Maximizing Cannabinoid and Polyphenol Extraction from Industrial Hemp (Cannabis sativa L. cv. Helena) Areal Parts: A Comparative Study of Ultrasound-Assisted and Conventional Methods at Two Harvest Stages
Abstract
1. Introduction
2. Results and Discussion
2.1. Impact of the Plant Stage Growth and Applied Solvent on the Profile of Phenolic Compounds, CBD and THC in Hemp Extracts
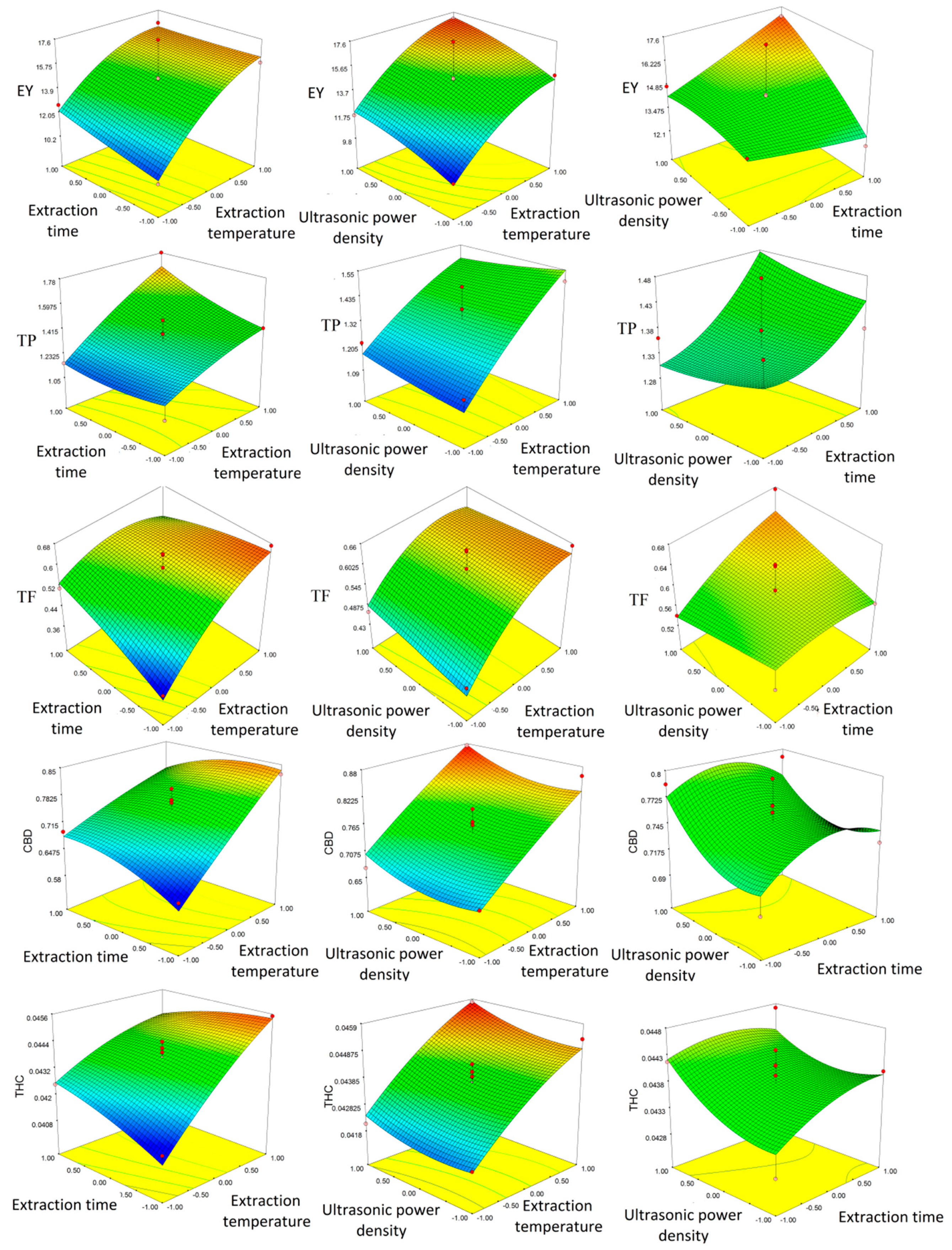
2.2. Ultrasound-Assisted Extraction (UAE) of Phenolic Compounds, CBD, and THC from the Areal Parts of the Hemp
3. Materials and Methods
3.1. Herbal Material
3.2. Chemicals
3.3. Conventional Extraction Procedure
3.4. UAE Procedure and Experimental Plan of Optimization
3.5. Characterization of Hemp Extracts
3.5.1. Total Phenol Content
3.5.2. Total Flavonoids Content
3.5.3. HPLC Analysis of Polyphenolic Constituents
3.5.4. GC/MS Analysis of CBD and THC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Odieka, A.E.; Obuzor, G.U.; Oyedeji, O.O.; Gondwe, M.; Hosu, Y.S.; Oyedeji, A.O. The Medicinal Natural Products of Cannabis sativa Linn.: A Review. Molecules 2022, 27, 1689. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef]
- Echeverry, C.; Reyes-Parada, M.; Scorza, C. Constituents of Cannabis sativa in Cannabinoids and Sleep. In Advances in Experimental Medicine and Biology; Monti, J.M., Pandi-Perumal, S.R., Murillo-Rodríguez, E., Eds.; Springer: Cham, Switzerland, 2021; Volume 1297, pp. 1–9. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. in Phytocannabinoids. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Cham, Switzerland, 2017; Volume 103, pp. 1–36. [Google Scholar] [CrossRef]
- Chandra, S.; Lata, H.; Khan, I.A.; ElSohly, M.A. Cannabis sativa L.: Botany and Horticulture. In Cannabis sativa L.—Botany and Biotechnology; Chandra, S., Lata, H., ElSohly, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 79–100. [Google Scholar] [CrossRef]
- Meijer, E.P.M.; de Hammond, K.M.; Micheler, M. The inheritance of chemical phenotype in Cannabis sativa L. (III): Variation in cannabichromene proportion. Euphytica 2009, 165, 293–311. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Hill, A.J.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Phytocannabinoids as novel therapeutic agents in {CNS} disorders. Pharmacol. Ther. 2012, 133, 79–97. [Google Scholar] [CrossRef]
- Schofs, L.; Sparo, M.D.; Sanchez Bruni, S.F. The antimicrobial effect behind Cannabis sativa. Pharmacol. Res. Perspect. 2021, 9, e00761. [Google Scholar] [CrossRef]
- Chesney, E.; Oliver, D.; McGuire, P. Cannabidiol (CBD) as a novel treatment in the early phases of psychosis. Psychopharmacology 2022, 239, 1179–1190. [Google Scholar] [CrossRef]
- Galanty, A.; Juncewicz, P.; Podolak, I.; Grabowska, K.; Służały, P.; Paśko, P. Comparative Analysis of Polyphenolic Profile and Chemopreventive Potential of Hemp Sprouts, Leaves, and Flowers of the Sofia Variety. Plants 2024, 13, 2023. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Taher, Z.M.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Sulejmanović, M.; Milić, N.; Mourtzinos, I.; Nastić, N.; Kyriakoudi, A.; Drljača, J.; Vidović, S. Ultrasound-assisted and subcritical water extraction techniques for maximal recovery of phenolic compounds from raw ginger herbal dust toward in vitro biological activity investigation. Food Chem. 2024, 437, 137774. [Google Scholar] [CrossRef] [PubMed]
- Živković, J.; Vladić, J.; Naffati, A.; Nastić, N.; Šavikin, K.; Tomić, M.; Vidović, S. Comparative chemical profiling of underexploited Arctostaphylos uva-ursi L. herbal dust extracts obtained by conventional, ultrasound-assisted and subcritical water extractions. Waste Biomass Valorization 2022, 13, 4147–4155. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Siddique, M.; Rashid, R.; Ali, A. Fundamentals of acoustic cavitation, ultrasound-assisted processes, and sonochemistry. In Modeling and Simulation of Sono-Processes; Kerboua, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 3–17. [Google Scholar] [CrossRef]
- Delmoral, R.K.; Hughes, B.; Pal, K.; Samuelsson, A. Cannabinoid Extraction Process Using Brin. U.S. Patent 10,406,453, 10 September 2019. [Google Scholar]
- Tegen, M.G.; Cho, J.; Sutterlin, W.R. Processes for Solvent Extraction of Cannabinoids, Terpenes and Flavonoids from Biomass. U.S. Patent 10,414,709, 17 September 2019. [Google Scholar]
- Chacon, F.T.; Raup-Konsavage, W.M.; Vrana, K.E.; Kellogg, J.J. Effect of Hemp Extraction Procedures on Cannabinoid and Terpenoid Composition. Plants 2024, 13, 2222. [Google Scholar] [CrossRef]
- Nahar, L.; Uddin, S.J.; Alam, M.A.; Sarker, S.D. Extraction of naturally occurring cannabinoids: An update. Phytochem. Anal. 2021, 32, 228–241. [Google Scholar] [CrossRef]
- Gallo-Molina, A.C.; Castro-Vargas, H.I.; Garzón-Méndez, W.F.; Ramírez, J.A.M.; Monroy, Z.J.R.; King, J.W.; Parada-Alfonso, F. Extraction, isolation and purification of tetrahydrocannabinol from the Cannabis sativa L. plant using supercritical fluid extraction and solid phase extraction. J. Supercrit. Fluids 2019, 146, 208–216. [Google Scholar] [CrossRef]
- Drinić, Z.; Vladić, J.; Koren, A.; Zeremski, T.; Stojanov, N.; Kiprovski, B.; Vidović, S. Microwave-assisted extraction of cannabinoids and antioxidants from Cannabis sativa aerial parts and processmodeling. J. Chem. Technol. Biotechnol. 2020, 95, 831–839. [Google Scholar] [CrossRef]
- Agarwal, C.; Mathe, K.; Hofmann, T.; Csoka, L. Ultrasound-Assisted Extraction of Cannabinoids from Cannabis Sativa L. Optimized by Response Surface Methodology. J. Food Sci. 2018, 83, 700–711. [Google Scholar] [CrossRef]
- Chepel, V.; Lisun, V.; Skrypnik, L. Changes in the Content of Some Groups of Phenolic Compounds and Biological Activity of Extracts of Various Parts of Heather (Calluna vulgaris (L.) Hull) at Different Growth Stages. Plants 2020, 9, 926. [Google Scholar] [CrossRef]
- Saffaryazdi, A.; Ganjeali, A.; Farhoosh, R.; Cheniany, M. Variation in phenolic compounds, α-linolenic acid and linoleic acid contents and antioxidant activity of purslane (Portulaca oleracea L.) during phenological growth stages. Physiol. Mol. Biol. Plants 2020, 26, 1519–1529. [Google Scholar] [CrossRef]
- Vlaisavljević, S.; Kaurinović, B.; Popović, M.; Vasiljević, S. Profile of phenolic compounds in Trifolium pratense L. extracts at different growth stages and their biological activities. Int. J. Food Prop. 2017, 20, 3090–3101. [Google Scholar] [CrossRef]
- Cobaleda-Velásco, M.; Alanís-Bañuelos, R.E.; Almaraz-Abarca, N.; Rojas-López, M.; González-Valdez, L.S.; Ávila-Reyes, J.A.; Rodrigo, S. Phenolic profiles and antioxidant properties of Physalis angulata L. as quality indicators. J. Pharm. Pharmacogn. Res. 2017, 5, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Bahorun, T.; Luximon-Ramma, A.; Crozier, A.; Aruoma, O.I. Total phenol, flavonoid, proanthocyanidin and vitamin C levels and antioxidant activities of Mauritian vegetables. J. Sci. Food Agric. 2004, 84, 1553–1561. [Google Scholar] [CrossRef]
- Durling, N.; Catchpole, O.; Grey, J.; Webby, R.; Mitchell, K.; Foo, L.; Perry, N. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Mkpenie, V.N.; Essien, E.E.; Udoh, I.I. Effect of extraction conditions on total polyphenol contents, antioxidant and antimicrobial activities of Cannabis sativa L. EJEAFChe 2012, 11, 300–307. [Google Scholar]
- Galvan d’Alessandro, L.; Kriaa, K.; Nikov, I.; Dimitrov, K. Ultrasound assisted extraction of polyphenols from black chokeberry. Sep. Purif. Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef]
- Nićiforović, N.; Abramovič, H. Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity. Compr. Rev. Food Sci. Food Saf. 2020, 13, 2014. [Google Scholar] [CrossRef]
- Gruz, J.; Ayaz, F.A.; Torun, H.; Strnad, M. Phenolic acid content and radical scavenging activity of extracts from medlar (Mespilus germanica L.) fruit at different stages of ripening. Food Chem. 2011, 124, 271–277. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Choi, S.Y.; Ko, H.C.; Ko, S.Y.; Hwang, J.H.; Park, J.G.; Kang, S.H.; Han, S.H.; Yun, S.H.; Kim, S.J. Correlation between flavonoid content and the NO production inhibitory activity of peel extracts from various citrus fruits. Biol. Pharm. Bull. 2007, 30, 772–778. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic Acid Derivatives and Their Biological Efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Mahlberg, P.G.; Kim, E.S. Accumulation of Cannabinoids in Glandular Trichomes of Cannabis (Cannabaceae). J. Ind. Hemp 2004, 9, 15–36. [Google Scholar] [CrossRef]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of Phenolic Compounds in Commercial Cannabis sativa L. Inflorescences Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631. [Google Scholar] [CrossRef]
- Biesiada, A.; Tomczak, A. Biotic and abiotic factors affecting the content of the chosen antioxidant compounds in vegetables. Veg. Crops Res. Bull. 2012, 76, 55–78. [Google Scholar] [CrossRef]
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp). J. Pharm. Biomed. Anal. 2017, 148, 228–236. [Google Scholar] [CrossRef]
- Krivošija, S.; Jerković, I.; Nastić, N.; Zloh, M.; Jokić, S.; Banožić, M.; Aladic, K.; Vidović, S. Green pathway for utilisation of orange peel dust and in silico evaluation of pharmacological potential. Microchem. J. 2023, 193, 109132. [Google Scholar] [CrossRef]
- Palma, M.; Barbero, G.F.; Pineiro, Z.; Liazid, A.; Barroso, C.G.; Rostagno, M.A.; Prado, J.M.; Meireles, M.A.A. Extraction of Natural Products: Principles and Fundamental Aspects. In Natural Product Extraction: Principles and Applications; Rostagno, M.A., Prado, J.M., Eds.; The Royal Society of Chemistry: London, UK, 2013; pp. 58–88. [Google Scholar] [CrossRef]
- Chemat, S.; Lagha, A.; AitAmar, H.; Bartels, P.V.; Chemat, F. Comparison of conventional and ultrasound-assisted extraction of carvone and limonene from caraway seeds. Flavour. Fragr. J. 2004, 19, 188–195. [Google Scholar] [CrossRef]
- Wei, X.; Chen, M.; Xiao, J.; Liu, Y.; Yu, L.; Zhang, H.; Wang, Y. Composition and bioactivity of tea flower polysaccharides obtained by different methods. Carbohydr. Polym. 2010, 79, 418–422. [Google Scholar] [CrossRef]
- Dahmoune, F.; Remini, H.; Dairi, S.; Aoun, O.; Moussi, K.; Bouaoudia-Madi, N.; Adjeroud, N.; Kadri, N.; Lefsih, K.; Boughani, L.; et al. Ultrasound assisted extraction of phenolic compounds from P. lentiscus L. leaves: Comparative study of artificial neural network (ANN) versus degree of experiment for prediction ability of phenolic compounds recovery. Ind. Crops Prod. 2015, 77, 251–261. [Google Scholar] [CrossRef]
- Matešić, N.; Jurina, T.; Benković, M.; Panić, M.; Valinger, D.; Gajdoš Kljusurić, J.; Jurinjak Tušek, A. Microwave-assisted extraction of phenolic compounds from Cannabis sativa L.: Optimization and kinetics study. Sep. Sci. Technol. 2020, 56, 2047–2060. [Google Scholar] [CrossRef]
- Mudrić, J.; Janković, T.; Šavikin, K.; Bigović, D.; Đukić-Ćosić, D.; Ibrić, S.; Đuriš, J. Optimization and modelling of gentiopicroside, isogentisin and total phenolics extraction from Gentiana lutea L. roots. Ind. Crops Prod. 2020, 155, 112767. [Google Scholar] [CrossRef]
- Bocsa, I.; Karus, M. The Cultivation of Hemp: Botany, Varieties, Cultivation and Harvesting; Hemptech: Sebastopol, CA, USA, 1998. [Google Scholar]
- Bas, D.; Boyacı, I.H. Modeling and optimization. I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Henonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods. In A Guide to Modern Techniques of Plant Analysis; Springer: Dordrecht, The Netherlands, 1994. [Google Scholar]
- Mišan, A.C.; Mimica-Dukić, N.M.; Mandić, A.I.; Sakač, M.B.; Milovanović, I.J.; Sedej, I.J. Development of a rapid resolution HPLC method for the separation and determination of 17 phenolic compounds in crude plant extracts. Cent. Eur. J. Chem. 2011, 9, 133–142. [Google Scholar] [CrossRef]
- Poortman-van der Meer, A.J.; Huizer, H. A contribution to the improvement of accuracy in the quantitation of THC. Forensic Sci. Int. 1999, 101, 1–8. [Google Scholar] [CrossRef]
| Solvent | Hemp at the Beginning of the Flowering Stage | Hemp in the Full Flowering Stage | ||||
|---|---|---|---|---|---|---|
| EY [%] | TP [mg GAE/mL] | TF [mg CE/mL] | EY [%] | TP [mg GAE/mL] | TF [mg CE/mL] | |
| 90% ethanol | 8.18 ± 0.41 e | 0.5962 ± 0.0298 e | 0.2370 ± 0.0119 ef | 7.54 ± 0.38 e | 0.5535 ± 0.0277 b | 0.3169 ± 0.02 b |
| 70% ethanol | 12.84 ± 0.64 d | 1.7045 ± 0.0853 a | 0.4451 ± 0.0223 bc | 11.66 ± 0.58 c | 1.0386 ± 0.0519 a | 0.3323 ± 0.02 ab |
| 50% ethanol | 16.96 ± 0.85 bc | 1.2565 ± 0.0629 b | 0.4817 ± 0.0241 b | 14.24 ± 0.71 b | 1.0476 ± 0.0524 a | 0.3584 ± 0.02 a |
| 30% ethanol | 17.34 ± 0.87 bc | 1.0981 ± 0.0549 c | 0.3022 ± 0.0151 d | 15.22 ± 0.76 ab | 1.0206 ± 0.0510 a | 0.2174 ± 0.01 d |
| Water | 17.94 ± 0.90 ab | 0.5759 ± 0.0288 e | 0.3950 ± 0.0198 c | 15.28 ± 0.76 ab | 0.4996 ± 0.0250 b | 0.1674 ± 0.01 e |
| Solvent | Protocatechuic Acid | Vanillic Acid | Syringic Acid | Epicatechin | Ferulic Acid | Sinapic Acid | Isovitexin | Rutin | Cinnamic Acid | Naringenin | Apigenin | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hemp areal parts at the beginning of the flowering stage | 90% Ethanol | 1.09 | 0.45 | nd a | nd | 2.57 | 1.13 | 1.14 | 4.13 | 0.81 | 1.21 | 0.31 |
| 70% Ethanol | 1.97 | 1.55 | 1.72 | 7.38 | 1.33 | 16.36 | 3.28 | 6.77 | 1.37 | nd | 7.73 | |
| 50% Ethanol | 1.50 | 1.44 | nd | nd | 3.61 | 30.02 | 1.84 | 0.19 | 1.44 | nd | 8.81 | |
| 30% Ethanol | 1.66 | 0.63 | nd | nd | 2.49 | 3.21 | 0.92 | 0.19 | 1.38 | 33.16 | 4.16 | |
| Water | 0.36 | nd | 0.42 | nd | 1.36 | 2.81 | nd | nd | 1.71 | 2.14 | 2.01 | |
| hemp areal in the full flowering stage | 90% Ethanol | 2.50 | 1.07 | nd | nd | nd | 4.02 | 0.64 | 2.28 | 7.32 | 1.60 | 0.31 |
| 70% Ethanol | 1.24 | 1.53 | 4.77 | 3.59 | nd | 1.19 | Nd | 2.63 | 8.84 | 1.51 | 0.96 | |
| 50% Ethanol | nd | 1.26 | nd | nd | nd | 1.03 | 6.21 | 2.74 | 3.28 | 1.33 | 1.10 | |
| 30% Ethanol | nd | 0.58 | nd | nd | nd | nd | Nd | nd | nd | 1.70 | 0.98 | |
| Water | nd | nd | nd | nd | nd | 1.46 | 0.98 | nd | nd | 1.46 | 0.96 |
| Independent Variables | Responses | |||||||
|---|---|---|---|---|---|---|---|---|
| Run | Extraction Temperature (°C) | Extraction Time [min] | Ultrasonic Power Density (W/L) | EY [%] | TP [mg GAE/mL Extract] | TF [mg CE/mL Extract] | CBD [mg/mL] | THC [mg/mL] |
| 1 | 60 | 40 | 42 | 13.34 | 1.3050 | 0.6391 | 0.7604 | 0.0439 |
| 2 | 60 | 20 | 60 | 14.78 | 1.3612 | 0.5405 | 0.7860 | 0.0442 |
| 3 | 80 | 20 | 42 | 15.88 | 1.4145 | 0.6722 | 0.8259 | 0.0455 |
| 4 | 60 | 40 | 42 | 14.74 | 1.2966 | 0.6426 | 0.7672 | 0.0444 |
| 5 | 60 | 60 | 60 | 17.02 | 1.4258 | 0.6749 | 0.7912 | 0.0447 |
| 6 | 40 | 60 | 42 | 12.66 | 1.1590 | 0.5137 | 0.6924 | 0.0425 |
| 7 | 80 | 40 | 60 | 17.04 | 1.4314 | 0.5808 | 0.8752 | 0.0458 |
| 8 | 40 | 20 | 42 | 10.20 | 1.0552 | 0.3793 | 0.6158 | 0.0412 |
| 9 | 60 | 40 | 42 | 13.66 | 1.3752 | 0.5620 | 0.7154 | 0.0431 |
| 10 | 40 | 40 | 60 | 11.72 | 1.2208 | 0.4680 | 0.6712 | 0.0421 |
| 11 | 60 | 40 | 42 | 17.54 | 1.4763 | 0.5925 | 0.7289 | 0.0437 |
| 12 | 60 | 20 | 24 | 14.10 | 1.3949 | 0.5378 | 0.6966 | 0.0428 |
| 13 | 80 | 40 | 24 | 14.94 | 1.4987 | 0.6543 | 0.8602 | 0.0453 |
| 14 | 60 | 60 | 24 | 12.16 | 1.3780 | 0.5656 | 0.7329 | 0.0440 |
| 15 | 40 | 40 | 24 | 10.00 | 1.1534 | 0.4563 | 0.6698 | 0.0420 |
| 16 | 80 | 60 | 42 | 16.56 | 1.7795 | 0.5459 | 0.7612 | 0.0440 |
| 17 | 60 | 40 | 42 | 14.68 | 1.2882 | 0.5262 | 0.7946 | 0.0441 |
| Optimal Conditionals | Predicted Values | |||||||
|---|---|---|---|---|---|---|---|---|
| Target | Extraction Temperature (°C) | Extraction Time [min] | Ultrasonic Power Density (W/L) | EY [%] | TP [mg GAE/mL Extract] | TF [mg CE/mL Extract] | CBD [mg/mL] | THC [mg/mL] |
| TP max TF max | 68.8 | 54.8 | 60 | 17.54 | 1.5245 | 0.6214 | 0.8077 | 0.0448 |
| CBD max THC none | 79.4 | 31.6 | 60 | 16.85 | 1.4435 | 0.6092 | 0.8827 | 0.0460 |
| CBD max THC min | 55.4 | 38.2 | 24 | 13.43 | 1.2370 | 0.5368 | 0.7339 | 0.0434 |
| Sample | Protocatechuic Acid | Vanillic Acid | Ferulic Acid | Sinapic Acid | Isovitexin | Rutin | Cinnamic Acid | Naringenin | Apigenin |
|---|---|---|---|---|---|---|---|---|---|
| Hemp aerial parts at the beginning of the flowering stage | 1.34 | 1.95 | 3.36 | 25.00 | 6.56 | 1.52 | 1.55 | 6.50 | 2.00 |
| Hemp aerial parts in the full flowering stage | 1.28 | 2.22 | 1.52 | 10.04 | 1.04 | 3.92 | 2.04 | 2.50 | 1.28 |
| Extraction Temperature (°C) | Extraction Time [min] | Ultrasonic Power Density (W/L) | Predicted Value | |
|---|---|---|---|---|
| EY [%] | 55.03 | 70.15 | 60 | 18.36 |
| TP [mg GAE/mL extract] | 60 | 80 | 41.53 | 1.7791 |
| TF [mg CE/mL extract] | 60 | 79.12 | 60 | 0.9108 |
| CBD [mg/mL] | 44.62 | 80 | 60 | 1.1347 |
| THC [mg/mL] | 20 | 80 | 60 | 0.1488 |
| Input Variable | Values of Input Variables | |||||
|---|---|---|---|---|---|---|
| Coded | Actual | Coded | Actual | Coded | Actual | |
| Extraction temperature [°C] | −1 | 40 | 0 | 60 | 1 | 80 |
| Extraction time [min] | −1 | 20 | 0 | 40 | 1 | 60 |
| Ultrasonic power density [W/L] | −1 | 24 | 0 | 42 | 1 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarević, Z.; Koren, A.; Zeremski, T.; Mišan, A.; Nastić, N.; Stojanov, N.; Vidović, S. Maximizing Cannabinoid and Polyphenol Extraction from Industrial Hemp (Cannabis sativa L. cv. Helena) Areal Parts: A Comparative Study of Ultrasound-Assisted and Conventional Methods at Two Harvest Stages. Plants 2025, 14, 816. https://doi.org/10.3390/plants14050816
Lazarević Z, Koren A, Zeremski T, Mišan A, Nastić N, Stojanov N, Vidović S. Maximizing Cannabinoid and Polyphenol Extraction from Industrial Hemp (Cannabis sativa L. cv. Helena) Areal Parts: A Comparative Study of Ultrasound-Assisted and Conventional Methods at Two Harvest Stages. Plants. 2025; 14(5):816. https://doi.org/10.3390/plants14050816
Chicago/Turabian StyleLazarević, Zorica, Anamarija Koren, Tijana Zeremski, Aleksandra Mišan, Nataša Nastić, Nadežda Stojanov, and Senka Vidović. 2025. "Maximizing Cannabinoid and Polyphenol Extraction from Industrial Hemp (Cannabis sativa L. cv. Helena) Areal Parts: A Comparative Study of Ultrasound-Assisted and Conventional Methods at Two Harvest Stages" Plants 14, no. 5: 816. https://doi.org/10.3390/plants14050816
APA StyleLazarević, Z., Koren, A., Zeremski, T., Mišan, A., Nastić, N., Stojanov, N., & Vidović, S. (2025). Maximizing Cannabinoid and Polyphenol Extraction from Industrial Hemp (Cannabis sativa L. cv. Helena) Areal Parts: A Comparative Study of Ultrasound-Assisted and Conventional Methods at Two Harvest Stages. Plants, 14(5), 816. https://doi.org/10.3390/plants14050816








