Comprehensive Comparison of the Nutrient and Phytochemical Compositions and Antioxidant Activities of Different Kiwifruit Cultivars in Korea
Abstract
1. Introduction
2. Results
2.1. Physicochemical Properties of Different Kiwifruit Cultivars
2.2. Free Sugar and Organic Acid Content of Different Kiwifruit Cultivars
2.3. Free Amino Acid Content in Different Kiwifruit Cultivars
2.4. Phenolic Acid and Flavonol Contents of Different Kiwifruit Cultivars
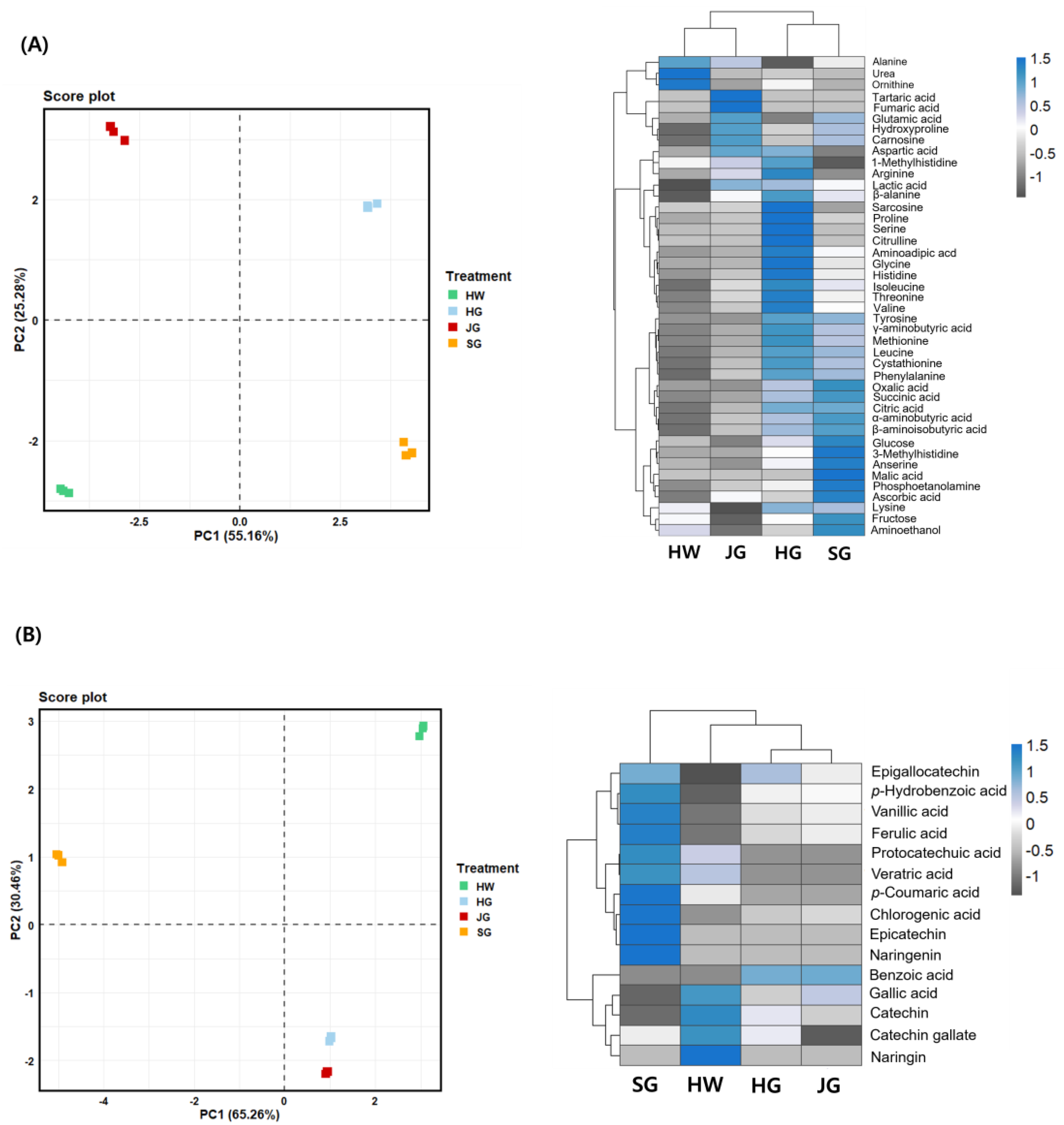
2.5. PCA Plot and Heatmap of Primary and Secondary Metabolites in Different Kiwifruit Cultivars
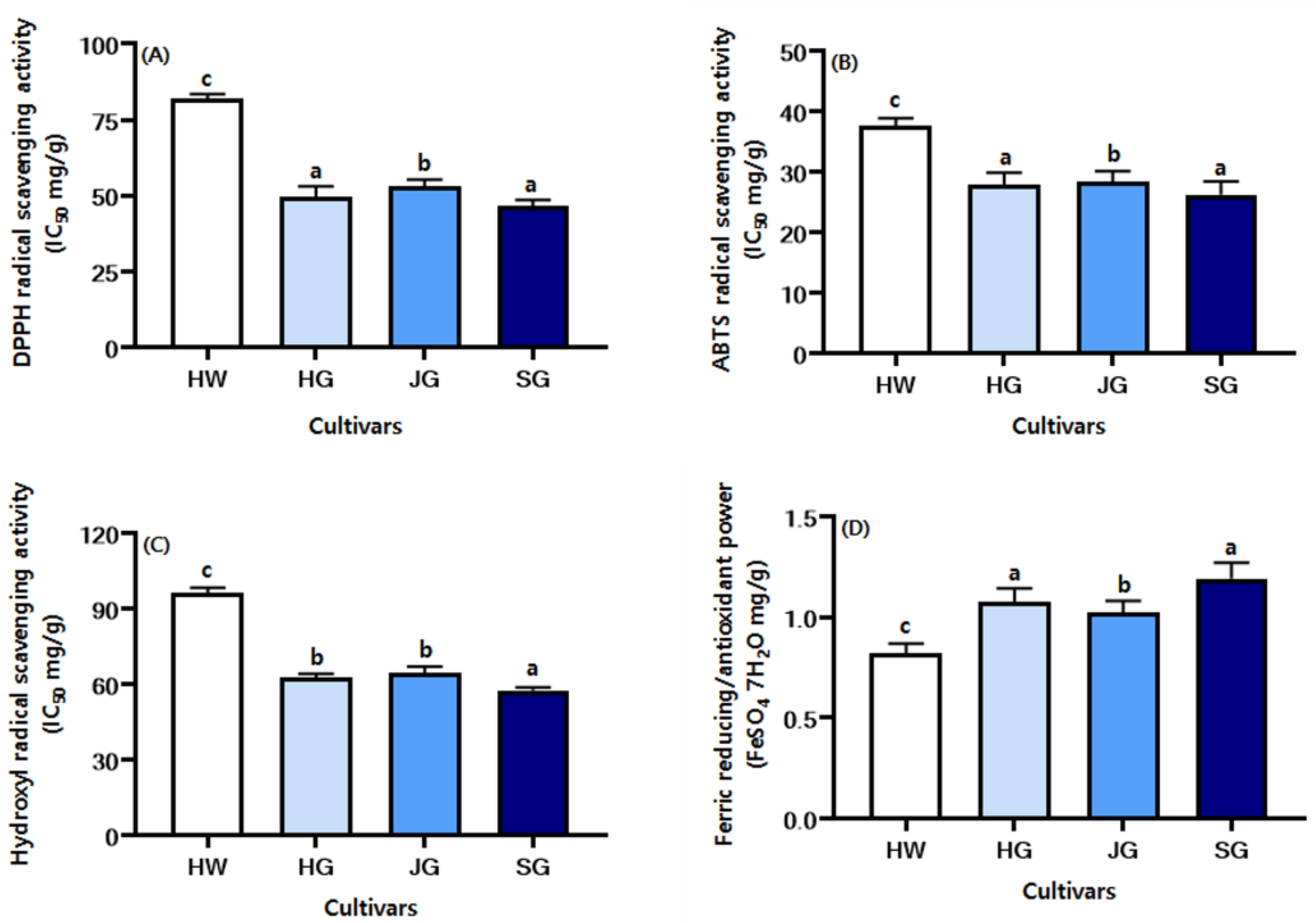
2.6. Comparison of the Antioxidant Activity of Kiwifruit Cultivars
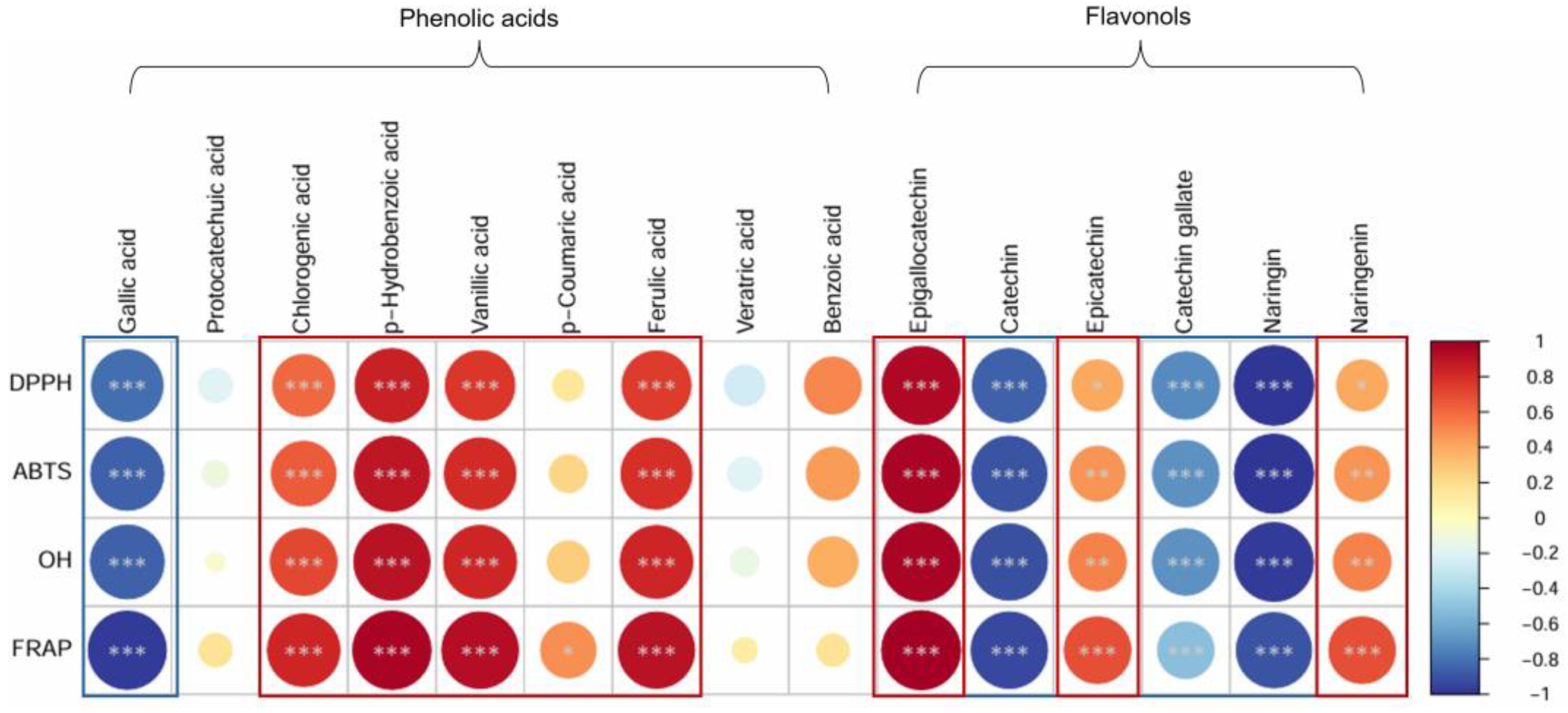
2.7. Correlation Between Antioxidant Activity and Phenolic Acid and Flavonol Compounds
3. Discussion
4. Materials and Methods
4.1. Kiwifruit, Reagents, and Instruments
4.1.1. Preparation of Kiwifruit Cultivars
4.1.2. Reagents
4.1.3. Instruments
4.2. Physicochemical Properties of Kiwifruit
4.2.1. pH and Total Acidity
4.2.2. Soluble Solids (Brix Values) and Reducing Sugars
4.2.3. Moisture
4.3. Free Sugar Content
4.4. Organic Acid Analysis
4.5. Free Amino Acid Content
4.6. Phenolic Acid and Flavonol Content
4.7. Antioxidant Activity
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Gao, N.; Shu, C.; Cheng, S.; Sun, X.; Liu, C.; Tian, J. Phenolics profile and antioxidant activity analysis of kiwi berry (Actinidia arguta) flesh and peel extracts from four regions in China. Front. Plant Sci. 2021, 12, 689038. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.S.; Kumar, V.A.; Arora, S.; Sharma, A.K.; Kumar, V.; Agrawal, S. Physicochemical and antioxidant properties of kiwifruit as a function of cultivar and fruit harvested month. Braz. Arch. Biol. Technol. 2015, 58, 262–271. [Google Scholar] [CrossRef]
- Ma, T.; Sun, X.; Zhao, J.; You, Y.; Lei, Y.; Gao, G.; Zhan, J. Nutrient compositions and antioxidant capacity of kiwifruit (Actinidia) and their relationship with flesh color and commercial value. Food Chem. 2017, 218, 294–304. [Google Scholar] [CrossRef]
- Eo, H.J.; Kim, C.W.; Lee, U.; Kim, Y. Comparative Analysis of the Characteristics of Two Hardy Kiwifruit Cultivars (Actinidia arguta cv. Cheongsan and Daebo) Stored at Low Temperatures. Plants 2024, 13, 2201. [Google Scholar] [CrossRef]
- Rajan, P.; Natraj, P.; Kim, M.; Lee, M.; Jang, Y.J.; Lee, Y.J.; Kim, S.C. Climate Change Impacts on and Response Strategies for Kiwifruit Production: A Comprehensive Review. Plants 2024, 13, 2354. [Google Scholar] [CrossRef]
- Liang, J.; Ren, Y.; Wang, Y.; Han, M.; Yue, T.; Wang, Z.; Gao, Z. Physicochemical, nutritional, and bioactive properties of pulp and peel from 15 kiwifruit cultivars. Food Biosci. 2021, 42, 101157. [Google Scholar] [CrossRef]
- Huang, C.H.; Tao, J.J.; Liao, G.L.; Xie, M.; Qu, X.Y.; Chen, L.; Xu, X.B. Dynamic changes of phenol and antioxidant capacity during fruit development of three Actinidia species (kiwifruit). Sci. Hortic. 2020, 273, 109571. [Google Scholar] [CrossRef]
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The nutritional and health attributes of kiwifruit: A review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef]
- Tavarini, S.; Degl’Innocenti, E.; Remorini, D.; Massai, R.; Guidi, L. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwifruit. Food Chem. 2008, 107, 282–288. [Google Scholar] [CrossRef]
- Rasheed, R.B.; Hussain, S.; Syed, S.K. Phytochemistry, nutritional and medicinal value of kiwi fruit. Postep. Biol. Komorki 2021, 48, 147–165. [Google Scholar]
- Nayik, G.A.; Gull, A. Antioxidants in Fruits: Properties and Health Benefits; Springer: Singapore, 2020; pp. 547–561. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Q.; Lan, T.; Geng, T.; Gao, C.; Yuan, Q.; Zhang, Q.; Xu, P.; Sun, X.; Liu, X.; et al. Comparative analysis of physicochemical characteristics, nutritional and functional components, and antioxidant capacity of fifteen kiwifruit (Actinidia) cultivars. Foods 2020, 9, 1267. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, H.; Fan, J.; Liu, F.; Li, J.; Zhong, C.; Zhang, Q. Comparative study on physicochemical and nutritional qualities of kiwifruit varieties. Foods 2022, 12, 108. [Google Scholar] [CrossRef]
- Towantakavanit, K.; Park, Y.S.; Gorinstein, S. Quality properties of wine from Korean kiwifruit new cultivars. Food Res. Int. 2011, 44, 1364–1372. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, S.C.; Jang, K.C.; Song, E.Y.; Kim, M.; Moon, D.Y.; Suh, H.D. A new kiwifruit cultivar, “Jecy Gold” with yellow flesh. Korean Soc. Breed. Sci. 2007, 39, 258–259. [Google Scholar]
- Kim, S.C.; Song, E.Y.; Kim, C.H. A new kiwifruit variety, ‘Halla Gold’ with high soluble solids content and early harvesting. Hortic. Sci. Technol. 2012, 30, 334–337. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, C.H.; Lim, C.K.; Song, E.Y. ‘Sweet Gold’, A Kiwifruit Variety with High Firmness. Korean Soc. Breed. Sci. 2018, 50, 245–248. [Google Scholar] [CrossRef]
- Dias, M.; Caleja, C.; Pereira, C.; Calhelha, R.C.; Kostic, M.; Sokovic, M.; Ferreira, I.C. Chemical composition and bioactive properties of byproducts from two different kiwi varieties. Food Res. Int. 2020, 127, 108753. [Google Scholar] [CrossRef]
- Zhu, C.; Chou, O.; Lee, F.Y.; Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Characterization of phenolics in rejected kiwifruit and their antioxidant potential. Processes 2021, 9, 781. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, B.; Luo, Z.; Yuan, Y.; Zhao, Z.; Liu, M. Composition analysis and nutritional value evaluation of amino acids in the fruit of 161 jujube cultivars. Plants 2023, 12, 1744. [Google Scholar] [CrossRef]
- Arivalagan, M.; Karunakaran, G.; Roy, T.K.; Dinsha, M.; Sindhu, B.C.; Shilpashree, V.M.; Shivashankara, K.S. Biochemical and nutritional characterization of dragon fruit (Hylocereus species). Food Chem. 2021, 353, 129426. [Google Scholar] [CrossRef]
- Latocha, P. The nutritional and health benefits of kiwiberry (Actinidia arguta)—A review. Plant Foods Hum. Nutr. 2017, 72, 325–334. [Google Scholar] [CrossRef]
- He, Y.; Qin, H.; Wen, J.; Wang, L.; Cao, W.; Fan, S.; Li, C. Characterization of Amino Acid Composition, Nutritional Value, and Taste of Fruits from Different Actinidia arguta Resources. J. Food Qual. 2024, 2024, 1005194. [Google Scholar] [CrossRef]
- Bare, A.; Thomas, J.; Etoroma, D.; Lee, S.G. Functional analysis of phosphoethanolamine N-methyltransferase in plants and parasites: Essential S-adenosylmethionine-dependent methyltransferase in choline and phospholipid metabolism. Methods Enzymol. 2023, 680, 101–137. [Google Scholar] [CrossRef]
- Qu, Z.; Liu, A.; Li, P.; Liu, C.; Xiao, W.; Huang, J.; Zhang, S. Advances in physiological functions and mechanisms of (−)-epicatechin. Crit. Rev. Food Sci. Nutr. 2021, 61, 211–233. [Google Scholar] [CrossRef]
- Hu, Y.K.; Kim, S.J.; Jang, C.S.; Lim, S.D. Antioxidant Activity Analysis of Native Actinidia arguta Cultivars. Int. J. Mol. Sci. 2024, 25, 1505. [Google Scholar] [CrossRef]
- Xi, Y.; Fan, X.; Zhao, H.; Li, X.; Cao, J.; Jiang, W. Postharvest fruit quality and antioxidants of nectarine fruit as influenced by chlorogenic acid. LWT 2017, 75, 537–544. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, D.; Chen, Y. Preparation and antioxidant activity of green tea extract enriched in epigallocatechin (EGC) and epigallocatechin gallate (EGCG). J. Agric. Food Chem. 2009, 57, 1349–1353. [Google Scholar] [CrossRef]
- Bursal, E.; Gülçin, İ. Polyphenol contents and in vitro antioxidant activities of lyophilised aqueous extract of kiwifruit (Actinidia deliciosa). Food Res. Int. 2011, 44, 1482–1489. [Google Scholar] [CrossRef]
- Addai, Z.R.; Abdullah, A.; Mutalib, S.A.; Musa, K.H.; Douqan, E.M. Antioxidant activity and physicochemical properties of mature papaya fruit (Carica papaya L. cv. Eksotika). Adv. J. Food Sci. Technol. 2013, 5, 859–865. [Google Scholar] [CrossRef]
- Tehranifar, A.; Zarei, M.; Esfandiyari, B.; Nemati, Z. Physicochemical properties and antioxidant activities of pomegranate fruit (Punica granatum) of different cultivars grown in Iran. Hortic. Environ. Biotechnol. 2010, 51, 573–579. [Google Scholar]
- Poudel, K.; Ansari, A.R.; Karki, S. Physico-chemical characteristics of different kiwifruit (Actinidia deliciosa) cultivars grown in the mid-hills region of eastern Nepal. In Proceedings of the Ninth National Horticulture Workshop, Lalitpur, Nepal, 31 May–1 June 2017; pp. 126–130. [Google Scholar]
- Smeekens, S.; Ma, J.; Hanson, J.; Rolland, F. Sugar signals and molecular networks controlling plant growth. Curr. Opin. Plant Biol. 2010, 13, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar signaling during fruit ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Xu, Z.; Chen, L.; Huang, Q.; Huang, C.; Tao, J.; Xu, X. Analysis of organic acid metabolism reveals citric acid and malic acid play major roles in determining acid quality during the development of kiwifruit (Actinidia eriantha). J. Sci. Food Agric. 2023, 103, 6055–6069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, Y.; Zhang, Z. The role of different natural organic acids in postharvest fruit quality management and its mechanism. Food Front. 2023, 4, 1127–1143. [Google Scholar] [CrossRef]
- Zheng, B.; Zhao, L.; Jiang, X.; Cherono, S.; Liu, J.; Ogutu, C.; Han, Y. Assessment of organic acid accumulation and its related genes in peach. Food Chem. 2021, 334, 127567. [Google Scholar] [CrossRef]
- Song, J.; Bi, J.; Chen, Q.; Wu, X.; Lyu, Y.; Meng, X. Assessment of sugar content, fatty acids, free amino acids, and volatile profiles in jujube fruits at different ripening stages. Food Chem. 2019, 270, 344–352. [Google Scholar] [CrossRef]
- Choi, H.R.; Baek, M.W.; Cheol, L.H.; Jeong, C.S.; Tilahun, S. Changes in metabolites and antioxidant activities of green ‘Hayward’ and gold ‘Haegeum’ kiwifruits during ripening with ethylene treatment. Food Chem. 2022, 384, 132490. [Google Scholar] [CrossRef]
- Ngo, D.H.; Vo, T.S. An updated review on pharmaceutical properties of gamma-aminobutyric acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef]
- Cömert, E.D.; Mogol, B.A.; Gökmen, V. Relationship between color and antioxidant capacity of fruits and vegetables. Curr. Res. Food Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Kustrin, E.; Morton, D.W. Phenolic acids contribution to antioxidant activities and comparative assessment of phenolic content in mango pulp and peel. S. Afr. J. Bot. 2018, 116, 158–163. [Google Scholar] [CrossRef]
- Kafkas, N.E.; Kosar, M.; Öz, A.T.; Mitchell, A.E. Advanced analytical methods for phenolics in fruits. J. Food Qual. 2018, 2018, 3836064. [Google Scholar] [CrossRef]
- Kiran, S.D.V.S.; Rohini, P.; Bhagyasree, P. Flavonoid: A review on Naringenin. J. Pharmacogn. Phytochem. 2017, 6, 2778–2783. [Google Scholar]
- Liang, N.N.; He, F.; Bi, H.Q.; Duan, C.Q.; Reeves, M.J.; Wang, J. Evolution of flavonols in berry skins of different grape cultivars during ripening and a comparison of two vintages. Eur. Food Res. Technol. 2012, 235, 1187–1197. [Google Scholar] [CrossRef]
- Aghofack-Nguemezi, J.; Schwab, W. Differential accumulation of flavonoids by tomato (Solanum lycopersicum) fruit tissues during maturation and ripening. J. Appl. Biosci. 2014, 84, 7682–7694. [Google Scholar] [CrossRef]
- Deng, W.; Yang, Q.-N.; Liu, H.-Y.; Xia, Y.; Yan, H.; Huang, J.-W.; Hu, Y.-C.; Zou, L.; Gan, R.-Y.; Wu, D.-T. Comparative analysis of phenolic compounds in different thinned unripe kiwifruits and their biological functions. Food Chem. X 2024, 24, 101815. [Google Scholar] [CrossRef] [PubMed]
- Taubert, D.; Breitenbach, T.; Lazar, A.; Censarek, P.; Harlfinger, S.; Berkels, R.; Roesen, R. Reaction rate constants of superoxide scavenging by plant antioxidants. Free Radic. Biol. Med. 2003, 35, 1599–1607. [Google Scholar] [CrossRef]
- Calado, J.C.P.; Albertão, P.A.; Oliveira, E.D.; Letra, M.H.S.; Sawaya, A.C.H.F.; Marcucci, M.C. Flavonoid contents and antioxidant activity in fruit, vegetables, and other types of food. Agric. Sci. 2015, 6, 426–435. [Google Scholar] [CrossRef]
- Den Hartogh, D.J.; Tsiani, E. Antidiabetic properties of naringenin: A citrus fruit polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Lee, J.G. Ripening-dependent changes in antioxidants, color attributes, and antioxidant activity of seven tomato (Solanum lycopersicum L.) cultivars. J. Anal. Methods Chem. 2016, 2016, 5498618. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Lee, H.Y.; Cho, K.M.; Joo, O.S. Kiwi-persimmon wine produced using wild Saccharomyces cerevisiae strains with sugar, acid, and alcohol tolerance. Food Sci. Preserv. 2023, 30, 52–64. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, D.Y.; Jang, K.J.; Jeong, J.B.; Lee, G.Y.; Jang, M.Y.; Cho, K.M. Changes in Nutrient Components and Digestive Enzymatic Inhibition Activities in Soy Leaves by Ethephon Treatment. Plants 2023, 12, 3640. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Cho, D.Y.; Kim, D.H.; Park, J.H.; Jeong, J.B.; Jeon, S.H.; Lee, J.H. Examining the Alterations in Metabolite Constituents and Antioxidant Properties in Mountain-Cultivated Ginseng (Panax ginseng C.A. Meyer) Organs during a Two-Month Maturation Period. Antioxidants 2024, 13, 612. [Google Scholar] [CrossRef] [PubMed]

| Contents 1 | Cultivars 2 | |||
|---|---|---|---|---|
| HW | HG | JG | SG | |
| pH | 3.34 ± 0.02 a | 3.26 ± 0.03 b | 3.31 ± 0.06 a | 3.23 ± 0.02 b |
| Acidity (%, lactic acid) | 2.16 ± 0.02 c | 2.21 ± 0.06 a | 2.19 ± 0.04 b | 2.25 ± 0.04 a |
| Soluble solids (°Bx) | 12.20 ± 0.24 c | 13.60 ± 0.13 b | 10.60 ± 0.21 d | 16.20 ± 0.48 a |
| Reducing sugars (mg/g) | 78.36 ± 0.78 c | 86.12 ± 3.44 b | 66.64 ± 2.00 d | 95.73 ± 1.91 a |
| Moisture (g/100 g) | 89.12 ± 1.08 c | 89.88 ± 1.02 b | 90.86 ± 1.10 a | 90.22 ± 1.04 a |
| Contents 1 (g/100 mL) | Cultivars 2 | |||
|---|---|---|---|---|
| HW | HG | JG | SG | |
| Free sugar | ||||
| Glucose | 4.67 ± 0.08 c | 5.20 ± 0.06 b | 4.38 ± 0.13 d | 6.27 ± 0.17 a |
| Fructose | 3.82 ± 0.04 b | 3.80 ± 0.04 b | 3.13 ± 0.03 c | 4.98 ± 0.21 a |
| Total | 8.49 | 9.00 | 7.51 | 11.25 |
| Organic acids | ||||
| Oxalic acid | 0.37 ± 0.01 c | 0.33 ± 0.01 b | 0.16 ± 0.02 c | 0.38 ± 0.01 a |
| Tartaric acid | nd 3 | nd | 0.38 ± 0.04 a | nd |
| Malic acid | 1.71 ± 0.01 d | 2.24 ± 0.04 b | 1.86 ± 0.04 c | 3.04 ± 0.05 a |
| Ascorbic acid | 0.14 ± 0.01 d | 0.30 ± 0.01 c | 0.43 ± 0.03 b | 0.63 ± 0.03 a |
| Lactic acid | 3.09 ± 0.03 d | 3.41 ± 0.05 b | 3.65 ± 0.03 a | 3.12 ± 0.06 c |
| Citric acid | 2.38 ± 0.02 c | 4.58 ± 0.03 a | 2.13 ± 0.03 c | 4.48 ± 0.02 b |
| Succinic acid | 1.11 ± 0.04 c | 1.59 ± 0.08 b | 1.17 ± 0.02 c | 1.73 ± 0.01 a |
| Fumaric acid | nd | nd | tr 4 | nd |
| Total | 8.8 | 12.45 | 9.78 | 13.08 |
| Contents 1 (g/100 mL) | Cultivars 2 | |||
|---|---|---|---|---|
| HW | HG | JG | SG | |
| Nonessential amino acids | ||||
| Taurine | nd 3 | nd | nd | nd |
| Phosphoethanolamine | nd | nd | nd | 30.30 ± 0.13 a |
| Urea | 172.44 ± 2.20 a | 14.56 ± 0.08 b | nd | nd |
| Proline | 1.08 ± 0.02 d | 91.38 ± 0.34 a | 11.26 ± 0.08 c | 15.21 ± 0.17 b |
| Aspartic acid | 40.24 ± 0.06 c | 76.34 ± 0.10 b | 80.51 ± 0.28 a | 33.01 ± 1.52 d |
| Serine | 30.24 ± 0.03 d | 172.42 ± 0.08 a | 36.83 ± 0.02 b | 33.21 ± 0.01 c |
| Glutamic acid | 76.94 ± 0.69 c | 50.61 ± 0.41 d | 209.17 ± 1.88 a | 182.65 ± 1.01 b |
| Sarcosine | 4.01 ± 0.03 c | 25.68 ± 0.14 a | 4.74 ± 0.04 b | nd |
| Aminoadipic acid | 4.43 ± 0.04 d | 36.48 ± 0.20 a | 6.86 ± 0.06 c | 17.51 ± 0.01 b |
| Glycine | 13.95 ± 0.13 d | 93.01 ± 0.51 a | 18.00 ± 0.16 c | 34.09 ± 0.19 b |
| Alanine | 73.78 ± 0.66 a | 8.68 ± 0.05 d | 59.47 ± 0.16 b | 43.98 ± 0.19 c |
| Citrulline | nd | 14.71 ± 0.09 a | nd | nd |
| α-aminobutyric acid | 5.30 ± 0.05 d | 14.56 ± 0.08 b | 8.99 ± 0.08 c | 17.45 ± 0.96 a |
| Cystine | nd | nd | nd | nd |
| Cystathionine | 10.52 ± 0.09 d | 22.11 ± 0.12 a | 13.78 ± 0.12 c | 19.32 ± 0.11 b |
| Tyrosine | 32.43 ± 0.26 c | 60.43 ± 0.16 a | 32.92 ± 0.27 c | 56.42 ± 0.17 b |
| β-alanine | 8.02 ± 0.06 c | 15.49 ± 0.03 a | 12.34 ± 0.10 b | 12.85 ± 0.03 b |
| β-aminoisobutyric acid | 19.44 ± 0.17 d | 33.38 ± 0.08 b | 24.56 ± 0.20 c | 36.43 ± 0.08 a |
| γ-aminobutyric acid | 30.96 ± 0.24 d | 241.39 ± 0.54 a | 63.20 ± 0.50 c | 179.53 ± 0.40 b |
| Aminoethanol | 9.68 ± 0.07 b | 8.93 ± 0.02 c | 7.72 ± 0.06 d | 11.10 ± 0.02 a |
| Hydroxyproline | 3.70 ± 0.02 d | 5.07 ± 0.01 c | 7.01 ± 0.06 a | 6.47 ± 0.01 b |
| Ornithine | 3.98 ± 0.03 a | 2.84 ± 0.02 b | 2.27 ± 0.02 c | 2.38 ± 0.01 c |
| 1-Methylhistidine | 2.54 ± 0.02 c | 4.67 ± 0.01 a | 3.35 ± 0.02 b | nd |
| 3-Methylhistidine | nd | 0.22 ± 0.00 b | nd | 0.66 ± 0.01 a |
| Anserine | 7.43 ± 0.05 c | 12.49 ± 0.02 b | 5.69 ± 0.05 d | 21.19 ± 0.05 a |
| Carnosine | 9.37 ± 0.07 d | 12.45 ± 0.02 c | 18.36 ± 0.15 a | 16.64 ± 0.04 b |
| Arginine | 114.99 ± 0.92 c | 253.47 ± 0.57 a | 180.86 ± 1.46 b | 102.38 ± 0.23 d |
| Total | 675.47 | 1256.80 | 807.88 | 872.78 |
| Essential amino acids | ||||
| Threonine | 31.96 ± 0.17 d | 94.18 ± 0.51 a | 51.05 ± 0.27 c | 56.48 ± 0.30 b |
| Valine | 45.31 ± 0.24 d | 148.98 ± 0.80 a | 69.49 ± 0.37 c | 87.93 ± 0.47 b |
| Methionine | 13.86 ± 0.07 d | 45.93 ± 0.25 a | 18.92 ± 0.10 c | 35.93 ± 0.19 b |
| Isoleucine | 24.96 ± 0.13 d | 84.57 ± 0.45 a | 47.41 ± 0.25 c | 58.49 ± 0.31 b |
| Leucine | 35.66 ± 0.12 d | 92.86 ± 0.33 a | 48.58 ± 0.17 c | 83.20 ± 0.29 b |
| Phenylalanine | 31.66 ± 0.11 d | 86.79 ± 0.30 a | 50.83 ± 0.18 c | 77.39 ± 0.27 b |
| Lysine | 48.55 ± 0.17 c | 59.14 ± 0.21 a | 22.77 ± 0.08 d | 55.94 ± 0.20 b |
| Histidine | 16.08 ± 0.06 d | 30.72 ± 0.11 a | 18.53 ± 0.06 c | 20.79 ± 0.07 b |
| Total | 8.8 | 12.45 | 9.78 | 13.08 |
| Contents 1 (μg/mL) | Cultivars 2 | |||
|---|---|---|---|---|
| HW | HG | JG | SG | |
| Phenolic acids | ||||
| Gallic acid | 6.03 ± 0.02 a | 5.73 ± 0.02 b | 5.89 ± 0.02 a | 5.55 ± 0.02 c |
| Protocatechuic acid | 1.68 ± 0.01 b | nd | nd | 2.85 ± 0.03 a |
| Chlorogenic acid | 338.18 ± 1.07 c | 364.68 ± 1.15 b | 374.59 ± 1.18 a | 385.19 ± 1.53 a |
| p-hydrobenzoic acid | 5.39 ± 0.20 c | 11.08 ± 0.03 b | 11.39 ± 0.04 b | 17.16 ± 0.05 a |
| Vanillic acid | 22.54 ± 0.07 c | 28.15 ± 0.09 b | 28.92 ± 0.09 b | 38.22 ± 0.12 a |
| p-coumaric acid | 2.16 ± 0.01 b | nd | nd | 8.05 ± 0.02 a |
| Ferulic acid | 5.87 ± 0.01 c | 6.80 ± 0.01 b | 6.99 ± 0.02 b | 8.63 ± 0.02 a |
| Veratric acid | 4.92 ± 0.01 b | nd | nd | 7.36 ± 0.02 a |
| Benzoic acid | nd 3 | 27.61 ± 0.06 b | 28.36 ± 0.06 a | nd |
| t-cinnamic acid | nd | nd | nd | nd |
| Total | 386.76 | 444.06 | 456.13 | 473.01 |
| Flavonols | ||||
| Epigallocatechin | nd | 62.85 ± 0.94 b | 42.97 ± 0.40 c | 70.02 ± 1.06 a |
| Catechin | 34.99 ± 0.33 a | 19.28 ± 0.29 b | 14.52 ± 0.11 c | 2.00 ± 0.03 d |
| Epicatechin | nd | nd | nd | 13.35 ± 0.35 a |
| Epigallocatechin gallate | nd | nd | nd | nd |
| Vanilin | nd | nd | nd | nd |
| Rutin | nd | nd | nd | nd |
| Catechin gallate | 8.27 ± 0.08 a | 6.33 ± 0.09 b | 3.74 ± 0.03 d | 5.82 ± 0.09 c |
| Naringin | 2.66 ± 0.02 a | nd | nd | nd |
| Quercetin | nd | nd | nd | nd |
| Naringenin | nd | nd | nd | 6.24 ± 0.20 a |
| Total | 45.93 | 88.45 | 61.23 | 96.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.-B.; Cho, D.-Y.; Lee, H.-Y.; Lee, A.-R.; Lee, G.-Y.; Jang, M.-Y.; Son, K.-H.; Cho, K.-M. Comprehensive Comparison of the Nutrient and Phytochemical Compositions and Antioxidant Activities of Different Kiwifruit Cultivars in Korea. Plants 2025, 14, 757. https://doi.org/10.3390/plants14050757
Jeong J-B, Cho D-Y, Lee H-Y, Lee A-R, Lee G-Y, Jang M-Y, Son K-H, Cho K-M. Comprehensive Comparison of the Nutrient and Phytochemical Compositions and Antioxidant Activities of Different Kiwifruit Cultivars in Korea. Plants. 2025; 14(5):757. https://doi.org/10.3390/plants14050757
Chicago/Turabian StyleJeong, Jong-Bin, Du-Yong Cho, Hee-Yul Lee, Ae-Ryeon Lee, Ga-Yong Lee, Mu-Yeun Jang, Ki-Ho Son, and Kye-Man Cho. 2025. "Comprehensive Comparison of the Nutrient and Phytochemical Compositions and Antioxidant Activities of Different Kiwifruit Cultivars in Korea" Plants 14, no. 5: 757. https://doi.org/10.3390/plants14050757
APA StyleJeong, J.-B., Cho, D.-Y., Lee, H.-Y., Lee, A.-R., Lee, G.-Y., Jang, M.-Y., Son, K.-H., & Cho, K.-M. (2025). Comprehensive Comparison of the Nutrient and Phytochemical Compositions and Antioxidant Activities of Different Kiwifruit Cultivars in Korea. Plants, 14(5), 757. https://doi.org/10.3390/plants14050757







