Abstract
In this study, the antifungal potential of chemical constituents of Piper ceanothifolium Kunth was determined against three phytopathogenic fungi associated with the cocoa crop. The methodology included the phytochemical study of the inflorescences of P. ceanothifolium, the synthesis of a chroman-4-one type derivative and the evaluation of the antifungal activity against Moniliophthora roreri, Fusarium solani, and Lasiodiplodia theobromae. The phytochemical study led to the isolation and identification of two new hydroquinones (1 and 5), together with three known compounds (hydroquinones 2 and 3, and chromene 4). The synthesis of a new chromone 6 obtained from 2 through an oxa-Michael type intramolecular cyclization is also reported. All compounds showed strong antifungal activity, with 6 (IC50 of 16.9 µM) standing out for its action against F. solani, while prenylated hydroquinones 1 (30.4 µM) and 2 (60.0 µM) were the most active against M. roreri and L. theobromae, respectively. The results of this research represent the first report of the chemical composition and antifungal properties for P. ceanotifolium, suggesting its potential use as a control method against M. roreri, F. solani, and L. theobromae.
1. Introduction
Theobroma cacao L., one of the most representative species of the Malvaceae family, is a source of various useful raw materials for the food, pharmaceutical, and cosmetic industries [1,2]. At the therapeutic level, it is characterized for its stimulating effect on the central nervous system and for its antioxidant, antibacterial, antipyretic, antianemic, antihypertensive, and anti-inflammatory properties [3,4]. Both the production and export of cacoa are tools for strengthening and increasing competitiveness in the agricultural sector for underdeveloped countries, so it is considered a product of great socioeconomic value [5,6]. On the other hand, according to the International Cocoa Organization, the demand for cocoa beans has increased by 2.5% per year, which allows for the forecast of a deficit of two million tons of cocoa by 2030. In addition, production yields are moderate to low in some regions due to several factors, including infestation by various pests [7,8,9].
Fungi and oomycetes are important phytopathogens associated with cocoa crops, for which it has been reported that they can reduce production yields by 30 to 80% [10,11]. These phytopathogens cause various diseases in cocoa plantations, altering their physiological state, and in extreme cases causing the death of the plant [10]. Among the most representative are, Moniliophthora roreri, which affects the fruits and causes the disease known as moniliasis [11,12], M. perniciosa, that affects the growing tissues of the plant causing the disease known as “witches broom” [13,14], and, Ceratocystis fimbriata, which causes the disease known as “machete disease” that affects the vascular system and can cause the death of the plant [15,16]. In addition, secondary pests have been reported, such as the Phytophthora species, which attack the fruit and vascular system and cause the disease known as “black ear” [17], and Fusarium solani and Lasiodiplodia theobromae, which attack the vascular system and cause the disease known as “vascular streak dieback” (VSD) [18,19]. There are different strategies for the control of these phytopathogens that involve integrated management of the crop and the use of commercial pesticides [20,21]. Although these chemicals are effective, many of them have been associated with environmental damage and health problems for farmers and consumers. In this way, it is necessary to develop new effective and safe products to control this type of pest.
Thus, plants can be considered as a potential alternative due to the great variety of specialized metabolites that they produce in response to different biotic and abiotic factors, and for which their properties for pest control have been determined [22,23,24]. The genus Piper, which belongs to the family Piperaceae, is recognized for the antifungal potential of its extracts, essential oils, and chemical constituents, which can be used for the control of phytopathogens that affect agricultural crops of commercial importance. Among the specialized metabolites described for the genus Piper that stand out for their antifungal properties are alkylbenzenes, amides, benzoic acid derivatives, phenylpropanoids, flavonoids, and terpenes [25,26,27,28,29,30,31,32,33]. There are very few studies on species of the genus Piper for the control of phytopathogens associated with cocoa cultivation, but generally there are reports on the preliminary antifungal potential of some extracts and essential oils against Phytophtora palmivora, P. capsici, M. roreri, L. theobromae, and F. solani [29,30,31,32,33]. Research on chemical constituents present in bioactive species is not very common, highlighting the study carried out on P. pesaresanum where the antifungal properties of the extract, fractions, and chemical constituents against M. roreri, Phytophtora sp., and F. solani were determined. In addition, some preliminary structure–activity relationships were determined from the most active constituents [33].
Piper ceanothifolium Kunth (synonyms Enckea ceanothifolia (Kunth) Kunth, P. amalago f. ceanothifolium (Humb., Bonpl. & Kunth) Steyerm., P. amalago var. ceanothifolium (Kunth) Yunck., and P. medium var. ceanothifolium (Kunth) Trel. & Yunck), is a native species distributed in tropical countries such as Colombia, Panama, Venezuela, and Brazil [34,35,36]. There are no reports in the literature on the chemical composition and biological properties for this species. The present research describes the antifungal potential of chemical constituents from P. ceanothifolium against three phytopathogenic fungi associated with the cocoa crop (M. roreri, M. perniciosa, and L. theobromae).
2. Results and Discussion
2.1. Phytochemical Study and Inhibition of Mycelial Growth Assays
The fractionation of ethanolic extract (EE) from P. ceanothifolium inflorescences using vacuum liquid chromatography (VLC) led to four fractions of different polarity being obtained (dichloromethane (DCM), ethyl acetate (EtOAc), isopropanol (IPA), and ethanol/water 8:2 (EtOH:H2O 8:2). EE and each of the fractions obtained were subjected to inhibition of mycelial growth (IMG) assays against F. solani, L. theobromae, and M. roreri. The results obtained show that the antifungal activity on the three fungi was concentrated in the DCM fraction (Table 1). The DCM fraction in comparison to the EE presented lower IC50 against L. theobromae and M. roreri, while on F. solani no significant differences were found. In this way, the DCM fraction was selected to undergo purification by chromatographic techniques to isolate and identify the bioactive constituents.

Table 1.
Determination of the antifungal activity of the ethanolic extract and fractions from P. ceanothifolium inflorescences against F. solani, L. theobromae, and M. roreri.
The phytochemical study carried out on the DCM fraction from P. ceanothifolium led to the isolation and identification of two new hydroquinones (ceanothifolione (1) and ceanothifoliol (5)), along with three known compounds (debromocymopolone (2), 1,4-dihydroxy-2-(3′,7′-dimethyl-1′-oxo-2′-Z-6′-octadienil)benzene (3) and lhotzcromene (4)). These chemical constituents are reported for the first time in the species; however, compounds 2 to 4 have been previously reported in other species of the Piper genus [37,38,39,40,41]. The structural characteristics of 1–5 are in accordance with the chemotaxonomy of the genus since some of its species have been reported to have prenylated hydroquinones with oxogeranyl chains and prenylated derivatives of cyclic benzoic acid (chromenes) [25,29,42]. On the other hand, chroman-4-one 6 was synthesized for the first time and in good yield from 2 through an intramolecular cyclization of oxa-Michael type with some modifications [43]. Figure 1 shows the chemical structures for the isolated and synthesized compounds.
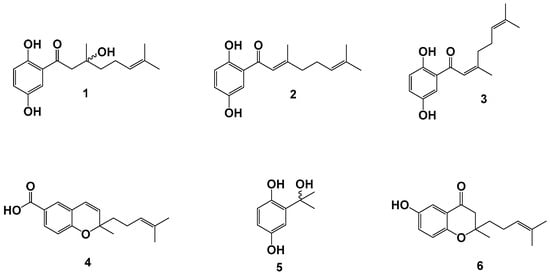
Figure 1.
Chemical constituents from P. ceanothifolium (1–5) and a synthesized derivative (6).
Compound 1 was obtained as a white solid with a m.p. of 82–83 °C, which produces a dark brown color when sprayed with the FeCl3 reagent on TLC, indicating the possible presence of phenolic hydroxyls [44]. According to the NMR analysis (Figure S1 and Figure S2 in Supplementary Materials), it was determined that 1 presents a 1,2,4 trisubstituted aromatic ring by the signals in 1H with δH 7.39 (d, J = 3.2 Hz, 1H), 7.09 (dd, J = 8.9; 3.2 Hz, 1H), and 6.84 (d, J = 8.9 Hz, 1H), together with the signals in APT at δC 154.2 (C), 150.1 (C), 125.4 (CH), 120.2 (C), 119.6 (CH), and 110.6 (CH). Additionally, it was determined that the substituents on the aromatic ring correspond to two hydroxyl groups (signals in APT with δC 154.2 (C) and 150.1 (C)) and a prenylated chain of the geranyl type (3′-hydroxy-3′,7′-dimethyl-1′-oxo-6′-octenyl) which is characterized by having a carbonyl group in the 1′ position and in the 3′ position a hydroxyl group. This prenylated substituent has been previously reported in the literature and is characterized spectroscopically by the signals at δH 5.06 (t, J = 7.2, 1H), 2.78 (d, J = 16.7, 1H), 2.66 (d, J = 16.7, 1H), 2.12–2.08 (q, 2H), 1.83–1.76 (m, 1H), 1.67 (s, 3H), and 1.57 (s, 3H), and the signals with δC 194.0 (C), 132.3 (C), 123.3 (CH), 80.8 (C), 47.4 (CH2), 39.0 (CH2), 25.6 (CH3), 22.2 (CH2), and 17.6 (CH3) [37,41,45]. The use of two-dimensional experiments (COSY, HMQC, and HMBC, see Figures S3–S5 in Supplementary Materials) allowed for the confirmation of the substructures and the location of the substituents on the aromatic ring. Using high-resolution mass spectrometry analysis (HR-MS) in positive mode, the molecular formula was established as C16H22O4 (m/z 278.1462 [M + H]+, calculated for C16H22O4, 278.1518) and its formula is consistent with the result of spectroscopic analysis. Thus, compound 1 is reported for the first time and we have named it ceanothifolione.
Compound 2 has an NMR profile like 1, where the characteristic signals for prenylated hydroquinone are observed (Figure S6 and Figure S7 in Supplementary Materials). The difference in their spectra occurs in the prenylated chain, being for 2 of the oxo-geranyl type by the signals at δH 6.68 (s, 1H), 5.13 (t, J = 7.0 Hz, 1H), 2.30–2.24 (m, 4H), 2.20 (s, 3H), 1.73 (s, 3H), and 1.64 (s, 3H), and the signals at δC 196.8 (C), 161.9 (C), 133.6 (C), 124.9 (CH), 119.8 (CH),115.7 (CH), 42.3 (CH2), 26.9 (CH2), 26.4 (CH3), 20.8 (CH3), and 17.9 (CH3). Based on this analysis and by comparison with the spectroscopic data described in the literature, 2 was identified as debromocymopolone. This compound was isolated for the first time in the algae Cymopolia barbata and from there it derives its name [41]. In the Piperaceae family it has been reported in the leaves and roots of P. crassinervium [40]. Debromocymopolone 2 has been reported to have antiparasitic activity against Trypanosoma cruzi (IC50 of 6.1 µg/mL) and moderate antioxidant activity by inhibiting lipoperoxidation (IC50 of 26.43 µM) [37]. Antifungal activity against Cladosporium cladosporioides and C. sphaerospermum has been reported for 2, with a minimum inhibitory quantity (MIQ) of 1.0 µg to inhibit both phytopathogens [38].
Compound 3 exhibits an NMR profile characteristic of prenylated hydroquinones, like compounds 1 and 2 (Figure S8 and Figure S9, Supplementary Materials). A comparison between 3 and 2 revealed comparable shifts in both the 1H and APT spectra, except for the signals corresponding to positions 4′ and 10′, suggesting that 3 could be a geometric isomer of 2. NOESY analysis confirmed the relative positioning of substituents on the double bond in both compounds (Figure S10, Supplementary Materials). Based on these findings and a comparison with previously reported spectroscopic data, compound 3 was identified as 1,4-dihydroxy-2-(3′,7′-dimethyl-1′-oxo-2′Z,6′-octadienyl) benzene, a compound previously described in the leaves and roots of P. crassinervium [44]. The antifungal action of 3 against C. cladosporioides and C. sphaerospermum (MIQ of 5.0 µg and 10.0 µg, respectively) has been described in the literature [38]. Additionally, antioxidant properties have been reported due to its ability to inhibit lipoperoxidation (IC50 of 63.11 µM) [40].
Compound 4 was obtained as an amorphous solid with a m.p. of 48–49 °C, which produces a dark blue color when sprayed with the vanillin/H2SO4 reagent on TLC. That is characteristic for chromenes present in species of the genus Piper [29,44]. Analysis of the NMR signals for 4 (Figure S11 and Figure S12 in Supplementary Materials) confirms the presence of a prenylated chromene, which was identified as lhotzchromene and which was previously reported in the leaves of P. lhotzkyanum and roots of P. crassinervium [45]. There are no previous reports in the literature on the biological properties of lhotzchromene.
Compound 5 was isolated as a yellow crystalline solid with a m.p. at 53–55 °C, and by analysis of its NMR spectra (Figure S13 and Figure S14 in Supplementary Materials) the presence of a 1,2,4-trisubstituted aromatic ring was determined (signals with δH 7.31 (d, J = 3.0 Hz, 1H), 7.11 (dd, J = 8.9, 3.0 Hz, 1H), and 6.82 (d, J = 8.8 Hz, 1H), and with δC 155.6 (C), 149.3 (C), 124.8 (C), 119.4 (CH), 118.5 (CH), and 115.5 (CH)). The substituents on the aromatic ring were determined as two hydroxyls located in positions 1 and 4 (signals in δc 155.6 (C) and 149.3 (C)) and a group of type 1′-hydroxy-1′-methylethyl located in position 2, (δH 2.86 (s, 3H), 2.63 (s, 3H) and δC 88.6 (C), 29.6 (CH3), 26.0 (CH3)). Thus, 5 is reported for the first time, and we have called it ceanothifoliol.
Starting from the major compound debromocymopolone 2, the chromone called ceanothichromone 6 was synthesized for the first time and with good yield, using typical conditions of an intramolecular cyclization of the oxa-Michael type (Figure 2) [43]. For the reaction carried out, the presence of a phenolic hydroxyl and a chain with α,β-unsaturated carbonyl was used for the formation of chromone-type compounds of which there are previous reports in the literature of their antifungal potential [37,38,39,40]. Compound 6 was characterized in NMR by the characteristic signals of the chromone base nucleus with δH 7.38 (s, 1H), 7.09 (dd, J = 9.1, 3.1 Hz, 1H), 6.87 (d, J = 9.1 Hz, 1H), and 2.80 (d, J = 16.7 Hz, 1H), together with the signals at δC 194.8 (CO), 157.1 (C), 154.5 (C), 125.0 (CH), 120.3 (C), 119.6 (CH), 110.7 (CH), 80.8 (C), and 47.5 (CH2). Additionally, some two-dimensional experiments (COSY, HMQC, and HMBC) were used for the proposal of each signal and the confirmation of the proposed structure (Figures S15–S19 in Supplementary Materials).
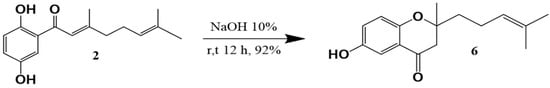
Figure 2.
Synthesis of ceanothichromone (6).
2.2. Antifungal Potential of Chemical Constituents of P. ceanothifolium
The antifungal potential of the chemical constituents 1–6 was determined by evaluating its inhibitory capacity on mycelial growth, fungicide–fungistatic effect, and inhibition of conidia germination against M. roreri, L. theobromae, and F. solani
2.2.1. Inhibitory Capacity on Mycelial Growth
The results of antifungal activity expressed as half-maximal inhibitory concentration (IC50) are summarized in Table 2. The results show that compound 5 was the only one that did not cause any inhibition on the three fungi evaluated. The IC50 values for active compounds against the three fungi range from 16.9 μM to 199.2 μM. These results constitute the first report of antifungal activity against M. roreri, L. theobromae, and F. solani for all evaluated compounds (1 to 6).

Table 2.
Inhibitory capacity on mycelial growth of chemical constituents from P. ceanothifolium.
The results of antifungal activity against F. solani show that chromenes 4 and 6 are the most active. Comparing the IC50 values of compounds 1, 2, and 3, it is observed that the presence of a double bond at position 2′ of the aliphatic chain of prenylated hydroquinones, forming an α, β-unsaturated carbonyl system, increases the antifungal activity. In addition, the geometry of the double bond has a significant effect as the E double bond enhances the antifungal activity, making compound 2 twice as active as compound 3. It is also found that the presence of the prenylated chain in hydroquinones is necessary to exert the antifungal activity, which is confirmed by the inactivity of compound 5 against F. solani. Furthermore, it can be concluded that the formation of a chromone significantly improves the antifungal activity against this phytopathogen when comparing the activity of compound 6 with its precursor 2, the latter being twice as inactive. These results are consistent with previous studies on compounds from Piper species, which have shown that prenylated chromenes and chromones tend to exhibit greater antifungal activity [29,32,38].
The results of antifungal activity against L. theobromae show that the prenylated hydroquinones 1 and 2 were the most active substances in inhibiting the growth of L. theobromae, with 1 being approximately twice as active as 2, which allows us to conclude that the presence of the hydroxyl group in the 3′ position of the prenylated chain has a positive effect on the antifungal activity. It is also observed that compound 2 is approximately twice as active as its isomer 3, demonstrating that the E-isomerism of the double bond at the 2′ position allows better antifungal effects, which has also been observed for F. solani. Furthermore, it is evident that cyclic compounds of the chromene 4 and chromone 6 type are not as promising compared to the results obtained against F. solani. In the case of the open-chain benzoic acid, compound 1 is the only one that does not present significant differences with respect to mancozeb, so it can be considered the one with the greatest potential against L. theobromae.
The results of antifungal activity against M. roreri indicate that the most active compound was prenylated hydroquinone 2, followed by its Z-isomer (compound 3). Prenylated hydroquinones with a double bond at the 2′ position of the oxo-geranyl chain (compounds 2 and 3) proved to be the most active, highlighting the importance of this double bond for antifungal activity against M. roreri. Like other pathogens, the E isomer was more active than the Z isomer. Chromene 4 was evaluated for the first time against this pathogen; however, a previous study by Chitiva et al. [32] identified two chromenes with activity comparable to mancozeb against M. roreri. One of these compounds was the chromene known as 2,2-dimethyl-8-(3′,3′-dimethylallyl)-2H-1-chromene-6-carboxylic acid (IC50 = 2.9 µM), a positional isomer of compound 4 that differs in the position of the isopentenyl group. The chromene evaluated by Chitiva et al. [32] was approximately 70 times more active than compound 4 (IC50 = 199.2 µM), suggesting that the position of the prenyl chain has a significant impact on the antifungal activity of these compounds. None of the compounds tested achieved inhibition levels like the positive controls, and M. roreri was the least susceptible fungus to treatment with these compounds.
2.2.2. Fungicide–Fungistatic Effect
The fungicidal or fungistatic activity of the compounds with inhibitory potential against the three microorganisms studied was evaluated using a maximum concentration of 100 μg/mL. The prenylated hydroquinones (1 to 3), the chromene (4), and the chromone (6) showed a fungistatic effect, while the controls (mancozeb and benomyl) showed a fungicidal effect against the three phytopathogens evaluated (see Figure S21 in the Supplementary Materials). The specific mechanism responsible for the fungicidal activity has not yet been determined, although some authors have linked it to the inhibition of ergosterol, chitin, and/or glucan biosynthesis in the fungal cell wall as these components are essential for cell structure and are considered key inhibition sites in fungi [46,47]. This study represents the first analysis of the fungicidal–fungistatic effect of the five bioactive compounds (1 to 4, and 6) on M. roreri, F. solani, and L. theobromae and contributes to the knowledge of the post-treatment effects of the evaluated compounds.
2.2.3. Inhibition of Conidia Germination
Conidia are the main vector for fungal dissemination to their host [47,48,49]; therefore, it is essential to evaluate the ability of the compounds to inhibit their germination. To this end, a conidial germination inhibition assay was performed using the compounds with the highest antifungal potential (1 to 4, and 6). The results, expressed as the percentage inhibition of conidia germination (% ICG), ranged from 25.5% to 85.2% (Table 3), indicating that the inhibitory capacity of the compounds also affects the reproductive structures of the fungi. Specifically, compound 6 was the most effective in inhibiting conidial germination in F. solani and L. theobromae, while compound 1 showed the best results in M. roreri. Although none of the compounds exceeded the inhibition percentage achieved by the positive controls at the concentration evaluated (IC50), most compounds were able to reduce conidial germination by more than 50% in all three microorganisms. This study represents the first report on the inhibition of conidial germination in M. roreri, F. solani, and L. theobromae of the evaluated compounds.

Table 3.
Percentages of inhibition of conidial germination (% ICG) of Piper derived compounds with antifungal activity against M. roreri, F. solani, and L. theobromae.
3. Materials and Methods
3.1. General Experimental Procedures
Vacuum liquid chromatography (VLC) was performed on SiliaPlateTM silica gel F254 of size 5–20 μm (SiliCycle® Inc., Quebec, QC, Canada). Flash chromatography (FC) was performed on SiliaFlash® silica gel P60 of size 40–63 μm (SiliCycle® Inc., Quebec, QC, Canada). Thin-layer chromatography (TLC) was performed on SiliaPlateTM alumina plates pre-coated with silica gel 60 F254 (SiliCycle® Inc., Quebec, Canada). The solvents used in the chromatographic techniques were of technical grade and were distilled before use. Melting points (m.p.s) were recorded on a Thermo Scientific 00590Q Fisher-Johns apparatus (Thermo Scientific®, Waltham, MA, USA). The 1H-NMR and APT, and 2D (COSY, HMQC, HMBC and NOESY) spectra were performed on a Bruker Advance AC-400 spectrometer (Bruker®, Leipzig, Germany) for the 1H-NMR and APT experiments, operating at 400 MHz for 1H-NMR and 100 MHz for APT. For high-resolution mass spectrometry analysis (HRMS), an LC-MS-TOF system (Shimadzu®, Duisburg, Germany) was used. The ionization method was operated with ESI in positive ion mode.
3.2. Strains and Fungal Growth Conditions
The fungi used for the bioassays were isolated from the organs of cacao plants with symptoms of vascular diseases. The molecular characterization of the fungi was carried out using the Sanger technique using the general primers ITS1 and ITS4. The sequences were compared with those reported in GenBank, finding that they have a 99% identity with Moniliophtora roreri, Lasiodiploidea theobromae, and Fusarium solani. The fungi were cryopreserved in 25% glycerol in cryovials at −80 °C, and for each bioassay they were activated following the methodology reported in the literature [47,48]. The strains were used after 5 days of growth for F. solani, 15 days for M. roreri, and 8 days for L. theobromae (Figure S21).
3.3. Plant Material
The inflorescences of P. ceanothifolium Kunth were collected in the rural part of the El Colegio municipality located in the department of Cundinamarca, Colombia (4.37392, −74.40591). The determination of the species was made by the biologist Ricardo Callejas, and a specimen has been deposited in the Herbario de la Universidad de Antioquia (HUA) with the collection number 217583.
3.4. Extraction and Isolation
The dried and ground inflorescences of P. ceanothifolium (450 g) were subjected to extraction with 96% ethanol using the maceration method at room temperature. In the extraction process, enough solvent was placed to cover the sample, and four extractions were carried out with changes of solvent every third day. The resulting solution was concentrated by distillation under reduced pressure to obtain 75 g of ethanolic extract. A part of the extract (74 g) was fractionated by VLC using solvents of different polarity: dichloromethane (DCM), ethyl acetate (EtOAc), isopropanol (IPA), and an ethanol–water mixture (EtOH:H2O 80:20). After evaporation of the solvents under reduced pressure, the fractions DCM (40 g), EtOAc (11 g), IPA (8 g), and EtOH-H2O (1 g) were obtained. These fractions were evaluated in the mycelial inhibition assay against F. solani, M. roreri, and L. theobromae, which led to the determination that the DCM fraction is active against both phytopathogens. The DCM fraction (40 g) was subjected to FC using a mixture of hexane/AcOEt with increasing polarity (80:20 to 50:50) as the mobile phase, which led to us obtaining six fractions (1–6). Fractions 1 and 2 were combined (29.4 g), and through successive FC with different mixtures of hexane/DCM/AcOEt (80:10:10; 70:15:15; 50:25:25), allowed the isolation of a white crystalline solid (1, 149 mg, m.p. 82–83 °C), an orange crystalline solid (2, 1.6 g, m.p. 74–75 °C), and a yellow oil (3, 215 mg). The pooled fractions of 3 and 4 (9.3 g) were subjected to FC with hexane/DCM/AcOEt (60:20:20), allowing the isolation of a white solid (4, 281 mg, m.p. 48–49 °C). Fractions 5 and 6 were combined (5.5 g) and subjected to FC using hexane/AcOEt (50:50) as the mobile phase, which led to the isolation of a yellow crystalline solid (5, 22 mg, m.p. 52–53 °C). The general purification diagram carried out on the DCM fraction from P. ceanothifolium is illustrated in Scheme S1 of the Supplementary Materials.
Ceanothifolione (1): A white solid with a m.p. of 82–83 °C. 1H-NMR (400 MHz, CDCl3): δH 7.39 (d, J = 3.2 Hz, 1H, H-3), 7.09 (dd, J = 8.9, 3.2 Hz, 1H, H-5), 6,84 (d, J = 8.9 Hz, 1H, H-6), 5.06 (t, J = 7.2 Hz, 1H, H-6′), 2.78 (d, J = 16.7 Hz, 1H, H-2′), 2.66 (d, J = 16.7 Hz, 1H, H-2′), 2.12–2.08 (q, 2H, H-5′), 1.83–1.76 (m, 1H, H-4′), 1.67 (s, 3H, H-8′), 1.57 (s, 3H, H-9′), and 1.40 (s, 3H, H-10′). APT (100 MHz, CDCl3): δC 194.0 (C-1′), 154.2 (C-1), 150.1 (C-4), 132.3 (C-7′), 125.4 (C-5), 123.3 (C-6′), 120.2 (C-2), 119.6 (C-6), 110.6 (C-3), 80.8 (C-3′), 47.4 (C-2′), 39.0 (C-4′), 25.6 (C-8′), 23.8 (C-10′), 22.2 (C-5′), and 17.6 (C-9′). HRMS (ESI) calc. for C16H22O4 [M + H]+: 278.1518, found: 278.1462. The spectroscopic data can be consulted in Figures S1–S5 in Supplementary Materials.
Debromocymopolone (2): An orange solid with a m.p. of 74–75 °C. 1H-NMR (400 MHz, CDCl3): δH 12.41 (s, 1H, OH), 7.25 (d, J = 3.0 Hz, 1H, H-3), 7.00 (dd, J = 8.9, 3.0 Hz, 1H, H-5), 6.88 (d, J = 8.9 Hz, 1H, H-6), 6.68 (s, 1H, H-2′), 5.35 (s, 1H, OH), 5.13 (t, J = 7.0 Hz, 1H, H-6′), 2.30–2.24 (m, 4H, H-5′y H-4′), 2.20 (s, 3H, H-10′), 1.73 (s, 3H, H-8′), and 1.64 (s, 3H, H-9′). APT (100 MHz, CDCl3): δC 196.9 (C-1′), 161.9 (C-3′), 157.9 (C-1), 148.1 (C-4), 133.6 (C-7′), 124.9 (C-5), 123.6 (C-6′), 121.2 (C-2), 120.3 (C-2′), 119.8 (C-6), 115.7 (C-3), 42.3 (C-4′), 26.9 (C-5′), 26.2 (C-10′), 20.8 (C-8′), and 18.8 (C-9′). The spectroscopic data were consistent with those reported in the literature for debromocypolone [42,43,44]. The spectroscopic data can be consulted in Figures S6 and S7 in Supplementary Materials.
1,4-dihydroxy-2-(3′,7′-dimethyl-1′-oxo-2′-Z-6′-octadienyl)-benzene (3): A yellow liquid. 1H- NMR (400 MHz, CDCl3): δH 12.50 (s, 1H, OH), 7.26 (d, J = 3.0 Hz, 1H, H-3), 7.02 (dd, J = 8.8, 3.0 Hz, 1H, H-5), 6.88 (d, J = 8.8 Hz, 1H, H-6), 6.65 (s, 1H, H-2′), 5.14 (t, J = 7.6 Hz, 1H, H-6′), 2.62 (t, J = 7.6 Hz, 2H, H-4′), 2.22 (q, J = 7.5 Hz, 2H, H-5′), 2.01 (s, 3H, H-10′), 1.65 (s, 3H, H-8′), and 1.63 (s, 3H, H-9′). APT (100 MHz, CDCl3): δC 195.5 (C-1′), 161.5 (C-3′), 156.9 (C-1), 147.2 (C-4), 132.4 (C-7′), 124.1 (C-5), 123.3 (C-6′), 120.3 (C-2), 120.0 (C-2′), 118.9 (C-6), 114.9 (C-3), 34.3 (C-4′), 26.6 (C-5′), 25.8 (C-8′), 25.4 (C-10′), and 17.5 (C-9′). The spectroscopic data were consistent with those reported in the literature [42,43,44]. The spectroscopic data can be consulted in Figures S8 and S9 in Supplementary Materials.
lhotzcromene (4): A white solid with a m.p. of 48–49 °C. 1H-NMR (400 MHz, CDCl3): δH 11.7 (s, 1H, OH), 7.89 (dd, J = 8.5, 2.2 Hz, 1H, H-7), 7.76 (d, J = 2.2 Hz, 1H, H-5), 6.82 (d, J = 8.5 Hz, 1H, H-8), 6.42 (d, J = 10.0 Hz, 1H, H-4), 5.63 (d, J = 10.0 Hz, 1H, H-3), 5.10 (t, J = 5.6, 1.4 Hz, 1H, H-3′), 2.14–2.11 (m, 2H, H-2′), 1.84–1.76 (m, 1H, H-1′), 1.72–1.65 (m, 1H, H-1′), 1.67 (s, 3H, H-1″),1.58 (s, 3H, H-5′), and 1.44 (s, 3H, H-6′). APT (100 MHz, CDCl3): δC 172.3 (C-9), 158.4 (C-8a), 131.9 (C-7), 131.8 (C-4′), 129.9 (C-3), 128.8 (C-5), 123.8 (C-3′), 122.2 (C-4), 121.4 (C-6), 120.5 (C-4a), 116.0 (C-8), 80.0 (C-2), 41.7 (C-1′), 27.1 (C-1″), 25.6 (C-5′), 22.7 (C-2′), and 17.6 (C-6′). The spectroscopic data were consistent with those reported in the literature for lhotzchromene [47]. The spectroscopic data can be consulted in Figures S11 and S12 in Supplementary Materials.
Ceanothifoliol (5): A yellow solid with a m.p. of 64–66 °C. 1H-NMR (400 MHz, acetone-d6): δH 11.75 (s, 1H, OH), 8.14 (s, 1H, OH), 7.31 (d, J = 3.0 Hz, 1H, H-3), 7.11 (dd, J = 8.8, 3.0 Hz, 1H, H-5), 6.82 (d, J = 8.8 Hz, 1H, H-6), 2.86 (s, 3H, H-2′), and 2.63 (s, 3H, H-1″). APT (100 MHz, acetone-d6): δC 155.6 (C-1), 149.3 (C-4), 124.8 (C-2), 119.4 (C-3), 118.5 (C-6), 115.5 (C-5), 88.6 (C-1′), 29.6 (C-2′), and 26.0 (C-1″). The spectroscopic data can be consulted in Figures S13 and S14 in Supplementary Materials.
3.5. Preparation of Ceanothichromone 6 Starting from 2
Compound 2 was subjected to an intramolecular cyclization reaction of the oxo-Michael type, adapting the methodology described in the literature for similar compounds [43]. In a typical experiment, 100.0 mg of 2 (0.100 mmol) and 10.0 mL of 10% NaOH were added to a round-bottomed flask. The resulting mixture was continuously stirred at room temperature for a period of approximately 12 h. The reaction was monitored by TLC to verify the disappearance of compound 2. Subsequently, 10% HCl was added slowly until the mixture was neutralized and then a liquid–liquid extraction was carried out with AcOEt (3 × 25 mL). The organic phases were combined, dried over anhydrous Na2SO4, and the solvent was distilled off under reduced pressure. In this way, a brown oily liquid corresponding to compound 6 (92.0 mg, 92.0%) was obtained.
Ceanothichromone (6): A brown oily liquid. 1H-NMR (400 MHz, CDCl3): δH 2.80 (d, J = 16.7 Hz, 1H, H-3), 7.38 (s, 1H, H-5), 7.09 (dd, J = 9.1, 3.1 Hz, 1H, H-7), and 6.87 (d, J = 9.1 Hz, 1H, H-8). APT (100 MHz, CDCl3): δC 194.8 (CO), 157.1 (C-8a), 154.5 (C-6), 125.0 (C-7), 120.3 (C-4a), 119.6 (C-3′), 110.7 (C-8), 80.8 (C-2), and 47.5 (C-3). The spectroscopic data can be consulted in Figure S15 and Figure S19 in Supplementary Materials.
3.6. Antifungal Potential of Chemical Constituents of P. ceanothifolium
3.6.1. Inhibition of Mycelial Growth Assay
The antifungal potential against M. roreri, F. solani, and L. theobromae of the compounds was determined by the inhibition of mycelial growth assay, adapting the poisoned food technique with some modifications [50]. Stock solutions of the compounds were prepared with concentrations between 2000 μg/mL and 62.5 μg/mL in EtOH and then these were mixed with the potato dextrose agar (PDA) medium to obtain final concentrations per well of 100 μg/mL, 50 μg/mL, 25 μg/mL, 12.5 μg/mL, 6.5 μg/mL, and 3.2 μg/mL. In the center of each well, a 2 mm-diameter plug of fungus was inoculated and the boxes were incubated at 25 °C (15 days for M. roreri, 3 days for F. solani, and 8 days for L. theobromae). 2% ethanol was used as the negative control, PDA medium was used as the blank, and benomyl and mancozeb were used as the positive controls. After the incubation time, the plates were scanned and the mycelial growth area in each well was determined using the IMAGE J image processing program. The data obtained were used to determine the mycelial growth inhibition percentage (% ICM) using Equation (1), which is as follows:
where C is the radial growth of the fungus in the blank and T the radial growth of the fungus with the treatment.
% ICM = [(C − T)/C] × 100
With the % ICM and the concentrations evaluated, the IC50 were determined by means of a non-linear regression analysis using the GraphPad Prism 8 program. All the reported results correspond to the average of nine independent replicates (n = 9), together with its 95% confidence interval.
3.6.2. Determination of the Fungicidal or Fungistatic Effect
The fungicidal or fungistatic effect was determined for the active compounds following the methodology reported in the literature with some modifications [51]. The plug of the fungus used in each of the wells where no apparent growth was observed when evaluating a concentration of 100 µg/mL of each compound in the mycelial growth inhibition assay was inoculated into sterile PDA medium. It was incubated at 25 °C for 15 days for M. roreri, 8 days for L. theobromae, and 5 days for F. solani. A fungistatic effect was defined as the development of a fungal colony from the inoculum, while the absence of colony growth was a fungicidal effect. For each compound, three independent tests were performed, each with nine independent replicates (n = 9). Benomyl and mancozeb were used as positive controls.
3.6.3. Inhibition of conidial germination
The percentage of inhibition of conidial germination for the active compounds for which the IC50 was determined was done following the methodology reported in the literature with some modifications [52]. The method involved mixing a test tube of culture medium (glucose-yeast extract for L. theobromae and agar-water for F. solani and M. roreri) with the solution of the compound to be evaluated, ensuring that the concentration end of the compound was the respective IC50. The mixture was allowed to solidify in a Petri dish, then 10 μL of a conidial suspension (105 conidia/mL) was applied and a coverslip was placed on top. Each assembly was incubated at 35 °C and the conidial germination reading was performed after 7 h for F. solani and 72 h for L. theobromae and M. roreri. For the conidial germination reading, 20 conidia were counted in 5 different fields, for a total of 100 conidia, and it was determined that the germinated conidia were those that presented twice the length of the germ tube with respect to the length of the same conidium. The percentage of inhibition of conidia germination (% ICG) was determined with Equation (2), which is as follows:
where B is the conidia germinated in the control (without treatment) and T is the conidia germinated in the treatment.
% ICG = [(B − T)/B] × 100
Benomyl and mancozeb were used as positive controls, evaluated at their respective IC50. For each trial, three independent trials were performed, each with nine independent replicates (n = 9). The % ICGs were calculated, which are reported together with their standard deviations.
3.7. Data Analysis
The one-way ANOVA statistical test was performed considering the assumptions of the test (normality, homogeneity of variances, independence, randomness, and outliers) to determine if there were significant differences in the trials. The data that presented significant differences were subjected to additional multiple comparison tests, such as a Dunnet for normal data, to confirm in which group the differences occurred. These analyses were performed in the Graphpad Prism 8 statistical program. All the results reported correspond to the mean of nine independent replicates (n = 9) and their respective standard deviation, using a statistical significance of p < 0.05, p < 0.005, p < 0.001, and p < 0.0001.
4. Conclusions
This study corresponds to the first report on the chemical composition and antifungal activity of substances from P. ceanothifolium, highlighting the first report in the literature for ceanothifolione 1, ceanothifoliol 5, and ceanothichromone 6. In addition, this research provides the first evidence of fungicidal and fungistatic effects, and the inhibition of conidial germination for compounds 1–6 against three phytopathogens of importance in cocoa cultivation (M. roreri, F. solani, and L. theobromae). The strong antifungal activity observed, particularly for 1, 2, and 6, indicates the potential of these bioactive compounds as promising biocontrol agents for the management of major diseases in cocoa crops.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14060934/s1, The Supplementary Materials are available online and correspond to the isolation scheme of compounds 1–5 from P. ceanothifolium (Scheme S1). 1D and D NMR Spectra of compounds 1 to 6 (Figures S1–S19). Finally, Strains of phytopathogenic fungi used in the bioassays: and the results of the fungicide and fungistatic assay of the compounds with the greatest porcentage inhibition of mycelial growth of phytopathogenic associated of cocoa (Figures S20 and S21).
Author Contributions
Conceptualization, O.J.P.-L. and J.A.P.-R.; methodology, Y.S.M.-J., O.J.P.-L. and J.A.P.-R.; formal analysis, Y.S.M.-J., O.J.P.-L. and J.A.P.-R.; study investigation, Y.S.M.-J.; data curation and interpretation, Y.S.M.-J., O.J.P.-L. and J.A.P.-R.; original draft preparation, J.A.P.-R. and O.J.P.-L.; supervision, O.J.P.-L. and J.A.P.-R.; funding acquisition, O.J.P.-L. and J.A.P.-R. All authors contributed to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad Nacional de Colombia (ID 42562), Pontificia Universidad Javeriana (ID PPTA 7748), and Ministerio de Ciencia Tecnología e Innovación (MINCIENCIAS) through the project with contract 403-2020, announcement 802 of 2018.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors extend their gratitude to the research groups (QUIPRONAB and BioMolUN belonging to the Universidad Nacional de Colombia, and GIFUJ belonging to the Pontificia Universidad Javeriana) for their collaboration in the development of the research. Moreover, to the contract of access to genetic resources and derived products No. 121 of 01/22/2016, with OTROSI No. 21 celebrated between the Universidad Nacional de Colombia and the Ministerio de Ambiente y Desarrollo Sostenible.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vásquez, Z.; De Calvalho Neto, D.; Pereira, G.; Vandenberghe, L.; De Oliveira, P.; Tiburcio, P.; Rogez, H.; Neto, A.; Soccol, C. Biotechnological approaches for cocoa waste management: A review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.S.; Ahmad, S.; Jan, K.; Bashir, K. Status, supply chain and processing of cocoa—A review. Trends Food Sci. Technol. 2017, 66, 108–116. [Google Scholar] [CrossRef]
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and dark chocolate polyphenols: From biology to clinical applications. Front. Immunol. 2017, 15, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, K.; Jegadeeswari, V. Evaluating the processed beans of different cocoa (Theobroma cacao L.) accessions for quality parameters. J. Phytol. 2019, 11, 1–4. [Google Scholar]
- Sriwana, I.K.; Arkeman, Y.; Syah, D.; Marimin, M. Sustainability improvement in cacao supply chain agro-industry. World Rev. Sci. Technol. Sustain. Dev. 2017, 13, 256–275. [Google Scholar] [CrossRef]
- Antolinez, E.; Almanza, P.J.; Barona, A.F.; Polanco, E.; Serrano, P.A. Current State of Cocoa Plantation: A Review of its Main Limitations. Cienc. Agric. 2020, 17, 1–11. [Google Scholar] [CrossRef]
- ICCO: International Cocoa Organization. Available online: https://www.icco.org/ (accessed on 9 September 2024).
- Cilas, C.; Bastide, P. Challenges to Cocoa Production in the Face of Climate Change and the Spread of Pests and Diseases. Agronomy 2020, 10, 1232. [Google Scholar] [CrossRef]
- Aneani, F.; Adu-Acheampong, R.; Sakyi-Dawson, O. Exploring opportunities for enhancing innovation in agriculture: The case of cocoa (Theobroma cacao L.) production in Ghana. J. Sustain. Agric. Res. 2018, 7, 33–53. [Google Scholar] [CrossRef][Green Version]
- Somarriba, E.; Peguero, F.; Cerda, R.; Orozco-Aguilar, L.; López-Sampson, A.; Leandro-Muñoz, M.E.; Jagoret, P.; Sinclair, F.L. Rehabilitation and renovation of cocoa (Theobroma cacao L.) agroforestry systems. A review. Agron. Sustain. Dev. 2021, 41, 64. [Google Scholar] [CrossRef]
- Perez, V. Moniliophthora roreri H.C. Evans et al. and Moniliophthora perniciosa (Stahel) Aime: Impact, symptoms, diagnosis, epidemiology and management. Rev. De Protección Veg. 2018, 33, 1–13. [Google Scholar]
- Flores, V.J.; Gomez, L.; Lopez, J.A. Mechanisms of endogenous infection in cocoa fruits with Moniliophthora roreri. Polibotanica 2022, 53, 197–209. [Google Scholar] [CrossRef]
- Evans, H.C. Witches’ broom disease (Moniliophthora perniciosa): History and biology. In Cacao Diseases: A History of Old Enemies and New Encounters; Springer Nature: Berlin/Heidelberg, Germany, 2016; pp. 137–177. [Google Scholar]
- Pérez, L. Moniliophthora roreri H.C. Evans et al. y Moniliophthora perniciosa (Stahel) Aime: Impacto, síntomas, diagnóstico, epidemiología y manejo. Rev. Protección 2018, 33, 1–13. [Google Scholar]
- Harrington, T.C.; Ferreira, M.A.; Somasekhara, Y.M.; Vickery, J.; Mayers, C.G. An expanded concept of Ceratocystis manginecans and five new species in the Latin American Clade of Ceratocystis. Mycologia 2023, 116, 184–212. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, O.G.; Molano, E.P.; José, J.; Alvarez, J.C.; Pereira, G.A. Ceratocystis Wilt Pathogens: History and Biology-Highlighting C. cacaofunesta, the Causal Agent of Wilt Disease of Cacao. In Cacao Diseases: A History of Old Enemies and New Encounters; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 383–428. [Google Scholar]
- Hernández, A.; Ruíz, Y.; Acebo, Y.; Miguélez, Y.; Heydrich, M. Antagonistas microbianos para el manejo de la pudrición negra del fruto en Theobroma cacao L. estado actual y perspectivas de uso en Cuba. Rev. Protección Veg. 2014, 29, 11–19. [Google Scholar]
- Moreira, A.; Cedeño, V.; Canchignia, F.; Garcés, R. Lasiodiplodia theobromae (Pat.) Griffon y Maubl [(syn.) Botryodiplodia theobromae Pat] in the cocoa crop: Symptoms, biological cycle, and strategies management. Sci. Agropecu. 2021, 12, 653–662. [Google Scholar] [CrossRef]
- Pisco, M.P.A. Lasiodiplodia theobromae causing dieback on Theobroma cacao in Colombia. New Dis. Rep. 2024, 49, e12266. [Google Scholar] [CrossRef]
- Kennard, N.; Vagnoni, C. Pesticides and health. In UK Parliament Post 2021; UK Parliament: London, UK, 2021. [Google Scholar] [CrossRef]
- Abbas, M.; Hussain, D.; Ramzan, M.; Saleem, M.J.; Abbas, S.; Hussain, N.; Irshad, M.; Hussain, K.; Ghouse, G.; Khaliq, M.; et al. Review on integrated disease and pest management of field crops. Int. J. Trop. Insect Sci. 2022, 42, 3235–3243. [Google Scholar] [CrossRef]
- Bello, L.A.; Hernandez, M.; Santana, R.; Romero, H. Bioactive compounds: Uses of plant extracts in plant-based foods. Handb. Plant-Based Food Drink. Des. 2024, 10, 45–57. [Google Scholar] [CrossRef]
- Gwinn, K.D. Bioactive Natural Products in Plant Disease Control. Stud. Nat. Prod. Chem. 2018, 56, 229–246. [Google Scholar] [CrossRef]
- Brusotti, G.; Cesari, I.; Dentamaro, A.; Caccialanza, G.; Massolini, G. Isolation and characterization of bioactive compounds from natural resources: Metabolomics and molecular approaches. Evol. Divers. Source Anticancer. Mol. 2021, 87, 77–101. [Google Scholar] [CrossRef]
- Ladino, C. Potencialidad del género Piper Como Fuente de Sustancias Para el Control de Hongos Fitopatógenos. Master’s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2018. [Google Scholar]
- Pineda, R.; Vizcaíno, S.; García, C.; Gil, J.; Durango, D. Chemical composition and antifungal activity of Piper auritum Kunth and Piper holtonii C. DC. against phytopathogenic fungi. Chil. J. Agric. Res. 2012, 72, 507. [Google Scholar] [CrossRef]
- Tangarife, V.; Correa, J.; Roa, V.; Pino, N.; Betancur, L.; Durán, D.; Mesa, A. Anti-Dermatophyte, Anti-Fusarium and Cytotoxic Activity of Essential Oils and Plant Extracts of Piper Genus. J. Essent. Oil Res. 2014, 26, 221–227. [Google Scholar] [CrossRef]
- Lago, J.; Ramos, C.; Casanova, D.; Morandim, A.; Bergamo, D.; Cavalheiro, A.; Kato, M. Benzoic Acid Derivatives from Piper Species and Their Fungitoxic Activity against Cladosporium cladosporioides and C. sphaerospermum. J. Nat. Prod. 2004, 67, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Parra, J.E.; Cuca, L.E.; González, A. Antifungal and phytotoxic activity of benzoic acid derivatives from inflorescences of Piper cumanense. Nat. Prod. Res. 2019, 35, 2763–2771. [Google Scholar] [CrossRef]
- Huaman, C.; Cabezas, O. Aceite de Matico (Piper aduncum) en el control de Moniliophthora roreri agente causal de la moniliasis en cacao. Peruv. Agric. Res. 2019, 1, 53–57. [Google Scholar] [CrossRef]
- Xu, W.-H.; Li, X.-C. Antifungal compounds from Piper Species. Curr Bioact Compd. 2011, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chitiva, L.C.; Ladino, C.; Cuca, L.E.; Prieto, J.A.; Patiño, O.J. Antifungal activity of chemical constituents from Piper pesaresanum C. DC. and derivatives against phytopathogen fungi of cocoa. Molecules 2021, 26, 32–56. [Google Scholar] [CrossRef]
- Cardenas, D.; Rincon, S.; Coy, E. Identification of Antifungal Compounds from Piper Plants Against Fusarium oxysporum: An Untargeted Metabolite Profiling-Based Approach. Nat. Prod. Commun. 2022, 17, 9995. [Google Scholar] [CrossRef]
- GBIF: Global Biodiversity Information Facility. Piper ceanothifolium Kunth. Available online: https://www.gbif.org/species/4185062 (accessed on 9 March 2025).
- Royal Botanic Gardens Kew. Piper ceanothifolium Kunth. Piper ceanothifolium Kunth. Plants of the World Online. Kew Science. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:282941-2 (accessed on 9 March 2025).
- Trujillo, W.; Hoyos, F. El Género Piper (Piperaceae) En La Reserva Natural Las Dalias, Municipio de La Montañita-Caquetá. Momentos Cienc. 2013, 10, 88–96. [Google Scholar]
- López, S.N.; Lopes, A.A.; Batista, J.M., Jr.; Flausino, O., Jr.; da Silva Bolzani, V.; Kato, M.J.; Furlan, M. Geranylation of benzoic acid derivatives by enzymatic extracts from Piper crassinervium (Piperaceae). Bioresour. Technol. 2010, 101, 4251–4260. [Google Scholar] [CrossRef]
- Danelutte, A.; João, G.; Lago, M.; Young and Kato, J. Antifungal Flavanones and Prenylated Hydroquinones from Piper crassinervium Kunth. Phytochemistry 2003, 64, 555–559. [Google Scholar] [CrossRef]
- Parra, J.E.; Delgado, W.A.; Cuca, L.E. Cumanensic acid, a new chromene isolated from Piper cf. cumanense Kunth (Piperaceae). Phytochem. Lett. 2011, 4, 280–282. [Google Scholar] [CrossRef]
- Yamaguchi, L.; Lago, J.; Tanizaki, T.; Di Mascio, P.; Kato, M. Antioxidant activity of prenylated hydroquinone and benzoic acid derivatives from Piper crassinervium Kunth. Phytochemistry 2006, 67, 1838–1843. [Google Scholar] [CrossRef] [PubMed]
- Gallimore, W.A.; Sambo, T.; Campbell, T. Debromocymopolone from the green alga, Cymopolia barbata. J. Chem. Res. 2009, 3, 160–161. [Google Scholar] [CrossRef]
- García, P.; Hernández, Á.; San Feliciano, A.; Castro, M. Bioactive prenyl-and terpenyl-quinones/hydroquinones of marine origin. Mar. Drugs 2018, 16, 292. [Google Scholar] [CrossRef]
- Stiasni, N.; Kappe, C. A tandem intramolecular Michael-addition/elimination sequence in dihydropyrimidone to quinoline rearrangements. Arkat USA 2012, 7, 71–79. [Google Scholar] [CrossRef]
- Marina, S.; Scott, S. Thin layer chromatography. Methods Enzymol. 2013, 533, 303–324. [Google Scholar] [CrossRef]
- De Moreira, D.; Guimarães, E.F.; Kaplan, M.A. A C-glucosylflavone from leaves of Piper lhotzkyanum. Phytochemistry 2000, 55, 783–786. [Google Scholar] [CrossRef]
- Hasim, S.; Coleman, J.J. Targeting the fungal cell wall: Current therapies and implications for development of alternative antifungal agents. Future Med. Chem. 2019, 11, 869–883. [Google Scholar] [CrossRef]
- Iskandarov, U.S.; Guzalova, A.G.; Davranov, K.D. Effects of nutrient medium composition and temperature on the germination of conidia and the entomopathogenic activity of the fungi. Prikl. Biokhimiia Mikrobiol. 2006, 42, 81–85. [Google Scholar]
- Amiri, A.; Cholodowski, D.; Bompeix, G. Adhesion and germination of waterborne and airborne conidia of Penicillium expansum to apple and inert surfaces. Physiol. Mol. Plant Pathol. 2005, 67, 40–48. [Google Scholar] [CrossRef]
- Osherov, G.; May, S. Los mecanismos moleculares de la germinación de conidios. FEMS Microbiol. Lett. 2001, 199, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Mohana, D.C.; Raveesha, K.A. Anti-fungal evaluation of some plant extracts against some plant pathogenic field and storage fungi. J. Agric. Technol. 2007, 4, 119–137. [Google Scholar]
- Jandaik, S.; Thakur, P.; Kumar, V. Efficacy of cow urine as plant growth enhancer and antifungal agent. Adv. Agric. 2015, 1, 1–7. [Google Scholar] [CrossRef]
- Catão, H.C.; Sales, N.; Azevedo, D.M.; Flavio, N.S.; Menezes, J.B.; Barbosa, L.V.; Martinez, R.A. Fungicides and alternative products in the mycelial growth and germination control of Alternaria tomatophila. Idesia 2013, 31, 21–28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).