Identification and Characterization of Lipid Droplet-Associated Protein (LDAP) Isoforms from Tung Tree (Vernicia fordii)
Abstract
1. Introduction
2. Results
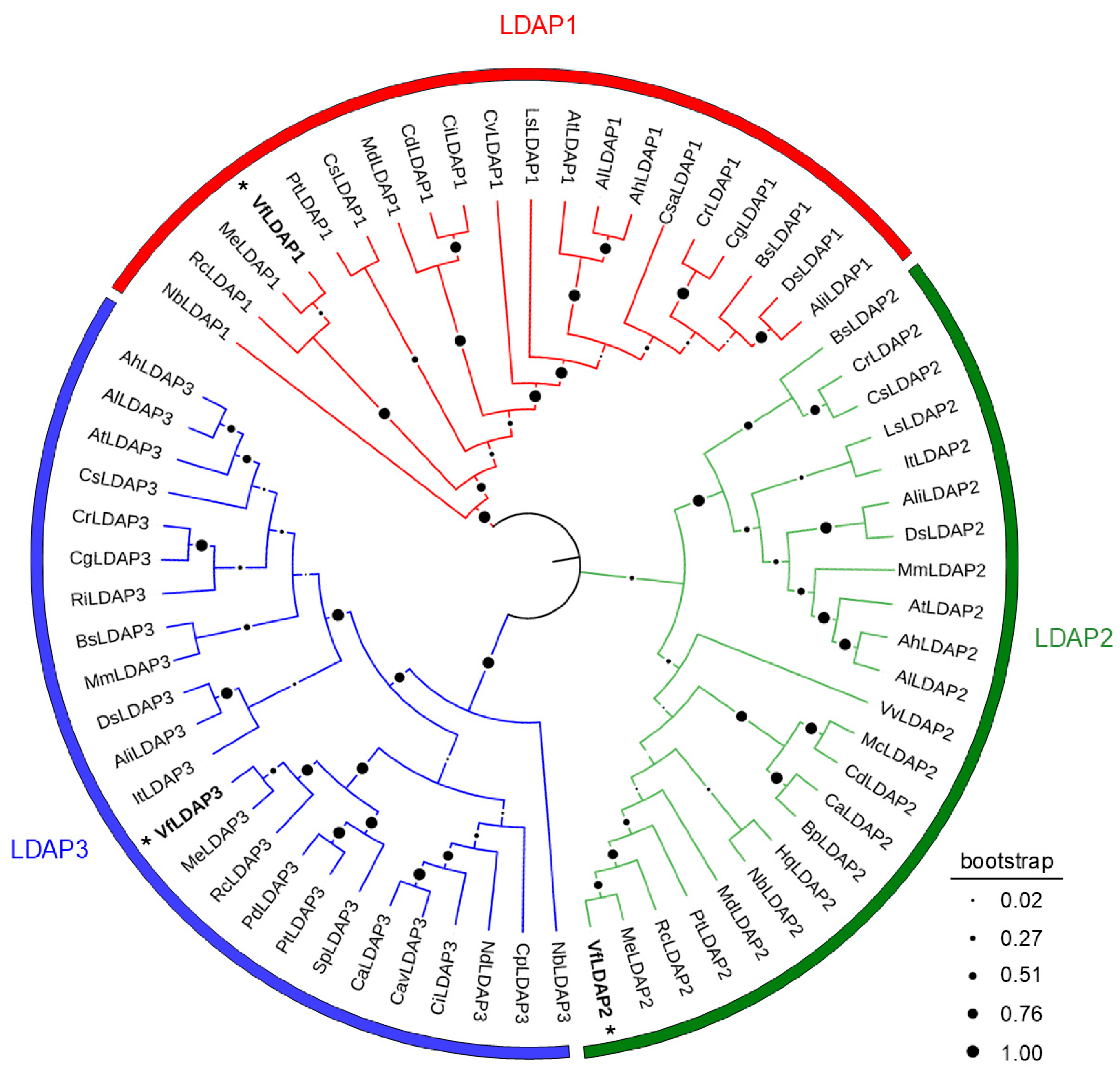
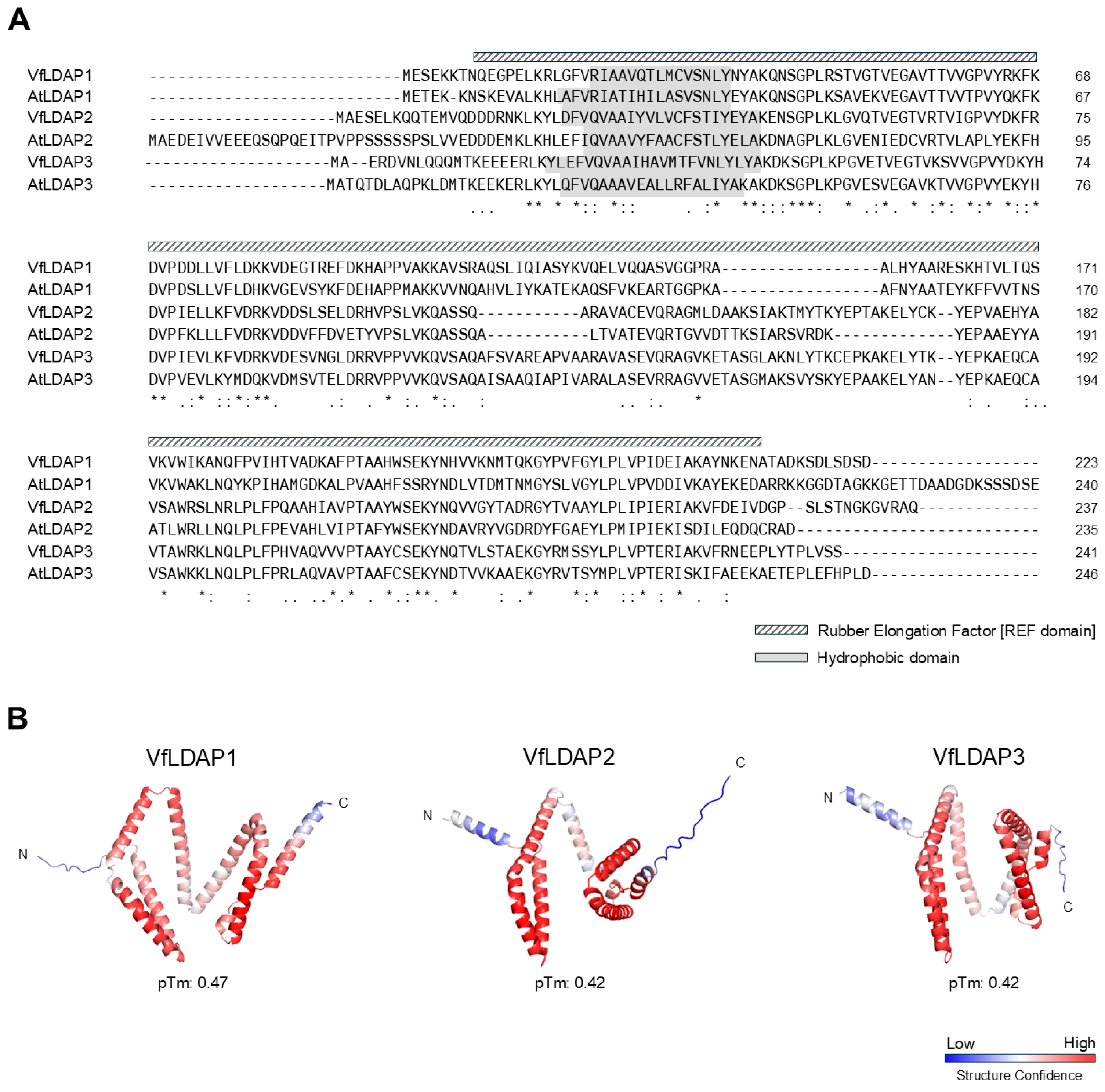
2.1. Tung Has Three Distinct LDAP Isoforms
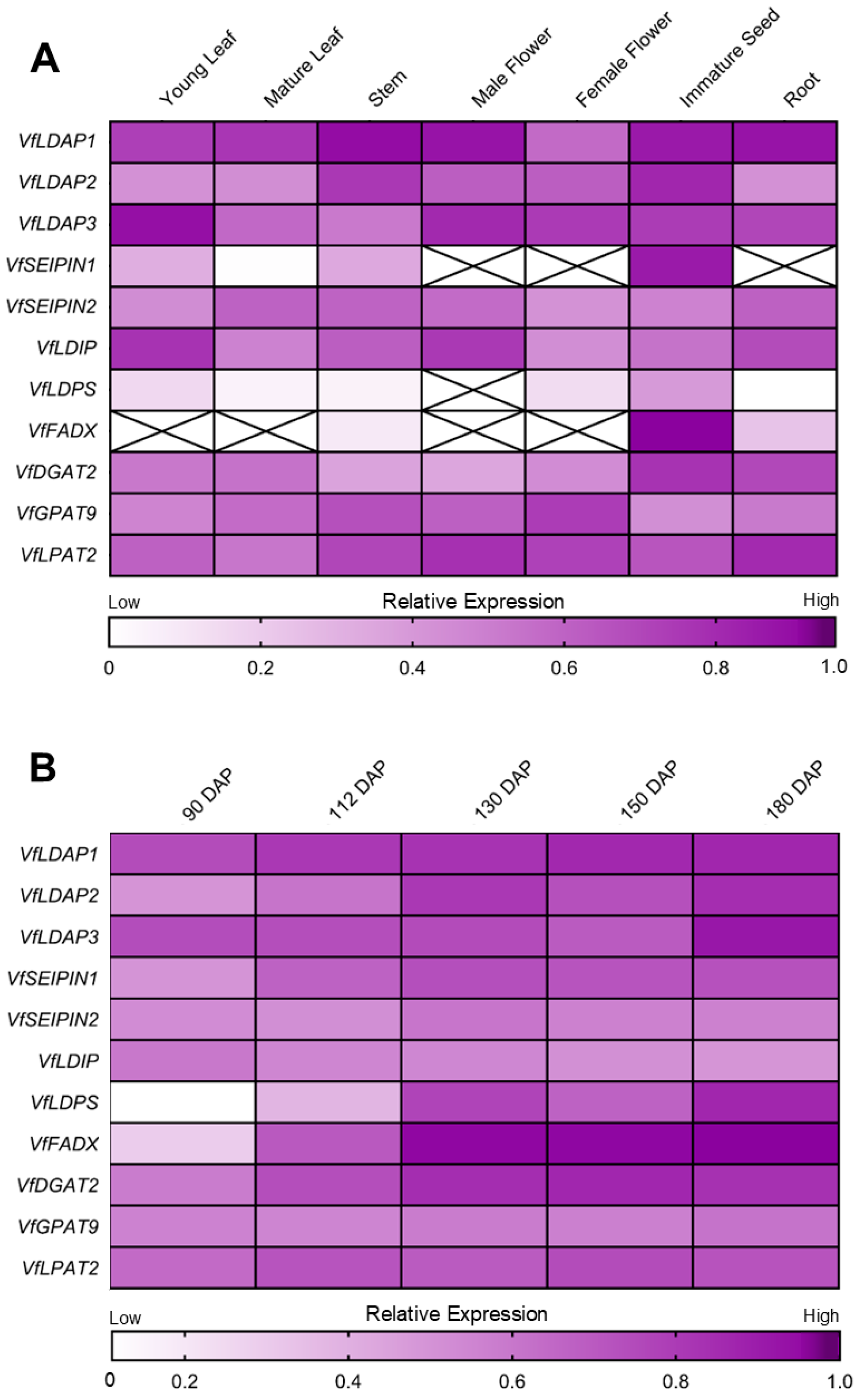
2.2. VfLDAPs Are Differentially Expressed in Various Tung Tree Organs and Developmental Stages
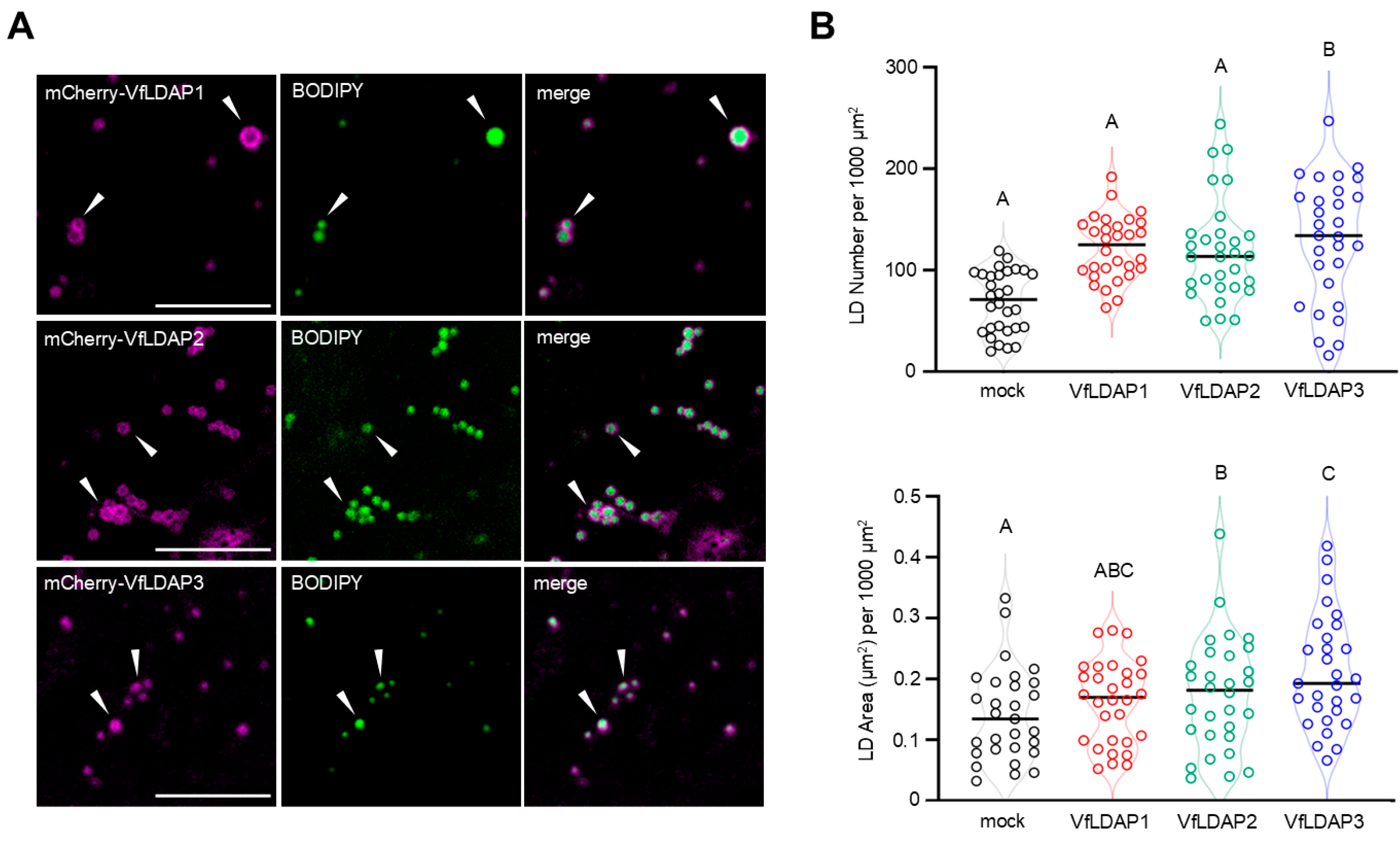
2.3. VfLDAP1, 2, and 3 Localize to LDs and Influence Their Numbers and Sizes When Transiently Expressed in N. benthamiana Leaves
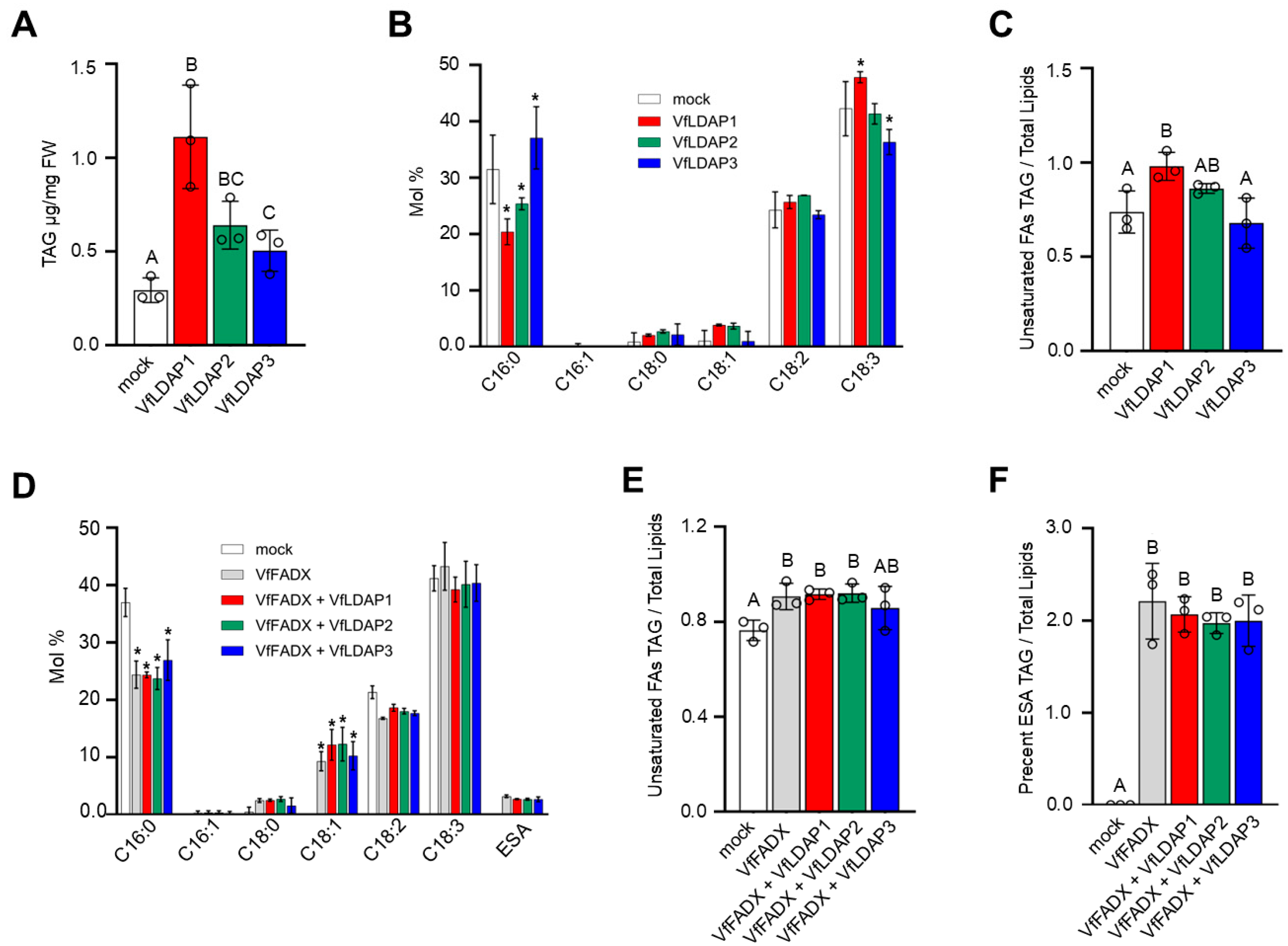
2.4. VfLDAPs Transiently Expressed in N. benthamiana Leaves Differentially Affect TAG Content and Fatty Acid Composition
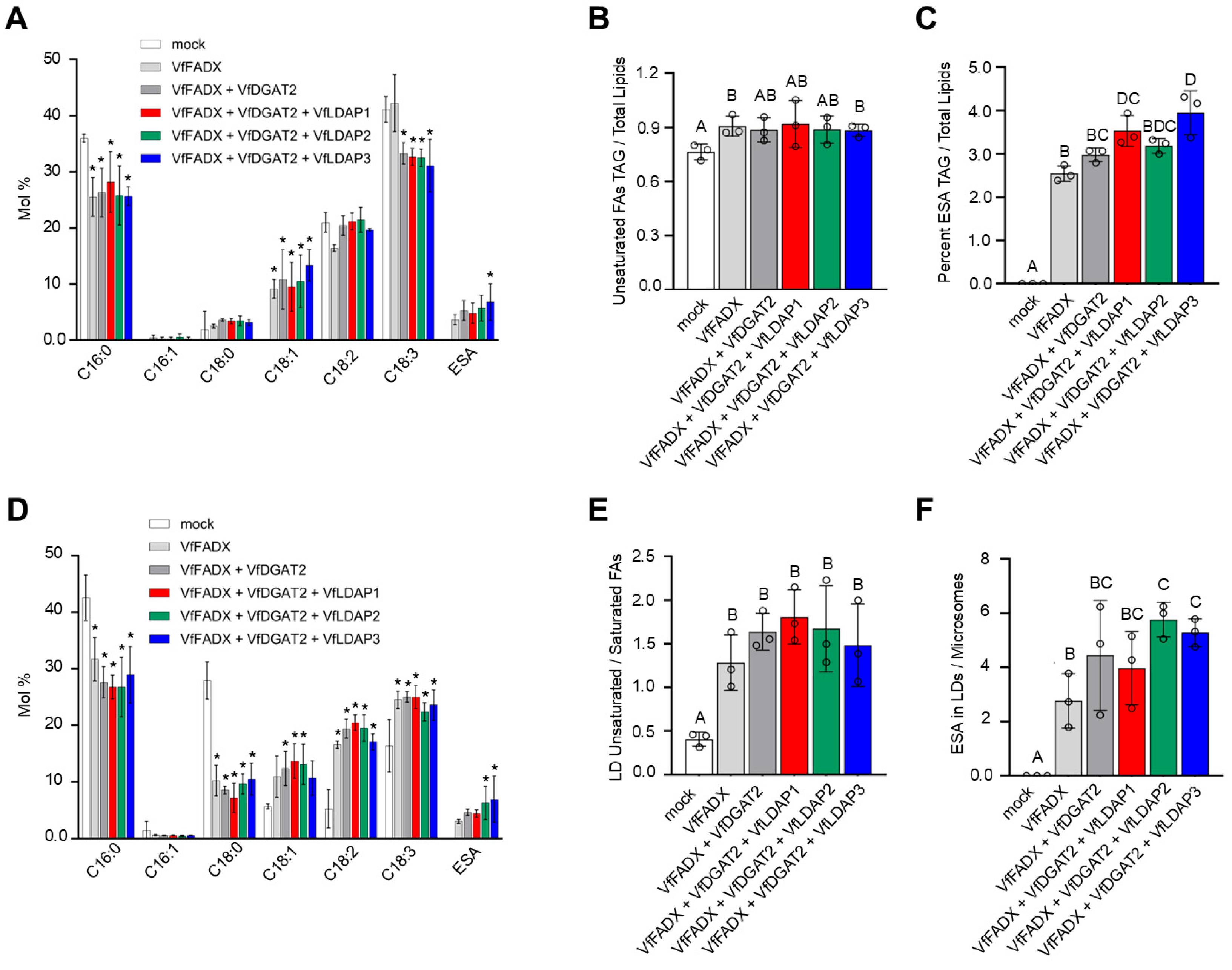
2.5. Transient Co-Expression of VfFADX, VfDGAT2, and VfLDAP2 or VfLDAP3 in N. benthamiana Leaves Results in an Increase in ESA Content in LDs
3. Discussion
3.1. Identification and Characterization of Three LDAP Isoforms (VfLDAP1-3) in Tung
3.2. Transient Expression of Tung LDAP Isoforms Differentially Alters TAG Content and Composition in Leaves and VfLDAP2 and VfLDAP3 Enrich ESA in a Partially Reconstituted TAG-ESA Biosynthetic Pathway
3.3. Potential Mechanisms by Which LD Coat Proteins Modulate TAG Content and Composition in LDs
4. Materials and Methods
4.1. Sequence, Phylogenetic, and Structural Analyses of VfLDAPs
4.2. Plasmid Construction
4.3. Transient Expression in N. benthamiana Leaves
4.4. RT-PCR
4.5. Confocal Microscopy
4.6. LD and ER Microsomal Isolations
4.7. Lipid Analysis
4.8. Statistical Analysis
4.9. Gene Accession Numbers
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LDAP | LIPID DROPLET-ASSOCIATED-PROTEIN |
| TAG | Triacylglycerol |
| LD | Lipid Droplet |
| ESA | Eleostearic Acid |
| FADX | FATTY ACID DESATURASE X |
| DGAT2 | DIACYLGLYCEROL ACYLTRANSFERASE 2 |
| ER | Endoplasmic Reticulum |
| DAP | Days Post Pollination |
| LDPS | LIPID DROPLET PROTEIN OF SEEDS |
| REF | Rubber Elongation Factor |
| FAD2 | FATTY ACID DESATURASE 2 |
| SRPP | SMALL RUBBER PARTICLE PROTEIN |
| PLIN | PERILIPIN |
| BLAST | Basic Local Alignment Search Tool |
| CLSM | Confocal Laser Scanning Microscopy |
| BODIPY | Boron-Dipyrromethene |
| LDIP | LIPID-DROPLET-INTERACTING-PROTEIN |
| GPAT9 | GLYCEROL-3-PHOSPHATE ACYLTRANSFERASE 9 |
| LPAT2 | LYSOPHOSPHATIDYL ACYLTRANSFERASE 2 |
References
- Li, W.; Liu, M.; Dong, X.; Cao, H.; Wu, Y.; Shang, H.; Huang, H.; Zhang, L. Flower Biology and Ontogeny of the Tung Tree (Vernicia fordii Hemsl.). Trees 2020, 34, 1363–1381. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chen, J.-H.; Chang, C.-Y.; Chang, C.-C. Biodiesel Production from Tung (Vernicia montana) Oil and Its Blending Properties in Different Fatty Acid Compositions. Bioresour. Technol. 2010, 101, 9521–9526. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Lin, Q.; Fang, D.; Zhang, L.; Li, R.; Cheng, J.; Gao, F.; Shockey, J.; Hu, S.; Lü, S. Tung Tree (Vernicia fordii Hemsl.) Genome and Transcriptome Sequencing Reveals Co-Ordinate Up-Regulation of Fatty Acid β-Oxidation and Triacylglycerol Biosynthesis Pathways During Eleostearic Acid Accumulation in Seeds. Plant Cell Physiol. 2018, 59, 1990–2003. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hammond, E.G.; Nikolau, B.J. In Vivo Studies of the Biosynthesis of α-Eleostearic Acid in the Seed of Momordica charantia L. Plant Physiol. 1997, 113, 1343–1349. [Google Scholar] [CrossRef]
- Shockey, J.; Rinehart, T.; Chen, Y.; Yangdong, W.; Zhiyong, Z.; Lisong, H. Tung (Vernicia fordii and Vernicia montana). In Industrial Oil Crops; Elsevier: Amsterdam, The Netherlands, 2016; pp. 243–273. ISBN 978-1-893997-98-1. [Google Scholar]
- He, Z.; Qian, J.; Qu, L.; Yan, N.; Yi, S. Effects of Tung Oil Treatment on Wood Hygroscopicity, Dimensional Stability and Thermostability. Ind. Crops Prod. 2019, 140, 111647. [Google Scholar] [CrossRef]
- He, Z.; Chapital, D.C.; Cheng, H.N.; Thomas Klasson, K.; Olanya, O.M.; Uknalis, J. Application of Tung Oil to Improve Adhesion Strength and Water Resistance of Cottonseed Meal and Protein Adhesives on Maple Veneer. Ind. Crops Prod. 2014, 61, 398–402. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Tokuyama, Y.; Igarashi, M.; Miyazawa, T. Tumor Growth Suppression by α-Eleostearic Acid, a Linolenic Acid Isomer with a Conjugated Triene System, via Lipid Peroxidation. Carcinogenesis 2004, 25, 1417–1425. [Google Scholar] [CrossRef]
- Zhuo, R.-J.; Wang, F.; Zhang, X.-H.; Zhang, J.-J.; Xu, J.; Dong, W.; Zou, Z.-Q. α-Eleostearic Acid Inhibits Growth and Induces Apoptosis in Breast Cancer Cells via HER2/HER3 Signaling. Mol. Med. Rep. 2014, 9, 993–998. [Google Scholar] [CrossRef][Green Version]
- Cao, Y.; Liu, M.; Long, H.; Zhao, Q.; Jiang, L.; Zhang, L. Hidden in Plain Sight: Systematic Investigation of Leucine-Rich Repeat Containing Genes Unveil Their Regulatory Network in Response to Fusarium Wilt in Tung Tree. Int. J. Biol. Macromol. 2020, 163, 1759–1767. [Google Scholar] [CrossRef]
- Bernál, M.; Schneider, R.; Machado, N. Environmental Assessment of the Tung Cultivation Through Life Cycle Analysis. IJET 2014, 3, 70–71. [Google Scholar] [CrossRef][Green Version]
- Shockey, J.M.; Gidda, S.K.; Chapital, D.C.; Kuan, J.-C.; Dhanoa, P.K.; Bland, J.M.; Rothstein, S.J.; Mullen, R.T.; Dyer, J.M. Tung Tree DGAT1 and DGAT2 Have Nonredundant Functions in Triacylglycerol Biosynthesis and Are Localized to Different Subdomains of the Endoplasmic Reticulum. Plant Cell 2006, 18, 2294–2313. [Google Scholar] [CrossRef] [PubMed]
- Dyer, J.M.; Chapital, D.C.; Kuan, J.-C.W.; Mullen, R.T.; Turner, C.; McKeon, T.A.; Pepperman, A.B. Molecular Analysis of a Bifunctional Fatty Acid Conjugase/Desaturase from Tung. Implications for the Evolution of Plant Fatty Acid Diversity. Plant Physiol. 2002, 130, 2027–2038. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, O.; Shockey, J.M.; Gidda, S.K.; Silver, M.I.; Chapman, K.D.; Mullen, R.T.; Dyer, J.M. Engineering the Production of Conjugated Fatty Acids in Arabidopsis thaliana Leaves. Plant Biotechnol. J. 2017, 15, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
- Shanklin, J.; Cahoon, E.B. Desaturation and Related Modifications of Fatty Acids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 611–641. [Google Scholar] [CrossRef]
- Xu, Y.; Mietkiewska, E.; Shah, S.; Weselake, R.J.; Chen, G. Punicic Acid Production in Brassica napus. Metab. Eng. 2020, 62, 20–29. [Google Scholar] [CrossRef]
- Clews, A.C.; Ulch, B.A.; Jesionowska, M.; Hong, J.; Mullen, R.T.; Xu, Y. Variety of Plant Oils: Species-Specific Lipid Biosynthesis. Plant Cell Physiol. 2024, 65, 845–862. [Google Scholar] [CrossRef]
- Thiam, A.R.; Ikonen, E. Lipid Droplet Nucleation. Trends Cell Biol. 2021, 31, 108–118. [Google Scholar] [CrossRef]
- Guzha, A.; Whitehead, P.; Ischebeck, T.; Chapman, K.D. Lipid Droplets: Packing Hydrophobic Molecules Within the Aqueous Cytoplasm. Annu. Rev. Plant Biol. 2023, 74, 195–223. [Google Scholar] [CrossRef]
- Kumari, R.M.; Khatri, A.; Chaudhary, R.; Choudhary, V. Concept of Lipid Droplet Biogenesis. Eur. J. Cell Biol. 2023, 102, 151362. [Google Scholar] [CrossRef]
- Gidda, S.; Park, S.; Pyc, M.; Yurchenko, O.; Cai, Y.; Wu, P.; Andrews, D.; Chapman, K.; Mullen, R. Lipid Droplet-Associated Proteins (LDAPs) Are Required for the Dynamic Regulation of Neutral Lipid Compartmentation in Plant Cells. Plant Physiol. 2016, 170, 2052–2071. [Google Scholar] [CrossRef]
- Horn, P.J.; James, C.N.; Gidda, S.K.; Kilaru, A.; Dyer, J.M.; Mullen, R.T.; Ohlrogge, J.B.; Chapman, K.D. Identification of a New Class of Lipid Droplet-Associated Proteins in Plants. Plant Physiol. 2013, 162, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.U.; Hsieh, K.; Ratnayake, C.; Huang, A.H.C. A Novel Group of Oleosins Is Present Inside the Pollen of Arabidopsis. J. Biol. Chem. 2002, 227, 22677–22684. [Google Scholar] [CrossRef] [PubMed]
- Tzen, J.T.C.; Lai, Y.-K.; Chan, K.-L.; Huang, A.H.C. Oleosin Isoforms of High and Low Molecular Weights Are Present in the Oil Bodies of Diverse Seed Species. Plant Physiol. 1990, 94, 1282–1289. [Google Scholar] [CrossRef]
- Miquel, M.; Trigui, G.; d’Andréa, S.; Kelemen, Z.; Baud, S.; Berger, A.; Deruyffelaere, C.; Trubuil, A.; Lepiniec, L.; Dubreucq, B. Specialization of Oleosins in Oil Body Dynamics During Seed Development in Arabidopsis Seeds. Plant Physiol. 2014, 164, 1866–1878. [Google Scholar] [CrossRef]
- Siloto, R.M.P.; Findlay, K.; Lopez-Villalobos, A.; Yeung, E.C.; Nykiforuk, C.L.; Moloney, M.M. The Accumulation of Oleosins Determines the Size of Seed Oilbodies in Arabidopsis. Plant Cell 2006, 18, 1961–1974. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, G.; Wang, Y.; Xu, L. F-Box and Oleosin: Additional Target Genes for Future Metabolic Engineering in Tung Trees? Ind. Crops Prod. 2010, 32, 684–686. [Google Scholar] [CrossRef]
- Huang, A.H.C. Plant Lipid Droplets and Their Associated Proteins: Potential for Rapid Advances. Plant Physiol. 2018, 176, 1894–1918. [Google Scholar] [CrossRef]
- Vanhercke, T.; Wood, C.C.; Stymne, S.; Singh, S.P.; Green, A.G. Metabolic Engineering of Plant Oils and Waxes for Use as Industrial Feedstocks. Plant Biotechnol. J. 2013, 11, 197–210. [Google Scholar] [CrossRef]
- Anaokar, S.; Liang, Y.; Yu, X.-H.; Cai, Y.; Cai, Y.; Shanklin, J. The Expression of Genes Encoding Novel Sesame Oleosin Variants Facilitates Enhanced Triacylglycerol Accumulation in Arabidopsis Leaves and Seeds. New Phytol. 2024, 243, 271–283. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, X.-H.; Anaokar, S.; Shi, H.; Dahl, W.B.; Cai, Y.; Luo, G.; Chai, J.; Cai, Y.; Mollá-Morales, A.; et al. Engineering Triacylglycerol Accumulation in Duckweed (Lemna japonica). Plant Biotechnol. J. 2023, 21, 317–330. [Google Scholar] [CrossRef]
- Liu, W.X.; Liu, H.L.; Qu, L.Q. Embryo-Specific Expression of Soybean Oleosin Altered Oil Body Morphogenesis and Increased Lipid Content in Transgenic Rice Seeds. Theor. Appl. Genet. 2013, 126, 2289–2297. [Google Scholar] [CrossRef] [PubMed]
- Yee, S.; Rolland, V.; Reynolds, K.B.; Shrestha, P.; Ma, L.; Singh, S.P.; Vanhercke, T.; Petrie, J.R.; El Tahchy, A. Sesamum indicum Oleosin L Improves Oil Packaging in Nicotiana benthamiana Leaves. Plant Direct. 2021, 5, e343. [Google Scholar] [CrossRef] [PubMed]
- Guzha, A.; Gautam, B.; Marchiafava, D.; Ver Sagun, J.; Garcia, T.; Jarvis, B.A.; Barbaglia-Hurlock, A.M.; Johnston, C.; Grotewold, E.; Sedbrook, J.C.; et al. Targeted Modulation of Pennycress Lipid Droplet Proteins Impacts Droplet Morphology and Seed Oil Content. Plant J. 2024, 120, 2151–2171. [Google Scholar] [CrossRef]
- Kim, E.Y.; Park, K.Y.; Seo, Y.S.; Kim, W.T. Arabidopsis Small Rubber Particle Protein Homolog SRPs Play Dual Roles as Positive Factors for Tissue Growth and Development and in Drought Stress Responses. Plant Physiol. 2016, 170, 2494–2510. [Google Scholar] [CrossRef] [PubMed]
- Shockey, J.; Regmi, A.; Cotton, K.; Adhikari, N.; Browse, J.; Bates, P.D. Identification of Arabidopsis GPAT9 (AT5G60620) as an Essential Gene Involved in Triacylglycerol Biosynthesis. Plant Physiol. 2016, 170, 163–179. [Google Scholar] [CrossRef]
- Gidda, S.K.; Shockey, J.M.; Rothstein, S.J.; Dyer, J.M.; Mullen, R.T. Arabidopsis thaliana GPAT8 and GPAT9 are Localized to the ER and Possess Distinct ER Retrieval Signals: Functional Divergence of the Dilysine ER Retrieval Motif in Plant Cells. Plant Physiol. Biochem. 2009, 47, 867–879. [Google Scholar] [CrossRef]
- Shockey, J.; Lager, I.; Stymne, S.; Kotapati, H.K.; Sheffield, J.; Mason, C.; Bates, P.D. Specialized Lysophosphatidic Acid Acyltransferases Contribute to Unusual Fatty Acid Accumulation in Exotic Euphorbiaceae Seed Oils. Planta 2019, 249, 1285–1299. [Google Scholar] [CrossRef]
- Cai, Y.; Goodman, J.M.; Pyc, M.; Mullen, R.T.; Dyer, J.M.; Chapman, K.D. Arabidopsis SEIPIN Proteins Modulate Triacylglycerol Accumulation and Influence Lipid Droplet Proliferation. Plant Cell 2015, 27, 2616–2636. [Google Scholar] [CrossRef]
- Pyc, M.; Cai, Y.; Gidda, S.K.; Yurchenko, O.; Park, S.; Kretzschmar, F.K.; Ischebeck, T.; Valerius, O.; Braus, G.H.; Chapman, K.D.; et al. Arabidopsis Lipid Droplet-Associated Protein (LDAP)–Interacting Protein (LDIP) Influences Lipid Droplet Size and Neutral Lipid Homeostasis in Both Leaves and Seeds. Plant J. 2017, 92, 1182–1201. [Google Scholar] [CrossRef]
- Kretzschmar, F.K.; Doner, N.M.; Krawczyk, H.E.; Scholz, P.; Schmitt, K.; Valerius, O.; Braus, G.H.; Mullen, R.T.; Ischebeck, T. Identification of Low-Abundance Lipid Droplet Proteins in Seeds and Seedlings. Plant Physiol. 2020, 182, 1326–1345. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, L.; Tan, X.; Long, H.; Shockey, J.M. Identification, Classification and Differential Expression of Oleosin Genes in Tung Tree (Vernicia fordii). PLoS ONE 2014, 9, e88409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, P.; Lu, W.; Lü, S. Molecular Mechanism of the Extended Oil Accumulation Phase Contributing to the High Seed Oil Content for the Genotype of Tung Tree (Vernicia fordii). BMC Plant Biol. 2018, 18, 248. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, Transient Expression of Fluorescent Fusion Proteins in Tobacco Plants and Generation of Stably Transformed. Plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef]
- Listenberger, L.L.; Ostermeyer-Fay, A.G.; Goldberg, E.B.; Brown, W.J.; Brown, D.A. Adipocyte Differentiation-Related Protein Reduces the Lipid Droplet Association of Adipose Triglyceride Lipase and Slows Triacylglycerol Turnover. J. Lipid Res. 2007, 48, 2751–2761. [Google Scholar] [CrossRef]
- Pyc, M.; Cai, Y.; Greer, M.S.; Yurchenko, O.; Chapman, K.D.; Dyer, J.M.; Mullen, R.T. Turning Over a New Leaf in Lipid Droplet Biology. Trends Plant Sci. 2017, 22, 596–609. [Google Scholar] [CrossRef]
- Chapman, K.D.; Aziz, M.; Dyer, J.M.; Mullen, R.T. Mechanisms of Lipid Droplet Biogenesis. Biochem. J. 2019, 476, 1929–1942. [Google Scholar] [CrossRef]
- Berthelot, K.; Lecomte, S.; Estevez, Y.; Zhendre, V.; Henry, S.; Thévenot, J.; Dufourc, E.J.; Alves, I.D.; Peruch, F. Rubber Particle Proteins, HbREF and HbSRPP, Show Different Interactions with Model Membranes. Biochim. Biophys. Acta Biomembr. 2014, 1838, 287–299. [Google Scholar] [CrossRef]
- Hillebrand, A.; Post, J.J.; Wurbs, D.; Wahler, D.; Lenders, M.; Krzyzanek, V.; Prüfer, D.; Gronover, C.S. Down-Regulation of Small Rubber Particle Protein Expression Affects Integrity of Rubber Particles and Rubber Content in Taraxacum brevicorniculatum. PLoS ONE 2012, 7, e41874. [Google Scholar] [CrossRef]
- Wolters, S.M.; Laibach, N.; Riekötter, J.; Roelfs, K.-U.; Müller, B.; Eirich, J.; Twyman, R.M.; Finkemeier, I.; Prüfer, D.; Schulze Gronover, C. The Interaction Networks of Small Rubber Particle Proteins in the Latex of Taraxacum koksaghyz Reveal Diverse Functions in Stress Responses and Secondary Metabolism. Front. Plant Sci. 2024, 15, 1498737. [Google Scholar] [CrossRef]
- Oh, S.K.; Kang, H.; Shin, D.H.; Yang, J.; Chow, K.-S.; Yeang, H.Y.; Wagner, B.; Breiteneder, H.; Han, K.-H. Isolation, Characterization, and Functional Analysis of a Novel cDNA Clone Encoding a Small Rubber Particle Protein from Hevea brasiliensis. J. Biol. Chem. 1999, 274, 17132–17138. [Google Scholar] [CrossRef]
- Pyc, M.; Gidda, S.K.; Seay, D.; Esnay, N.; Kretzschmar, F.K.; Cai, Y.; Doner, N.M.; Greer, M.S.; Hull, J.J.; Coulon, D.; et al. LDIP Cooperates with SEIPIN and LDAP to Facilitate Lipid Droplet Biogenesis in Arabidopsis. Plant Cell 2021, 33, 3076–3103. [Google Scholar] [CrossRef] [PubMed]
- López-Ribera, I.; La Paz, J.L.; Repiso, C.; García, N.; Miquel, M.; Hernández, M.L.; Martínez-Rivas, J.M.; Vicient, C.M. The Evolutionary Conserved Oil Body Associated Protein OBAP1 Participates in the Regulation of Oil Body Size. Plant Physiol. 2014, 164, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Kishikawa, A.; Yoshida, M. Proteomic Analysis of Lipid Droplets in Sesamum indicum. Protein J. 2020, 39, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Miklaszewska, M.; Zienkiewicz, K.; Klugier-Borowska, E.; Rygielski, M.; Feussner, I.; Zienkiewicz, A. CALEOSIN 1 Interaction with AUTOPHAGY-RELATED PROTEIN 8 Facilitates Lipid Droplet Microautophagy in Seedlings. Plant Physiol. 2023, 193, 2361–2380. [Google Scholar] [CrossRef]
- Galli, V.; Guzman, F.; Messias, R.S.; Körbes, A.P.; Silva, S.D.A.; Margis-Pinheiro, M.; Margis, R. Transcriptome of Tung Tree Mature Seeds with an Emphasis on Lipid Metabolism Genes. Tree Genet. Genomes 2014, 10, 1353–1367. [Google Scholar] [CrossRef]
- Parchuri, P.; Bhandari, S.; Azeez, A.; Chen, G.; Johnson, K.; Shockey, J.; Smertenko, A.; Bates, P.D. Identification of Triacylglycerol Remodeling Mechanism to Synthesize Unusual Fatty Acid Containing Oils. Nat. Commun. 2024, 15, 3547. [Google Scholar] [CrossRef]
- Kory, N.; Farese, R.V.; Walther, T.C. Targeting Fat: Mechanisms of Protein Localization to Lipid Droplets. Trends Cell Biol. 2016, 26, 535–546. [Google Scholar] [CrossRef]
- Olarte, M.-J.; Swanson, J.M.J.; Walther, T.C.; Farese, R.V. The CYTOLD and ERTOLD Pathways for Lipid Droplet–Protein Targeting. Trends Biochem. Sci. 2022, 47, 39–51. [Google Scholar] [CrossRef]
- Prévost, C.; Sharp, M.E.; Kory, N.; Lin, Q.; Voth, G.A.; Farese, R.V.; Walther, T.C. Mechanism and Determinants of Amphipathic Helix-Containing Protein Targeting to Lipid Droplets. Dev. Cell. 2018, 44, 73–86. [Google Scholar] [CrossRef]
- Brocard, L.; Immel, F.; Coulon, D.; Esnay, N.; Fouillen, L.; Bessoule, J.-J.; Bréhélin, C. Proteomic Analysis of Lipid Droplets from Arabidopsis Aging Leaves Brings New Insight into Their Biogenesis and Functions. Front. Plant Sci. 2017, 8, 894. [Google Scholar] [CrossRef]
- Dias Araújo, A.R.; Bello, A.A.; Bigay, J.; Franckhauser, C.; Gautier, R.; Cazareth, J.; Kovács, D.; Brau, F.; Fuggetta, N.; Čopič, A.; et al. Surface Tension–Driven Sorting of Human Perilipins on Lipid Droplets. J. Cell Biol. 2024, 223, e202403064. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-D.; Huang, A.H.C. Subcellular Lipid Droplets in Vanilla Leaf Epidermis and Avocado Mesocarp are Coated with Oleosins of Distinct Phylogenic Lineages. Plant Physiol. 2016, 171, 1867–1878. [Google Scholar] [CrossRef] [PubMed]
- Itabe, H.; Yamaguchi, T.; Nimura, S.; Sasabe, N. Perilipins: A Diversity of Intracellular Lipid Droplet Proteins. Lipids Health Dis. 2017, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Vanhercke, T.; Divi, U.K.; El Tahchy, A.; Liu, Q.; Mitchell, M.; Taylor, M.C.; Eastmond, P.J.; Bryant, F.; Mechanicos, A.; Blundell, C.; et al. Step Changes in Leaf Oil Accumulation via Iterative Metabolic Engineering. Metab. Eng. 2017, 39, 237–246. [Google Scholar] [CrossRef]
- Busta, L.; Chapman, K.D.; Cahoon, E.B. Better Together: Protein Partnerships for Lineage-Specific Oil Accumulation. Curr. Opin. Plant Biol. 2022, 66, 102191. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. The Clustal Omega Multiple Alignment Package. In Multiple Sequence Alignment: Methods and Protocols; Katoh, K., Ed.; Springer: New York, NY, USA, 2021; pp. 3–16. ISBN 978-1-07-161036-7. [Google Scholar]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L.L. Predicting Transmembrane Protein Topology with a Hidden Markov Model: Application to Complete Genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2022, 51, D418–D427. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A Comparative Platform for Green Plant Genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Kumar, S.; Nei, M.; Dudley, J.; Tamura, K. MEGA: A Biologist-Centric Software for Evolutionary Analysis of DNA and Protein Sequences. Brief. Bioinform. 2008, 9, 299–306. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent Updates to the Phylogenetic Tree Display and Annotation Tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Senior, A.W.; Evans, R.; Jumper, J.; Kirkpatrick, J.; Sifre, L.; Green, T.; Qin, C.; Žídek, A.; Nelson, A.W.R.; Bridgland, A.; et al. Improved Protein Structure Prediction Using Potentials from Deep Learning. Nature 2020, 577, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.D.; Grossniklaus, U. A Gateway Cloning Vector Set for High-Throughput Functional Analysis of Genes in Planta. Plant Physiol. 2003, 133, 462. [Google Scholar] [CrossRef] [PubMed]
- Doner, N.M.; Seay, D.; Mehling, M.; Sun, S.; Gidda, S.K.; Schmitt, K.; Braus, G.H.; Ischebeck, T.; Chapman, K.D.; Dyer, J.M.; et al. Arabidopsis thaliana Early Responsive to Dehydration 7 Localizes to Lipid Droplets via Its Senescence Domain. Front. Plant Sci. 2021, 12, 658961. [Google Scholar] [CrossRef]
- Petrie, J.R.; Shrestha, P.; Liu, Q.; Mansour, M.P.; Wood, C.C.; Zhou, X.-R.; Nichols, P.D.; Green, A.G.; Singh, S.P. Rapid Expression of Transgenes Driven by Seed-Specific Constructs in Leaf Tissue: DHA Production. Plant Methods 2010, 6, 8. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef]
- Izquierdo, Y.; Fernández-Santos, R.; Cascón, T.; Castresana, C. Lipid Droplet Isolation from Arabidopsis thaliana Leaves. BioProtoc. 2020, 10, e3867. [Google Scholar] [CrossRef]
- Wang, Z.; Benning, C. Arabidopsis thaliana Polar Glycerolipid Profiling by Thin Layer Chromatography (TLC) Coupled with Gas-Liquid Chromatography (GLC). J. Vis. Exp. 2011, 49, e2518. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clews, A.C.; Whitehead, P.S.; Zhang, L.; Lü, S.; Shockey, J.M.; Chapman, K.D.; Dyer, J.M.; Xu, Y.; Mullen, R.T. Identification and Characterization of Lipid Droplet-Associated Protein (LDAP) Isoforms from Tung Tree (Vernicia fordii). Plants 2025, 14, 814. https://doi.org/10.3390/plants14050814
Clews AC, Whitehead PS, Zhang L, Lü S, Shockey JM, Chapman KD, Dyer JM, Xu Y, Mullen RT. Identification and Characterization of Lipid Droplet-Associated Protein (LDAP) Isoforms from Tung Tree (Vernicia fordii). Plants. 2025; 14(5):814. https://doi.org/10.3390/plants14050814
Chicago/Turabian StyleClews, Alyssa C., Payton S. Whitehead, Lingling Zhang, Shiyou Lü, Jay M. Shockey, Kent D. Chapman, John M. Dyer, Yang Xu, and Robert T. Mullen. 2025. "Identification and Characterization of Lipid Droplet-Associated Protein (LDAP) Isoforms from Tung Tree (Vernicia fordii)" Plants 14, no. 5: 814. https://doi.org/10.3390/plants14050814
APA StyleClews, A. C., Whitehead, P. S., Zhang, L., Lü, S., Shockey, J. M., Chapman, K. D., Dyer, J. M., Xu, Y., & Mullen, R. T. (2025). Identification and Characterization of Lipid Droplet-Associated Protein (LDAP) Isoforms from Tung Tree (Vernicia fordii). Plants, 14(5), 814. https://doi.org/10.3390/plants14050814






