Herbal Immunostimulants and Their Phytochemicals: Exploring Morinda citrifolia, Echinacea purpurea, and Phyllanthus niruri
Abstract
1. Introduction
2. Methods
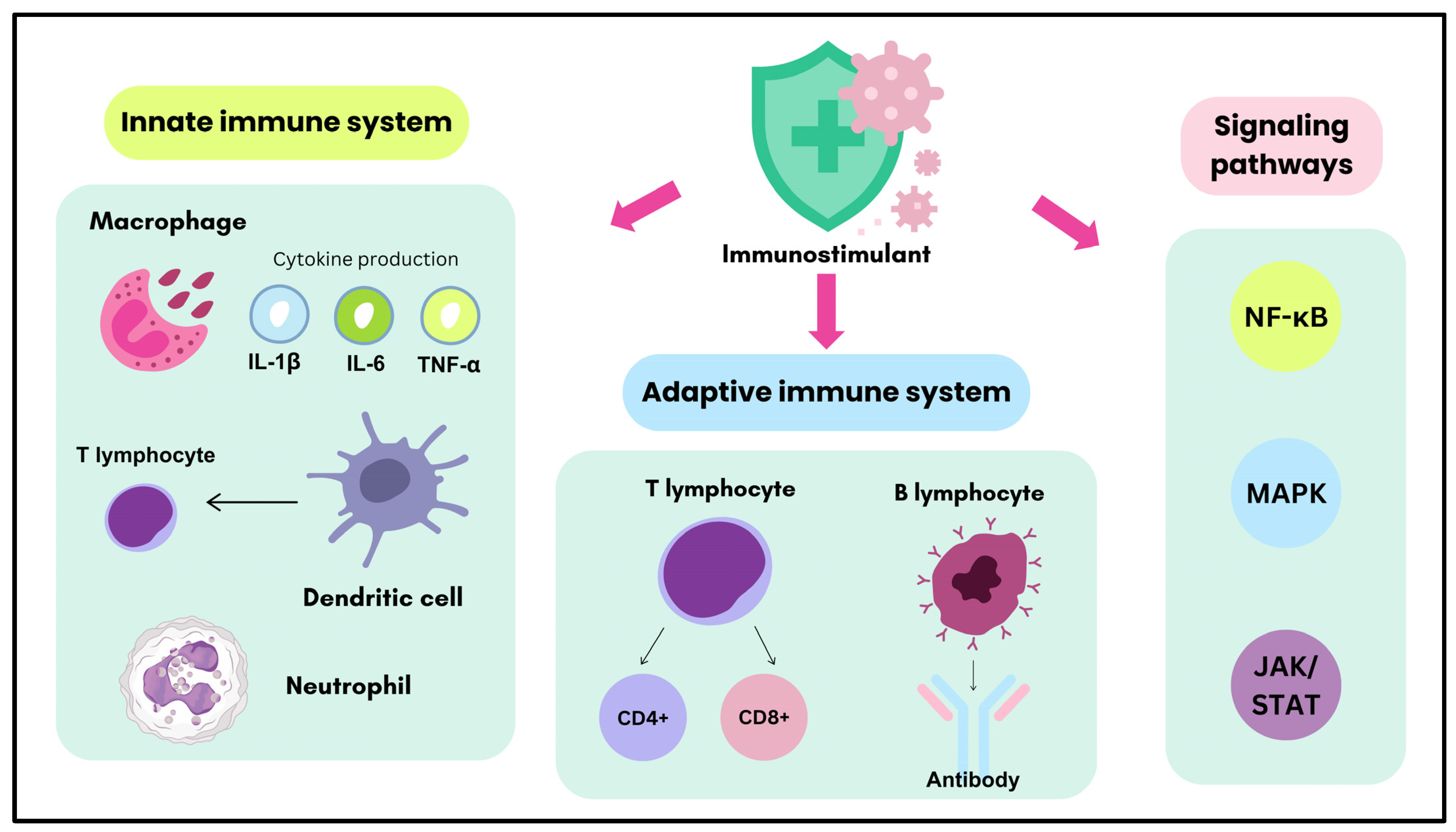
3. Biological Targets of Immunostimulants in Immune System Modulation
3.1. Innate Immune System
3.2. Adaptive Immune System
3.3. Immune Signaling Pathways
4. Morinda citrifolia
4.1. Innate Immune System
4.2. Adaptive Immune System
4.3. Immune Signaling Pathways
5. Echinacea purpurea
5.1. Innate Immune System
5.2. Adaptive Immune System
5.3. Immune Signaling Pathways
6. Phyllanthus niruri
6.1. Innate Immune System
6.2. Adaptive Immune System
6.3. Immune Signaling Pathways
7. Future Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization (WHO). The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 17 November 2024).
- World Health Organization (WHO). Tuberculosis. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 17 November 2024).
- World Health Organization (WHO). 14.9 Million Excess Deaths Associated with the COVID-19 Pandemic in 2020 and 2021. 2022. Available online: https://www.who.int/news/item/05-05-2022-14.9-million-excess-deaths-were-associated-with-the-covid-19-pandemic-in-2020-and-2021 (accessed on 17 November 2024).
- Lerner, A.; Jeremias, P.; Matthias, T. The World Incidence and Prevalence of Autoimmune Diseases Is Increasing. Int. J. Celiac Dis. 2016, 3, 151–155. [Google Scholar] [CrossRef]
- Chew, N.; Ng, C.H.; Tan, D.; Kong, G.; Lin, C.X.; Chin, Y.H.; Foo, R.; Chan, M.; Muthiah, M. Global Burden of Metabolic Diseases: Data from Global Burden of Disease 2000–2019. A Cosortium of Metabolic Disease. Eur. Heart J. 2023, 44, ehac779.131. [Google Scholar] [CrossRef]
- Yang, J.-W.; Fan, L.-C.; Miao, X.-Y.; Mao, B.; Li, M.-H.; Lu, H.-W.; Liang, S.; Xu, J.-F. Corticosteroids for the Treatment of Human Infection with Influenza Virus: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2015, 21, 956–963. [Google Scholar] [CrossRef]
- Kintoko, K.; Ananda, A.N.; Rido, A.R.; Makati, A.C.; Yanti, A.A.; Wahyuni, D.; Maghfirah, Y.S.; Isnaini, I. Herbal-Synthetic Drug Interactions. Magna Med. 2023, 10, 211–220. [Google Scholar] [CrossRef]
- Li, C.; Jia, W.; Yang, J.; Cheng, C.; Olaleye, O.E. Multi-Compound and Drug-Combination Pharmacokinetic Research on Chinese Herbal Medicines. Acta Pharmacol. Sin. 2022, 43, 3080–3095. [Google Scholar] [CrossRef]
- Di Sotto, A.; Vitalone, A.; Di Giacomo, S. Plant-Derived Nutraceuticals and Immune System Modulation: An Evidence-Based Overview. Vaccines 2020, 8, 468. [Google Scholar] [CrossRef]
- Yuandani; Jantan, I.; Rohani, A.S.; Sumantri, I.B. Immunomodulatory Effects and Mechanisms of Curcuma Species and Their Bioactive Compounds: A Review. Front. Pharmacol. 2021, 12, 643119. [Google Scholar] [CrossRef]
- Hikmah, U.; Triastuti, A. Phyllanthus niruri (Meniran) Sebagai Imunomodulator: Mekanisme Aksi Dan Senyawa Bioaktif. J. Ilm. Farm. 2022, 18, 205–218. [Google Scholar] [CrossRef]
- Febriyanti, R.; Levita, J.; Diantini, A. Immunomodulatory Role of Plants and Their Constituents on the Management of Metabolic Disorders: An Evidence-Based Review. Drug Des. Dev. Ther. 2024, 18, 513–534. [Google Scholar] [CrossRef]
- Susilawati, S.; Anwar, C.; Saleh, M.I.; Salni, S.; Hermansyah, H.; Oktiarni, D. Chemical Composition and Antifungal Activity of Morinda citrifolia Fruit Extract. Biosci. J. 2023, 39, e39076. [Google Scholar] [CrossRef]
- Algenstaedt, P.; Stumpenhagen, A.; Westendorf, J. The Effect of Morinda citrifolia L. Fruit Juice on the Blood Sugar Level and Other Serum Parameters in Patients with Diabetes Type 2. Evid.-Based Complement. Altern. Med. 2018, 2018, 3565427. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Kenganora, M.; Manjula, S.N. Health Benefits of Morinda citrifolia (Noni): A Review. Pharmacogn. J. 2016, 8, 321–334. [Google Scholar] [CrossRef]
- Nayak, S.; Mengi, S. Immunostimulant Activity of Noni (Morinda citrifolia) on T and B Lymphocytes. Pharm. Biol. 2010, 48, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Yee, M. Investigation of Phytochemical, Chemical Composition and Antimicrobial Activities of Noni Leaf (Morinda citrifolia Linn). Int. J. Curr. Innov. Adv. Res. 2019, 2, 35–45. [Google Scholar]
- Kim, H.; Rahmawati, L.; Hong, Y.H.; Choi, S.-Y.; Cho, J.Y. NK Cell-Mediated Immunostimulatory Effects of Ethanol Extract of Morinda citrifolia (Noni) Fruit. BMC Complement. Med. Ther. 2022, 22, 222. [Google Scholar] [CrossRef]
- Gerwick, W.H. Plant Sources of Drugs and Chemicals ☆. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Miroshina, T.; Poznyakovskiy, V. Echinacea Purpurea as a Medicinal Plant: Characteristics, Use as a Biologically Active Component of Feed Additives and Specialized Foods (Review). E3S Web Conf. 2023, 380, 01005. [Google Scholar] [CrossRef]
- Burger, R.A.; Torres, A.R.; Warren, R.P.; Caldwell, V.D.; Hughes, B.G. Echinacea-Induced Cytokine Production by Human Macrophages. Int. J. Immunopharmacol. 1997, 19, 371–379. [Google Scholar] [CrossRef]
- Tjandrawinata, R.R.; Susanto, L.W.; Nofiarny, D. The Use of Phyllanthus niruri L. as an Immunomodulator for the Treatment of Infectious Diseases in Clinical Settings. Asian Pac. J. Trop. Dis. 2017, 7, 132–140. [Google Scholar] [CrossRef]
- Pucci, N.D.; Marchini, G.S.; Mazzucchi, E.; Reis, S.T.; Srougi, M.; Evazian, D.; Nahas, W.C. Effect of Phyllanthus niruri on Metabolic Parameters of Patients with Kidney Stone: A Perspective for Disease Prevention. Int. Braz. J. Urol. 2018, 44, 758–764. [Google Scholar] [CrossRef]
- Kusumaningrum, A.D.R.; Sumadiono, S.; Soenarto, Y. Effects of Phyllanthus niruri on the Severity of the Common Cold in Children. Paediatr. Indones. 2012, 52, 346–351. [Google Scholar] [CrossRef]
- Jantan, I.; Haque, M.A.; Ilangkovan, M.; Arshad, L. An Insight Into the Modulatory Effects and Mechanisms of Action of Phyllanthus Species and Their Bioactive Metabolites on the Immune System. Front. Pharmacol. 2019, 10, 878. [Google Scholar] [CrossRef] [PubMed]
- Marhaeny, H.D.; Aliyah, A.N.; Miatmoko, A.; Khotib, J. Investigating the Anti-Allergic Activity of Phyllanthus niruri via MALT1 Protease Inhibition: An in Silico Approach. Pharm. Educ. 2023, 23, 196–202. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Ling, Z.; Li, J.; Hu, J.; He, F.; Chen, Q. The Role of Dendritic Cells in the Immunomodulation to Implanted Biomaterials. Int. J. Oral Sci. 2022, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.; O’Neill, L.A. Metabolic Reprogramming in Macrophages and Dendritic Cells in Innate Immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef]
- Katholnig, K.; Linke, M.; Pham, H.; Hengstschläger, M.; Weichhart, T. Immune Responses of Macrophages and Dendritic Cells Regulated by MTOR Signalling. Biochem. Soc. Trans. 2013, 41, 927–933. [Google Scholar] [CrossRef]
- Lee, M.; Du, H.; Winer, D.A.; Clemente-Casares, X.; Tsai, S. Mechanosensing in Macrophages and Dendritic Cells in Steady-State and Disease. Front. Cell Dev. Biol. 2022, 10, 1044729. [Google Scholar] [CrossRef]
- Roquilly, A.; Mintern, J.D.; Villadangos, J.A. Spatiotemporal Adaptations of Macrophage and Dendritic Cell Development and Function. Annu. Rev. Immunol. 2022, 40, 525–557. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. BioMed Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef]
- Zareinejad, M.; Mehdipour, F.; Roshan-Zamir, M.; Faghih, Z.; Ghaderi, A. Dual Functions of T Lymphocytes in Breast Carcinoma: From Immune Protection to Orchestrating Tumor Progression and Metastasis. Cancers 2023, 15, 4771. [Google Scholar] [CrossRef]
- Ritzau-Jost, J.; Hutloff, A. T Cell/B Cell Interactions in the Establishment of Protective Immunity. Vaccines 2021, 9, 1074. [Google Scholar] [CrossRef]
- Nisar, A.; Akhtar, N.; Hassan, A.; Banday, T.; Wani, B.; Zargar, M.A. Effect of Ajuga bracteosa on Systemic T-Cell Immunity in Balb/C Mice: Dual Th1/Th2 Immunostimulatory Effects. Am. J. Chin. Med. 2014, 42, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Darji, P.; Thakur, B.S.; Jain, A.; Jain, P.K.; Khare, B. Immunostimulants: Concepts, Types and Functions. Asian J. Dent. Health Sci. 2022, 2, 26–34. [Google Scholar] [CrossRef]
- Ahmad, W.; Jantan, I.; Haque, M.A.; Arsyad, L. Magnoflorine from Tinospora crispa Upregulates Innate and Adaptive Immune Responses in Balb/c Mice. Int. Immunopharmacol. 2022, 111, 109081. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lin, Q.; Zhang, Z.; Zhang, L. Therapeutic Strategies for the Costimulatory Molecule OX40 in T-Cell-Mediated Immunity. Acta Pharm. Sin. B 2020, 10, 414–433. [Google Scholar] [CrossRef]
- Xing, J.; Tian, H.; Tang, X.; Sheng, X.; Zhan, W. Kinetics of T Lymphocyte Subsets and B Lymphocytes in Response to Immunostimulants in Flounder (Paralichthys olivaceus): Implications for CD4+ T Lymphocyte Differentiation. Sci. Rep. 2020, 10, 13827. [Google Scholar] [CrossRef]
- Rahimi, K.; Hassanzadeh, K.; Khanbabaei, H.; Haftcheshmeh, S.M.; Ahmadi, A.; Izadpanah, E.; Mohammadi, A.; Sahebkar, A. Curcumin: A Dietary Phytochemical for Targeting the Phenotype and Function of Dendritic Cells. Curr. Med. Chem. 2021, 28, 1549–1564. [Google Scholar] [CrossRef]
- Qu, X.; Tang, Y.; Hua, S. Immunological Approaches Towards Cancer and Inflammation: A Cross Talk. Front. Immunol. 2018, 9, 563. [Google Scholar] [CrossRef]
- Renda, G.; Gökkaya, İ.; Şöhretoğlu, D. Immunomodulatory Properties of Triterpenes. Phytochem. Rev. 2022, 21, 537–563. [Google Scholar] [CrossRef]
- Costagliola, G.; Nuzzi, G.; Spada, E.; Comberiati, P.; Verduci, E.; Peroni, D.G. Nutraceuticals in Viral Infections: An Overview of the Immunomodulating Properties. Nutrients 2021, 13, 2410. [Google Scholar] [CrossRef]
- Kahkhaie, K.R.; Mirhosseini, A.; Aliabadi, A.; Mohammadi, A.; Mousavi, M.J.; Haftcheshmeh, S.M.; Sathyapalan, T.; Sahebkar, A. Curcumin: A Modulator of Inflammatory Signaling Pathways in the Immune System. Inflammopharmacology 2019, 27, 885–900. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.; Niu, M.; Zhang, H.; Wu, Y.; Wu, K.; Dai, Z. Targeting Cytokine and Chemokine Signaling Pathways for Cancer Therapy. Signal Transduct. Target. Ther. 2024, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Tabrah, F.L.; Eveleth, B.M. Evaluation of the Effectiveness of Ancient Hawaiian Medicine. Hawaii Med. J. 1966, 25, 223–230. [Google Scholar] [PubMed]
- Eelager, M.P.; Masti, S.P.; Chougale, R.B.; Dalbanjan, N.P.; Praveen Kumar, S.K. Noni (Morinda citrifolia) Leaf Extract Incorporated Methylcellulose Active Films: A Sustainable Strategy for Browning Inhibition in Apple Slice Packaging. Int. J. Biol. Macromol. 2024, 269, 132270. [Google Scholar] [CrossRef] [PubMed]
- Potterat, O.; Hamburger, M. Morinda citrifolia (Noni) Fruit—Phytochemistry, Pharmacology, Safety. Planta Med. 2007, 73, 191–199. [Google Scholar] [CrossRef]
- Chan-Blanco, Y.; Vaillant, F.; Mercedes Perez, A.; Reynes, M.; Brillouet, J.-M.; Brat, P. The Noni Fruit (Morinda citrifolia L.): A Review of Agricultural Research, Nutritional and Therapeutic Properties. J. Food Compos. Anal. 2006, 19, 645–654. [Google Scholar] [CrossRef]
- Ross, I.A. Medicinal Plants of the World. In Chemical Constituent, Traditional and Modern Medicinal Use; Humana Press: New York, NY, USA, 2001; Volume 2. [Google Scholar]
- Dixon, A.; McMillen, H.; Etkin, N. Ferment This: The Transformation of Noni, a Traditional Polynesian Medicine (Morinda citrifolia, Rubiaceae). Econ. Bot. 1999, 53, 51–68. [Google Scholar] [CrossRef]
- Morton, J. The Ocean-Going Noni, or Indian Mulberry (Morinda citrifolia, Rubiaceae) and Some of Its “Colorful” Relatives. Econ. Bot. 1992, 46, 241–256. [Google Scholar] [CrossRef]
- Motshakeri, M.; Ghazali, H.M. Nutritional, Phytochemical and Commercial Quality of Noni Fruit: A Multi-Beneficial Gift from Nature. Trends Food Sci. Technol. 2015, 45, 118–129. [Google Scholar] [CrossRef]
- Dittmar, A. Morinda citrifolia L.: Use in Indigenous Samoan Medicine. J. Herbs Spices Med. Plants 1993, 1, 77–92. [Google Scholar] [CrossRef]
- Nelson, S. Noni Cultivation and Production in Hawai‘i. In Proceedings of the 2002 Hawai‘i Noni Conference; Agriculture and Human Resources: Honolulu, HI, USA, 2003; pp. 33–50. [Google Scholar]
- Hirazumi, A.; Furusawa, E.; Chou, S.C.; Hokama, Y. Immunomodulation Contributes to the Anticancer Activity of Morinda citrifolia (Noni) Fruit Juice. Proc. West. Pharmacol. Soc. 1996, 39, 7–9. [Google Scholar]
- Purwianingsih, W.; Hidayat, R.Y.; Rahmat, A. Increasing Anthraquinone Compounds on Callus Leaf Morinda citrifolia (L.) by Elicitation Method Using Chitosan Shell of Shrimps (Penaeus monodon). J. Phys. Conf. Ser. 2019, 1280, 022001. [Google Scholar] [CrossRef]
- Royani, A.; Hanafi, M.; Julistiono, H.; Manaf, A. The Total Phenolic and Flavonoid Contents of Aloe vera and Morinda citrifolia Extracts as Antibacterial Material against Pseudomonas aeruginosa. Mater. Today Proc. 2023, 72, 2796–2802. [Google Scholar] [CrossRef]
- Manobharathi, V.; Surya, C.; Shobanadevi, P.; Umadevi, P.; Rabiya, S.; Vedhapriya, R.; Sangavai, C. Phytochemical Screening and Antiobesity Activity of Morinda citrifolia Leaves Extract. J. Xidian Univ. 2024, 18, 240–252. [Google Scholar]
- Vashti, E.; Fernando Oen, B.C.; Yonatan, E.R.; Sabatina, V.B.; Sean, S.; Rukmini, E. Antioxidant and Anti-Inflammatory Effect of Morinda citrifolia: A Meta-Analysis and Systematic Review. FITOFARMAKA J. Ilm. Farm. 2022, 12, 35–45. [Google Scholar] [CrossRef]
- Choi, J.; Lee, K.-T.; Choi, M.-Y.; Nam, J.-H.; Jung, H.-J.; Park, S.-K.; Park, H.-J. Antinociceptive Anti-Inflammatory Effect of Monotropein Isolated from the Root of Morinda officinalis. Biol. Pharm. Bull. 2005, 28, 1915–1918. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Ballal, S.; Velmurugan, N. Comparative Evaluation of the Antimicrobial Activity of Natural Extracts of Morinda citrifolia, Papain and Aloe vera (All in Gel Formulation), 2% Chlorhexidine Gel and Calcium Hydroxide, against Enterococcus Faecalis: An in Vitro Study. J. Conserv. Dent. 2012, 15, 293. [Google Scholar] [CrossRef]
- Palu, A.K.; Kim, A.H.; West, B.J.; Deng, S.; Jensen, J.; White, L. The Effects of Morinda citrifolia L. (Noni) on the Immune System: Its Molecular Mechanisms of Action. J. Ethnopharmacol. 2008, 115, 502–506. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Huda, N.; Zhang, B.; Deng, S. Ethanolic Noni (Morinda citrifolia L.) Leaf Extract Dechlorophyllised Using Sedimentation Process: Antioxidant, Antibacterial Properties and Efficacy in Extending the Shelf-life of Striped Catfish Slices. Int. J. Food Sci. Technol. 2021, 56, 2804–2819. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Ozpinar, A.; Alturfan, E.E.; Elmaci, I. Vinorelbine’s Anti-Tumor Actions May Depend on the Mitotic Apoptosis, Autophagy and Inflammation: Hypotheses with Implications for Chemo-Immunotherapy of Advanced Cancers and Pediatric Gliomas. J. Chemother. 2018, 30, 203–212. [Google Scholar] [CrossRef]
- Thipwong, J.; Kongton, K.; Nupan, B. In Vitro Xanthine Oxidase Inhibitory Activity of Morinda citrifolia L. (Noni) Leaf and Identification of Its Xanthine Oxidase Inhibitors. Trends Sci. 2022, 20, 4201. [Google Scholar] [CrossRef]
- Jin, Z.; Mendu, S.K.; Birnir, B. GABA Is an Effective Immunomodulatory Molecule. Amino Acids 2013, 45, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Potterat, O.; Von Felten, R.; Dalsgaard, P.W.; Hamburger, M. Identification of TLC Markers and Quantification by HPLC-MS of Various Constituents in Noni Fruit Powder and Commercial Noni-Derived Products. J. Agric. Food Chem. 2007, 55, 7489–7494. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hou, Y.; Zou, C.; Liang, H.; Mu, J.; Jiao, X.; Zhu, Y.; Su, L.; Liu, M.; Chen, X.; et al. Alizarin, a Nature Compound, Inhibits the Growth of Pancreatic Cancer Cells by Abrogating NF-ΚB Activation. Int. J. Biol. Sci. 2022, 18, 2759–2774. [Google Scholar] [CrossRef] [PubMed]
- Alitheen, N.B.; Manaf, A.A.; Yeap, S.K.; Shuhaimi, M.; Nordin, L.; Mashitoh, A.R. Immunomodulatory Effects of Damnacanthal Isolated from Roots of Morinda elliptica. Pharm. Biol. 2010, 48, 446–452. [Google Scholar] [CrossRef]
- Leung, K.; Leung, P.; Kong, L.; Leung, P. Immunomodulatory Effects of Esculetin (6,7-Dihydroxycoumarin) on Murine Lymphocytes and Peritoneal Macrophages. Cell. Mol. Immunol. 2005, 2, 181–189. [Google Scholar]
- Wan Osman, W.N.; Che Ahmad Tantowi, N.A.; Lau, S.F.; Mohamed, S. Epicatechin and Scopoletin Rich Morinda citrifolia (Noni) Leaf Extract Supplementation, Mitigated Osteoarthritis via Anti-Inflammatory, Anti-Oxidative, and Anti-Protease Pathways. J. Food Biochem. 2019, 43, e12755. [Google Scholar] [CrossRef]
- Alanazi, H.H.; Elasbali, A.M.; Alanazi, M.K.; El Azab, E.F. Medicinal Herbs: Promising Immunomodulators for the Treatment of Infectious Diseases. Molecules 2023, 28, 8045. [Google Scholar] [CrossRef]
- Erdinest, N.; Shmueli, O.; Grossman, Y.; Ovadia, H.; Solomon, A. Anti-Inflammatory Effects of Alpha Linolenic Acid on Human Corneal Epithelial Cells. Investig. Opthalmol. Vis. Sci. 2012, 53, 4396. [Google Scholar] [CrossRef]
- Lv, L.; Chen, H.; Ho, C.-T.; Sang, S. Chemical Components of the Roots of Noni (Morinda citrifolia) and Their Cytotoxic Effects. Fitoterapia 2011, 82, 704–708. [Google Scholar] [CrossRef]
- O’Shea, M.; Bassaganya-Riera, J.; Mohede, I.C. Immunomodulatory Properties of Conjugated Linoleic Acid. Am. J. Clin. Nutr. 2004, 79, 1199S–1206S. [Google Scholar] [CrossRef]
- Su, B.-N.; Pawlus, A.D.; Jung, H.-A.; Keller, W.J.; McLaughlin, J.L.; Kinghorn, A.D. Chemical Constituents of the Fruits of Morinda citrifolia (Noni) and Their Antioxidant Activity. J. Nat. Prod. 2005, 68, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Kah Hui, C.; Majid, N.I.; Mohd Yusof, H.; Mohd Zainol, K.; Mohamad, H.; Mohd Zin, Z. Catechin Profile and Hypolipidemic Activity of Morinda citrifolia Leaf Water Extract. Heliyon 2020, 6, e04337. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. Protective Effect of Catechin on Humoral and Cell Mediated Immunity in Rat Model. Int. Immunopharmacol. 2018, 54, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, C.; Dong, X.; Cheng, D.; Zhou, T. Immunomodulatory Effects of Epicatechin-(2β →O→7, 4β →8)-Ent-Epicatechin Isolated from Rhododendron spiciferum in Vitro. Immunopharmacol. Immunotoxicol. 2015, 37, 527–534. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, J.; Li, C.; Liu, S.; Wang, L. Morinda citrifolia L. Leaves Extracts Obtained by Traditional and Eco-Friendly Extraction Solvents: Relation between Phenolic Compositions and Biological Properties by Multivariate Analysis. Ind. Crops Prod. 2020, 153, 112586. [Google Scholar] [CrossRef]
- Lee, C.-C.; Wang, C.-C.; Huang, H.-M.; Lin, C.-L.; Leu, S.-J.; Lee, Y.-L. Ferulic Acid Induces Th1 Responses by Modulating the Function of Dendritic Cells and Ameliorates Th2-Mediated Allergic Airway Inflammation in Mice. Evid.-Based Complement. Altern. Med. 2015, 2015, 678487. [Google Scholar] [CrossRef]
- Fontes, R.F.; Andrade, J.K.S.; Rajan, M.; Narain, N. Chemical Characterization of Different Parts of Noni (Morinda citrifolia) Fruit and Its Freeze-Dried Pulp Powder with Emphasis on Its Bioactive Compounds and Antioxidant Activities. Food Sci. Technol. 2023, 43, 103722. [Google Scholar] [CrossRef]
- Hofer, S.; Geisler, S.; Lisandrelli, R.; Nguyen Ngoc, H.; Ganzera, M.; Schennach, H.; Fuchs, D.; Fuchs, J.E.; Gostner, J.M.; Kurz, K. Pharmacological Targets of Kaempferol Within Inflammatory Pathways—A Hint Towards the Central Role of Tryptophan Metabolism. Antioxidants 2020, 9, 180. [Google Scholar] [CrossRef]
- Oo, A.M.; Nor, M.N.M.; Lwin, O.M.; Simbak, N.; Mohd Adnan, L.H.; Rao, U.S.M. Immunomodulatory Effects of Apigenin, Luteolin, and Quercetin through Natural Killer Cell Cytokine Secretion. J. Appl. Pharm. Sci. 2022, 12, 120914. [Google Scholar] [CrossRef]
- Khalil, H.E.; Ibrahim, H.-I.M.; Ahmed, E.A.; Emeka, P.M.; Alhaider, I.A. Orientin, a Bio-Flavonoid from Trigonella hamosa L., Regulates COX-2/PGE-2 in A549 Cell Lines via MiR-26b and MiR-146a. Pharmaceuticals 2022, 15, 154. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Q.; Yang, Q.; Yan, X.; Feng, S.; Wang, Z. Comparison of Anthraquinones, Iridoid Glycosides and Triterpenoids in Morinda officinalis and Morinda citrifolia Using UPLC/Q-TOF-MS and Multivariate Statistical Analysis. Molecules 2019, 25, 160. [Google Scholar] [CrossRef] [PubMed]
- Qiang, J.; Yang, R.; Li, X.; Xu, X.; Zhou, M.; Ji, X.; Lu, Y.; Dong, Z. Monotropein Induces Autophagy through Activation of the NRF2/PINK Axis, Thereby Alleviating Sepsis-Induced Colonic Injury. Int. Immunopharmacol. 2024, 127, 111432. [Google Scholar] [CrossRef] [PubMed]
- Gavrila, A.I.; Zalaru, C.M.; Tatia, R.; Seciu-Grama, A.-M.; Negrea, C.L.; Calinescu, I.; Chipurici, P.; Trifan, A.; Popa, I. Green Extraction Techniques of Phytochemicals from Hedera Helix L. and In Vitro Characterization of the Extracts. Plants 2023, 12, 3908. [Google Scholar] [CrossRef] [PubMed]
- Pongkitwitoon, B.; Putalun, W.; Triwitayakorn, K.; Kitisripanya, T.; Kanchanapoom, T.; Boonsnongcheep, P. Anti-Inflammatory Activity of Verbascoside- and Isoverbascoside-Rich Lamiales Medicinal Plants. Heliyon 2024, 10, e23644. [Google Scholar] [CrossRef]
- Zieniuk, B. Dihydrocaffeic Acid—Is It the Less Known but Equally Valuable Phenolic Acid? Biomolecules 2023, 13, 859. [Google Scholar] [CrossRef]
- da Fontoura Sprenger, R.; Cass, Q.B. Characterization of Four Phyllanthus Species Using Liquid Chromatography Coupled to Tandem Mass Spectrometry. J. Chromatogr. A 2013, 1291, 97–103. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, W.; Jin, X.; Li, J.; Yu, Y. Vitexin-2-O-Rhamnoside Improves Immunosuppression, Oxidative Stress, and Phosphorylation of PI3K/Akt Signal Pathway in Cyclophosphamide Treated Mice. Eur. J. Pharmacol. 2022, 925, 174999. [Google Scholar] [CrossRef]
- Zhu, M.; Tang, X.; Zhu, Z.; Gong, Z.; Tang, W.; Hu, Y.; Cheng, C.; Wang, H.; Sarwar, A.; Chen, Y.; et al. STING Activation in Macrophages by Vanillic Acid Exhibits Antineoplastic Potential. Biochem. Pharmacol. 2023, 213, 115618. [Google Scholar] [CrossRef]
- Wolska, K.; Gorska, A.; Antosik, K.; Lugowska, K. Immunomodulatory Effects of Propolis and Its Components on Basic Immune Cell Functions. Indian J. Pharm. Sci. 2019, 81, 548. [Google Scholar] [CrossRef]
- Shen, M.; Yuan, L.; Zhang, J.; Wang, X.; Zhang, M.; Li, H.; Jing, Y.; Zeng, F.; Xie, J. Phytosterols: Physiological Functions and Potential Application. Foods 2024, 13, 1754. [Google Scholar] [CrossRef]
- Lolok, N.; Sumiwi, S.; Sahidin, I.; Levita, J. Stigmasterol Isolated from the Ethyl Acetate Fraction of Morinda citrifolia Fruit (Using the Bioactivity-guided Method) Inhibits A-amylase Activity: In Vitro and in Vivo Analyses. World Acad. Sci. J. 2023, 5, 25. [Google Scholar] [CrossRef]
- Singh, D.; Singh, S.; Banu, V. Changes in Antioxidants and Minerals in Noni (Morinda citrifolia L.) Fruits during Development Process. Br. J. Pharm. Res. 2016, 10, 1–11. [Google Scholar] [CrossRef]
- Hughes, D.A. Effects of Carotenoids on Human Immune Function. Proc. Nutr. Soc. 1999, 58, 713–718. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, J.; de Oliveira, R.N.; Costa, J.P.; Junior, A.L.G.; de Sousa, D.P.; Freitas, R.M.; Allegretti, S.M.; Pinto, P.L.S. Phytol, a Diterpene Alcohol from Chlorophyll, as a Drug against Neglected Tropical Disease Schistosomiasis Mansoni. PLoS Negl. Trop. Dis. 2014, 8, e2617. [Google Scholar] [CrossRef]
- Harun, N.H.; Ahmad, W.A.N.W.; Suppian, R. Immunostimulatory Effects of Asiatic Acid and Madecassoside on the Phagocytosis Activities of Macrophages Cell Line (J774A.1). J. Appl. Pharm. Sci. 2021, 11, 104–111. [Google Scholar] [CrossRef]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent Anti-Inflammatory Activity of Ursolic Acid, a Triterpenoid Antioxidant, Is Mediated through Suppression of NF-ΚB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef]
- Sasidharan Nair, V.; Huehn, J. Impact of Vitamin C on the Development, Differentiation and Functional Properties of T Cells. Eur. J. Microbiol. Immunol. 2024, 14, 67–74. [Google Scholar] [CrossRef]
- Saah, S.A.; Adu-Poku, D. Phytochemical, Proximate, and Vitamin C Content in Morinda citrifolia (Noni). J. Trop. Pharm. Chem. 2021, 5, 182–187. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Yin, G.; Li, Y.; Zhao, Y.; Guo, X.; Guo, Y.; Yan, S. The Effects of Supplementation of Noni (Morinda citrifolia L.) Fruit Polysaccharides-Rich Extract on Antioxidant Status and Immune Function in Cashmere Goats. J. Anim. Sci. 2022, 100, skac276. [Google Scholar] [CrossRef]
- Wiedmann, S.; Eudy, J.D.; Zempleni, J. Biotin Supplementation Increases Expression of Genes Encoding Interferon-γ, Interleukin-1β, and 3-Methylcrotonyl-CoA Carboxylase, and Decreases Expression of the Gene Encoding Interleukin-4 in Human Peripheral Blood Mononuclear Cells. J. Nutr. 2003, 133, 716–719. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.; dos Santos, A.; Celestino, A.; Sampaio, N.; Baldez, J.; Melecchi, M.; Bjerk, T.; Krause, L.; Caramão, E. Ultrasonic Extracts of Morinda citrifolia L.: Characterization of Volatile Compounds by Gas Chromatography-Mass Spectrometry. J. Braz. Chem. Soc. 2018, 30, 132–139. [Google Scholar] [CrossRef]
- Hong, Y.H.; Yi, Y.; Han, S.Y.; Aziz, N.; Kim, H.G.; Park, S.H.; Hossain, M.A.; Baik, K.S.; Choi, S.Y.; Lee, J.; et al. Morinda citrifolia Noni Water Extract Enhances Innate and Adaptive Immune Responses in Healthy Mice, Ex Vivo, and in Vitro. Phytother. Res. 2019, 33, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.M.; Prajitno, A. Aqueous Morinda citrifolia Leaves Extract Enhances Glutathione Peroxidase Activity and A2-Macroglobulin Gene Expression in Macrobrachium rosenbergii. Res. J. Life Sci. 2017, 4, 25–34. [Google Scholar] [CrossRef]
- Choi, S.-I.; La, I.-J.; Han, X.; Men, X.; Lee, S.-J.; Oh, G.; Kwon, H.-Y.; Kim, Y.-D.; Seong, G.-S.; Kim, S.-H.; et al. Immunomodulatory Effect of Polysaccharide from Fermented Morinda citrifolia L. (Noni) on RAW 264.7 Macrophage and Balb/c Mice. Foods 2022, 11, 1925. [Google Scholar] [CrossRef]
- de Sousa, B.C.; Miguel, C.B.; Rodrigues, W.F.; Machado, J.R.; da Silva, M.V.; da Costa, T.A.; Lazo-Chica, J.E.; do Prado Degasperi, T.; Sales-Campos, H.; Bucek, E.U.; et al. Effects of Short-Term Consumption of Morinda citrifolia (Noni) Fruit Juice on Mice Intestine, Liver and Kidney Immune Modulation. Food Agric. Immunol. 2017, 28, 528–542. [Google Scholar] [CrossRef]
- Ma, A.; Shi, W.; Niu, X.; Wang, M.; Zhong, X. Effects of Echinacea Purpurea Extract on the Immunological Response to Infectious Bursal Disease Vaccine in Broilers. Front. Agric. China 2009, 3, 452–456. [Google Scholar] [CrossRef]
- Gharieb, M.M.; Youssef, F.M. Effect of Echinacea Purpurea and Garlic on Growth Performance, Immune Response, Biochemical and Hematological Parameters in Broiler Chicks. Assiut Vet. Med. J. 2014, 60, 218–228. [Google Scholar] [CrossRef]
- Balciunaite, G.; Haimi, P.-J.; Mikniene, Z.; Savickas, G.; Ragazinskiene, O.; Juodziukyniene, N.; Baniulis, D.; Pangonyte, D. Identification of Echinacea purpurea (L.) Moench Root LysM Lectin with Nephrotoxic Properties. Toxins 2020, 12, 88. [Google Scholar] [CrossRef]
- Ross, S.M. Echinacea Purpurea. Holist. Nurs. Pract. 2016, 30, 54–57. [Google Scholar] [CrossRef]
- Penzak, S.R.; Robertson, S.M.; Hunt, J.D.; Chairez, C.; Malati, C.Y.; Alfaro, R.M.; Stevenson, J.M.; Kovacs, J.A. Echinacea purpurea Significantly Induces Cytochrome P450 3A Activity but Does Not Alter Lopinavir-Ritonavir Exposure in Healthy Subjects. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2010, 30, 797–805. [Google Scholar] [CrossRef] [PubMed]
- C Yin, A.; H Goldberg, K.; Mupparapu, A.; P Retzbach, E.; Yin, K.; F Yang, C.; Goldberg, G.S. Low Molecular Weight Components in Aqueous Echinacea Purpurea Leaf Extract Inhibit Melanoma Cell Growth. J. Cancer Biol. Ther. 2016, 2, 109. [Google Scholar] [CrossRef]
- Fusco, D.; Liu, X.; Savage, C.; Taur, Y.; Xiao, W.; Kennelly, E.; Yuan, J.; Cassileth, B.; Salvatore, M.; Papanicolaou, G.A. Echinacea Purpurea Aerial Extract Alters Course of Influenza Infection in Mice. Vaccine 2010, 28, 3956–3962. [Google Scholar] [CrossRef]
- Petkova, N.; Petrova, A.; Ivanov, I.; Hambarlyiska, I.; Tumbarski, Y.; Dincheva, I.; Ognyanov, M.; Denev, P. Chemical Composition of Different Extracts from Echinacea purpurea (L.) Moench Roots and Evaluation of Their Antimicrobial Activity. ChemEngineering 2023, 7, 94. [Google Scholar] [CrossRef]
- Dongdong, Z.; Jin, Y.; Yang, T.; Yang, Q.; Wu, B.; Chen, Y.; Luo, Z.; Liang, L.; Liu, Y.; Xu, A.; et al. Antiproliferative and Immunoregulatory Effects of Azelaic Acid Against Acute Myeloid Leukemia via the Activation of Notch Signaling Pathway. Front. Pharmacol. 2019, 10, 1396. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Liu, Y.; Liu, Z.; Xu, Q.; Zhang, Y.; Liu, L.; Yang, X.; Li, L.; Xue, C. Effects of Caprylic Acid and Eicosapentaenoic Acid on Lipids, Inflammatory Levels, and the JAK2/STAT3 Pathway in ABCA1-Deficient Mice and ABCA1 Knock-Down RAW264.7 Cells. Nutrients 2023, 15, 1296. [Google Scholar] [CrossRef]
- Mazza, G.; Cottrell, T. Volatile Components of Roots, Stems, Leaves, and Flowers of Echinacea Species. J. Agric. Food Chem. 1999, 47, 3081–3085. [Google Scholar] [CrossRef]
- Lin, J.-J.; Lin, J.H.; Hsu, S.-C.; Weng, S.-W.; Huang, Y.-P.; Tang, N.-Y.; Lin, J.-G.; Chung, J.-G. Alpha-Phellandrene Promotes Immune Responses in Normal Mice Through Enhancing Macrophage and Natural Killer Cell Activities. In Vivo 2013, 2013, 809–814. [Google Scholar]
- Kusuhara, M.; Maruyama, K.; Ishii, H.; Masuda, Y.; Sakurai, K.; Tamai, E.; Urakami, K. A Fragrant Environment Containing α-Pinene Suppresses Tumor Growth in Mice by Modulating the Hypothalamus/Sympathetic Nerve/Leptin Axis and Immune System. Integr. Cancer Ther. 2019, 18, 1534735419845139. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene—What Are the Potential Health Benefits of This Flavouring and Aroma Agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef]
- Moharregh-Khiabani, D.; Linker, R.; Gold, R.; Stangel, M. Fumaric Acid and Its Esters: An Emerging Treatment for Multiple Sclerosis. Curr. Neuropharmacol. 2009, 7, 60–64. [Google Scholar] [CrossRef]
- Lappas, C.M.; Lappas, N.T. D-Limonene Modulates T Lymphocyte Activity and Viability. Cell. Immunol. 2012, 279, 30–41. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, J.M.S.; Nunes, T.A.d.L.; Rodrigues, R.R.L.; de Sousa, J.P.A.; Val, M.d.C.A.; Coelho, F.A.d.R.; dos Santos, A.L.S.; Maciel, N.B.; de Souza, V.M.R.; Machado, Y.A.A.; et al. Cytotoxic and Antileishmanial Effects of the Monoterpene β-Ocimene. Pharmaceuticals 2023, 16, 183. [Google Scholar] [CrossRef]
- Li, H.; Tan, H.; Liu, Z.; Pan, S.; Tan, S.; Zhu, Y.; Wang, Q.; Su, G.; Zhou, C.; Cao, Q.; et al. Succinic Acid Exacerbates Experimental Autoimmune Uveitis by Stimulating Neutrophil Extracellular Traps Formation via SUCNR1 Receptor. Br. J. Ophthalmol. 2023, 107, 1744–1749. [Google Scholar] [CrossRef] [PubMed]
- Waidyanatha, S.; Pierfelice, J.; Cristy, T.; Mutlu, E.; Burback, B.; Rider, C.V.; Ryan, K. A Strategy for Test Article Selection and Phytochemical Characterization of Echinacea Purpurea Extract for Safety Testing. Food Chem. Toxicol. 2020, 137, 111125. [Google Scholar] [CrossRef]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic Acid: A Review on Its Mechanisms of Anti-Inflammation, Disease Treatment, and Related Delivery Systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From Preclinical Evidence to Potential Clinical Applications. Front. Pharmacol. 2021, 11, 599959. [Google Scholar] [CrossRef]
- Viet, T.D.; Xuan, T.D.; Anh, L.H. α-Amyrin and β-Amyrin Isolated from Celastrus Hindsii Leaves and Their Antioxidant, Anti-Xanthine Oxidase, and Anti-Tyrosinase Potentials. Molecules 2021, 26, 7248. [Google Scholar] [CrossRef]
- Yi, J.; Obminska-Mrukowicz, B.; Yuan, L.; Yuan, H. Immunomodulatory Effects of Betulinic Acid from the Bark of White Birch on Mice. J. Vet. Sci. 2010, 11, 305. [Google Scholar] [CrossRef]
- Araldi, E.; Fernández-Fuertes, M.; Canfrán-Duque, A.; Tang, W.; Cline, G.W.; Madrigal-Matute, J.; Pober, J.S.; Lasunción, M.A.; Wu, D.; Fernández-Hernando, C.; et al. Lanosterol Modulates TLR4-Mediated Innate Immune Responses in Macrophages. Cell Rep. 2017, 19, 2743–2755. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rezaei, M.; Tseng, T.-M.; Mastinu, A. Effects of Metribuzin Herbicide on Some Morpho-Physiological Characteristics of Two Echinacea Species. Horticulturae 2022, 8, 169. [Google Scholar] [CrossRef]
- Cichello, S.A.; Yao, Q.; He, X.Q. Proliferative Activity of a Blend of Echinacea Angustifolia and Echinacea Purpurea Root Extracts in Human Vein Epithelial, HeLa, and QBC-939 Cell Lines, but Not in Beas-2b Cell Lines. J. Tradit. Complement. Med. 2016, 6, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Wang, Y.; Wu, Y.; Chen, H.; Zheng, S.; Li, Y.; Xu, X.; Li, W. Echinacea purpurea Extract Polarizes M1 Macrophages in Murine Bone Marrow-Derived Macrophages Through the Activation of JNK. J. Cell. Biochem. 2017, 118, 2664–2671. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, F.; Mohammadzadeh, R.; Nazemiyeh, H.; Mesgari-Abbasi, M.; Barzegar-Jalali, M.; Eskandani, M.; Adibkia, K. Electrosprayed Nanoparticles Containing Hydroalcoholic Extract of Echinacea purpurea (L.) Moench Stimulates Immune System by Increasing Inflammatory Factors in Male Wistar Rats. Adv. Pharm. Bull. 2023, 13, 283–289. [Google Scholar] [CrossRef]
- Enany, M.E.; Algammal, A.M.; Solimane, R.T.; El-Sissi, A.F.; Hebashy, A.A. The Immunostimulatory Effects of Dietary Echinacea Purpurea Supplementation in Broiler Chickens. Suez Canal Vet. Med. J. 2017, 22, 119–134. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; El-Zamarany, E.A.; Salem, M.L.; El-Bahrawy, H.A.; Al-Ashmawy, G.M. In Vitro and in Vivo Studies of the Immunomodulatory Effect of Echinacea Purpurea on Dendritic Cells. J. Genet. Eng. Biotechnol. 2015, 13, 185–192. [Google Scholar] [CrossRef]
- Yao, L.; Bai, L.; Tan, Y.; Sun, J.; Qu, Q.; Shi, D.; Guo, S.; Liu, C. The Immunoregulatory Effect of Sulfated Echinacea Purpurea Polysaccharide on Chicken Bone Marrow-Derived Dendritic Cells. Int. J. Biol. Macromol. 2019, 139, 1123–1132. [Google Scholar] [CrossRef]
- Senchina, D.; Wu, L.; Flinn, G.; Konopka, D.; McCoy, J.-A.; Widrelechner, M.; Wurtele, E.; Kohut, M. Year-and-a-Half Old, Dried Echinacea Roots Retain Cytokine-Modulating Capabilities in an in Vitro Human Older Adult Model of Influenza Vaccination. Planta Med. 2006, 72, 1207–1215. [Google Scholar] [CrossRef]
- Dapas, B.; Dall’Acqua, S.; Bulla, R.; Agostinis, C.; Perissutti, B.; Invernizzi, S.; Grassi, G.; Voinovich, D. Immunomodulation Mediated by a Herbal Syrup Containing a Standardized Echinacea Root Extract: A Pilot Study in Healthy Human Subjects on Cytokine Gene Expression. Phytomedicine 2014, 21, 1406–1410. [Google Scholar] [CrossRef]
- Fonseca, F.N.; Papanicolaou, G.; Lin, H.; Lau, C.B.S.; Kennelly, E.J.; Cassileth, B.R.; Cunningham-Rundles, S. Echinacea purpurea (L.) Moench Modulates Human T-Cell Cytokine Response. Int. Immunopharmacol. 2014, 19, 94–102. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wu, Y.; Wang, B.; Chen, X.; Xu, X.; Chen, H.; Li, W.; Xu, X. Echinacea pupurea Extracts Promote Murine Dendritic Cell Maturation by Activation of JNK, P38 MAPK and NF-ΚB Pathways. Dev. Comp. Immunol. 2017, 73, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Paulovičová, E.; Paulovičová, L.; Pawlaczyk-Graja, I.; Gancarz, R.; Kopáčová, M.; Capek, P. Effectivity of Polyphenolic Polysaccharide-Proteins Isolated from Medicinal Plants as Potential Cellular Immune Response Modulators. Biologia 2022, 77, 3581–3593. [Google Scholar] [CrossRef] [PubMed]
- Pathania, R.; Najda, A.; Chawla, P.; Kaushik, R.; Khan, M.A. Low-Energy Assisted Sodium Alginate Stabilized Phyllanthus niruri Extract Nanoemulsion: Characterization, in Vitro Antioxidant and Antimicrobial Application. Biotechnol. Rep. 2022, 33, e00711. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kaur, B.; Sirhindi, G. Phytochemistry and Pharmacology of Phyllanthus niruri L.: A Review. Phytother. Res. 2017, 31, 980–1004. [Google Scholar] [CrossRef]
- Rithikha; Yokesh, S.; Aravindan, S.; Maha swetha, K.; Gokul raj, G.; Divya, C.; Harish kannan, S.; Praveen kumar, P.; Udhaya kumar, T.; Aslakkhan, D.; et al. Review on Medicinal Plant Phyllanthus Niruri and Its Effects. Int. J. Membr. Sci. Technol. 2023, 10, 436–443. [Google Scholar] [CrossRef]
- Nigam, R.; Arnold, R. Qualitative and Quantitative Phytochemical Screening and Chemical Fingerprint Analysis of Herbal Plant Phyllanthus niruri Using HPTLC. J. Sci. Res. 2021, 13, 623–633. [Google Scholar] [CrossRef]
- Hasan, M.; Safarianti, S.; Ramadhani, A.F.; Khilfi, S.; Suryawati, S.; Husna, F. Bioactive Compounds and In Vitro Evaluation of Phyllanthus Niruri Extract as Antioxidant and Antimicrobial Activities. Trends Sci. 2024, 21, 7130. [Google Scholar] [CrossRef]
- Damasak, A.A. Phytochemical Components and In Vitro Antioxidant Activity of Methanol Leaves Extract of Phyllanthus Niruri Linn. (Chanca piedra). Arid-Zone J. Basic. Appl. Res. 2023, 2, 105–111. [Google Scholar] [CrossRef]
- Tiwana, G.; Cock, I.E.; Cheesman, M.J. Phyllanthus Niruri Linn.: Antibacterial Activity, Phytochemistry, and Enhanced Antibiotic Combinatorial Strategies. Antibiotics 2024, 13, 654. [Google Scholar] [CrossRef]
- Mao, X.; Gu, C.; Ren, M.; Chen, D.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, J.; Luo, Y.; et al. L-Isoleucine Administration Alleviates Rotavirus Infection and Immune Response in the Weaned Piglet Model. Front. Immunol. 2018, 9, 01654. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.; Kumar, B. Identification and Characterization of Phenolics and Terpenoids from Ethanolic Extracts of Phyllanthus Species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Faheem, M.A.; Akhtar, T.; Naseem, N.; Aftab, U.; Zafar, M.S.; Hussain, S.; Shahzad, M.; Gobe, G.C. Chrysin Is Immunomodulatory and Anti-Inflammatory against Complete Freund’s Adjuvant-Induced Arthritis in a Pre-Clinical Rodent Model. Pharmaceutics 2023, 15, 1225. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.; Samanta, S.; Dash, R.; Karpiński, T.M.; Habibi, E.; Sadiq, A.; Ahmadi, A.; Bungau, S. The Neuroprotective Effects of Fisetin, a Natural Flavonoid in Neurodegenerative Diseases: Focus on the Role of Oxidative Stress. Front. Pharmacol. 2022, 13, 1015835. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, H.; Yuan, L.; Li, X.; Cai, Y. Potential Implications of Hyperoside on Oxidative Stress-Induced Human Diseases: A Comprehensive Review. J. Inflamm. Res. 2023, 16, 4503–4526. [Google Scholar] [CrossRef]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef]
- Li, H.; Gu, Y.; Jin, R.; He, Q.; Zhou, Y. Effects of Dietary Rutin Supplementation on the Intestinal Morphology, Antioxidant Capacity, Immunity, and Microbiota of Aged Laying Hens. Antioxidants 2022, 11, 1843. [Google Scholar] [CrossRef]
- Ranjan, R.; Kishore, K.; TJ, S.; Jha, A.K.; Ojha, B.K.; Kumar, S.; Kumar, R. Nutraceutical Potential of Vitexin: A Flavone Glycoside. J. Phytopharm. 2023, 12, 44–50. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mukherjee, T.; Mukhopadhyay, R.; Mukherjee, B.; Sengupta, S.; Chattopadhyay, S.; Jaisankar, P.; Roy, S.; Majumder, H.K. The Lignan Niranthin Poisons Leishmania donovani Topoisomerase IB and Favours a Th1 Immune Response in Mice. EMBO Mol. Med. 2012, 4, 1126–1143. [Google Scholar] [CrossRef]
- Wan, F.; Zhong, R.; Wang, M.; Zhou, Y.; Chen, Y.; Yi, B.; Hou, F.; Liu, L.; Zhao, Y.; Chen, L.; et al. Caffeic Acid Supplement Alleviates Colonic Inflammation and Oxidative Stress Potentially Through Improved Gut Microbiota Community in Mice. Front. Microbiol. 2021, 12, 784211. [Google Scholar] [CrossRef]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and Anti-Inflammatory Effect of p-Coumaric Acid, a Common Dietary Polyphenol on Experimental Inflammation in Rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef]
- Li, R.; Narita, R.; Ouda, R.; Kimura, C.; Nishimura, H.; Yatagai, M.; Fujita, T.; Watanabe, T. Structure-Dependent Antiviral Activity of Catechol Derivatives in Pyroligneous Acid against the Encephalomycarditis Virus. RSC Adv. 2018, 8, 35888–35896. [Google Scholar] [CrossRef]
- Cornélio Favarin, D.; Martins Teixeira, M.; Lemos de Andrade, E.; de Freitas Alves, C.; Lazo Chica, J.E.; Artério Sorgi, C.; Faccioli, L.H.; Paula Rogerio, A. Anti-Inflammatory Effects of Ellagic Acid on Acute Lung Injury Induced by Acid in Mice. Mediat. Inflamm. 2013, 2013, 164202. [Google Scholar] [CrossRef] [PubMed]
- Cordiano, R.; Gammeri, L.; Di Salvo, E.; Gangemi, S.; Minciullo, P.L. Pomegranate (Punica granatum L.) Extract Effects on Inflammaging. Molecules 2024, 29, 4174. [Google Scholar] [CrossRef] [PubMed]
- Mektrirat, R.; Chuammitri, P.; Navathong, D.; Khumma, T.; Srithanasuwan, A.; Suriyasathaporn, W. Exploring the Potential Immunomodulatory Effects of Gallic Acid on Milk Phagocytes in Bovine Mastitis Caused by Staphylococcus Aureus. Front. Vet. Sci. 2023, 10, 1255058. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Costa, J.F.; Meira, C.S.; das Neves, M.V.G.; Dos Reis, B.P.Z.C.; Soares, M.B.P. Anti-Inflammatory Activities of Betulinic Acid: A Review. Front. Pharmacol. 2022, 13, 883857. [Google Scholar] [CrossRef]
- Das, A.; Jawed, J.J.; Das, M.C.; Sandhu, P.; De, U.C.; Dinda, B.; Akhter, Y.; Bhattacharjee, S. Antileishmanial and Immunomodulatory Activities of Lupeol, a Triterpene Compound Isolated from Sterculia Villosa. Int. J. Antimicrob. Agents 2017, 50, 512–522. [Google Scholar] [CrossRef]
- Amin, Z.A.; Abdulla, M.A.; Ali, H.M.; Alshawsh, M.A.; Qadir, S.W. Assessment of In Vitro Antioxidant, Antibacterial and Immune Activation Potentials of Aqueous and Ethanol Extracts of Phyllanthus niruri. J. Sci. Food Agric. 2012, 92, 1874–1877. [Google Scholar] [CrossRef]
- Murugaiyah, V.; Chan, K.-L. Mechanisms of Antihyperuricemic Effect of Phyllanthus Niruri and Its Lignan Constituents. J. Ethnopharmacol. 2009, 124, 233–239. [Google Scholar] [CrossRef]
- Nworu, C.S.; Akah, P.A.; Okoye, F.B.C.; Proksch, P.; Esimone, C.O. The Effects of Phyllanthus niruri Aqueous Extract on the Activation of Murine Lymphocytes and Bone Marrow-Derived Macrophages. Immunol. Invest. 2010, 39, 245–267. [Google Scholar] [CrossRef]
- Hutomo, S.; Putri, D.U.; Suryanto, Y.I.; Susilowati, H. Potential Immunomodulatory Activity of Phyllanthus Niruri Aqueous Extract on Macrophage Infected with Streptococcus Sanguinis. Dent. J. 2018, 51, 124–128. [Google Scholar] [CrossRef]
- Putri, D.U.; Rintiswati, N.; Soesatyo, M.H.; Haryana, S.M. Immune Modulation Properties of Herbal Plant Leaves: Phyllanthus niruri Aqueous Extract on Immune Cells of Tuberculosis Patient—In Vitro Study. Nat. Prod. Res. 2018, 32, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, E.T.; Soetrisno, S.; Purwanto, B.; Reviono, R.; Wasita, B.; Laqif, A. Impacts of Phyllanthus Niruri Extract on Biomarker Levels, Macrophage Count, and Lesion Area in an Endometriotic Rat Model. Narra J. 2024, 4, e1002. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, M.; Subramani, P.A.; Michael, R.D. Immunostimulatory Effect of the Aqueous Leaf Extract of Phyllanthus Niruri on the Specific and Nonspecific Immune Responses of Oreochromis Mossambicus Peters. Iran. J. Vet. Res. 2016, 17, 200–202. [Google Scholar] [PubMed]
- Tendean, M.; Riwanto, I. The Effects of Phyllanthus Niruri Linn on Infiltrating Dendritic Cell and Ratio of Neutrophile/Lymphocytes in Chemotherapy of Sprague-Dawley Rats with Colorectal Cancer. Asian Pac. J. Cancer Prev. 2021, 22, 3561–3568. [Google Scholar] [CrossRef]
- Akintola, O. Ben Immunomodulatory Activities of Methanol Extract of the Whole Aerial Part of Phyllantus niruri L. Int. J. Biochem. Biotechnol. 2019, 8, 001–006. [Google Scholar]
- Aldi, Y.; Rasyadi, Y.; Handayani, D. Aktivitas Imunomodulator Dari Ekstrak Etanol Meniran (Phyllanthus niruri Linn.) Terhadap Ayam Broiler. J. Sains Farm. Klin. 2014, 1, 20–26. [Google Scholar] [CrossRef][Green Version]
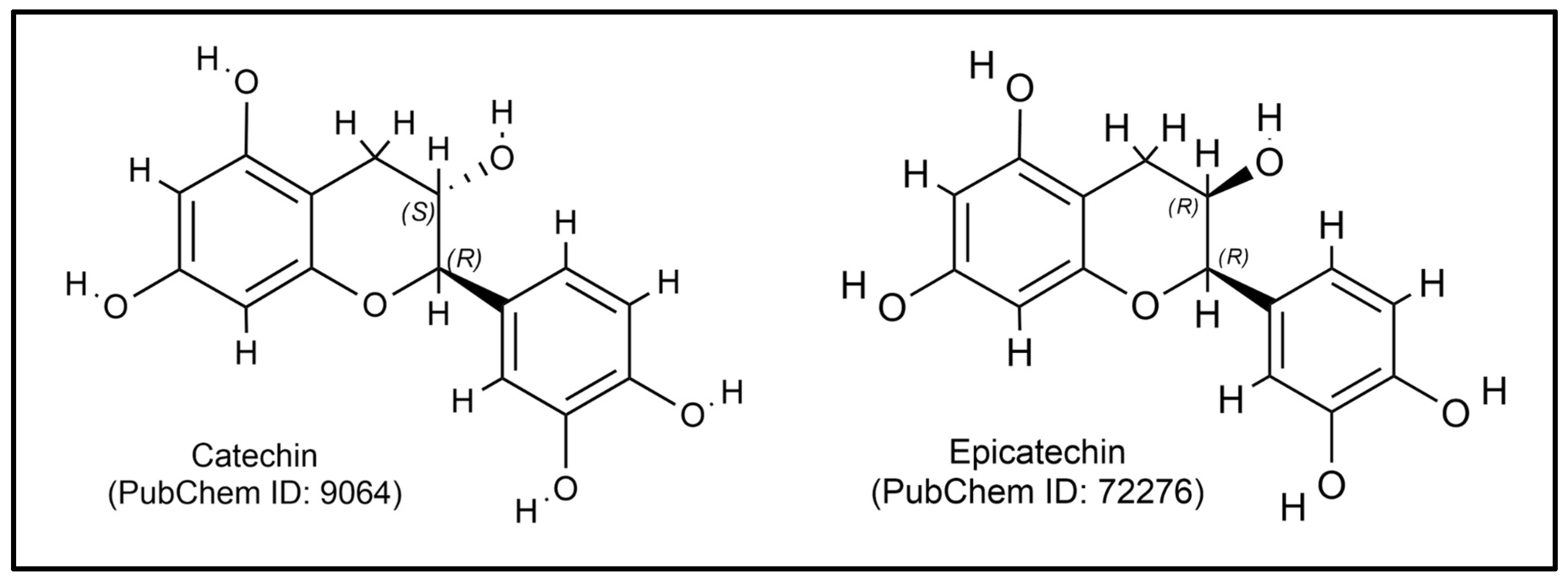
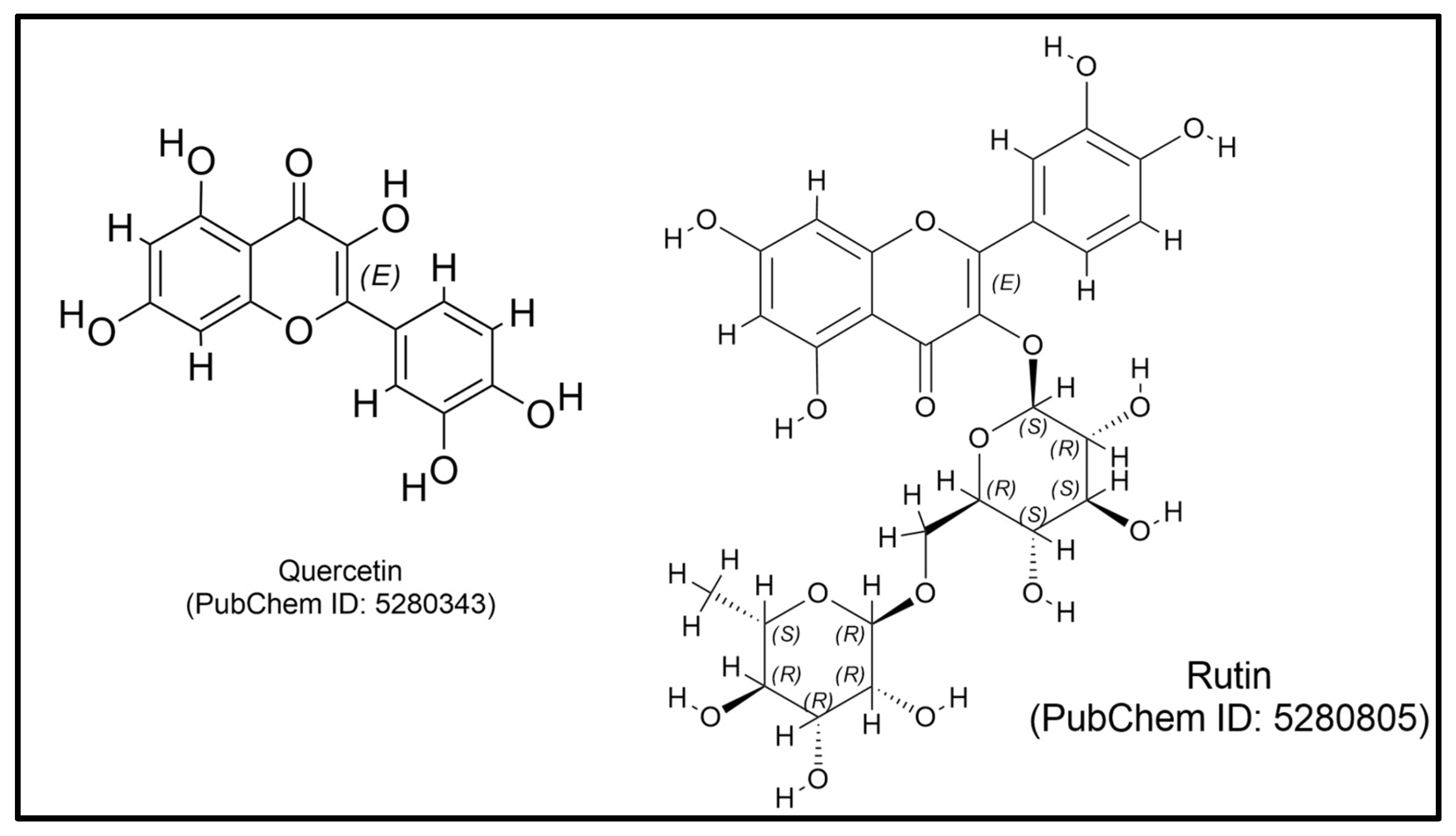
| Class | Compound | Plant Part | Function | Ref. |
|---|---|---|---|---|
| Alkaloid | Vinorelbine | Leaf | Induces Toll-Like Receptor 4 signaling | [64,65] |
| Amino acid | γ-aminobutyric acid | Leaf | Modulates phagocytic activity and chemotaxis in human peripheral monocytes | [66,67] |
| Anthraquinone | Alizarin | Fruit | Induces apoptosis and cell cycle arrest by targeting the NF-κB signaling pathway | [68,69] |
| Damnacanthal | Root | Induces higher levels of IL-2 and reduces IL-12 production | [70] | |
| Coumarin | Esculetin | Leaf | Combined with LPS, it enhances macrophage migration, phagocytosis, and nitric oxide (NO) production, aiding tumor defense | [66,71] |
| Scopoletin | Leaf | Stimulates macrophage activation | [72,73] | |
| Fatty acid | α-Linolenic acid | Leaf | Modulates NF-κB signaling pathways | [64,74] |
| Ibericin | Root | Enhances the activity of macrophages and T lymphocytes | [9,75] | |
| Linolenic acid | Fruit | Enhances lymphocyte proliferation and IL-2 production | [68,76] | |
| Flavonoid | 6α-hydroxyadoxoside | Fruit | Enhances the activation of splenocytes | [77] |
| Catechin | Leaf | Stimulates delayed-type hypersensitivity response, promotes leukocyte migration, and increases phagocytic activity and neutrophil adhesion | [78,79] | |
| Epicatechin | Leaf | Increases levels of Th1 cytokines (IL-2, IL-12, IFN-γ, and TNF-α) | [72,80] | |
| Ferulic acid | Leaf | Promotes Th1 differentiation by enhancing dendritic cell maturation and increasing IL-12 production | [81,82] | |
| Kaempferol | Fruit | Stimulates the expression of IL-1β and TNF-α | [83,84] | |
| Luteolin | Leaf | Enhances the secretion of cytokines such as IL-2 and IFN-γ from NK cells | [66,85] | |
| Orientin | Leaf | Regulates immune markers like BCL-2 and iNOS | [81,86] | |
| Glycoside | Geniposidic acid | Leaf | Enhances the activity of immune cells, including macrophages and T cells | [87,88] |
| Hamamelose | Leaf | Enhances immune function by stimulating cytokine production and activating immune cells | [64,89] | |
| Monotropein | Leaf | Induces autophagy through the activation of the NRF2-PINK signaling axis | [87,88] | |
| Verbasoside | Leaf | Enhances the production of anti-inflammatory cytokines | [64,90] | |
| Phenolic acid | Dihydrocaffeic acid | Leaf | Enhances the proliferation and activation of macrophages and increases the secretion of pro-inflammatory cytokines | [64,91] |
| Gallic acid | Leaf | Regulates Th17/Treg cell balance, reduces MMP overexpression, and modulates inflammation-related cytokines (increasing IL-10 and TGF-β) in the CIA mouse model | [92,93] | |
| Vanillic acid | Fruit | Stimulates the Stimulator of Interferon Genes pathway in macrophages, leading to the production of type I interferons | [83,94] | |
| Sesquiterpene | Artepilin C | Fruit | Stimulates the production of anti-inflammatory cytokines such as IL-10 | [83,95] |
| Sterol | Phytosterols | Fruit | Influences immunomodulatory activity by altering T cell populations, including CD3+, CD4+, and CD8+ T cells | [96,97] |
| Terpenoid | Carotenoid | Fruit | Increases percentages of CD4+ T cells and improves expression of MHC class II molecules on monocytes | [98,99] |
| E-phytol | Leaf | An adjuvant that enhances both humoral and cellular immune responses | [92,100] | |
| Madecassic acid | Leaf | Enhances phagocytosis and nitric oxide production in macrophages | [64,101] | |
| Ursolic acid | Fruit | Activates T cells, B cells, and macrophages | [68,102] | |
| Vitamin | Ascorbic acid (Vitamin C) | Fruit | Increases levels of IL-12 and IFN-γ | [103,104] |
| Biotin (Vitamin B) | Fruit | Increases activity of mRNA encoding interferon-gamma (IFN-γ) and interleukin-1 beta (IL-1β) | [105,106] | |
| Tocopherol (Vitamin C) | Fruit | Augments IL-2 production | [107,108] |
| Class | Compound | Plant Part | Function | Ref. |
|---|---|---|---|---|
| Fatty acid | Azelaic acid | Root | Promotes the activation of natural killer (NK) cells and T cells and increases the secretion of TNF-α and IFN-γ | [120,121] |
| Caproic acid | Root | Increases the expression of anti-inflammatory cytokines like IL-10 and IL-4 | [120,122] | |
| Caprylic acid | Root | Upregulates the p-JAK2/p-STAT3 pathway | [120,122] | |
| Monoterpene | α-phellandrene | Root | Increases the percentage of CD3+ T cells, CD11b+ monocytes, and MAC3+ macrophages | [123,124] |
| α-pinene | Root, flower, leaf, stem | Stimulates the activity of immune cells such as B cells, CD4+ T cells, CD8+ T cells, and NK cells | [123,125] | |
| α-terpinene | Root, flower, leaf, stem | Enhances NK-cell activity | [123] | |
| β-myrcene | Flower, leaf, stem | Stimulates the proliferation of lymphocytes and activates macrophages | [123,126] | |
| β-pinene | Root, flower, leaf, stem | Increases the proliferation of lymphocytes and enhances the activity of NK cells | [123,125] | |
| Geranyl acetate | Leaf | Increases hemagglutinating antibody titers and delayed-type hypersensitivity (DTH) responses | [123,127] | |
| Limonene | Root, flower, leaf, stem | Influences the upregulation of activation markers on T lymphocytes | [123,128] | |
| Ocimene | Root, flower, leaf, stem | Increases the production of pro-inflammatory cytokines such as TNF-α and nitric oxide (NO) | [123,129] | |
| Organic acid | Fumaric acid | Root | Increases the production of Th2 cytokines (IL-4 and IL-5) | [120,127] |
| Succinic acid | Root | It increases the frequencies of Th1 and Th17 cells and elevates levels of pro-inflammatory cytokines such as IFN-γ and IL-17A. | [120,130] | |
| Phenolic acid | Chlorogenic acid | Root | Shifts macrophage polarization from a pro-inflammatory (M1) phenotype to an anti-inflammatory (M2) phenotype | [131,132] |
| Phytosterol | β-Sitosterol | Root | Promotes the activity of T-lymphocytes and NK cells | [120] |
| Campesterol | Root | Promotes of regulatory T-cell responses | [120,133] | |
| Triterpenoid | α-amyrin | Root | Enhances the activity of immune cells and normalizes the functioning of specific T-helper lymphocytes (Th1 and Th2) | [120,134] |
| Betulin | Root | Enhances the percentage of CD4+ T cells and increases the ratios of CD4+/CD8+ T cells in spleen tissue | [120,135] | |
| Lanosterol | Root | Improves phagocytosis and bacterial clearance in immune cells | [120,136] |
| Class | Compound | Plant Part | Function | Ref. |
|---|---|---|---|---|
| Amino acid | Isoleucine | Leaf | Activates pattern recognition receptor (PRR) signaling pathways | [155,156] |
| Flavonoid | Chrysin | Leaf | Induces cell proliferation | [157,158] |
| Fisetin | Leaf | Increases the production of IL-10 | [155,159] | |
| Hyperoside | Leaf | Promotes anti-inflammatory cytokines | [155,160] | |
| Kaempferol | Leaf | Induces NF-κB activation in THP1-Blue cells | [84,155] | |
| Quercetin-3-O-glucoside | Leaf | Stimulates the production of interferon-gamma (IFN-γ) | [92,161] | |
| Rutin | Leaf | Increases the levels of secretory immunoglobulin A (sIgA), immunoglobulin M (IgM), and interleukin-10 (IL-10) | [92,162] | |
| Vitexin | Leaf | Promotes the production of IL-10 | [155,163] | |
| Vitexin-2″-O-rhamnoside | Leaf | Promotes the proliferation of T and B lymphocytes | [92,93] | |
| Lignan | Niranthin | Leaf, root, stem | Induces a switch from a Th2-type immune response to a Th1-type immune response | [164] |
| Organic acid | Fumaric acid | Leaf | Promotes the production of anti-inflammatory cytokines like IL-4 and IL-5 | [127,155] |
| Phenolic acid | Caffeic acid | Leaf | Increases IL-10 production | [157,165] |
| Coumaric acid | Leaf | Increases serum immunoglobulin levels and enhances macrophage phagocytic activity | [157,166] | |
| Polyphenol | Catechol | Leaf | Increases expression levels of pro-inflammatory cytokines such as IL-6 and IFN-β | [155,167] |
| Ellagic acid | Leaf | Increases the anti-inflammatory cytokine IL-10 | [155,168] | |
| Ellagitannin | Leaf | Stimulates the production of IL-10 | [92,169] | |
| Gallic acid | Leaf | Enhances phagocytic activity and the release of neutrophil extracellular traps (NETs) | [92,170] | |
| Triterpenoid | Betulinic acid | Leaf | Stimulates lymphocyte proliferation and increases the percentage of CD4+ and CD19+ B cells. | [157,171] |
| Lupeol | Bark, leaf, root, stem | Stimulates nitric oxidation generation in macrophages and upregulates pro-inflammatory cytokines: TNF-α, IL-12, or IFN-γ | [157,172] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivadila, T.; Iswantini, D.; Rahminiwati, M.; Rafi, M.; Salsabila, A.P.; Sianipar, R.N.R.; Indariani, S.; Murni, A. Herbal Immunostimulants and Their Phytochemicals: Exploring Morinda citrifolia, Echinacea purpurea, and Phyllanthus niruri. Plants 2025, 14, 897. https://doi.org/10.3390/plants14060897
Trivadila T, Iswantini D, Rahminiwati M, Rafi M, Salsabila AP, Sianipar RNR, Indariani S, Murni A. Herbal Immunostimulants and Their Phytochemicals: Exploring Morinda citrifolia, Echinacea purpurea, and Phyllanthus niruri. Plants. 2025; 14(6):897. https://doi.org/10.3390/plants14060897
Chicago/Turabian StyleTrivadila, Trivadila, Dyah Iswantini, Min Rahminiwati, Mohamad Rafi, Adisa Putri Salsabila, Rut Novalia Rahmawati Sianipar, Susi Indariani, and Anggia Murni. 2025. "Herbal Immunostimulants and Their Phytochemicals: Exploring Morinda citrifolia, Echinacea purpurea, and Phyllanthus niruri" Plants 14, no. 6: 897. https://doi.org/10.3390/plants14060897
APA StyleTrivadila, T., Iswantini, D., Rahminiwati, M., Rafi, M., Salsabila, A. P., Sianipar, R. N. R., Indariani, S., & Murni, A. (2025). Herbal Immunostimulants and Their Phytochemicals: Exploring Morinda citrifolia, Echinacea purpurea, and Phyllanthus niruri. Plants, 14(6), 897. https://doi.org/10.3390/plants14060897








