Exploring the Anti-Chagas Activity of Zanthoxylum chiloperone’s Seedlings Through Metabolomics and Protein–Ligand Docking
Abstract
1. Introduction
2. Materials and Methods
2.1. Seedling Production
2.2. Plant Material
2.3. Extract Preparation
2.4. Metabolomic Study
2.4.1. Derivatization Procedure
2.4.2. GC × GC-TOFMS Method
2.5. Data Processing and Analysis
2.6. Biological Assays
2.6.1. Cytotoxic Activity
2.6.2. Trypanocidal Activity
2.7. Computational Methods
3. Results
3.1. Seedling Production and Extraction Work
3.2. Metabolomics Analysis
3.3. Results of Cytotoxicity and Anti-Trypanosoma cruzi Activity In Vitro
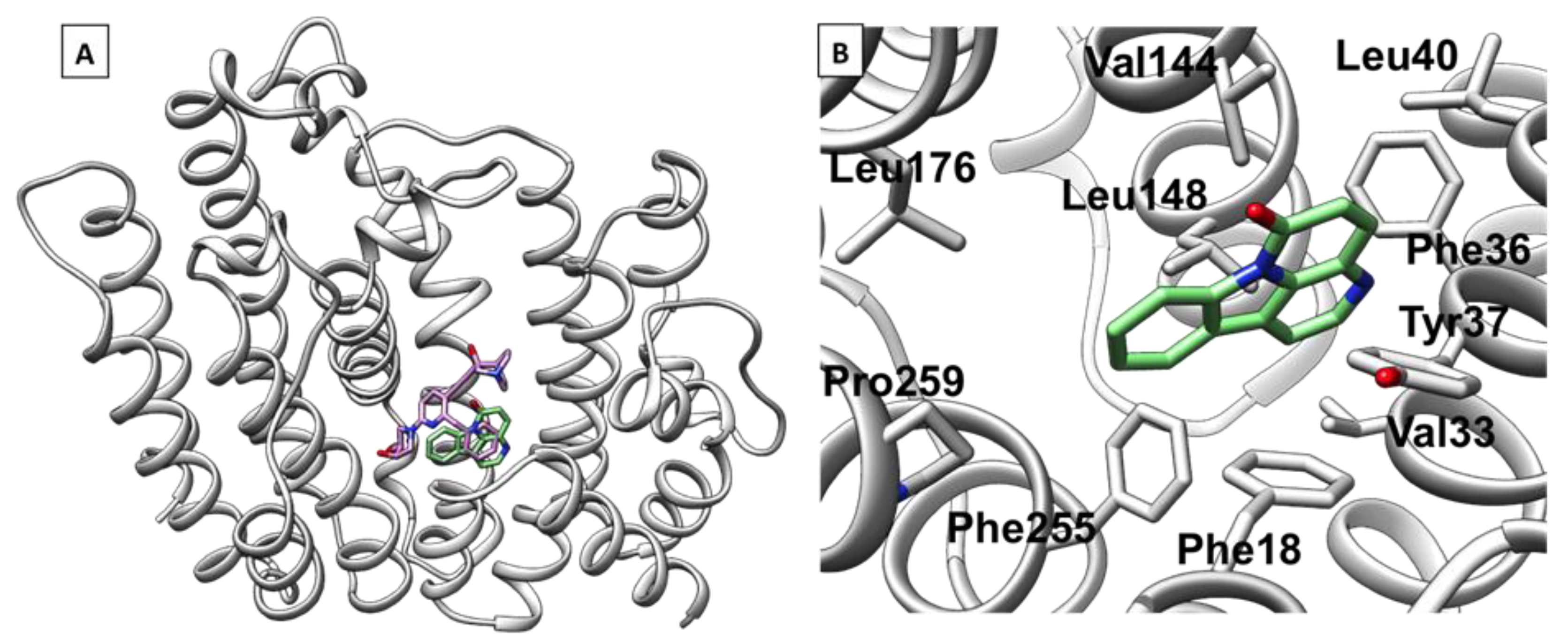
3.4. Computational Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Balb/mice | albino laboratory-bred strain house mice |
| CYP450 | cytochrome 450 |
| DMSO | dimethyl sulfoxide |
| GC | tgas chromatography |
| HPLC | high performance liquid chromatography |
| MPS | multi purpose sampler |
| NIST | National Institute of Science and Technology, USA |
| PDB | protein data bank |
| RPMI | Roswell Park Memorial Institute medium |
| Strain CL | clone Brener |
References
- Ribeiro, V.; Dias, N.; Paiva, T.; Hagström-Bex, L.; Nitz, N.; Pratesi, R.; Hecht, M. Current trends in the pharmacological management of Chagas disease. Int. J. Parasitol. Drug 2020, 12, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-K.; Leung, S.S.F.; Guilbert, C.; Jacobson, M.P.; McKerrow, J.H.; Podust, L.M. Structural characterization of CYP51 from Trypanosoma cruzi and Trypanosoma brucei bound to the antifungal drugs posaconazole and fluconazole. PLoS Negl. Trop. Dis. 2010, 4, e651. [Google Scholar] [CrossRef] [PubMed]
- Sales Junior, P.A.; Molina, I.; Fonseca Murta, S.M.; Sánchez-Montalvá, A.; Salvador, F.; Corrêa-Oliveira, R.; Carneiro, C.M. Experimental and clinical treatment of Chagas disease: A review. Am. J. Trop. Med. Hyg. 2017, 97, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Olivera, M.J.; Cucunubá, Z.M.; Valencia-Hernández, C.A.; Herazo, R.; Agreda-Rudenko, D.; Flórez, C.; Duque, S.; Nicholls, R.S. Risk factors for treatment interruption and severe adverse effects to benznidazole in adult patients with Chagas disease. PLoS ONE 2017, 12, e0185033. [Google Scholar] [CrossRef]
- Vermelho, A.B.; Rodrigues, G.C.; Supuran, C.T. Why hasn’t there been more progress in new Chagas disease drug discovery? Expert Opin. Drug Dis. 2020, 15, 145–158. [Google Scholar] [CrossRef]
- Ferreira, M.E.; Cebrián-Torrejón, G.; Corrales, A.S.; Vera De Bilbao, N.; Rolón, M.; Gomez, C.V.; Leblanc, K.; Yaluf, G.; Schinini, A.; Torres, S.; et al. Zanthoxylum chiloperone leaves extract: First sustainable Chagas disease treatment. J. Ethnopharmacol. 2011, 133, 986–993. [Google Scholar] [CrossRef]
- Porta, E.O.J.; Kalesh, K.; Steel, P.G. Navigating drug repurposing for Chagas disease: Advances challenges, and opportunities. Front. Pharmacol. 2023, 14, 1233253. [Google Scholar] [CrossRef]
- Freire, E.S.; da Silva, L.P.; Silva, A.D.; de Castro, P.A.S.V.; de Araujo, G.R.; Otta, D.A.; Braz, D.C.; Bezerra, J.M.T. New drugs and promising drugs combination in the treatment of Chagas Disease in Brazil: A systematic review and meta-analysis. Arch. Med. Res 2025, 56, 103084. [Google Scholar] [CrossRef]
- Hamid, A.; Mäser, P.; Mahmoud, A.B. Drug repurprosing in the chemotherapy of infectious diseases. Molecules 2024, 29, 635. [Google Scholar] [CrossRef]
- Torchelsen, F.K.V.D.; Mazetti, A.L.; Mosqueira, V.C.F. Drugs in preclinical and early clinical development for the treatment of Chagas ’s disease: The current status. Expert Opin. Investig. Drugs 2024, 33, 575–590. [Google Scholar] [CrossRef]
- Ferreira, M.E.; Nakayama, H.; De Arias, A.R.; Schinini, A.; De Bilbao, N.V.; Serna, E.; Lagoutte, D.; Soriano-Agatón, F.; Poupon, E.; Hocquemiller, R.; et al. Effects of canthin-6-one alkaloids from Zanthoxylum chiloperone on Trypanosoma cruzi-Infected Mice. J. Ethopharmacol. 2007, 109, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Agatón, F.; Lagoutte, D.; Poupon, E.; Roblot, F.; Fournet, A.; Gantier, J.-C.; Hocquemiller, R. Extraction, hemisynthesis, and synthesis of canthin-6-one analogues. Evaluation of their antifungal activities. J. Nat. Prod. 2005, 68, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Thouvenel, C.; Gantier, J.-C.; Duret, P.; Fourneau, C.; Hocquemiller, R.; Ferreira, M.-E.; De Arias, A.R.; Fournet, A. Antifungal Compounds from Zanthoxylum chiloperone var. angustifolium. Phytother. Res. 2003, 17, 678–680. [Google Scholar] [CrossRef]
- Rasooli, I. Bioactive Compounds in Phytomedicine; IntechOpen: London, UK, 2012; ISBN 978-953-307-805-2. [Google Scholar]
- Nooreen, Z.; Tandon, S.; Yadav, N.P.; Kumar, P.; Xuan, T.D.; Ahmad, A. Zanthoxylum: A review of its traditional uses, naturally occurring constituents and pharmacological properties. COC 2019, 23, 1307–1341. [Google Scholar] [CrossRef]
- Spichiger, R.; Bocquet, G.; Ramella, L.; Perret, P. Flora del Paraguay. 8: Angiospermae Rutaceae; Conservatoire et Jardin Botaniques: Genève, Switzerland, 1987; ISBN 978-2-8277-0510-8. [Google Scholar]
- Fournet, J. Flore Illustrée des Phanérogames de Guadeloupe et de Martinique; Nouv. éd. rev. et augm; CIRAD, Centre de coopération internationale en recherche agronomique pour le développement Gondwana éd: Montpellier, France, 2002; ISBN 978-2-87614-489-7. [Google Scholar]
- Farouil, L.; Sylvestre, M.; Fournet, A.; Cebrián-Torrejón, G. Review on canthin-6-one alkaloids: Distribution, chemical aspects and biological activities. Eur. J. Med. Chem. Rep. 2022, 5, 100049. [Google Scholar] [CrossRef]
- Cebrián-Torrejón, G.; Doménech-Carbó, A.; Figadère, B.; Poupon, E.; Fournet, A. Phytoelectrochemical analysis of Zanthoxylum Chiloperone: An electrochemical approach to phytochemical screening. Phytochem. Anal. 2017, 28, 171–175. [Google Scholar] [CrossRef]
- Farouil, L.; Dias, R.P.; Popotte-Julisson, G.; Bibian, G.; Adou, A.I.; De La Mata, A.P.; Sylvestre, M.; Harynuk, J.J.; Cebrián-Torrejón, G. The metabolomic profile of the essential oil from Zanthoxylum caribaeum (Syn chiloperone) growing in Guadeloupe FWI using GC × GC-TOFMS. Metabolites 2022, 12, 1293. [Google Scholar]
- Dias, R.P.; Johnson, T.A.; Ferrão, L.F.V.; Munoz, P.R.; De La Mata, A.P.; Harynuk, J.J. Improved sample storage, preparation and extraction of blueberry aroma volatile organic compounds for gas chromatography. J. Chromatogr. Open 2023, 3, 100075. [Google Scholar] [CrossRef]
- Tarazona Carrillo, K.; Béziat, N.S.; Cebrián-Torrejón, G.; Gros, O.; De La Mata, A.P.; Harynuk, J.J. Metabolomic analysis of secondary metabolites from Caribbean crab gills using comprehensive two-dimensional gas chromatography—Time-of-flight mass spectrometry—New inputs for a better understanding of symbiotic associations in crustaceans. J. Chromatogr. Open 2022, 2, 100069. [Google Scholar] [CrossRef]
- Stilo, F.; Tredici, G.; Bicchi, C.; Robbat, A.; Morimoto, J.; Cordero, C. Climate and processing effects on tea (Camellia sinensis L. Kuntze) Metabolome: Accurate profiling and fingerprinting by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. Molecules 2020, 25, 2447. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Z.; Ye, G.; Zhao, C.; Lu, X.; Xu, G. Non-targeted metabolomics study for the analysis of chemical compositions in three types of tea by using gas chromatography-mass spectrometry and liquid chromatography-mass spectrometry. Chin. J. Chromatogr. 2014, 32, 804. [Google Scholar] [CrossRef] [PubMed]
- Cebrián-Torrejón, G.; Kablan, L.; Ferreira, M.E.; Rodríguez De La Cruz, D.; Doménech-Carbó, A.; Vera De Bilbao, N.; Rojas De Arias, A.; Figadère, B.; Poupon, E.; Fournet, A. Harvesting canthinones: Identification of the optimal seasonal point of harvest of Zanthoxylum chiloperone leaves as a source of 5-methoxycanthin-6-one. Nat. Prod. Res. 2015, 29, 2054–2058. [Google Scholar] [CrossRef] [PubMed]
- Cordero, J.; Boshier, D.H. (Eds.) Árboles de Centroamérica: Un Manual para Extensionistas. CATIE: Turrialba, Costa Rica, 2003; ISBN 978-0-85074-161-2. [Google Scholar]
- Black, L.; Berenbaum, M.C. Factors Affecting the dye exclusion test for cell viability. Exp. Cell Res. 1964, 35, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Chiari, E.; Oliveira, A.B.; Prado, M.A.; Alves, R.J.; Galvão, L.M.; Araujo, F.G. Potential use of WR6026 as prophylaxis against transfusion-transmitted American trypanosomiasis. Antimicrob. Agents Chemother. 1996, 40, 613–615. [Google Scholar] [CrossRef]
- Rolón, M.; Seco, E.M.; Vega, C.; Nogal, J.J.; Escario, J.A.; Gómez-Barrio, A.; Malpartida, F. Selective activity of polyene macrolides produced by genetically modified Streptomyces on Trypanosoma cruzi. Int. J. Antimicrob. Agents. 2006, 28, 104–109. [Google Scholar] [CrossRef]
- Hamuy, R.; Acosta, N.; López, E.; Ferreira, M.; Vera de Bilbao, N. Determinación de la sensibilidad in vitro de diferentes cepas de Trypanosoma cruzi al benznidazol y al extracto de hoja de la planta Zanthoxylum chiloperone. Mem. Inst. Investig. Cienc. Salud 2013, 11, 16–25. [Google Scholar]
- Buckner, F.S.; Verlinde, C.L.; La Flamme, A.C.; Van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar] [CrossRef]
- Rodríguez-Guerra Pedregal, J.; Sciortino, G.; Guasp, J.; Municoy, M.; Maréchal, J.-D.; Gaudi, M.M. A modular multi-objective platform for molecular modeling. J. Comput. Chem. 2017, 38, 2118–2126. [Google Scholar] [CrossRef]
- Eldridge, M.D.; Murray, C.W.; Auton, T.R.; Paolini, G.V.; Mee, R.P. Empirical scoring functions: I. The development of a fast empirical scoring function to estimate the binding affinity of ligands in receptor complexes. J. Comput. Aid Mol. Des. 1997, 11, 425–445. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Nam, S.; de la Mata, A.P.; Harynuk, J. Automated screening and filtering scripts for GC×GC-TOFMS metabolomics data. Separations 2021, 8, 84. [Google Scholar] [CrossRef]
- Gillmor, S.A.; Craik, C.S.; Fletterick, R.J. Structural determinants of specificity in the cysteine protease cruzain. Protein Sci. 1997, 6, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Shang, N.; Li, Q.; Ko, T.-P.; Chan, H.-C.; Li, J.; Zheng, Y.; Huang, C.-H.; Ren, F.; Chen, C.-C.; Zhu, Z.; et al. Squalene synthase as a target for Chagas disease therapeutics. PLoS Pathog. 2014, 10, e1004114. [Google Scholar] [CrossRef] [PubMed]

| In Vitro Assays at Different Concentrations of Zanthoxylum chiloperone Against Trypomastigotes of Trypanosoma cruzi.* and Cytotoxicity in Peritoneal Murine Macrophages. | ||||||
|---|---|---|---|---|---|---|
| Concentrations of Extract # (µg/mL) | % of Lysis Trypomastigotes | % of Lysis Murine Macrophages | % of Lysis Trypomastigotes | % of Lysis Murine Macrophages | % of Lysis Trypomastigotes | % of Lysis Murine Macrophages |
| 12 Months Old IC50 ** = 119 ± 10 µg/mL | 18 Months Old IC50 ** = 141 ± 14 µg/mL | 24 Months Old IC50 ** = 71 ± 8 µg/mL | ||||
| 250 | 63 ± 5 | ND *** | 59 ± 7 | ND | 77 | ND |
| 100 | 54 ± 5 | 3 ± 1 | 48 ± 5 | 4 ± 1 | 59 ± 8 | 3 ± 1 |
| 50 | 27 ± 3 | 0 ± 1 | 30 ± 3 | 1 ± 1 | 36 ± 6 | 1 ± 1 |
| 25 | 0 ± 2 | ND | 0 ± 1 | ND | 23 ± 5 | ND |
| Canthinone Derivative | Cruzain Protease (PDB ID: 3KKU) | Dihydroorotate Dehydrogenase (PDB ID: 2DJX) | Lanosterol 14-Alpha-Demethylase (PDB ID: 2WX2) | Dihydrofolate Reductase-Thymidylate (PDB ID: 3IRM) | Farnesyl Diphosphate Synthase (PDB ID: 4E1E) | Squalene Synthase (PDB ID: 3WCC) |
|---|---|---|---|---|---|---|
| 1: canthin-6-one | 25.75 | 27.2 | 30.37 | 28.11 | 23.6 | 33.65 |
| 2: 5-methoxy-canthin-6-one | 23.82 | 25.38 | 29.1 | 28.77 | 24.27 | 32.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Bilbao, N.V.; Giebelhaus, R.T.; Dias, R.P.; Ferreira, M.E.; Martínez, M.; Velasco-Carneros, L.; Nam, S.L.; de la Mata, A.P.; Maréchal, J.-D.; Adou, A.I.; et al. Exploring the Anti-Chagas Activity of Zanthoxylum chiloperone’s Seedlings Through Metabolomics and Protein–Ligand Docking. Plants 2025, 14, 954. https://doi.org/10.3390/plants14060954
de Bilbao NV, Giebelhaus RT, Dias RP, Ferreira ME, Martínez M, Velasco-Carneros L, Nam SL, de la Mata AP, Maréchal J-D, Adou AI, et al. Exploring the Anti-Chagas Activity of Zanthoxylum chiloperone’s Seedlings Through Metabolomics and Protein–Ligand Docking. Plants. 2025; 14(6):954. https://doi.org/10.3390/plants14060954
Chicago/Turabian Stylede Bilbao, Ninfa Vera, Ryland T. Giebelhaus, Ryan P. Dias, Maria Elena Ferreira, Miguel Martínez, Lorea Velasco-Carneros, Seo Lin Nam, A. Paulina de la Mata, Jean-Didier Maréchal, Ahissan Innocent Adou, and et al. 2025. "Exploring the Anti-Chagas Activity of Zanthoxylum chiloperone’s Seedlings Through Metabolomics and Protein–Ligand Docking" Plants 14, no. 6: 954. https://doi.org/10.3390/plants14060954
APA Stylede Bilbao, N. V., Giebelhaus, R. T., Dias, R. P., Ferreira, M. E., Martínez, M., Velasco-Carneros, L., Nam, S. L., de la Mata, A. P., Maréchal, J.-D., Adou, A. I., Yaluff, G., Serna, E., Sylvestre, M., Torres, S., Schinini, A., Galeano, R., Fournet, A., Harynuk, J. J., & Cebrián-Torrejón, G. (2025). Exploring the Anti-Chagas Activity of Zanthoxylum chiloperone’s Seedlings Through Metabolomics and Protein–Ligand Docking. Plants, 14(6), 954. https://doi.org/10.3390/plants14060954












