Fig Seeds as a Novel Oil Source: Investigating Lipochemodiversity Through Fatty Acids Profiling and FTIR Spectral Fingerprints
Abstract
1. Introduction
2. Results and Discussion
2.1. Oil Content
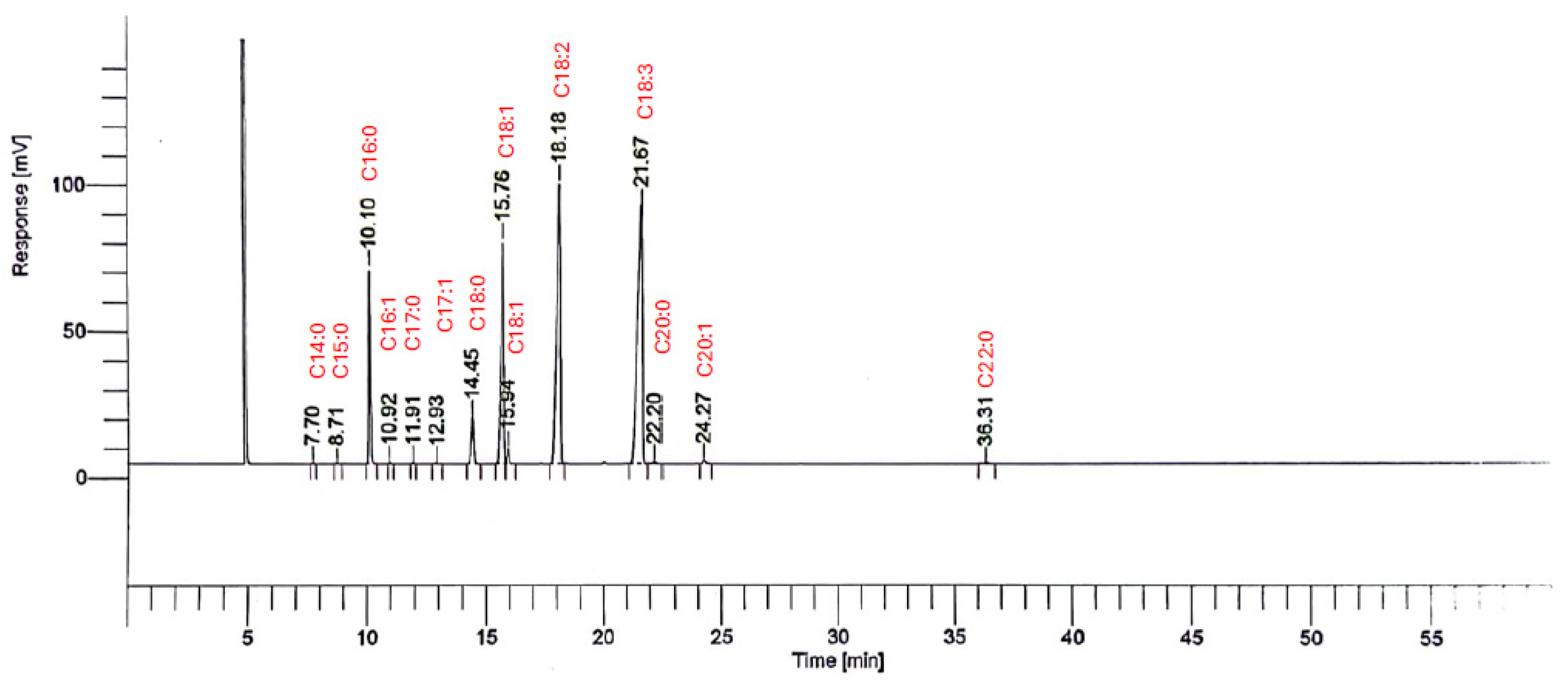
2.2. Fatty Acids Composition of Seeds Oil
2.3. Fatty Acids Ratios
2.4. Assessing Lipochemodiversity Through ANOVA
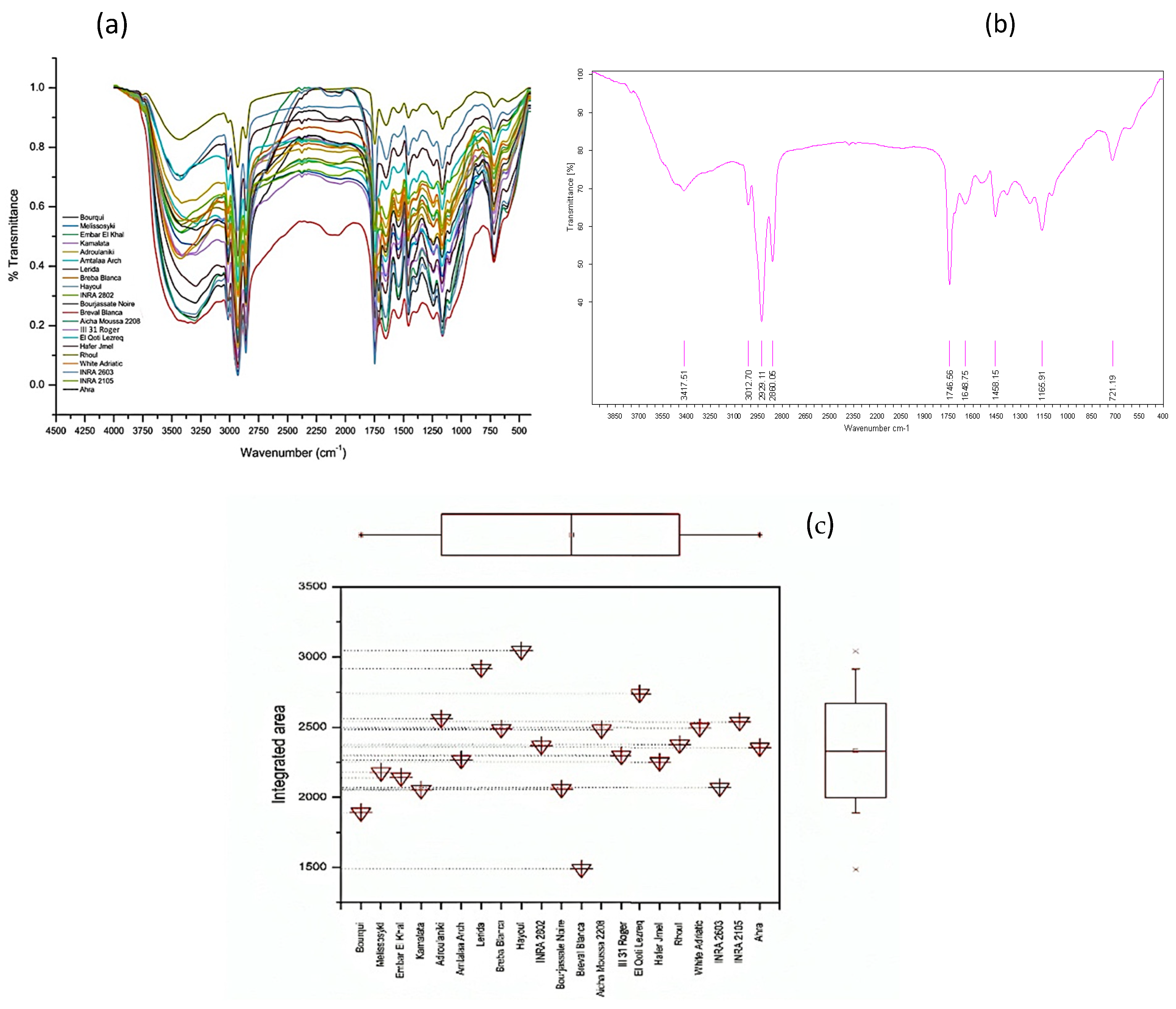
2.5. Characteristics of FTIR-ATR Fingerprints
| Wavenumber (cm−1) | Functional Groups | Modes of Vibration | Assignments | References |
|---|---|---|---|---|
| 3417 | δ(O–H) | Stretching vibration | Intramolecular hydrogen bond between C(3)OH• • •O(5) and C(6)O• • •O(2)H | [36,48,49] |
| 3071 | ν(=C–H) | Bending vibration | Unsaturated fatty acids | [49,50,51] |
| 3012 | δ(C–H) | Stretching vibration | Unsaturated fatty acids particularly present in oleic acid or linoleic acid | [49] |
| 2929 2860 | δ(C–H), δ(–CH2–) | Stretching vibration | Methylene and methyl groups (lipids) | [50,51,52] |
| 1746 | δ(C=O-H), δ(O=C-H) | Stretching vibration | Ester carbonyl functional group of the triglycerides and fatty acids | [38,40,41,42,43,44,45,46,47,48,49,50,51,52,53] |
| 1648 | δ(C=C) | Stretching vibration | Saturated fatty acids | [39,40] |
| 1544 | δ(C=C) | Stretching vibration | Aromatric rings from minor bioactive compounds (i.e., phenols) | [17] |
| 1458 | δ(C–H), ν(C–H) | Stretching and bending vibrations | Lipids and cholesterol esters | [40,53,54] |
| 1386 1239 | ν(C–H), δ(C–O) | Stretching and bending vibrations | Esters | [39,50,51,52,53,54,55] |
| 1165 | δ(C–O), ν(C–C) | Bending vibration | Esters | [50,51,52,53] |
| 1102 | δ(C–O) | Stretching vibration | Unsaturated esters and esters derived from secondary alcohols | [39] |
| 721 | -(CH2)n- | Rocking | Long chain fatty acids | [43,44] |
| 611 | ν(–(CH2)n–), δ(–HC=CH–(cis-)) | Bending vibration (rocking) | Disubstituted olefinic cis-alkenes | [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] |
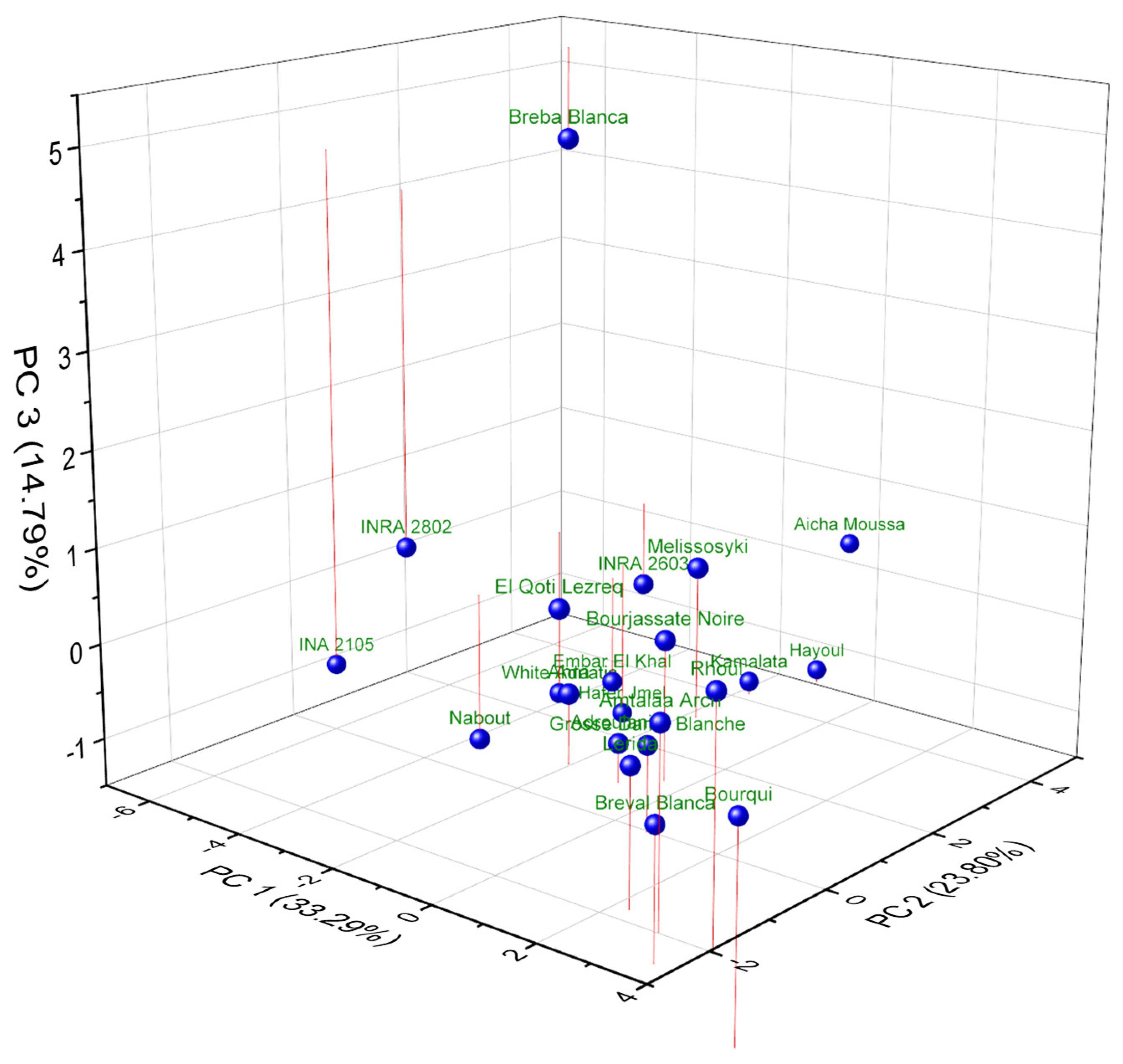
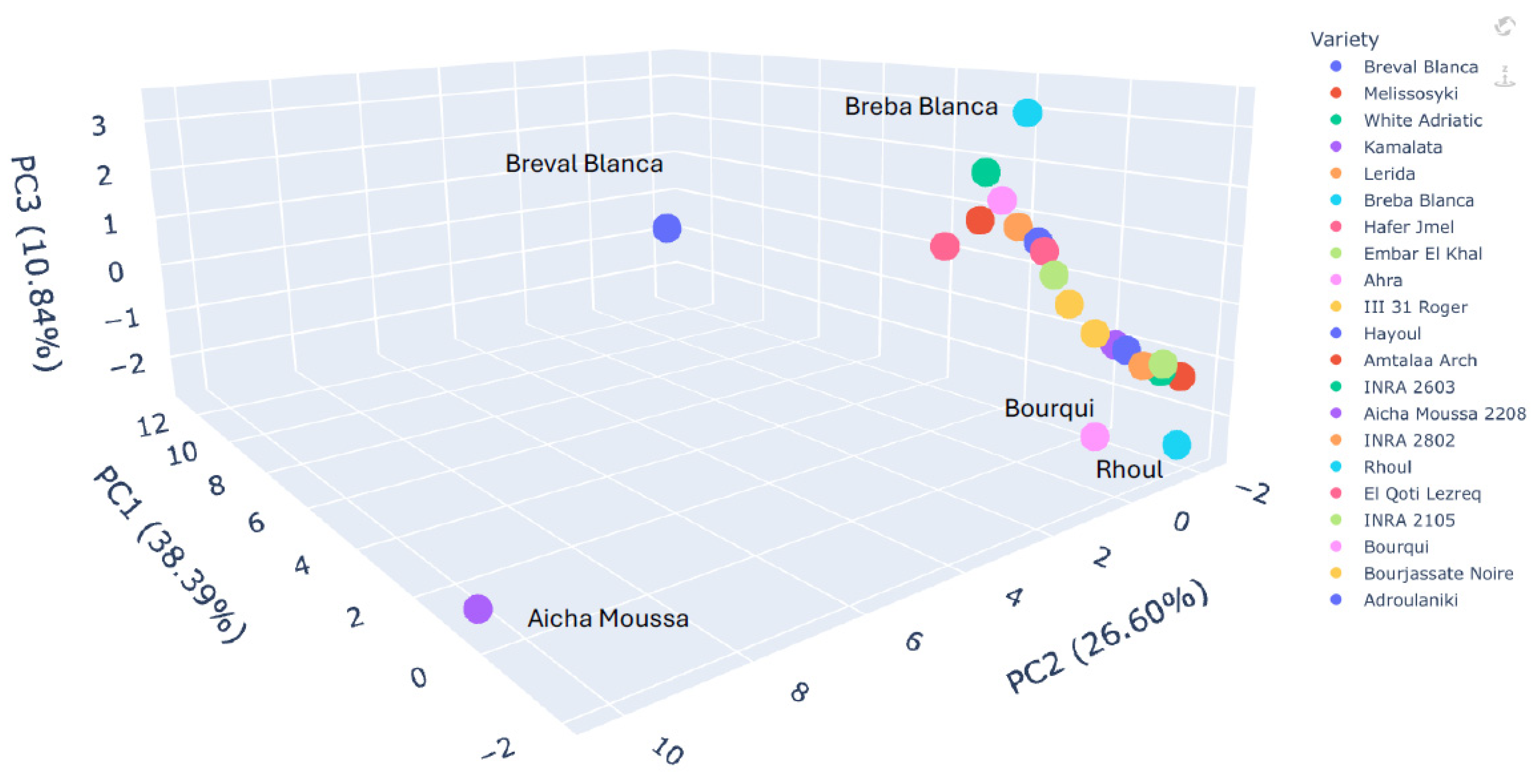
2.6. Principal Component Analysis
3. Materials and Methods
3.1. Plant Material and Experimental Design
3.2. Fruit Sampling
3.3. Seed and Oil Extraction
3.4. Seed Oil Analysis
3.4.1. Chemical Inputs
3.4.2. Fatty Acids Profiling
3.4.3. FTIR-ATR Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ODR | Oleic Desaturation Ratio |
| LDR | Linoleic Desaturation Ratio |
| TUFA | Total Unsaturated Fatty Acids |
| TSFA | Total Saturated Fatty Acids |
| UFA | Unsaturated Fatty Acids |
| OA | Oleic Acid |
| LDL | Low-Density Lipoprotein |
| WHO | World Health Organization |
| FAO | Food and Agriculture Organization |
| GC | Gas Chromatography |
| FAME | Fatty Acid Methyl Esters |
| EU | European Union |
| FTIR-ATR | Fourier-Transform Infrared Spectroscopy- |
| ANOVA | Analysis of Variance |
| PCA | Principal Component Analysis |
| PC | Principal Component |
| FID | Flame Ionization Detector |
References
- Aksoy, U.; Sen, F.; Meyvaci, K.B. Effect of magnesium phosphide, an alternative to methyl bromide, on dried fig quality. Acta Hortic. 2008, 798, 285–292. [Google Scholar] [CrossRef]
- Hssaini, L.; Ouaabou, R.; Hanine, H.; Razouk, R.; Idlimam, A. Kinetics, energy efficiency and mathematical modeling of thin layer solar drying of figs (Ficus carica L.). Sci. Rep. 2021, 11, 1–21. [Google Scholar]
- Hssaini, L.; Hanine, H.; Charafi, J.; Razouk, R.; Elantari, A.; Ennahli, S.; Hernández, F.; Ouaabou, R. A First report on fatty acids composifour fig cultivars (Ficus carica L.) grown in Morocco. OCL 2020, 27, 8. [Google Scholar] [CrossRef]
- Hssaini, L.; Hanine, H.; Razouk, R.; Ennahli, S.; Mekaoui, A.; Ejjilani, A.; Charafi, J. Assessment of genetic diversity in Moroccan fig (Ficus carica L.) collection by combining morphological and physicochemical descriptors. Genet. Resour. Crop Evol. 2020, 67, 457–474. [Google Scholar] [CrossRef]
- FAO. Global Food Losses and Waste. Extent, Causes and Prevention. 2011. Available online: http://www.fao.org/docrep/014/mb060e/mb060e00.pdf (accessed on 17 February 2025).
- FAO. Global Initiative on Food Loss and Waste Reduction. 2015. Available online: http://www.fao.org/3/a-i4068e.pdf (accessed on 18 February 2025).
- Lipinski, B.; Hanson, C.; Lomax, J.; Kitinoja, L.; Waite, R.; Searchinger, T. Reducing Food Loss and Waste. Working Paper, Instalment 2 of Creating a Sustainable Food Future; World Resources Institute: Washington, DC, USA, 2013; Available online: http://pdf.wri.org/reducing_food_loss_and_waste.pdf (accessed on 19 February 2025).
- NRDC. Wasted: How America Is Losing up to 40 Percent of Its Food from Farm to Fork. NRDC Issue PAPER, iP:12–06-B. 2012. Available online: https://www.nrdc.org/sites/default/files/wasted-food-IP.pdf (accessed on 19 February 2025).
- Raihana, A.R.N.; Marikkar, J.M.N.; Amin, I.; Shuhaimi, M. A review on food values of selected tropical fruits’ Seeds. Int. J. Food Prop. 2015, 18, 2380–2392. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M. Seeds recovered from industry by-products of nine fruit species with a high potential utility as a source of unconventional oil for biodiesel and cosmetic and pharmaceutical sectors. Ind. Crops Prod. 2016, 83, 329–338. [Google Scholar] [CrossRef]
- Hssaini, L.; Razouk, L.; Irchad, A.; Aboutayeb, R.; Ouaabou, R. Do pollination and pollen sources affect fig seed set and quality? First attempt using chemical and vibrational fingerprints coupled with chemometrics. J. Chem. 2022, 2022, 3969165. [Google Scholar] [CrossRef]
- Berry, S.K. Cyclopropene fatty acids in some Malaysian edible seeds and nuts: I. Durian (Durio zibethinus Murr.). Lipids 1980, 15, 452–455. [Google Scholar] [CrossRef]
- Taoufik, F.; Zine, S.; El Hadek, M.; Idrissi Hassani, L.; Gharby, S.; Harhar, H.; Matthäus, B. Oil content and main constituents of cactus seed oils Opuntia ficus indica of different origin in Morocco. Mediterr. J. Nutr. Metab. 2015, 8, 85–92. [Google Scholar] [CrossRef]
- Prasad, N.B.L.; Azeemoddin, G. Characteristics and composition of guava (Psidium guajava L.) seed and oil. J. Am. Oil Chem. Soc. 1994, 71, 457–458. [Google Scholar] [CrossRef]
- Güven, N.; Gökyer, A.; Koç, A.; Temiz, N.N.; Selvi, S.; Koparal, B.; Erman, C. Physiochemical composition of fig seed oil from Turkey. J. Pharm. Pharmacol. 2019, 7, 541–545. [Google Scholar]
- Wagner, K.H.; Kamal-Eldin, A.; Elmadfa, I. Gamma-tocopherol—An underestimated vitamin. Ann. Nutr. Metab. 2004, 48, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Hssaini, L.; Razouk, R.; Charafi, J.; Houmanat, K.; Hanine, H. Fig seeds:Combined approach of lipochemical assessment using gas chromatography and FTIRATR spectroscopy using chemometrics. Vibration. Spectrosc. 2021, 114, 103251. [Google Scholar] [CrossRef]
- Irchad, A.; Ouaabou, R.; Aboutayeb, R.; Razouk, R.; Houmanat, K.; Hssaini, L. Lipidomic profiling reveals phenotypic diversity and nutritional benefits in Ficus carica L.(Fig.) seed cultivars. Front. Plant Sci. 2023, 14, 1229994. [Google Scholar] [CrossRef]
- Nakilcioğlu-Taş, E. Biochemical characterization of fig (Ficus carica L.) seeds. J. Agric. Sci. 2019, 25, 232–237. [Google Scholar] [CrossRef]
- Naoui, H.; Benalia, M.; Hachani, S.; Djeridane, A.; Ahmed, Z.B.; Seidel, V.; Yousfi, M. Chemical composition and antioxidant activity of lipids from Ficus carica L. fruits. Chem. J. Mold. 2023, 18, 92–101. [Google Scholar] [CrossRef]
- Vosoughkia, M.; Hossainchi Ghareaghag, L.; Ghavami, M.; Gharachorloo, M.; Delkhosh, B. Evaluation of oil content and fatty acid composition in seeds of different genotypes of safflower. Int. J. Agric. Sci. Res. 2012, 2, 59–66. [Google Scholar]
- Ramadan, M.F. Chemistry and functionality of fruit oils: An introduction. In Fruit Oils: Chemistry and Functionality; Springer: Cham, Switzerland, 2019; pp. 3–8. [Google Scholar]
- Chougui, N.; Tamendjari, A.; Hamidj, W.; Hallal, S.; Barras, A.; Richard, T.; Larbat, R. Oil composition and characterisation of phenolic compounds of Opuntia ficusindica seeds. Food Chem. 2013, 139, 796–803. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowe diseases. Mol. Nutr. Food Res. 2008, 52, 885–897. [Google Scholar] [CrossRef]
- Ramadan, M.F. (Ed.) Fruit Oils: Chemistry and Functionality; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Kris-Etherton, P.M. Monounsaturated fatty acids and risk of cardiovascular disease. Circulation 1999, 100, 1253–1258. [Google Scholar] [CrossRef]
- Park, H.G.; Engel, M.G.; Vogt-Lowell, K.; Lawrence, P.; Kothapalli, K.S.; Brenna, J.T. The role of fatty acid desaturase (FADS) genes in oleic acid metabolism: FADS1 Δ7 desaturates 11–20: 1 to 7, 11–20: 2. Prostaglandins Leukot. Essent. Fat. Acids 2018, 128, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-de-Albuquerque, C.F.; Medeiros-de-Moraes, I.M.; Oliveira, F.M.D.J.; Burth, P.; Bozza, P.T.; Castro Faria, M.V.; Castro-Faria-Neto, H.C.D. Omega-9 oleic acid induces fatty acid oxidation and decreases organ dysfunction and mortality in experimental sepsis. PLoS ONE 2016, 11, e0153607. [Google Scholar]
- Velasco, L.; Goffman, F.D.; Becker, H.C. Variability for the fatty acid composition of the seed oil in a germplasm collection of the genus Brassica. Genet. Resour. Crop Evol. 1998, 45, 371–382. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of the omega-6/omega-3 ratio and cardiovascular disease. Nutrients 2016, 8, 18. [Google Scholar]
- DACH. In Empfehlungen zur Ernährung; Deutsche Gesellschaft für Ernährung: Bonn, Germany, 2022.
- WHO; FAO Joint Consultation. Fats and Oils in Human Nutrition; World Health Organization and Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 1994. [Google Scholar]
- Hu, F.B. Omega-3 fatty acids and cardiovascular disease: A review. Curr. Atheroscler. Rep. 2001, 3, 215–220. [Google Scholar]
- Carvalho, I.S.D.; Miranda, I.; Pereira, H. Evaluation of oil composition of some crops suitable for human nutrition. Ind. Crop Prod. 2006, 24, 75–78. [Google Scholar] [CrossRef]
- Cassani, L.; Santos, M.; Gerbino, E.; Del Rosario Moreira, M.; Gómez-Zavaglia, A. A combined approach of infrared spectroscopy and multivariate analysis forthe simultaneous determination of sugars and fructans in strawberry juices during storage. J. Food Sci. 2017, 83, 631–638. [Google Scholar] [CrossRef]
- Anjos, O.; Campos, M.G.; Ruiz, P.C.; Antunes, P. Application of FTIRATR spectroscopy to the quantification of sugar in honey. Food Chem. 2015, 169, 218–223. [Google Scholar] [CrossRef]
- De la Mata, P.; Dominguez-Vidal, A.; Bosque-Sendra, J.M.; Ruiz-Medina, A.; Cuadros Rodrıguez, L.; Ayora-Cañada, M.J. Olive oil assessment inedible oil blends by means of ATR-FTIR and chemometrics. Food Control 2012, 23, 449–455. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Infrared spectroscopy in the study of edible oils and fats. J. Sci. Food Agric. 1997, 75, 1–11. [Google Scholar] [CrossRef]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta 2006, 573–574, 459–465. [Google Scholar] [CrossRef]
- Jeong, W.S.; Lachance, P.A. Phytosterols and fatty acids in fig (Ficus carica var. Mission) fruit and tree components. J. Food Sci. 2001, 66, 278–281. [Google Scholar] [CrossRef]
- Lerma-García, M.J.; Ramis-Ramos, G.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Authentication of extra virgin olive oils by Fourier-transform infrared spectroscopy. Food Chem. 2010, 118, 78–83. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y.B. Application of Fourier transform infrared spectroscopy for authentication of functional food oils. Appl. Spectrosc. Rev. 2012, 47, 1–13. [Google Scholar] [CrossRef]
- Soltana, H.; Tekaya, M.; Amri, Z.; El-Gharbi, S.; Nakbi, A.; Harzallah, A.; Mechri, B.; Hammami, M. Characterization of fig achenes’ oil of Ficus carica grown in Tunisia. Food Chem. 2016, 196, 1125–1130. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Safar, M.; Bertrand, D.; Robert, P.; Devaux, M.F.; Genot, C. Characterization of edible oils, butters and margarines by Fourier transform infrared spectroscopy with attenuated total reflectance. J. Am. Oil Chem. Soc. 1994, 71, 371–377. [Google Scholar] [CrossRef]
- Yang, H.; Irudayaraj, J.; Paradkar, M.M. Discriminant analysis of edible oils and fats by FTIR, FT-NIR and FT-Raman spectroscopy. Food Chem. 2005, 93, 25–32. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vibration. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef]
- Niu, S.; Zhou, Y.; Yu, H.; Lu, C.; Han, K. Investigation on thermaldegradation properties of oleic acid and its methyl and ethyl esters through TG-FTIR. Energy Conver. Manag. 2017, 149, 495–504. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Raghu, K. Biosynthesis and characterization of gold and silver nanoparticles using milk thistle (Silybum marianum) seed extract. J. Nanosci. 2014, 2014, 905404. [Google Scholar] [CrossRef]
- Tulukcu, E.; Cebi, N.; Sagdic, O. Chemical fingerprinting of seeds of some salvia species in Turkey by using GC-MS and FTIR. Foods 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.K.; Daun, J.K.; Przybylski, R. FT-IR based methodology forquantitation of total tocopherols, tocotrienols and plastochromanol-8 in vegetable oils. J. Food Compos. Anal. 2005, 18, 359–364. [Google Scholar] [CrossRef]
- Liu, X.; Renard, C.M.G.C.; Bureau, S.; Le Bourvellec, C. Revisiting the contribution of ATR-FTIR spectroscopy to characterize plant cell wall polysaccharides. Carbohydr. Polym. 2021, 262, 117935. [Google Scholar] [CrossRef]
- Sylverstein, R.M.; Bassler, G.C.; Morrill, T.C. Spectrophotometric Identification of Organic Compounds, 5th ed.; Wiley: New York, NY, USA, 1991. [Google Scholar]
- Van de Voort, F.R.; Ismail, A.A.; Sedman, J. A rapid automated method for the determination of cis and trans content of fats and oils by Fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 1995, 72, 873–880. [Google Scholar] [CrossRef]
| Genotypes | Seeds Yield (g/Fruit) | Seed Yield in (g/kg) | Oil Yield (%) |
|---|---|---|---|
| Breval Blanca | 2.04 ± 0.3 | 117.95 ± 2.89 | 31.24 ± 0.09 |
| Melissosyki | 1.97 ± 0.48 | 114.81 ± 3.17 | 30.89 ± 0.14 |
| White Adriatic | 2.27 ± 0.93 | 89.03 ± 1.48 | 22.49 ± 0.42 |
| Kamalata | 2.10 ± 0.14 | 112.02 ± 2.11 | 28.87 ± 0.40 |
| Lerida | 2.25 ± 0.38 | 92.07 ± 2.92 | 30.28 ± 0.12 |
| Breba Blanca | 2.30 ± 0.18 | 78.68 ± 2.85 | 23.75 ± 0.28 |
| Hafer Jmel | 2.23 ± 0.4 | 107.23 ± 2.19 | 31.50 ± 0.81 |
| Embar El Khal | 2.09 ± 0.75 | 107.17 ± 3.18 | 30.77 ± 0.26 |
| Ahra 2870 | 2.24 ± 0.05 | 101.10 ± 2.48 | 30.75 ± 0.44 |
| III 31 Roger | 1.93 ± 0.40 | 120.52 ± 1.22 | 29.18 ± 0.30 |
| Hayoul 2265 | 2.18 ± 0.87 | 104.54 ± 2.55 | 28.67 ± 0.40 |
| Amtalaa Arch | 2.08 ± 0.12 | 116.87 ± 1.86 | 30.20 ± 0.0 |
| INRA 2603 | 1.99 ± 0.55 | 109.35 ± 1.70 | 23.29 ± 0.15 |
| Aicha Moussa | 1.39 ± 0.89 | 107.75 ± 1.81 | 31.08 ± 0.70 |
| INRA 2802 | 1.79 ± 0.2 | 131.62 ± 1.37 | 16.68 ± 0.69 |
| Rhoul 2216 | 2.07 ± 0.78 | 110.46 ± 3.75 | 31.86 ± 0.13 |
| El Qoti Lezreq | 2.46 ± 0.02 | 58.18 ± 1.00 | 28.97 ± 0.55 |
| INRA 2105 | 1.96 ± 0.45 | 121.95 ± 2.41 | 6.69 ± 0.14 |
| Bourqui | 1.39 ± 0.54 | 111.23 ± 2.52 | 39.97 ± 0.01 |
| Bourjassate Noire | 1.99 ± 0.51 | 114.72 ± 3.50 | 30.89 ± 0.42 |
| Adroulaniki | 1.95 ± 0.48 | 128.23 ± 2.98 | 26.54 ± 0.91 |
| Cultivars | C14:0 | C15:0 | C16:0 | C16:1 | C17:0 | C17:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C20:1 | C22:0 | MUFA | PUFA | TSFA | TUFA | MUFA/PUFA | TSFA/TUFA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breval Blanca | 0.021 ± 0.001 | 0.028 ± 0.003 | 8.145 ± 0.007 | 0.060 ± 0.000 | 0.063 ± 0.004 | 0.230 ± 0.0269 | 3.325 ± 0.007 | 20.235 ± 0.035 | 31.670 ± 0.001 | 35.785 ± 0.007 | 0.230 ± 0.001 | 0.300 ± 0.001 | 0.086 ± 0.001 | 5.206 ± 0.076 | 29.230 ± 0.014 | 1.7 ± 0.0031 | 14.7133 ± 0.0518 | 0.1781 ± 0.05375 | 0.1156 ± 0.0603 |
| Melissosyki | 0.023 ± 0.001 | 0.031 ± 0.001 | 8.908 ± 0.005 | 0.067 ± 0.001 | 0.069 ± 0.005 | 0.047 ± 0.001 | 3.643 ± 0.003 | 8.925 ± 0.023 | 36.518 ± 0.018 | 32.286 ± 0.052 | 0.260 ± 0.002 | 0.259 ± 0.000 | 0.089 ± 0.003 | 2.324 ± 0.071 | 25.909 ± 0.031 | 1.86007 ± 0.002727 | 13.0167 ± 0.0158 | 0.0897 ± 0.002303 | 0.14383 ± 0.00173 |
| White Adriatic | 0.040 ± 0.003 | 0.020 ± 0.004 | 7.834 ± 0.106 | 0.074 ± 0.005 | 0.060 ± 0.001 | 0.038 ± 0.001 | 3.312 ± 0.046 | 8.675 ± 0.100 | 29.417 ± 0.170 | 41.292 ± 0.231 | 0.239 ± 0.004 | 0.131 ± 0.0151 | 0.087 ± 0.004 | 2.229 ± 0.064 | 26.461 ± 0.167 | 1.656 ± 0.028 | 13.270 ± 0.110 | 0.084 ± 0.00384 | 0.1257 ± 0.002552 |
| Kamalata | 0.018 ± 0.001 | 0.021 ± 0.001 | 7.104 ± 0.030 | 0.064 ± 0.004 | 0.061 ± 0.006 | 0.044 ± 0.001 | 3.179 ± 0.004 | 8.849 ± 0.099 | 31.634 ± 0.033 | 39.607 ± 0.209 | 0.237 ± 0.000 | 0.254 ± 0.000 | 0.081 ± 0.001 | 2.303 ± 0.026 | 26.697 ± 0.113 | 1.528 ± 0.006 | 13.408 ± 0.0573 | 0.0862 ± 0.00228 | 0.114 ± 0.00105 |
| Lerida | 0.020 ± 0.000 | 0.025 ± 0.001 | 8.272 ± 0.022 | 0.058 ± 0.001 | 0.065 ± 0.002 | 0.042 ± 0.001 | 3.337 ± 0.013 | 10.237 ± 0.038 | 30.671 ± 0.006 | 36.421 ± 0.102 | 0.244 ± 0.02 | 0.287 ± 0.001 | 0.080 ± 0.008 | 2.656 ± 0.010 | 25.776 ± 0.049 | 1.720 ± 0.007 | 12.952 ± 0.0248 | 0.1030 ± 0.00211 | 0.133 ± 0.00275 |
| Breba Blanca | 0.023 ± 0.001 | 0.039 ± 0.001 | 7.883 ± 0.030 | 0.051 ± 0.001 | 0.082 ± 0.001 | 0.037 ± 0.001 | 3.670 ± 0.006 | 7.247 ± 0.012 | 34.741 ± 0.047 | 38.369 ± 0.053 | 0.269 ± 0.011 | 0.255 ± 0.001 | 0.089 ± 0.002 | 1.897 ± 0.004 | 26.785 ± 0.037 | 1.722 ± 0.007 | 13.449 ± 0.0189 | 0.071 ± 0.0094 | 0.1280 ± 0.00373 |
| Hafer Jmel | 0.084 ± 0.0092 | 0.031 ± 0.011 | 7.411 ± 0.194 | 0.070 ± 0.016 | 0.059 ± 0.011 | 0.040 ± 0.005 | 3.870 ± 0.062 | 12.534 ± 0.054 | 30.639 ± 0.245 | 32.102 ± 0.259 | 0.147 ± 0.0170 | 0.275 ± 0.001 | 0.085 ± 0.003 | 3.229 ± 0.019 | 25.092 ± 0.186 | 1.669 ± 0.077 | 12.610 ± 0.0967 | 0.1287 ± 0.00104 | 0.1324 ± 0.008017 |
| Embar El Khal | 0.021 ± 0.001 | 0.027 ± 0.001 | 7.518 ± 0.000 | 0.061 ± 0.000 | 0.064 ± 0.001 | 0.040 ± 0.001 | 3.394 ± 0.001 | 9.215 ± 0.025 | 33.375 ± 0.001 | 36.490 ± 0.045 | 0.232 ± 0.002 | 0.273 ± 0.001 | 0.078 ± 0.006 | 2.397 ± 0.006 | 26.360 ± 0.024 | 1.618857 ± 0.001818 | 13.242 ± 0.012 | 0.0910 ± 0.0027 | 0.1222 ± 0.00153 |
| Ahra | 0.028 ± 0.001 | 0.033 ± 0.001 | 7.971 ± 0.005 | 0.059 ± 0.002 | 0.072 ± 0.001 | 0.041 ± 0.001 | 3.452 ± 0.009 | 9.262 ± 0.007 | 33.570 ± 0.121 | 35.659 ± 0.093 | 0.233 ± 0.003 | 0.270 ± 0.001 | 0.090 ± 0.003 | 2.408 ± 0.002 | 26.163 ± 0.074 | 1.697 ± 0.003 | 13.14317 ± 0.037241 | 0.092 ± 0.0336 | 0.129 ± 0.0087 |
| III 31 Roger | 0.019 ± 0.000 | 0.023 ± 0.001 | 7.996 ± 0.008 | 0.055 ± 0.001 | 0.063 ± 0.001 | 0.037 ± 0.001 | 3.175 ± 0.003 | 8.394 ± 0.041 | 31.778 ± 0.095 | 39.462 ± 0.018 | 0.243 ± 0.013 | 0.273 ± 0.001 | 0.089 ± 0.005 | 2.190 ± 0.011 | 26.545 ± 0.051 | 1.658 ± 0.004 | 13.333 ± 0.0262 | 0.0825 ± 0.00213 | 0.124 ± 0.00166 |
| Hayoul | 0.025 ± 0.001 | 0.026 ± 0.001 | 8.128 ± 0.011 | 0.061 ± 0.001 | 0.066 ± 0.002 | 0.038 ± 0.001 | 3.311 ± 0.004 | 9.607 ± 0.019 | 30.014 ± 0.011 | 38.490 ± 0.060 | 0.255 ± 0.011 | 0.285 ± 0.001 | 0.090 ± 0.002 | 2.498 ± 0.064 | 26.037 ± 0.030 | 1.699929 ± 0.04748 | 13.08217 ± 0.015556 | 0.095923031 ± 0.002129006457 | 0.129942433 ± 0.00305194805 |
| Amtalaa Arch | 0.019 ± 0.002 | 0.017 ± 0.001 | 7.510 ± 0.035 | 0.078 ± 0.000 | 0.051 ± 0.001 | 0.026 ± 0.013 | 3.515 ± 0.019 | 8.187 ± 0.020 | 29.096 ± 0.049 | 42.762 ± 0.173 | 0.229 ± 0.018 | 0.234 ± 0.001 | 0.090 ± 0.001 | 2.131 ± 0.008 | 26.682 ± 0.080 | 1.632 ± 0.011 | 13.397 ± 0.042 | 0.080 ± 0.00103 | 0.122 ± 0.002571 |
| INRA 2603 | 0.025 ± 0.000 | 0.031 ± 0.001 | 8.197 ± 0.004 | 0.059 ± 0.000 | 0.066 ± 0.001 | 0.038 ± 0.001 | 4.160 ± 0.011 | 10.099 ± 0.024 | 27.752 ± 0.019 | 38.733 ± 0.056 | 0.352 ± 0.016 | 0.271 ± 0.001 | 0.118 ± 0.002 | 2.617 ± 0.007 | 25.528 ± 0.033 | 1.850 ± 0.005 | 12.825 ± 0.016 | 0.1024 ± 0.001982 | 0.144 ± 0.00294 |
| Aicha Moussa | 0.123 ± 0.141 | 0.027 ± 0.001 | 8.103 ± 0.060 | 0.044 ± 0.000 | 0.075 ± 0.001 | 0.037 ± 0.001 | 3.114 ± 0.020 | 7.589 ± 0.014 | 36.683 ± 0.046 | 18.117 ± 0.25547 | 18.173 ± 0.25378 | 0.250 ± 0.027 | 0.177 ± 0.133 | 1.980 ± 0.010 | 20.796 ± 0.536 | 4.257 ± 0.3676 | 10.453 ± 0.4272 | 0.095 ± 0.0001 | 0.4075 ± 0.00860 |
| INRA 2802 | 0.018 ± 0.001 | 0.022 ± 0.001 | 7.942 ± 0.008 | 0.067 ± 0.000 | 0.059 ± 0.004 | 0.035 ± 0.001 | 3.134 ± 0.005 | 7.400 ± 0.012 | 33.451 ± 0.022 | 39.935 ± 0.067 | 0.220 ± 0.003 | 0.237 ± 0.001 | 0.081 ± 0.010 | 1.935 ± 0.004 | 26.928 ± 0.034 | 1.639 ± 0.004 | 13.520 ± 0.0172 | 0.072 ± 0.00105 | 0.121 ± 0.00258 |
| Rhoul | 0.018 ± 0.001 | 0.022 ± 0.001 | 6.671 ± 0.006 | 0.069 ± 0.001 | 0.055 ± 0.005 | 0.041 ± 0.003 | 2.562 ± 0.009 | 7.685 ± 0.053 | 34.070 ± 0.047 | 40.618 ± 0.172 | 0.186 ± 0.005 | 0.236 ± 0.001 | 0.083 ± 0.009 | 2.008 ± 0.014 | 27.457 ± 0.091 | 1.370 ± 0.005 | 13.786 ± 0.046 | 0.0731 ± 0.00160 | 0.099 ± 0.00111 |
| El Qoti Lezreq | 0.021 ± 0.001 | 0.024 ± 0.001 | 8.238 ± 0.014 | 0.056 ± 0.001 | 0.059 ± 0.004 | 0.039 ± 0.001 | 3.206 ± 0.015 | 9.365 ± 0.025 | 33.853 ± 0.047 | 35.199 ± 0.117 | 0.225 ± 0.011 | 0.271 ± 0.001 | 0.079 ± 0.004 | 2.433 ± 0.007 | 26.139 ± 0.063 | 1.693 ± 0.006 | 13.130 ± 0.032 | 0.093 ± 0.00112 | 0.1299 ± 0.00217 |
| INRA 2105 | 0.022 ± 0.001 | 0.025 ± 0.001 | 7.562 ± 0.021 | 0.070 ± 0.002 | 0.059 ± 0.004 | 0.036 ± 0.000 | 2.995 ± 0.006 | 7.356 ± 0.026 | 33.161 ± 0.062 | 40.808 ± 0.023 | 0.221 ± 0.001 | 0.239 ± 0.001 | 0.092 ± 004 | 1.925 ± 0.007 | 27.108 ± 0.037 | 1.57 ± 0.005 | 13.611 ± 0.019 | 0.0710 ± 0.00196 | 0.115 ± 0.00271 |
| Bourqui | 0.019 ± 0.002 | 0.022 ± 0.001 | 6.815 ± 0.086 | 0.069 ± 0.001 | 0.063 ± 0.004 | 0.040 ± 0.001 | 3.183 ± 0.032 | 8.440 ± 0.304 | 35.387 ± 0.008 | 36.970 ± 0.725 | 0.234 ± 0.001 | 0.246 ± 0.001 | 0.075 ± 0.003 | 2.199 ± 0.077 | 26.932 ± 0.346 | 1.487 ± 0.018183 | 13.52517 ± 0.173477 | 0.0816 ± 0.00223 | 0.110 ± 0.00105 |
| Bourjassate Noire | 0.017 ± 0.001 | 0.019 ± 0.001 | 7.833 ± 0.030 | 0.064 ± 0.001 | 0.065 ± 0.002 | 0.041 ± 0.001 | 3.294 ± 0.005 | 8.682 ± 0.052 | 31.894 ± 0.042 | 38.835 ± 0.098 | 0.259 ± 0.001 | 0.234 ± 0.001 | 0.083 ± 0.001 | 2.255 ± 0.013 | 26.470 ± 0.064 | 1.653 ± 0.005 | 13.291 ± 0.032 | 0.085 ± 0.00207 | 0.124 ± 0.00169 |
| Adroulaniki | 0.018 ± 0.001 | 0.018 ± 0.001 | 7.886 ± 0.001 | 0.065 ± 0.001 | 0.058 ± 0.004 | 0.037 ± 0.001 | 3.223 ± 0.006 | 8.514 ± 0.008 | 29.245 ± 0.028 | 41.858 ± 0.037 | 0.228 ± 0.007 | 0.247 ± 0.001 | 0.089 ± 0.004 | 2.216 ± 0.002 | 26.539 ± 0.025 | 1.645 ± 0.003 | 13.327 ± 0.012 | 0.083 ± 0.00101 | 0.123 ± 0.00267 |
| Cultivars | ODR | LDR | ω-6/ω-3 |
|---|---|---|---|
| Breval Blanca | 0.592 ± 0.0833 | 0.530 ± 0.01 | 0.885 ± 0.00 |
| Melissosyki | 0.584 ± 0.0442 | 0.469 ± 0.0745 | 1.131 ± 0.0342 |
| White Adriatic | 0.480 ± 0.0538 | 0.584 ± 0.0577 | 0.712 ± 0.0723 |
| Kamalata | 0.505 ± 0.0387 | 0.556 ± 0.0865 | 0.799 ± 0.0156 |
| Lerida | 0.529 ± 0.0300 | 0.542 ± 0.0947 | 0.842 ± 0.055 |
| Breba Blanca | 0.522 ± 0.0525 | 0.524 ± 0.0532 | 0.905 ± 0.088 |
| Hafer Jmel | 0.573 ± 0.0536 | 0.512 ± 0.0514 | 0.954 ± 0.0945 |
| Embar El Khal | 0.538 ± 0.037 | 0.522 ± 0.0967 | 0.915 ± 0.0317 |
| Ahra | 0.547 ± 0.0580 | 0.515 ± 0.0434 | 0.941 ± 0.01305 |
| III 31 Roger | 0.505 ± 0.0880 | 0.554 ± 0.0162 | 0.805 ± 0.05154 |
| Hayoul | 0.507 ± 0.0330 | 0.531 ± 0.085 | 0.780 ± 0.176 |
| Amtalaa Arch | 0.466 ± 0.0284 | 0.595 ± 0.0779 | 0.680 ± 0.0283 |
| INRA 2603 | 0.494 ± 0.0435 | 0.582 ± 0.0745 | 0.717 ± 0.0342 |
| Aicha Moussa | 0.710 ± 0.002 | 0.330 ± 0.0998 | 2.025 ± 0.002 |
| INRA 2802 | 0.507 ± 0.0336 | 0.544 ± 0.0753 | 0.838 ± 0.0326 |
| Rhoul | 0.506 ± 0.0369 | 0.544 ± 0.0783 | 0.839 ± 0.0276 |
| El Qoti Lezreq | 0.551 ± 0.0381 | 0.510 ± 0.0715 | 0.9612 ± 0.0397 |
| INRA 2105 | 0.498 ± 0.0796 | 0.551 ± 0.0267 | 0.813 ± 0.0275 |
| Bourqui | 0.542 ± 0.0300 | 0.511 ± 0.0989 | 0.957 ± 0.011 |
| Bourjassate Noire | 0.511 ± 0.0487 | 0.549 ± 0.0702 | 0.821 ± 0.0424 |
| Adroulaniki | 0.474 ± 0.05 | 0.589 ± 0.0565 | 0.699 ± 0.0769 |
| Variable | Mean | Std | CV (%) | F-Value | p-Value |
|---|---|---|---|---|---|
| Oil content (%) | 27.77 | 6.45717 | 23.2507 | 8.154251 | 0.000000 |
| C22:0 | 0.090762 | 0.027205 | 29.97435 | 3.225485 | 0.000685 |
| C20:1 | 0.253429 | 0.03869 | 15.26648 | 6.14741 | 3.93 × 10−7 |
| C20:0 | 1.091019 | 5.021421 | 460.2505 | 2.997145 | 0.001351 |
| C18:3 | 37.13293 | 6.072545 | 16.35353 | 5.247349 | 3.11 × 10−6 |
| C18:2 | 32.31495 | 2.443698 | 7.562128 | 5879.885 | 5.77 × 10−66 |
| C18:1 | 9.356762 | 2.72365 | 29.1089 | 7458.747 | 3.91 × 10−68 |
| C18:0 | 3.335619 | 0.321377 | 9.634691 | 1549.288 | 8.17 × 10−54 |
| C17:1 | 0.047595 | 0.053607 | 112.6307 | 3.067134 | 0.001096 |
| C17:0 | 0.063214 | 0.007155 | 11.31923 | 19.50827 | 3.27 × 10−15 |
| C16:1 | 0.062714 | 0.008074 | 12.87443 | 24.09722 | 6.73 × 10−17 |
| C16:0 | 7.805857 | 0.508504 | 6.514384 | 514.2585 | 8.61 × 10−44 |
| C15:0 | 0.024929 | 0.005696 | 22.84857 | 24.0548 | 6.95 × 10−17 |
| C14:0 | 0.029488 | 0.033477 | 113.5289 | 2.864197 | 0.002017 |
| TUFA | 13.07052 | 0.879598 | 6.729634 | 3.426055 | 0.000382 |
| TSFA | 2.770699 | 4.521168 | 163.1778 | 194.8151 | 5.04 × 10−35 |
| PUFA | 26.26821 | 1.829967 | 6.966468 | 3.892632 | 0.000102 |
| MUFA | 2.430125 | 0.693972 | 28.55705 | 5610.327 | 1.54 × 10−65 |
| TSFA/TUFA | 0.224631 | 0.384561 | 171.1972 | 56.52292 | 4.54 × 10−24 |
| MUFA/PUFA | 0.093914 | 0.028918 | 30.7924 | 56.15978 | 5.16 × 10−24 |
| ω-6/ω-3 | 13.01706 | 88.6718 | 681.1967 | 1.004605 | 0.477061 |
| LDR | 1.409186 | 3.96565 | 281.4144 | 16,088.53 | 3.83 × 10−75 |
| ODR | 1.052425 | 2.357667 | 224.0224 | 6978.209 | 1.58 × 10−67 |
| Variables | PC1 | PC2 | PC3 |
|---|---|---|---|
| Oil content | 0.005051 | 0.003635 | 0.003602 |
| Seeds yield | 0.000035 | 0.029898 | 0.138464 |
| C14:0 | 0.000860 | 0.107673 | 0.015517 |
| C15:0 | 0.007966 | 0.022617 | 0.202530 |
| C16:0 | 0.007560 | 0.015093 | 0.107982 |
| C16:1 | 0.005515 | 0.074496 | 0.042504 |
| C17:0 | 0.001142 | 0.060858 | 0.107100 |
| C17:1 | 0.101614 | 0.004711 | 0.003983 |
| C18:0 | 0.002135 | 0.002384 | 0.190771 |
| C18:1 | 0.097336 | 0.004885 | 0.001834 |
| C18:2 | 0.000176 | 0.034054 | 0.006347 |
| C18:3 | 0.006989 | 0.130976 | 0.001287 |
| C20:0 | 0.000386 | 0.129819 | 0.051809 |
| C20:1 | 0.022229 | 0.003229 | 0.062323 |
| C22:0 | 0.000529 | 0.120594 | 0.013864 |
| MUFA | 0.097632 | 0.004855 | 0.001893 |
| PUFA | 0.007696 | 0.130228 | 0.000575 |
| TSFA | 0.102715 | 0.000601 | 0.009100 |
| TUFA | 0.025287 | 0.106803 | 0.007967 |
| MUFA/PUFA | 0.100334 | 0.000056 | 0.000604 |
| TSFA/TUFA | 0.102703 | 0.000109 | 0.010479 |
| ODR | 0.101551 | 0.004135 | 0.006180 |
| LDR | 0.100341 | 0.005895 | 0.005824 |
| ω-6/ω-3 | 0.102216 | 0.002395 | 0.007461 |
| Exotic Varieties | Local Genotypes |
|---|---|
| White Adriatic | Ahra |
| Breval Blanca | Aicha Moussa |
| Rhoul | El Qoti Lezreq |
| Adroulaniki | Hafer Jmel |
| Kamalata | Lerida |
| Melissosyki | Embar El Khal |
| III 31 Roger | INRA 2105 |
| Bourqui | INRA 2603 |
| Amtalaa Arch | INRA 2802 |
| Hayoul | |
| Breba Blanca | |
| Bourjassate Noire |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassimi, C.E.-d.; Houmanat, K.; Irchad, A.; Aboutayeb, R.; Ben Moumen, A.; Fadlaoui, A.; Guirrou, I.; Diai, F.; Hajji, L.; Hssaini, L. Fig Seeds as a Novel Oil Source: Investigating Lipochemodiversity Through Fatty Acids Profiling and FTIR Spectral Fingerprints. Plants 2025, 14, 945. https://doi.org/10.3390/plants14060945
Kassimi CE-d, Houmanat K, Irchad A, Aboutayeb R, Ben Moumen A, Fadlaoui A, Guirrou I, Diai F, Hajji L, Hssaini L. Fig Seeds as a Novel Oil Source: Investigating Lipochemodiversity Through Fatty Acids Profiling and FTIR Spectral Fingerprints. Plants. 2025; 14(6):945. https://doi.org/10.3390/plants14060945
Chicago/Turabian StyleKassimi, Charaf Ed-dine, Karim Houmanat, Ahmed Irchad, Rachid Aboutayeb, Abdessamad Ben Moumen, Aziz Fadlaoui, Ibtissame Guirrou, Fedoua Diai, Lhoussain Hajji, and Lahcen Hssaini. 2025. "Fig Seeds as a Novel Oil Source: Investigating Lipochemodiversity Through Fatty Acids Profiling and FTIR Spectral Fingerprints" Plants 14, no. 6: 945. https://doi.org/10.3390/plants14060945
APA StyleKassimi, C. E.-d., Houmanat, K., Irchad, A., Aboutayeb, R., Ben Moumen, A., Fadlaoui, A., Guirrou, I., Diai, F., Hajji, L., & Hssaini, L. (2025). Fig Seeds as a Novel Oil Source: Investigating Lipochemodiversity Through Fatty Acids Profiling and FTIR Spectral Fingerprints. Plants, 14(6), 945. https://doi.org/10.3390/plants14060945







