Characteristics of Growth, Photosynthesis, C/N Ratio, and Antioxidant Capacity in the Seedling Stage of Aquilaria sinensis ‘Qinan’
Abstract
1. Introduction
2. Results
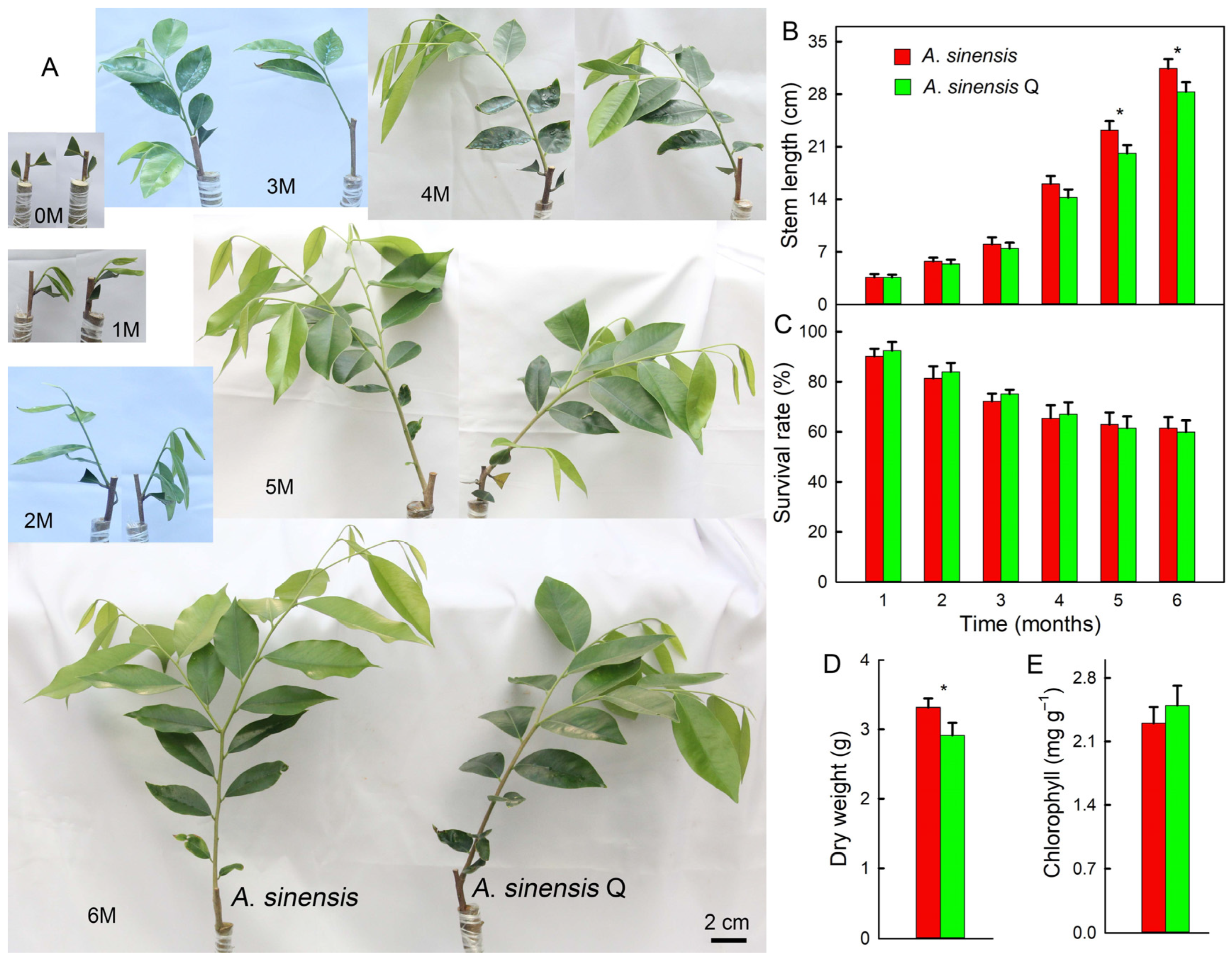
2.1. Growth
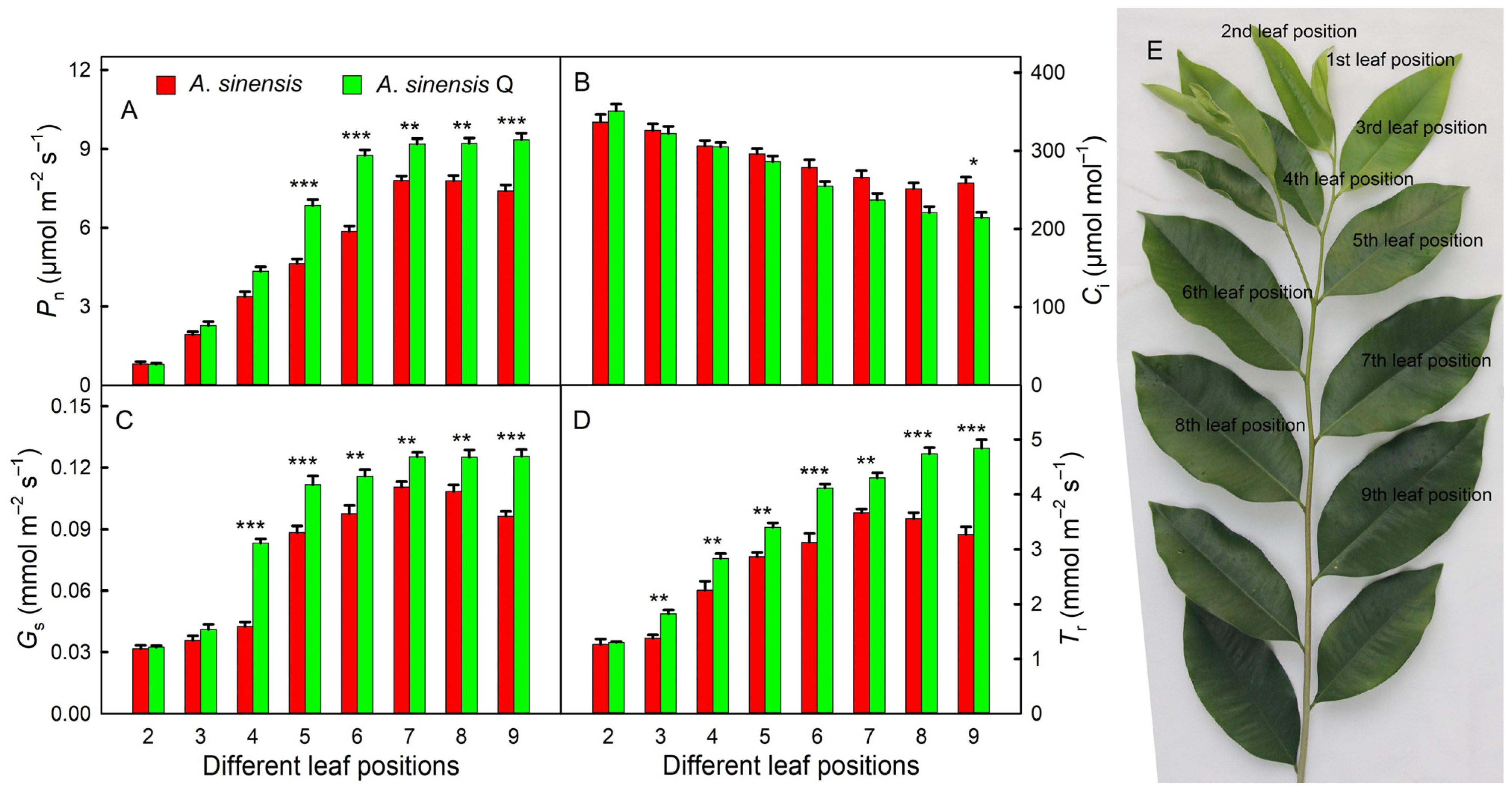
2.2. Gas Exchange Parameters
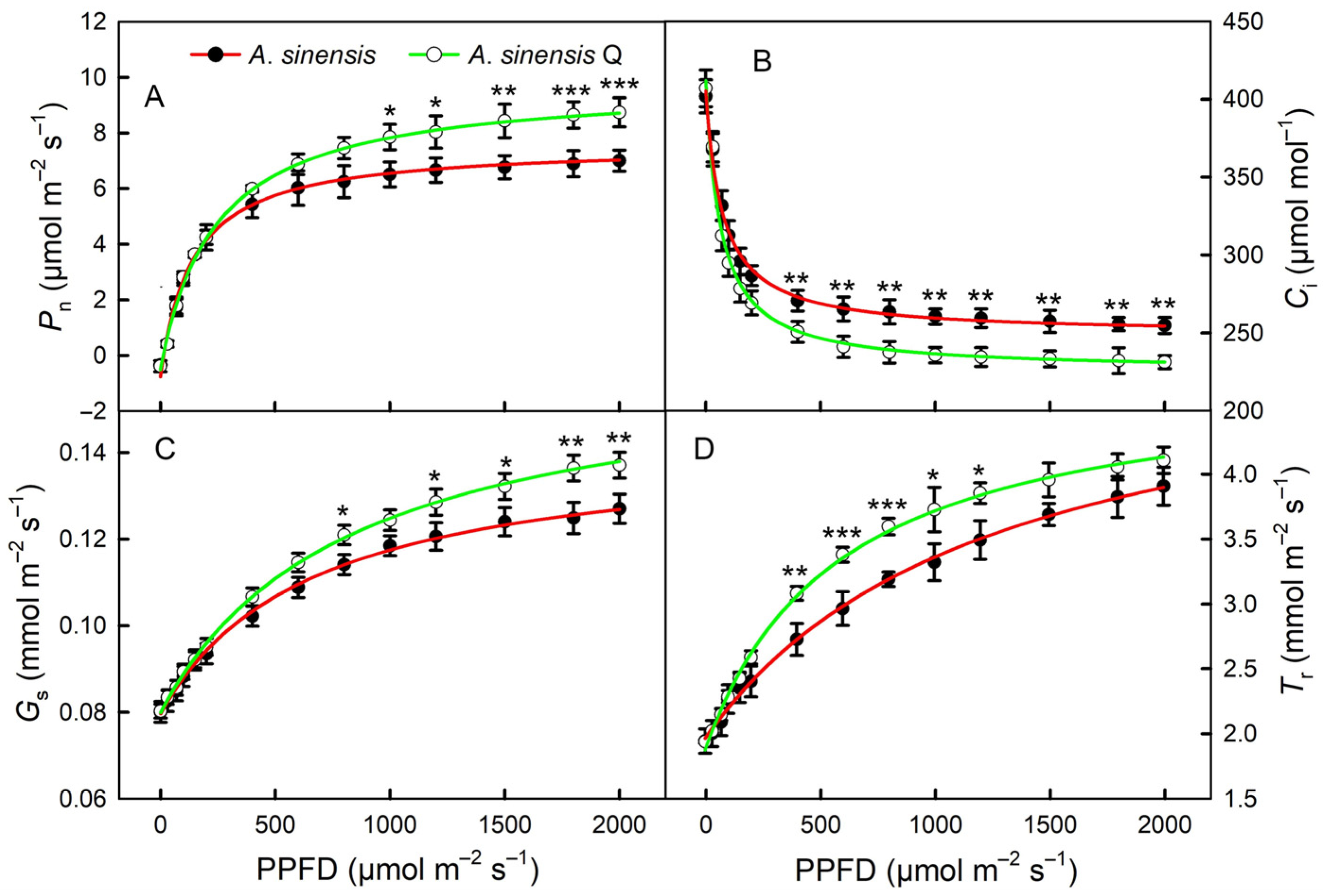
2.3. Light Response Curves
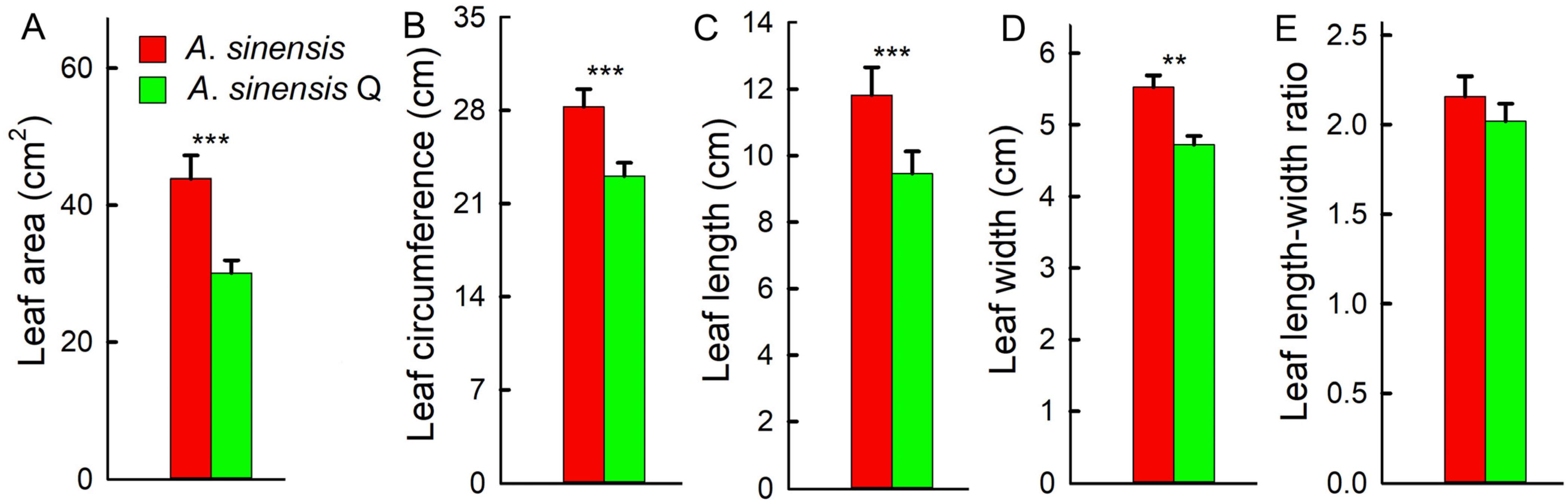
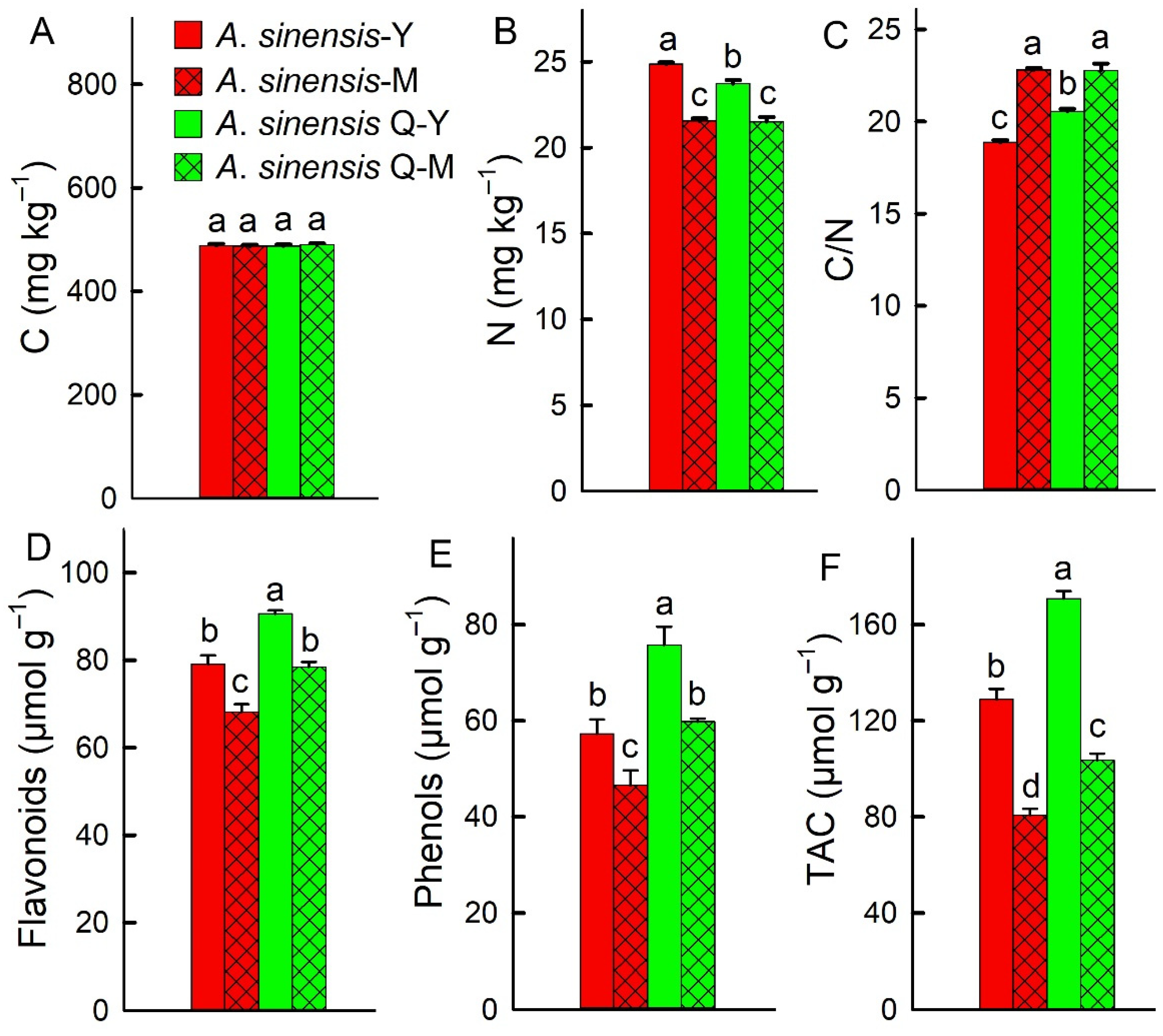
2.4. Leaf Parameters, C/N Ratio, and Antioxidant Capacity
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Gas Exchange, Leaf Parameters, and Chlorophyll Measurement
4.3. Carbohydrate and Nitrogen Content Measurement
4.4. Flavonoids, Phenols, and Total Antioxidant Capacity Measurement
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, P.; Li, X.F.; Cui, Z.Y.; Xu, D.P. Morphological, physiological, biochemical and molecular analyses reveal wounding-induced agarwood formation mechanism in two types of Aquilaria sinensis (Lour.) Spreng. Ind. Crops Prod. 2022, 178, 114603. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Jiang, C.; Zhou, J.H.; Zhao, Y.Y.; Huang, L.Q. Volatile organic compound and endogenous phytohormone characteristics during callus browning in Aquilaria sinensis. Ind. Crops Prod. 2021, 168, 113603. [Google Scholar] [CrossRef]
- Tan, C.S.; Isa, N.M.; Ismail, I.; Zainal, Z. Agarwood induction: Current developments and future perspectives. Front. Plant Sci. 2019, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Azren, P.D.; Lee, S.Y.; Emang, D.; Mohamed, R. History and perspectives of induction technology for agarwood production from cultivated Aquilaria in Asia: A review. J. For. Res. 2019, 30, 1–11. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Y.Y.; Feng, J.; Chen, D.L.; Yang, Y.; Liu, P.W.; Yu, Z.X.; Wei, J.H. Remarkable phytochemical characteristics of Chi-Nan agarwood induced from new-found Chi-Nan germplasm of Aquilaria sinensis compared with ordinary agarwood. Int. J. Anal. Chem. 2021, 2021, 5593730. [Google Scholar] [CrossRef]
- Hou, W.C.; Liu, P.W.; Liu, Y.Y.; Kang, Y.; Yang, Y.; Zhang, Y.X.; Gao, Z.H.; Yu, M.; Feng, J.; Lv, F.F.; et al. Chi-Nan agarwood germplasms constitute a new chemotype of Aquilaria sinensis (Lour.) Spreng. Ind. Crops Prod. 2022, 187, 115494. [Google Scholar] [CrossRef]
- Wang, X.H.; Gao, B.W.; Nakashima, Y.; Mori, T.; Zhang, Z.X.; Kodama, T.; Lee, Y.E.; Zhang, Z.K.; Wong, C.P.; Liu, Q.Q.; et al. Identification of a diarylpentanoid-producing polyketide synthase revealing an unusual biosynthetic pathway of 2-(2-phenylethyl) chromones in agarwood. Nat. Commun. 2022, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.N.; Xue, S.Y.; Song, J.; Zhou, X.R.; Zhou, D.H.; Liu, X.J.; Hong, Z.; Xu, D.P. Effects of various artificial agarwood-induction techniques on the metabolome of Aquilaria sinensis. BMC Plant Biol. 2021, 21, 591. [Google Scholar] [CrossRef]
- Lv, F.F.; Yang, Y.; Sun, P.W.; Zhang, Y.; Liu, P.W.; Fan, X.H.; Xu, Y.H.; Wei, J.H. Comparative transcriptome analysis reveals different defence responses during the early stage of wounding stress in germplasm and ordinary Aquilaria sinensis. BMC Plant Biol. 2022, 22, 464. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, X.Y.; Guo, X.R.; Xu, M.Y.; Tang, Z.H. A source-sink model explains the difference in the metabolic mechanism of mechanical damage to young and senescing leaves in Catharanthus roseus. BMC Plant Biol. 2021, 21, 154. [Google Scholar] [CrossRef]
- Fauset, S.; Freitas, H.C.; Galbraith, D.R.; Sullivan, M.J.P.; Aidar, M.P.M.; Joly, C.A.; Phillips, O.L.; Vieira, S.A.; Gloor, M.U. Differences in leaf thermoregulation and water use strategies between three co-occurring Atlantic forest tree species. Plant Cell Environ. 2018, 41, 1618–1631. [Google Scholar] [CrossRef]
- Leigh, A.; Sevanto, S.; Close, J.D.; Nicotra, A.B. The influence of leaf size and shape on leaf thermal dynamics: Does theory hold up under natural conditions? Plant Cell Environ. 2017, 40, 237–248. [Google Scholar] [CrossRef]
- Zhang, T.J.; Chow, W.S.; Liu, X.T.; Zhang, P.; Liu, N.; Peng, C.L. A magic red coat on the surface of young leaves: Anthocyanins distributed in trichome layer protect Castanopsis fissa leaves from photoinhibition. Tree Physiol. 2016, 36, 1296–1306. [Google Scholar] [CrossRef]
- Wu, Y.S.; Gong, W.Z.; Wang, Y.M.; Yong, T.W.; Yang, F.; Liu, W.G.; Wu, X.L.; Du, J.B.; Shu, K.; Liu, J.; et al. Leaf area and photosynthesis of newly emerged trifoliolate leaves are regulated by mature leaves in soybean. J. Plant Res. 2018, 131, 671–680. [Google Scholar] [CrossRef]
- Osnas, J.L.D.; Lichstein, J.W.; Reich, P.B.; Pacala, S.W. Global Leaf Trait Relationships: Mass, Area, and the Leaf Economics Spectrum. Science 2013, 340, 741–744. [Google Scholar] [CrossRef]
- Shang, B.; Xu, Y.S.; Dai, L.L.; Yuan, X.Y.; Feng, Z.Z. Elevated ozone reduced leaf nitrogen allocation to photosynthesis in poplar. Sci. Total Environ. 2019, 657, 169–178. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Wang, H.; Harrison, S.P.; Prentice, I.C.; Wright, I.J.; Peng, C.H.; Lin, G.H. Quantifying leaf-trait covariation and its controls across climates and biomes. New Phytol. 2019, 221, 155–168. [Google Scholar] [CrossRef]
- Chauvin, K.M.; Asner, G.P.; Martin, R.E.; Kress, W.J.; Wright, S.J.; Field, C.B. Decoupled dimensions of leaf economic and anti-herbivore defense strategies in a tropical canopy tree community. Oecologia 2018, 186, 765–782. [Google Scholar] [CrossRef]
- Gómez-Ocampo, G.; Cascales, J.; Medina-Fraga, A.L.; Ploschuk, E.L.; Mantese, A.I.; Crocco, C.D.; Matsusaka, D.; Sánchez, D.H.; Botto, J.F. Transcriptomic and physiological shade avoidance responses in potato (Solanum tuberosum) plants. Physiol. Plant. 2023, 175, e13991. [Google Scholar] [CrossRef]
- Weraduwagel, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar]
- Li, P.F.; Cheng, Z.G.; Ma, B.L.; Palta, J.A.; Kong, H.Y.; Mo, F.; Wang, J.Y.; Zhu, Y.; Lv, G.C.; Batool, A.; et al. Dryland wheat domestication changed the development of aboveground architecture for a well-structured canopy. PLoS ONE 2014, 9, e95825. [Google Scholar] [CrossRef] [PubMed]
- Medyouni, I.; Zouaoui, R.; Rubio, E.; Serino, S.; Ben Ahmed, H.; Bertin, N. Effects of water deficit on leaves and fruit quality during the development period in tomato plant. Food Sci. Nutr. 2021, 9, 1949–1960. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C.; John, G.P.; Poorter, H.; Mason, C.M.; Mendez-Alonzo, R.; Donovan, L.A. How do leaf veins influence the worldwide leaf economic spectrum? Review and synthesis. J. Exp. Bot. 2013, 64, 4053–4080. [Google Scholar] [CrossRef]
- Yu, Z.C.; Lin, W.; He, W.; Yan, G.Z.; Zheng, X.T.; Luo, Y.N.; Zhu, H.; Peng, C.L. Dynamic changes of the contents of photoprotective substances and photosynthetic maturation during leaf development of evergreen tree species in subtropical forests. Tree Physiol. 2023, 43, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Cascaes, M.M.; Guilhon, G.M.S.P.; Zoghbi, M.D.; Andrade, E.H.A.; Santos, L.S.; da Silva, J.K.R.; Uetanabaro, A.P.T.; Araújo, I.S. Flavonoids, antioxidant potential and antimicrobial activity of Myrcia rufipila mcvaugh leaves (myrtaceae). Nat. Prod. Res. 2021, 35, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Mu, P.Y.; Hu, Y.M.; Ma, X.; Shi, J.R.; Zhong, Z.D.; Huang, L.Y. Total flavonoids of Rhizoma Drynariae combined with calcium attenuate osteoporosis by reducing reactive oxygen species generation. Exp. Ther. Med. 2021, 21, 618. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Liu, X.J.; Li, X.F.; Fang, X.Y.; Xiong, Y.M.; Xu, D.P. Inducing Aquilaria sinensis (Lour.) Spreng “Qinan” agarwood formation with chemical reagents. Agronomy 2024, 14, 1727. [Google Scholar] [CrossRef]
- Yu, Z.C.; Zheng, X.T.; Lin, W.; Yan, G.Z.; He, W.; Luo, Y.N.; Lin, X.L.; Zhu, H.; Peng, C.L. Differences in leaf photoprotection strategies of tree species at different successional stages in subtropical forests under seasonal climate change and their relationship to construction cost strategies. Environ. Exp. Bot. 2023, 207, 105230. [Google Scholar] [CrossRef]
- Adam, A.Z.; Lee, S.Y.; Mohamed, R. Pharmacological properties of agarwood tea derived from Aquilaria (Thymelaeaceae) leaves: An emerging contemporary herbal drink. J. Herb. Med. 2017, 10, 37–44. [Google Scholar] [CrossRef]
- Kang, Y.F.; Chien, S.L.; Wu, H.M.; Li, W.J.; Chen, C.T.; Li, H.T.; Chen, H.L.; Chao, D.; Chen, S.J.; Huang, C.T.; et al. Secondary metabolites from the leaves of Aquilaria sinensis. Chem. Nat. Compd. 2014, 50, 1110–1112. [Google Scholar] [CrossRef]
- Coruzzi, G.M.; Zhou, L. Carbon and nitrogen sensing and signaling in plants: Emerging ‘matrix effects’. Curr. Opin. Plant Biol. 2001, 4, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Montes, E.; Tomás, M.; Escalona, J.M.; Bota, J.; Medrano, H. Leaf growth rate and nitrogen content determine respiratory costs during leaf expansion in grapevines. Physiol. Plant. 2019, 165, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Tian, L.D.; Querejeta, J.I. Tight coupling between leaf δ13C and N content along leaf ageing in the N2-fixing legume tree black locust (Robinia pseudoacacia L.). Physiol. Plant. 2024, 176, e14235. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.; Garcia, S.; Grandis, A.; Nascimento, H.; Domingues, T.F.; Guedes, A.; Aleixo, I.; Camargo, P.; Campos, J.; Damasceno, A.; et al. Changes in leaf functional traits with leaf age: When do leaves decrease their photosynthetic capacity in Amazonian trees? Tree Physiol. 2022, 42, 922–938. [Google Scholar] [CrossRef]
- Prieto, J.A.; Louarn, G.; Peña, J.P.; Ojeda, H.; Simonneau, T.; Lebon, E. A leaf gas exchange model that accounts for intra-canopy variability by considering leaf nitrogen content and local acclimation to radiation in grapevine (Vitis vinifera L.). Plant Cell Environ. 2012, 35, 1313–1328. [Google Scholar] [CrossRef]
- Oikawa, S.; Ainsworth, E.A. Changes in leaf area, nitrogen content and canopy photosynthesis in soybean exposed to an ozone concentration gradient. Environ. Pollut. 2016, 215, 347–355. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophyll a and chlorophhyll b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Clegg, K.M. The application of the anthrone reagent to the estimation of starch in cereals. J. Sci. Food Agric. 1986, 7, 40–44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Ma, N.; Su, Y.; Liu, X. Characteristics of Growth, Photosynthesis, C/N Ratio, and Antioxidant Capacity in the Seedling Stage of Aquilaria sinensis ‘Qinan’. Plants 2025, 14, 896. https://doi.org/10.3390/plants14060896
Zhang Q, Ma N, Su Y, Liu X. Characteristics of Growth, Photosynthesis, C/N Ratio, and Antioxidant Capacity in the Seedling Stage of Aquilaria sinensis ‘Qinan’. Plants. 2025; 14(6):896. https://doi.org/10.3390/plants14060896
Chicago/Turabian StyleZhang, Qilei, Ning Ma, Yu Su, and Xiaojin Liu. 2025. "Characteristics of Growth, Photosynthesis, C/N Ratio, and Antioxidant Capacity in the Seedling Stage of Aquilaria sinensis ‘Qinan’" Plants 14, no. 6: 896. https://doi.org/10.3390/plants14060896
APA StyleZhang, Q., Ma, N., Su, Y., & Liu, X. (2025). Characteristics of Growth, Photosynthesis, C/N Ratio, and Antioxidant Capacity in the Seedling Stage of Aquilaria sinensis ‘Qinan’. Plants, 14(6), 896. https://doi.org/10.3390/plants14060896





