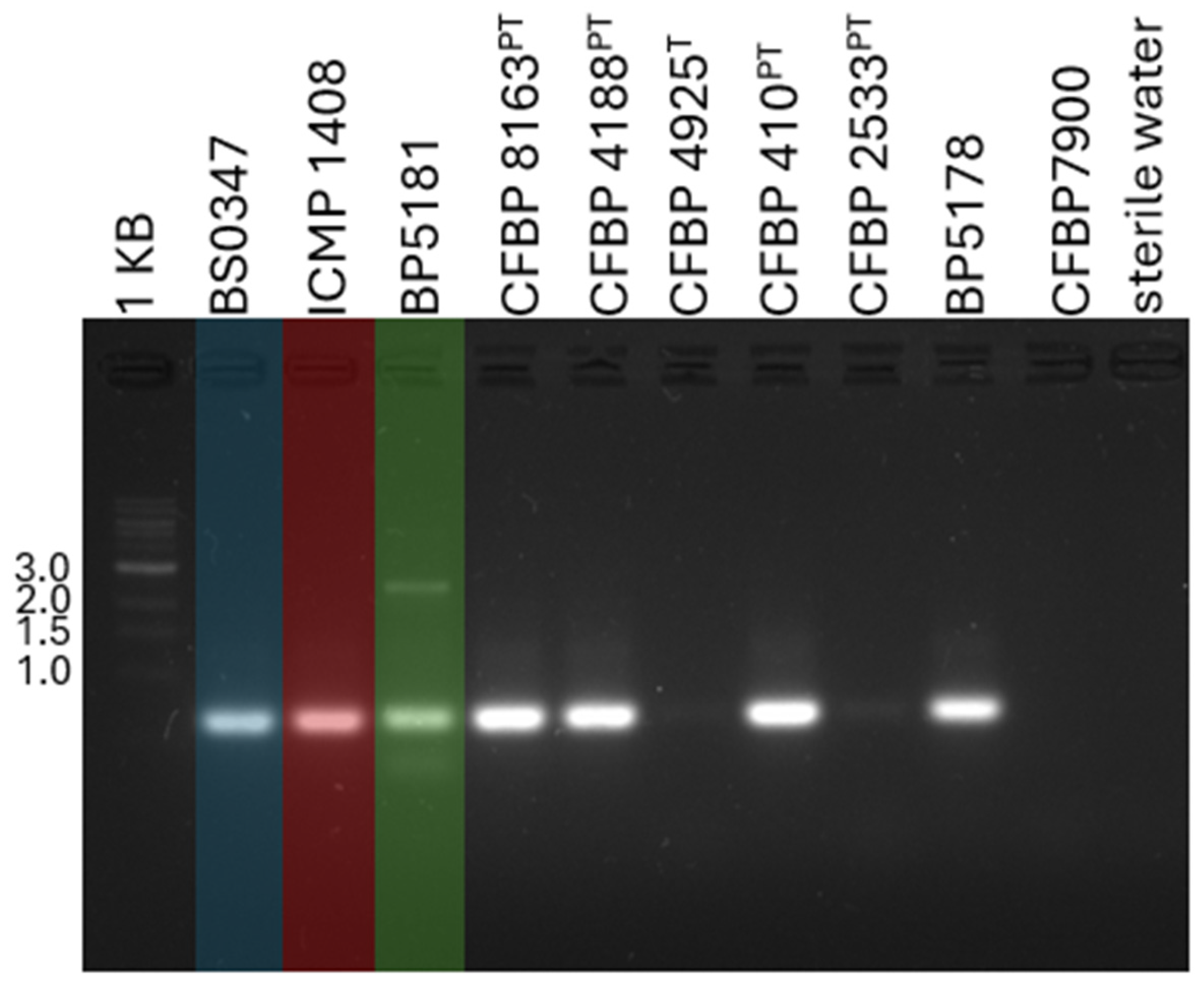
2.3. The Development of an Xhv-Specific Detection Method
2.4. Evaluating the Xhv-Specific Detection Method Using Environmental Samples
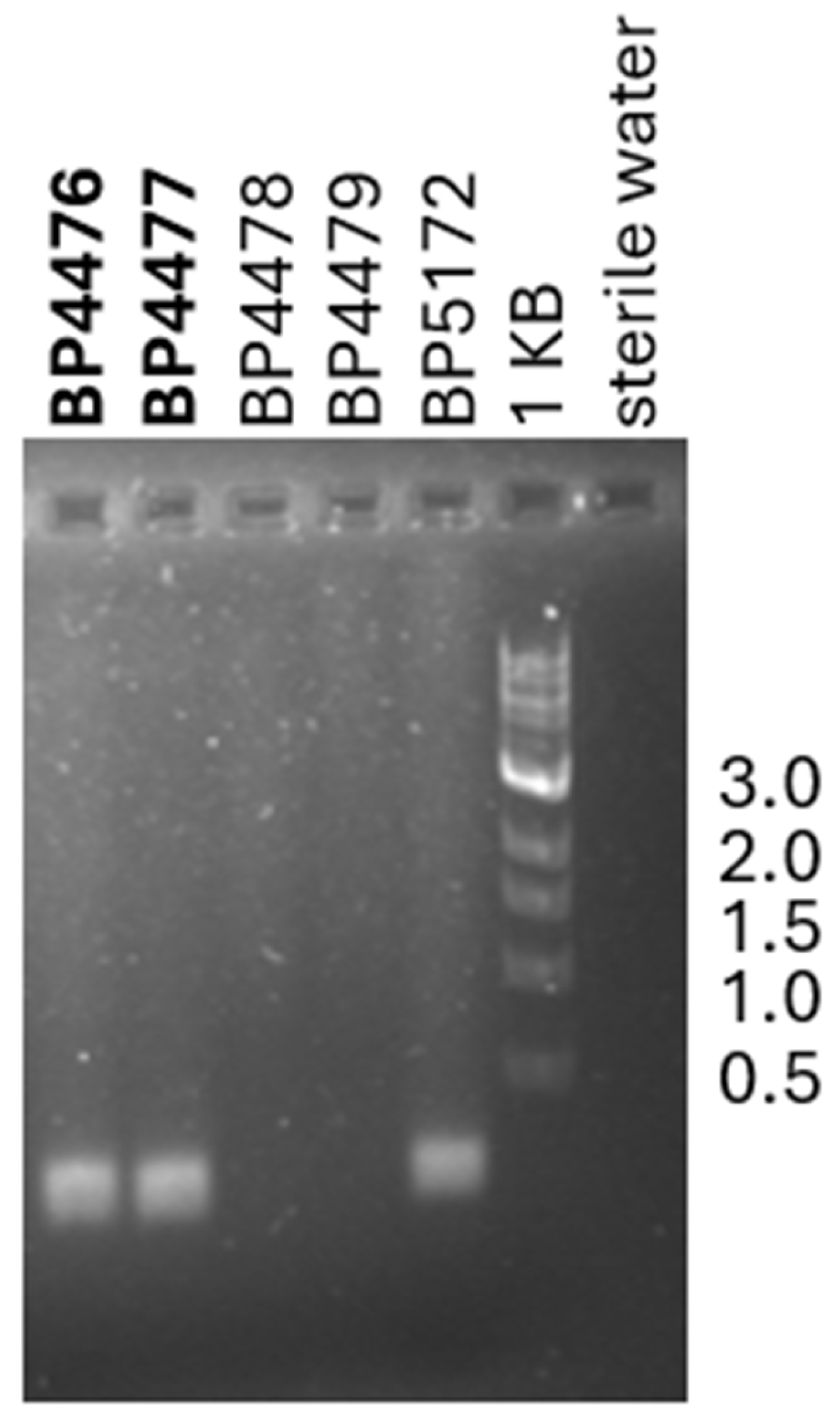
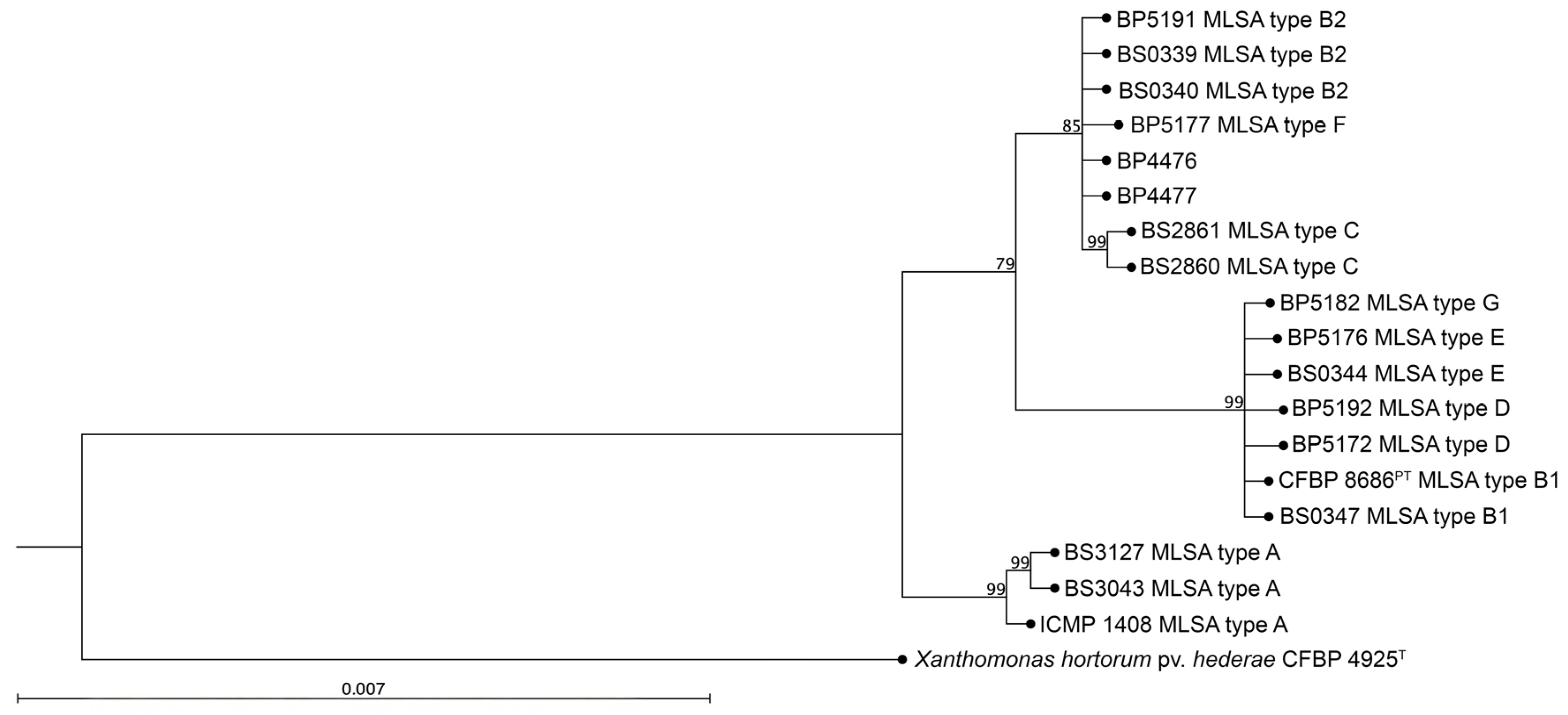
2.5. Progress Toward Xhv Race-Specific Detection
3. Discussion
4. Materials and Methods
4.1. Isolation, Culturing, and Storage of the Bacterial Strains
4.2. Evaluation of the B162 Primer Set
4.3. Genome Alignments and Target Selection
4.4. DNA Isolation and Sequencing
4.5. Primer Development
4.6. Touchdown PCR Development
4.7. The Multilocus Sequence Analysis of the PA Isolates
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Xhv | Xanthomonas hortorum pv. vitians |
| LAMP | Loop-mediated isothermal amplification |
| HR | Hypersensitive response |
| SNP | Single-nucleotide polymorphism |
| PCR | Polymerase chain reaction |
| DNA | Deoxyribonucleic acid |
| bp | Base pair |
| KB | Kilobase |
| NCBI | National Center for Biotechnology Information |
| BLAST | Basic local alignment search tool |
| ANI | Average nucleotide identity |
| gDDH | Genome-based DNA-DNA hybridization |
| NEB | New England Biolabs |
| MLSA | Multilocus sequence analysis |
References
- National Agricultural Statistics Service, U.S. Department of Agriculture. Quick Stats. Available online: https://quickstats.nass.usda.gov/results/3D366F3F-8B5A-3F99-8270-950C58D38DAA (accessed on 19 April 2024).
- Pernezny, K.; Raid, R.N.; Stall, R.E.; Hodge, N.; Collins, J. An outbreak of bacterial spot of lettuce in Florida caused by Xanthomonas campestris pv. vitians. Plant Dis. 1995, 79, 359–360. [Google Scholar] [CrossRef]
- Sahin, F.; Miller, S. Identification of the bacterial leaf spot pathogen of lettuce, Xanthomonas campestris pv. vitians, in Ohio, and assessment of cultivar resistance and seed treatment. Plant Dis. 1997, 81, 1443–1446. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.T.; Koike, S.T. Evaluating the efficacy of commercial products for management of bacterial leaf spot on lettuce. Plant Health Prog. 2005, 6, 3. [Google Scholar] [CrossRef]
- Carisse, O.; Ouimet, A.; Toussaint, V.; Philion, V. Evaluation of the effect of seed treatments, bactericides, and cultivars on bacterial leaf spot of lettuce caused by Xanthomonas campestris pv. vitians. Plant Dis. 2000, 84, 295–299. [Google Scholar] [CrossRef]
- Morinière, L.; Burlet, A.; Rosenthal, E.R.; Nesme, X.; Portier, P.; Bull, C.T.; Lavire, C.; Fischer-Le Saux, M.; Bertolla, F. Clarifying the taxonomy of the causal agent of bacterial leaf spot of lettuce through a polyphasic approach reveals that Xanthomonas cynarae Trébaol et al. 2000 emend. Timilsina et al. 2019 is a later heterotypic synonym of Xanthomonas hortorum Vauterin et al. 1995. Syst. Appl. Microbiol. 2020, 43, 126087. [Google Scholar]
- Barak, J.D.; Koike, S.T.; Gilbertson, R.L. Role of crop debris and weeds in the epidemiology of bacterial leaf spot of lettuce in California. Plant Dis. 2001, 85, 169–178. [Google Scholar] [CrossRef]
- Vauterin, L.; Hoste, B.; Kersters, K.; Swings, J. Reclassification of Xanthomonas. Int. J. Syst. Evol. Microbiol. 1995, 45, 472–489. [Google Scholar] [CrossRef]
- Dia, N.C.; Cottyn, B.; Blom, J.; Smits, T.H.; Pothier, J.F. Differentiation of the Xanthomonas hortorum–Xanthomonas hydrangeae species complex using sensitive and selective LAMP assays. Front. Agron. 2022, 4, 898778. [Google Scholar] [CrossRef]
- Bull, C.; Trent, M.; Hayes, R. Three races of Xanthomonas campestris pv. vitians causing bacterial leaf spot on lettuce identified. Phytopathology 2016, 106, 100. [Google Scholar]
- Sandoya Miranda, G.V.; Trent, M.; Hayes, R.J.; Lebeda, A.; Rosenthal, E.; Simko, I.; Bull, C.T. Differential Sources of Resistance from Lactuca serriola Against Three Races of Xanthomonas hortorum pathovar vitians (Brown, 1918) Morinière et al. 2020 Causing Bacterial Leaf Spot of Lettuce. Plant Dis. 2024. advanced online publication. [Google Scholar] [CrossRef]
- Rosenthal, E.; Potnis, N.; Bull, C.T. Comparative genomic analysis of the lettuce bacterial leaf spot pathogen, Xanthomonas hortorum pv. vitians, to investigate race specificity. Front. Microbiol. 2022, 13, 840311. [Google Scholar]
- Bradley, E.L.; Ökmen, B.; Doehlemann, G.; Henrissat, B.; Bradshaw, R.E.; Mesarich, C.H. Secreted glycoside hydrolase proteins as effectors and invasion patterns of plant-associated fungi and oomycetes. Front. Plant Sci. 2022, 13, 853106. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, N.; Cowie, C.; Heeney, J.; Stead, D. Phylogenetic structure of Xanthomonas determined by comparison of gyrB sequences. Int. J. Syst. Evol. Mirobiol. 2009, 59, 264–274. [Google Scholar] [CrossRef]
- Tambong, J.T.; Xu, R.; Cuppels, D.; Chapados, J.; Gerdis, S.; Eyres, J.; Koziol, A.; Dettman, J. Whole-genome resources and species-level taxonomic validation of 89 plant-pathogenic Xanthomonas strains isolated from various host plants. Plant Dis. 2022, 106, 1558–1565. [Google Scholar] [CrossRef]
- Baldwin, W.S. Phase 0 of the Xenobiotic Response: Nuclear Receptors and Other Transcription Factors as a First Step in Protection from Xenobiotics. Nucl. Recept. Res. 2019, 6, 101447. [Google Scholar] [CrossRef]
- Zacaroni, A.; Koike, S.; De Souza, R.; Bull, C. Bacterial leaf spot of radicchio (Cichorium intybus) is caused by Xanthomonas hortorum. Plant Dis. 2012, 96, 1820. [Google Scholar] [CrossRef]
- Sahin, F.; Abbasi, P.; Ivey, M.L.; Zhang, J.; Miller, S. Diversity among strains of Xanthomonas campestris pv.vitians from lettuce. Phytopathology 2003, 93, 64–70. [Google Scholar] [CrossRef]
- Arnaud, G. Une maladie bactérienne du lierre (Hedera helix L.). [A bacterial disease of ivy (Hedera helix L.)]. CR Acad. Sci. 1920, 171, 121–122. [Google Scholar]
- Dye, D. The genus Xanthomonas Dowson 1939. In A Proposed Nomenclature and classification for Plant Pathogenic Bacteria. NZ J. Agric. Res. 1978, 21, 153–177. [Google Scholar]
- Niederhauser, J. A bacterial leaf spot and blight of the Russian Dandelion. Phytopathology 1943, 33, 959–961. [Google Scholar]
- Brown, N.A. Bacterial leaf spot of Geranium in the eastern United States. J. Agric. Res. 1923, 23, 361–372. [Google Scholar]
- Jones, J.B.; Lacy, G.H.; Bouzar, H.; Stall, R.E.; Schaad, N.W. Reclassification of the anthomonads associated with bacterial spot disease of tomato and pepper. Syst. Appl. Microbiol. 2004, 27, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Šutic, D. Bakterioze crvenog patlidzana [tomato bacteriosis]. Posebna Izdanja Institut za Zastitu Bilja Beograd [Spec. Ed. Inst. Plant Prot. Belgrade] 1957, 6, 1–65. [Google Scholar]
- Trébaol, G.; Gardan, L.; Manceau, C.; Tanguy, J.; Tirilly, Y.; Boury, S. Genomic and phenotypic characterization of Xanthomonas cynarae sp. nov., a new species that causes bacterial bract spot of artichoke (Cynara scolymus L.). Int. J. Syst. Evol. Mirobiol. 2000, 50, 1471–1478. [Google Scholar] [CrossRef]
- Kendrick, J.B. Bacterial blight of carrot. J. Agric. Res. 1934, 49, 493–510. [Google Scholar]
- Srinivasan, M.C.; Patel, M.K.; Thirumalachar, M.J. A bacterial blight disease of coriander. Proc. Indian Natl. Sci. Acad. B 1961, 53, 298–301. [Google Scholar] [CrossRef]
- Dia, N.C.; Van Vaerenbergh, J.; Van Malderghem, C.; Blom, J.; Smits, T.H.; Cottyn, B.; Pothier, J.F. Xanthomonas hydrangeae sp. nov., a novel plant pathogen isolated from Hydrangea arborescens. Int. J. Syst. Evol. Microbiol. 2021, 71, 005163. [Google Scholar] [CrossRef]
- Starr, M.; Garces, O.; Rejuela, C. El agente causante de la gomosis bacterial del pasto imperial en Colombia. [The causal agent of bacterial gummosis of the imperial pasture grass in Colombia]. Rev. Fac. Nac. Agron. Medellin. 1950, 12, 73–83. [Google Scholar]
- Dowson, W. On the systematic position and generic names of the gram-negative bacterial plant pathogens. Trans. Br. Mycol. Soc. 1939, 26, 4–14. [Google Scholar] [CrossRef]
- Pammel, L.H. Notes on the flora of western Iowa. Proc. Iowa Acad. Sci. 1895, 3, 106–135. [Google Scholar]
- Jones, L.; Johnson, A.; Reddy, C. A bacterial disease of barley. J. Agric. Res. 1917, 11, 625–644. [Google Scholar]
- Pierce, N.B. Walnut bacteriosis. Bot. Gaz. 1901, 31, 272–273. [Google Scholar] [CrossRef]
- Bryan, M.K. Bacterial leaf spot of squash. J. Agric. Res. 1930, 40, 385. [Google Scholar]
- Riker, A.; Davis, G.C. Bacterial leaf spot of alfalfa. J. Agric. Res. 1935, 51, 177. [Google Scholar]
- Schaad, N.W.; Postnikova, E.; Lacy, G.H.; Sechler, A.; Agarkova, I.; Stromberg, P.E.; Stromberg, V.K.; Vidaver, A.K. Reclassification of Xanthomonas campestris pv. citri (ex Hasse 1915) Dye 1978 forms A, B/C/D, and E as X. smithii subsp. citri (ex Hasse) sp. nov. nom. rev. comb. nov., X. fuscans subsp. aurantifolii (ex Gabriel 1989) sp. nov. nom. rev. comb. nov., and X. alfalfae subsp. citrumelo (ex Riker and Jones) Gabriel et al., 1989 sp. nov. nom. rev. comb. nov.; X. campestris pv. malvacearum (ex Smith 1901) Dye 1978 as X. smithii subsp. smithii nov. comb. nov. nom. nov.; X. campestris pv. alfalfae (ex Riker and Jones, 1935) Dye 1978 as X. alfalfae subsp. alfalfae (ex Riker et al., 1935) sp. nov. nom. rev.; and “var. fuscans” of X. campestris pv. phaseoli (ex Smith, 1987) Dye 1978 as X. fuscans subsp. fuscans sp. nov. Syst. Appl. Microbiol. 2005, 28, 494–518. [Google Scholar]
- Wakker, J.H. Algemeene vereeniging voor bloombollencultuur. In Onderzoek der Ziekten van Hyacinthen en Andere bol- en Knolgewassen Gedurende de Jaren 1883, 1884 en 1885; Gedrukt voor de Leden der Algemeene Vereeniging voor Bloembollencultuur: Haarlem, The Netherlands, 1887. [Google Scholar]
- Gabriel, D.; Kingsley, M.; Hunter, J.; Gottwald, T. Reinstatement of Xanthomonas citri (ex Hasse) and X. phaseoli (ex Smith) to species and reclassification of all X. campestris pv. citri strains. Int. J. Syst. Evol. Microbiol. 1989, 39, 14–22. [Google Scholar] [CrossRef]
- Smith, E. Description of Bacillus phaseoli n. sp. Bot. Gaz. 1897, 24, 192. [Google Scholar]
- Elliott, C. Bacterial streak disease of Sorghums. J. Agric. Res. 1930, 40, 963–976. [Google Scholar]
- Wiehe, P.; Dowson, W. A bacterial disease of Cassava (Manihot utilissima) in Nyasaland. Emp. J. Exp. Agric. 1953, 21, 141–143. [Google Scholar]
- Ridé, M. Sur l’étiologie du chancre suintant du peuplier. [On the etiology of poplar weeping canker]. CR Acad. Sci. 1958, 246, 2795–2798. [Google Scholar]
- Ridé, M.; Ridé, S. Xanthomonas populi (ex Ridé 1958) sp. nov., nom. rev. Int. J. Syst. Evol. Microbiol. 1992, 42, 652–653. [Google Scholar] [CrossRef][Green Version]
- Doidge, E.M. A tomato canker. Ann. Appl. Biol. 1921, 7, 407–430. [Google Scholar] [CrossRef]
- Goto, M.; Okabe, N. Bacterial plant diseases in Japan IX. 1. Bacterial stem rot of Pea. 2. Halo blight of Bean. 3. Bacterial spot of Physalis plant. Rep. Fac. Agric. Shizuoka Univ. 1958, 8, 33–49. [Google Scholar]
- Young, J.; Wilkie, J.; Park, D.C.; Watson, D. New Zealand strains of plant pathogenic bacteria classified by multi-locus sequence analysis; proposal of Xanthomonas dyei sp. nov. Plant Pathol. 2010, 59, 270–281. [Google Scholar] [CrossRef]
- Ah-You, N.; Gagnevin, L.; Grimont, P.A.; Brisse, S.; Nesme, X.; Chiroleu, F.; Bui Thi Ngoc, L.; Jouen, E.; Lefeuvre, P.; Vernière, C. Polyphasic characterization of xanthomonads pathogenic to members of the Anacardiaceae and their relatedness to species of Xanthomonas. Int. J. Syst. Evol. Microbiol. 2009, 59, 306–318. [Google Scholar] [CrossRef]
- Hasse, C.H. Pseudomonas citri, the cause of citrus canker. J. Agric. Res. 1915, 4, 97–104. [Google Scholar]
- Ashby, S. Gumming disease of Sugar-cane. Trop. Agric. 1929, 6, 5. [Google Scholar]
- Dowson, W. On the generic names Pseudomonas, Xanthomonas and Bacterium for certain bacterial plant pathogens. Trans. Br. Mycol. Soc. 1943, 26, 4–14. [Google Scholar] [CrossRef]
- Skerman, V.B.D.; McGowan, V.; Sneath, P.H.A. Approved lists of bacterial names. Int. J. Syst. Evol. Microbiol. 1980, 30, 225–420. [Google Scholar] [CrossRef]
- Fayette, J.; Raid, R.; Roberts, P.D.; Jones, J.B.; Pernezny, K.; Bull, C.T.; Goss, E.M. Multilocus sequence typing of strains of bacterial spot of lettuce collected in the United States. Phytopathology 2016, 106, 1262–1269. [Google Scholar] [CrossRef]
- Eren, A.M.; Esen, Ö.C.; Quince, C.; Vineis, J.H.; Morrison, H.G.; Sogin, M.L.; Delmont, T.O. Anvi’o: An advanced analysis and visualization platform for ‘omics data. PeerJ 2015, 3, e1319. [Google Scholar] [CrossRef] [PubMed]
- Don, R.; Cox, P.; Wainwright, B.; Baker, K.; Mattick, J. Touchdown PCR to circumvent spurious priming during gene amplification. Nucl. Acids Res. 1991, 19, 4008. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Park, D.-C.; Shearman, H.; Fargier, E. A multilocus sequence analysis of the genus Xanthomonas. Syst. Appl. Microbiol. 2008, 31, 366–377. [Google Scholar] [CrossRef] [PubMed]
| Primer Set | Forward Sequence | Reverse Sequence | Amplicon Size (bp) | Target Strains |
|---|---|---|---|---|
| GC3906-152 | GTTCGGTCGCCATTTCGATG | AGATAACCTCCCAGACCGCT | 152 | Xhv |
| GC4021-112 | GGTGGCCTACTTTCATGCGA | GAGCAAGCCCTTCACAAGGT | 112 | Xhv race 1 |
| GC4381-178 | TATGATGCGGCACACAACCT | CGTATTGCGGTGCGAACTTT | 178 | Xhv race 2 |
| GC4980-138 | TCACTCAAAAGCCCACCCTC | ACATTCCTCGGCTATCCCCT | 138 | Xhv race 3 |
| Organism | Strain * | Other Strain IDs | Host of Isolation | Known or Hypothesized Race | GC3906-152 Detection | GC4021-112 Detection | GC4381-178 Detection | GC4980-138 Detection | Origin | Collector or Citation |
|---|---|---|---|---|---|---|---|---|---|---|
| X. hortorum pv. vitians | BP5172 ‡ | Xav 98-37 2/01 | Lactuca sativa | Xhv race 1 | Yes | No | No | No | Salinas, CA, USA | J. Barak |
| X. hortorum pv. vitians | BS0339 ‡ | Salinas 2/01 | Lactuca sativa | Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | J. Barak |
| X. hortorum pv. vitians | BS0340 ‡ | Xav 98-23 2/01 | Lactuca sativa | Xhv race 1 | Yes | No | No | No | Salinas, CA, USA | J. Barak |
| X. hortorum pv. vitians | BS0347 ‡ | Xcv 5/01 | Lactuca sativa | Xhv race 1 | Yes | Faint | No | No | Salinas, CA, USA | J. Barak |
| X. hortorum pv. vitians | BP5176 ‡ | Xcv 5/01 | Lactuca sativa | Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | J. Barak |
| X. hortorum pv. vitians | BP5177 ‡ | “Edge A” | Lactuca sativa | Xhv race 1 | Yes | No | No | No | CO, USA | S. Koike |
| X. hortorum pv. vitians | BP5179 ‡ | “Daniel Rom” | Lactuca sativa | Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | J. Barak |
| X. hortorum pv. vitians | BP5182 ‡ | “Moreno Let” | Lactuca sativa | Xhv race 1 | Yes | Yes | Yes | No | Santa Maria, CA, USA | J. Barak |
| X. hortorum pv. vitians | NCPPB 4058 ‡ | N/A | Lactuca sativa | Xhv race 1 | Yes | Yes | No | No | UK | H. Stanford |
| X. hortorum pv. vitians | CFBP 8686PT ‡ | LMG 938PT, NCPPB 2248PT, MR20213PT | Lactuca sativa | Xhv race 1 | Yes | Yes | No | No | Zimbabwe | [6,8] |
| X. hortorum pv. vitians | BP5191 ‡ | VT111 | Lactuca sativa | Xhv race 1 | Yes | No | No | No | Canada | V. Toussaint |
| X. hortorum pv. vitians | BP5192 ‡ | Xcv-2 | Lactuca sativa | Xhv race 1 | Yes | Yes | No | No | CA, USA | C. T. Bull |
| X. hortorum pv. vitians | ICMP 1408 ‡ | PDDCC 1408, Burkholder XL5 | Lactuca sativa | Xhv race 2 | Yes | No | Yes | No | Ithaca, NY, USA | W. H. Burkholder |
| X. hortorum pv. vitians | ICMP 4165 ‡ | LMG 7508, PDDCC 4165 | Lactuca sativa | Xhv race 2 | Yes | No | Yes | No | New Zealand | H. J. Boesewinkel |
| X. hortorum pv. vitians | BS3127 ‡ | VT106 | Lactuca sativa | Xhv race 2 | Yes | No | Yes | No | Canada | V. Toussaint |
| X. hortorum pv. vitians | BP5194 ‡ | 917 | Lactuca sativa | Xhv race 2 | Yes | No | Yes | No | OH, USA | [18] |
| X. hortorum pv. vitians | BS2861 ‡ | “Christy BuLet 2” | Lactuca sativa | Xhv race 3 | Yes | No | No | Yes | King City, CA, USA | S. Koike and Rianda |
| X. hortorum pv. vitians | BP5181 ‡ | “Christy BuLet 3” | Lactuca sativa | Xhv race 3 | Yes | No | No | Yes | King City, CA, USA | S. Koike and Rianda |
| X. hortorum pv. vitians | BS0313 | A674-2B (e1) | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | HI, USA | A. Alvarez |
| X. hortorum pv. vitians | BS0341 | C 5/20/01 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | J. Barak |
| X. hortorum pv. vitians | BS0342 | Xcv 5/20/01 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | J. Barak |
| X. hortorum pv. vitians | BS0343 | Xcv 5/01 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | J. Barak |
| X. hortorum pv. vitians | BS0345 | Xcv 5/01 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | J. Barak |
| X. hortorum pv. vitians | BS0346 | Xcv 5/01 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | J. Barak |
| X. hortorum pv. vitians | BS2849 | “Mike Lombard Reeves” | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS2850 | “Mike Lombard Harden” | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS2852 | “Daniel Rom” | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS2855 | “Frank Let” | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS2857 | “Frank Let” | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS2858 | John DeCarli Rom1-1 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS2859 | John DeCarli Rom1-2 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS2870 | “Moreno Let” | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Santa maria, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS2871 | “Keller Rom” | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS2872 | “Keller Rom” | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS2873 | “Greg greenleaf” | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS2874 | “Greg greenleaf” | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS2875 | “Greg redleaf” | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS2876 | “Greg let” | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS2908 | “Matt romaine” | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Watsonville, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS2994 | ICMP 6656, DAR 30547 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | New South Wales, Australia | R. Fitzell |
| X. hortorum pv. vitians | BS2996 | ICMP 6735, Watson B2578 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | Yes | No | Palmerston North, WI, New Zealand | D. R. W. Watson |
| X. hortorum pv. vitians | BS2997 | ICMP 7423, Watson J2928. | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Patumahoe, AK, New Zealand | D. R. W. Watson |
| X. hortorum pv. vitians | BS3050 | LMG 8688; ICMP 6461; Watson D2538 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | New Zealand | D. R. W. Watson |
| X. hortorum pv. vitians | BS3051 | LMG 8690; Hill AS3997; ICMP 6857 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | New Zealand | D. R. W. Watson |
| X. hortorum pv. vitians | BS3054 | CFBP 3980, Audusseau 11.72 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | Vaucluse, France | C. Audusseau |
| X. hortorum pv. vitians | BS3056 | CFBP 3996 Audusseau 17.09 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Isère, France | C. Audusseau |
| X. hortorum pv. vitians | BS3128 | 105 VT107 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Canada | V. Toussaint |
| X. hortorum pv. vitians | BS3131 | 119 VT41 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Canada | V. Toussaint |
| X. hortorum pv. vitians | BS3132‡ | B07-007, ID200707A | Lactuca sativa | Xhv race 1 | Yes | Yes | No | No | Canada | V. Toussaint |
| X. hortorum pv. vitians | BS3272 | L11 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | Yes | FL, USA | [2] |
| X. hortorum pv. vitians | BS3300 | Xcv-4 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | CA, USA | C. T. Bull |
| X. hortorum pv. vitians | BS3301 | Xcv-5 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | CA, USA | C. T. Bull |
| X. hortorum pv. vitians | BS3303 | Xcv-7 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | CA, USA | C. T. Bull |
| X. hortorum pv. vitians | BS3304 | Xcv-8 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | CA, USA | C. T. Bull |
| X. hortorum pv. vitians | BS3306 | Xcv-10 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | CA, USA | C. T. Bull |
| X. hortorum pv. vitians | BS0301 | 10S7-2 (a2) | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | HI, USA | A. Alvarez |
| X. hortorum pv. vitians | BS0302 | 10TB7 (a4) | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | HI, USA | A. Alvarez |
| X. hortorum pv. vitians | BS0304 | 10TB9 (a6) | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | HI, USA | A. Alvarez |
| X. hortorum pv. vitians | BS0305 | 10TB12-1 (a7) | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | HI, USA | A. Alvarez |
| X. hortorum pv. vitians | BS0309 | S4-1(K) (c5) | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | HI, USA | A. Alvarez |
| X. hortorum pv. vitians | BS0310 | S5-2 (c6) | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Faint | No | No | HI, USA | A. Alvarez |
| X. hortorum pv. vitians | BS0311 | S5-2(K) (c7) | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | HI, USA | A. Alvarez |
| X. hortorum pv. vitians | BS0314 | A674-4B (e2) | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | HI, USA | A. Alvarez |
| X. hortorum pv. vitians | BS0316 | 10TB9 (e4) | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | HI, USA | A. Alvarez |
| X. hortorum pv. vitians | BS0318 | QR71B (e8) | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | HI, USA | A. Alvarez |
| X. hortorum pv. vitians | BS0335 | Xav 98-05 2/01 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | Salinas, CA, USA | J. Barak |
| X. hortorum pv. vitians | BS0337 | Xav 98-67 2/01 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | J. Barak |
| X. hortorum pv. vitians | BS0338 | Xav 98-76 2/01 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | J. Barak |
| X. hortorum pv. vitians | BS0542 | 10S7-2 (a2) | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | Salinas, CA, USA | J. Barak |
| X. hortorum pv. vitians | BS0543 | “Spot A” | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | Salinas, CA, USA | J. Barak |
| X. hortorum pv. vitians | BS2946 | Julio Rodrigues Neto IBSBF 1553 Embrapa K 532 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | Brazil | I. M. G. Almeida |
| X. hortorum pv. vitians | BS2998 | ICMP 7465, IBSBF 325, C.F. Robbs: ENA2008 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | Brazil | C. F. Robbs |
| X. hortorum pv. vitians | BS3035 | NCPPB 970, ICPB XL 102, Thornberry 1-49 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | USA | H. H. Thornberry |
| X. hortorum pv. vitians | BS3036 | NCPPB 1839, ICPB XV169, Robbs ENA-250 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | Brazil | A. P. Viegas |
| X. hortorum pv. vitians | BS3041 | NCPPB 3663, Neto ISBF 473 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | J. R. Neto | |
| X. hortorum pv. vitians | BS3042 | NCPPB3931, Sellwood/Wilson A6520/1 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | The UK | J.E. Sellwood and J.K. Wilson |
| X. hortorum pv. vitians | BS3047 | LMG 7453; ATCC 11525; Burkholder XL3; CNBP 500; Dye YA3; ICMP 337; ICPB XL3; NCPPB 992; PDDCC 337 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | USA | H. Anderson |
| X. hortorum pv. vitians | BS3049 | LMG 7510; Fahy DAR30526; ICMP 6655; PDDCC 6655 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | Australia | P. Fahy |
| X. hortorum pv. vitians | BS3053 | CFBP 3973, Audusseau 11.08 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | C. Audusseau | |
| X. hortorum pv. vitians | BS3055 | CFBP 3983, Audusseau 14.28 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | Isère, France | C. Audusseau |
| X. hortorum pv. vitians | BS3130 | 118 VT25 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | V. Toussaint | |
| X. hortorum pv. vitians | BS3271 | L43 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | FL, USA | C. T. Bull |
| X. hortorum pv. vitians | BS3526 | B55 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | CA, USA | [18] |
| X. hortorum pv. vitians | BS3527 | B57 | Lactuca sativa | Hypothesized Xhv race 1 | Yes | No | No | No | CA, USA | [18] |
| X. hortorum pv. vitians | BS0344 ‡ | Xcv 5/01 | Lactuca sativa | Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | J. Barak |
| X. hortorum pv. vitians | BS2851 ‡ | “Mike Lombard Harden” | Lactuca sativa | Xhv race 1 | Yes | Yes | No | No | Salinas, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS2909 | “Matt romaine” | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Yes | No | No | Watsonville, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS3528 ‡ | B59 | Lactuca sativa | Xhv race 1 | Yes | Yes | No | No | CA, USA | [18] |
| X. hortorum pv. vitians | BS3034 ‡ | NCPPB 2969, ICPB XL6 | Lactuca sativa | Xhv race 2 | Yes | No | Yes | No | USA | W. H. Burkholder |
| X. hortorum pv. vitians | BS3043 ‡ | NCPPB 4033, Sahin/Miller 700a | Lactuca sativa | Xhv race 2 | Yes | No | Yes | No | USA | F. Sahin |
| X. hortorum pv. vitians | BS3126 ‡ | 99 VT101 | Lactuca sativa | Xhv race 2 | No | No | Yes | No | Isère, France | C. Audusseau |
| X. hortorum pv. vitians | BS3529 ‡ | 906 | Lactuca sativa | Xhv race 2 | Yes | No | Yes | No | OH, USA | S. Miller |
| X. hortorum pv. vitians | BS3531 ‡ | 923 | Lactuca sativa | Xhv race 2 | Yes | No | No | No | OH, USA | [18] |
| X. hortorum pv. vitians | BS3532 ‡ | 924 | Lactuca sativa | Xhv race 2 | Yes | No | No | No | OH, USA | [18] |
| X. hortorum pv. vitians | BS2860 | Christy BuLet 1 | Lactuca sativa | Hypothesized Xhv race 3 | Yes | No | No | Yes | King City, CA, USA | S. Koike |
| X. hortorum pv. vitians | BS2863 | Christy BuLet 4 | Lactuca sativa | Hypothesized Xhv race 3 | Yes | No | No | Yes | King City, CA, USA | S. Koike |
| X. hortorum pv. vitians | BP4476 | N/A | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Not tested | Not tested | Not tested | PA, USA | This study |
| X. hortorum pv. vitians | BP4477 | N/A | Lactuca sativa | Hypothesized Xhv race 1 | Yes | Not tested | Not tested | Not tested | PA, USA | This study |
| Pseudomonas viridiflava | BP4478 | N/A | Lactuca sativa | N/A | No | Not tested | Not tested | Not tested | PA, USA | This study |
| Pseudomonas allivorans | BP4479 | N/A | Lactuca sativa | N/A | No | Not tested | Not tested | Not tested | PA, USA | This study |
| X. hortorum | BP5178 | N/A | Cichorium intybus (radicchio) | N/A | No | No | No | No | Salinas, CA, USA | [17] |
| X. hortorum pv. hederae | CFBP 4925T | ICMP 453T, NCPPB 939T, LMG 733T | Hedera helix (English ivy) | N/A | No | No | No | No | USA | [8,19,20] |
| X. hortorum pv. taraxaci | CFBP 410PT | ATCC 19318PT, NCPPB 940PT, LMG 870PT | Taraxacum kok-sahgyz (Russian dandelion) | N/A | No | No | No | No | Ithaca, NY, USA | [8,19,20,21] |
| X. hortorum pv. pelargonii | CFBP 2533PT | ICMP 4321PT, LMG 7314PT, NCPPB 2985PT | Pelargonium peltatum (pelargonium) | N/A | No | No | No | No | Auckland, New Zealand | [20,22] |
| X. hortorum pv. gardneri | CFBP 8163PT | LMG 962PT, ATCC19865PT, NCPPB 881PT, PDCC 1620PT | Solanum lycopersicum (tomato) | N/A | No | No | No | No | Yugoslavia | [23,24] |
| X. hortorum pv. cynarae | CFBP 4188PT | ICMP 16775PT | Cynara scolymus (artichoke) | N/A | No | No | No | No | France | [25] |
| X. hortorum pv. carotae | CFBP 7900 | M081 | Daucus carota (carrot) | N/A | No | No | No | Faint | Hungary | [8,20,26] |
| X. campestris pv. coriandri | CFBP 8452PT | LMG 687PT, ATCC 17996PT, ICMP 5725PT, NCPPB 1758PT, PDDCC 5725PT | Coriandrum sativum (coriander) | N/A | No | No | No | No | India | [8,20,27] |
| X. hydrangeae | LMG 31884T | Van Vaerenbergh gbbc963T; GBBC 2123T, CCOS 1956T | Hydrangea arborescens (smooth hydrangea) | N/A | No | Not tested | Not tested | Not tested | Flanders, Belgium | [28] |
| X. axonopodis | CFBP 4924T | ATCC 19312T, CFBP 2156T, ICMP 50T, ICPB Xa103T, LMG 538T, LMG 982T, NCPPB 457T | Axonopus scoparius (carpet grass) | N/A | No | Not tested | Not tested | Not tested | Colombia | [29] |
| X. campestris | CFBP 5251T | ATCC 33913T, CFBP 2350T, CFBP 5241T, DSM 3586T, ICMP 13T, LMG 568T, Labo 11405T, NCPPB 528T | Brassica oleracea var. gemmifera (Brussels sprout) | N/A | No | Not tested | Not tested | Not tested | The UK | [30,31] |
| X. translucens | CFBP 2054T | ATCC 19319T, ICMP 5752T, ICPB XT2T, LMG 876T, NCPPB 973T | Hordeum vulgare (barley) | N/A | No | Not tested | Not tested | Not tested | USA | [8,32] |
| X. theicola | CFBP 4691T | ICMP 6774T, LMG 8684T | Camellia sinensis (tea tree) | N/A | No | Not tested | Not tested | Not tested | Japan | [8] |
| X. arboricola | CFBP 2528T | ATCC 49083T, ICMP 35T, LMG 747T, NCPPB 411T | Juglans regia (walnut) | N/A | No | Not tested | Not tested | Not tested | New Zealand | [8,33] |
| X. cucurbitae | CFBP 2542T | ICMP 2299T, LMG 690T, NCPPB 2597T | Cucurbita maxima (squash) | N/A | No | Not tested | Not tested | Not tested | New Zealand | [8,34] |
| X. alfalfae | CFBP 7686T | ATCC 11765T, ICPB 10701T, ICPB XA 121T, LMG 495T | Medicago sativa (alfalfa) | N/A | No | Not tested | Not tested | Not tested | India | [35,36] |
| X. sacchari | CFBP 4641T | LMG 471T | Saccharum officinarum (sugarcane) | N/A | No | Not tested | Not tested | Not tested | Guadeloupe, France | [8] |
| X. melonis | CFBP 4644T | IBSBF 68T, ICMP 8682T, LMG 8670T, NCPPB 3434T | Cucumis melo (melon) | N/A | No | Not tested | Not tested | Not tested | Brazil | [8] |
| X. hyacinthi | CFBP 1156T | ATCC 19314T, ICMP 189T, LMG 739T, NCPPB 599T | Hyacinthus orientalis (hyacinth) | N/A | No | Not tested | Not tested | Not tested | Netherlands | [8,37] |
| X. phaseoli | CFBP 8462T | ATCC 49119T, LMG 29033T | Phaseolus vulgaris (common bean) | N/A | No | Not tested | Not tested | Not tested | NE, USA | [38,39] |
| X. bromi | CFBP 1976T | ICMP 12545T, LMG 947T | Bromus carinatus (bromegrass) | N/A | No | Not tested | Not tested | Not tested | France | [8] |
| X. euvesicatoria | CFBP 6864T | ATCC 11633T, DSM 19128T, ICMP 109T, ICMP 98T, NCPPB 2968T | Capsicum frutescens (wild chili pepper) | N/A | No | Not tested | Not tested | Not tested | USA | [23] |
| X. vasicola | CFBP 2543T | ICMP 3103T, LMG 736T, NCPPB 2417T | Sorghum vulgare (sorghum) | N/A | No | Not tested | Not tested | Not tested | New Zealand | [8,40] |
| X. cassavae | CFBP 4642T | ICMP 204T, LMG 673T, NCPPB 101T | Manihot esculenta (cassava) | N/A | No | Not tested | Not tested | Not tested | Malawi | [8,41] |
| X. populi | CFBP 1817T | ATCC 51165T, ICMP 5816T, ICPB XP 240T, LMG 5743T | Populus x canadensis cv. Regenerata (Canadian poplar) | N/A | No | Not tested | Not tested | Not tested | Noyon, Oise, France | [42,43] |
| X. vesicatoria | CFBP 2537T | ATCC 35937T, CFBP 4645T, ICMP 63T, LMG 911T, NCPPB 422T | Lycopersicon esculentum (tomato) | N/A | No | Not tested | Not tested | Not tested | New Zealand | [8,44] |
| X. pisi | CFBP 4643T | ATCC 35936T, ICMP 570T, ICMP 570T, LMG 847T, Labo 13356T, NCPPB 762T | Pisum sativum (pea) | N/A | No | Not tested | Not tested | Not tested | Japan | [8,45] |
| X. dyei | CFBP 7245T | DSM 110537T, ICMP 12167T, NCPPB 4446T | Metrosideros excelsa (New Zealand Christmas tree) | N/A | No | Not tested | Not tested | Not tested | Omahanui, Bay of Plenty, New Zealand | [46] |
| X. citri | CFBP 3369T | ATCC 49118T, LMG 9322T | Citrus aurantifolia (key lime) | N/A | No | Not tested | Not tested | Not tested | FL, USA | [38,47,48] |
| X. albilineans | CFBP 2523T | ATCC 33915T, ICMP 196T, LMG 494T, NCPPB 2969T | Saccharum officinarum (sugarcane) | N/A | No | Not tested | Not tested | Not tested | Fiji | [49,50,51] |
| X. perforans | CFBP 7293T | DSM 18975T, NCPPB 4321T | Solanum lycopersicum L. (tomato) | N/A | No | Not tested | Not tested | Not tested | FL, USA | [23] |
| X. codiae | CFBP 4690T | ICMP 9513T, LMG 8678T | Codiacum variegatum (croton) | N/A | No | Not tested | Not tested | Not tested | FL, USA | [8] |
| Step | Instruction | Purpose |
|---|---|---|
| 1 | 95 °C for 1 min | Taq polymerase activation |
| 2 * | 95 °C for 30 s | Denaturation |
| 3 * | 68 °C for 30 s, −1 °C every cycle | Annealing |
| 4 * | 72 °C for 30 s | Extension |
| 5 * | GOTO Step 2, 10 times | Cycling |
| 6 | 95 °C for 30 s | Denaturation |
| 7 | 58 °C for 30 s | Annealing |
| 8 | 72 °C for 30 s | Extension |
| 9 | GOTO Step 6, 23 times | Cycling |
| 10 | 72 °C for 5 min | Final extension |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, E.R.; Hamidizade, M.; Zacaroni, A.B.; Bull, C.T. Novel PCR-Based Detection Methods for the Lettuce Bacterial Leaf Spot Pathogen, Xanthomonas hortorum pv. vitians Morinière et al., 2020. Plants 2025, 14, 964. https://doi.org/10.3390/plants14060964
Martinez ER, Hamidizade M, Zacaroni AB, Bull CT. Novel PCR-Based Detection Methods for the Lettuce Bacterial Leaf Spot Pathogen, Xanthomonas hortorum pv. vitians Morinière et al., 2020. Plants. 2025; 14(6):964. https://doi.org/10.3390/plants14060964
Chicago/Turabian StyleMartinez, Emma R., Mozhde Hamidizade, Ana B. Zacaroni, and Carolee T. Bull. 2025. "Novel PCR-Based Detection Methods for the Lettuce Bacterial Leaf Spot Pathogen, Xanthomonas hortorum pv. vitians Morinière et al., 2020" Plants 14, no. 6: 964. https://doi.org/10.3390/plants14060964
APA StyleMartinez, E. R., Hamidizade, M., Zacaroni, A. B., & Bull, C. T. (2025). Novel PCR-Based Detection Methods for the Lettuce Bacterial Leaf Spot Pathogen, Xanthomonas hortorum pv. vitians Morinière et al., 2020. Plants, 14(6), 964. https://doi.org/10.3390/plants14060964





