Response of a Benthic Sargassum Population to Increased Temperatures: Decline in Non-Photochemical Quenching of Chlorophyll a Fluorescence (NPQ) Precedes That of Maximum Quantum Yield of PSII
Abstract
1. Introduction
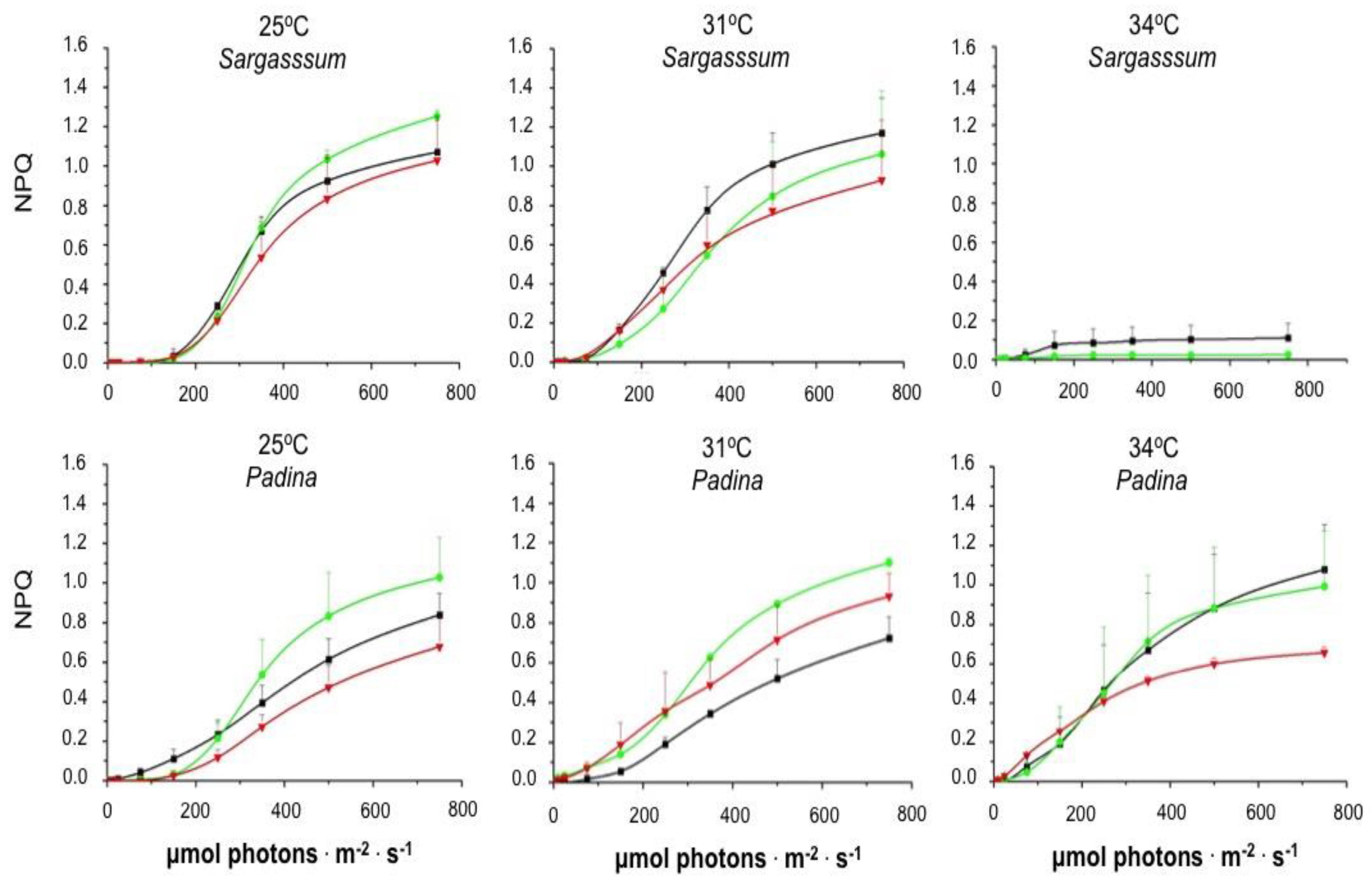
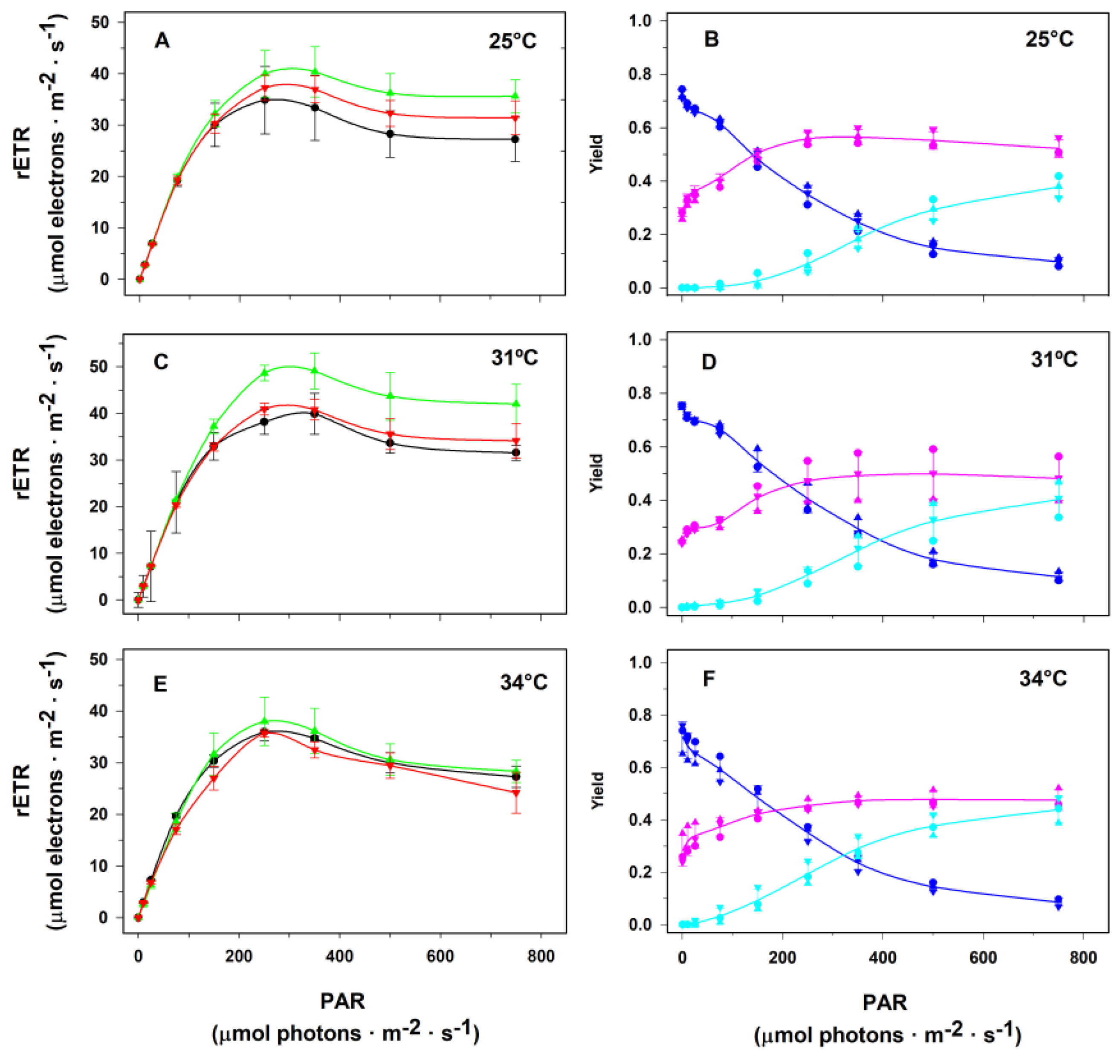
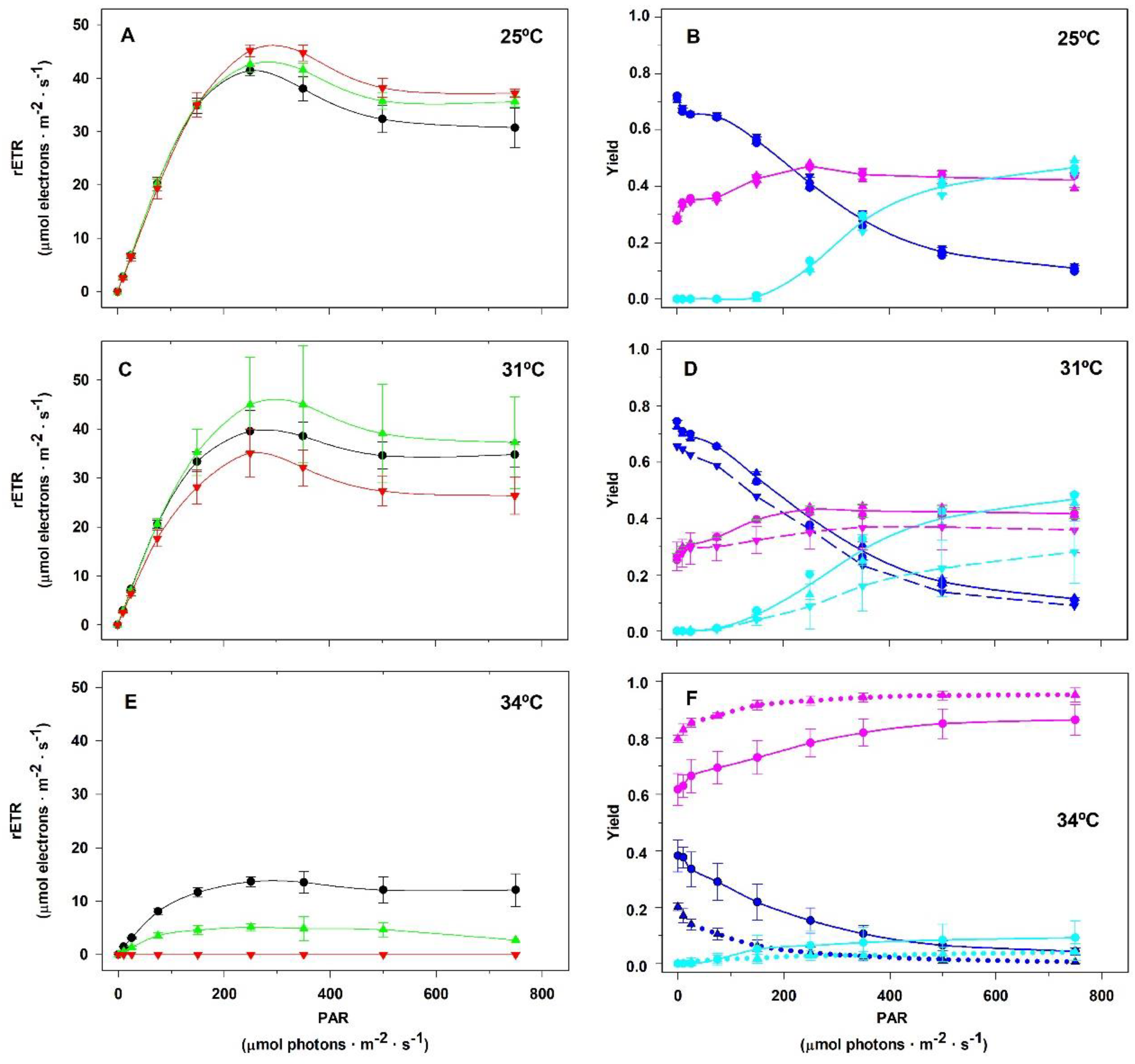
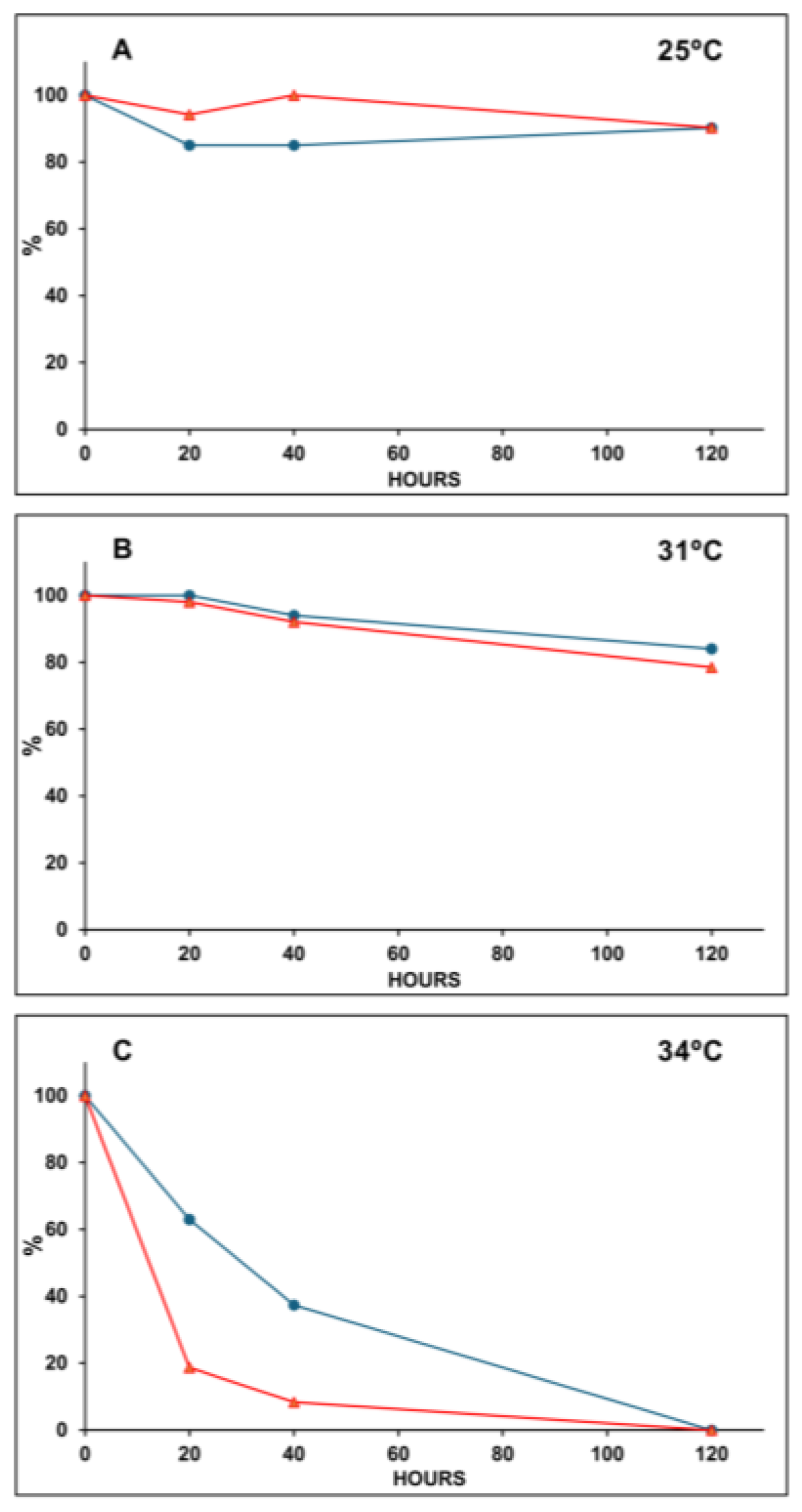
2. Results
2.1. Assays in the Field
Underwater Net Photosynthesis and Respiration
2.2. Assays in the Laboratory
3. Discussion
4. Materials and Methods
4.1. Biological Material
4.2. Field Studies
4.2.1. Measurements on a Pier
4.2.2. Underwater Measurements
4.3. Laboratory Assays
4.4. Photosynthesis Measurements
4.4.1. Chlorophyll a Fluorescence
4.4.2. Net Photosynthesis and Respiration
4.5. Abiotic Measurements
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yip, Z.T.; Quek, R.Z.B.; Huang, D. Historical biogeography of the widespread macroalga Sargassum (Fucales, Phaeophyceae). J. Phycol. 2020, 56, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Castillo, A.N.; Riosmena-Rodriguez, R.; Hernandez-Carmona, G.; Méndez-Trejo, M.D.C.; López-Vivas, J.M.; Sánchez-Ortiz, C.; Lara-Uc, M.M.; Torre-Cosio, J. Biodiversity associated to Sargassum forest at the Gulf of California. In Invertebrates: Classification, Evolution and Biodiversity; Riosmena-Rodriguez, R., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2013; Chapter 9; pp. 205–223. [Google Scholar]
- Tano, S.; Eggertsen, M.; Wikström, S.A.; Berkström, C.; Buriyo, A.S.; Halling, C. Tropical seaweed beds are important habitats for mobile invertebrate epifauna. Estuar. Coast. Shelf Sci. 2016, 183, 1–12. [Google Scholar] [CrossRef]
- Duarte, M.; Gattuso, J.-P.; Hancke, K.; Gundersen, H.; Filbee-Dexter, K.; Pedersen, M.F.; Middelburg, J.J.; Burrows, M.T.; Krumhans, K.A.; Wernberg, T.; et al. Global estimates of the extent and production of macroalgal forests. Global Ecol. Biogeogr. 2022, 31, 1422–1439. [Google Scholar] [CrossRef]
- Eggert, A. Seaweed responses to temperature. In Seaweed Biology: Novel Insights into Ecophysiology, Ecology and Utilization; Wiencke, C., Bischof, K., Eds.; Springer: New York, NY, USA, 2012; pp. 47–66. [Google Scholar] [CrossRef]
- Charan, H.; Inomata, E. Decreased irradiance and nutrient enrichment mitigate the negative effect of ocean warming on growth and biochemical compositions of a canopy-forming marine macroalga. J. Mar. Sci. Eng. 2022, 10, 479. [Google Scholar] [CrossRef]
- Kokubu, S.; Nishihara, G.N.; Watanabe, Y.; Tsuchiya, Y.; Amamo, Y.; Terada, R. The effect of irradiance and temperature on the photosynthesis of a native alga Sargassum fusiforme (Fucales) from Kagoshima, Japan. Phycologia 2015, 54, 235–247. [Google Scholar] [CrossRef]
- Liu, L.; Lin, L. Effect of heat stress on Sargassum fusiforme leaf metabolome. J. Plant Biol. 2020, 63, 229–241. [Google Scholar] [CrossRef]
- Phelps, C.M.; Boyce, M.C.; Huggett, M.J. Future climate change scenarios differentially affect three abundant algal species in southwestern Australia. Mar. Envir. Res. 2017, 126, 69–80. [Google Scholar] [CrossRef]
- Urrea-Victoria, V.; Nardelli, A.E.; Floh, E.I.S.; Chow, F. Sargassum stenophyllum (Fucales, Ochrophyta) responses to temperature short-term exposure photosynthesis and chemical composition. Braz. J. Bot. 2020, 43, 733–745. [Google Scholar] [CrossRef]
- Gouvêa, L.P.; Horta, P.A.; Fragkopoulou, E.; Gurgel, C.F.D.; Peres, L.M.C.; Bastos, E.; Ramlov, F.; Burle, G.; Koerich, G.; Martins, C.D.L.; et al. Phenotypic plasticity in Sargassum forests may not counteract projected biomass losses along a broad latitudinal gradient. Ecosystems 2023, 26, 29–41. [Google Scholar] [CrossRef]
- Carneiro, I.M.; Széchy, M.T.M.; Bertocci, I.; Paiva, P.C. Impact of a nuclear power station effluent on marine forests: A case study in SE Brazil and insights for global warming scenarios. Envir. Poll. 2024, 344, 123323. [Google Scholar] [CrossRef]
- Széchy, M.T.M.; Koutsoukos, V.S.; Barboza, C.A.M. Long-term decline of brown algal assemblages from southern Brazil under the influence of a nuclear power plant. Ecol. Indic. 2017, 80, 258–267. [Google Scholar] [CrossRef]
- Bernal-Ibánez, A.; Gestoso, I.; Wirtz, P.; Kaufmann, M.; Serrao, E.A.; Canning-Clode, J.; Cacabelos, E. The collapse of marine forests: Drastic reduction in populations of the family Sargassaceae in Madeira Island (NE Atlantic). Reg. Environ. Chang. 2021, 21, 71. [Google Scholar] [CrossRef]
- Phillips, J.A.; Blackshaw, J.K. Extirpation of macroalgae (Sargassum spp.) on the subtropical east Australian coast. Conser. Bio. 2011, 25, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Liu, Y.; Lia, X.; Fenga, Z.; Xu, Z.; Wu, H.; Xu, J. Expected CO2-induced ocean acidification modulates copper toxicity in the green tide alga Ulva prolifera. Envir. Exp. Bot. 2017, 135, 63–72. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. Photochem. Photobiol. B: Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Inhibition of photosynthesis by heat stress: The activation state of Rubisco as a limiting factor in photosynthesis. Physiol. Plant. 2004, 120, 179–186. [Google Scholar] [CrossRef]
- Yamamoto, Y. Quality control of photosystem II: The mechanisms for avoidance and tolerance of light and heat stresses are closely linked to membrane fluidity of the thylakoids. Front. Plant Sci. 2016, 7, 1136. [Google Scholar] [CrossRef]
- Wang, W.; Lin, Y.; Teng, F.; Ji, D.; Xu, Y.; Chen, C.; Xie, C. Comparative transcriptome analysis between heat-tolerant and sensitive Pyropia haitanensis strains in response to high stress. Algal Res. 2018, 29, 104–112. [Google Scholar] [CrossRef]
- Terada, R.; Vo, T.D.; Nishihara, G.N.; Matsumoto, K.; Kokubu, S.; Watanabe, Y.; Kawaguchi, S. The effect of photosynthetically active radiation and temperature on the photosynthesis of two Vietnamese species of Sargassum, S. mcclurei and S. oligocystum, based on the field and laboratory measurements. Phycological Res. 2016, 64, 230–240. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, Z.; Qin, S.; Li, J.; Li, J.; Liu, Z. Effects of temperature and light on growth rate and photosynthetic characteristics of Sargassum horneri. J. Oceanogr. Univ. China 2021, 20, 101–110. [Google Scholar] [CrossRef]
- Zhong, Z.H.; Wang, Y.; Qin, S.; Zhuang, L.C.; Li, J.J.; Song, W.L.; Liu, Z.Y. Short-term photoacclimation and photoregulation strategies of Sargassum horneri in response to temperature and light. Photosynthetica 2021, 59, 268–276. [Google Scholar] [CrossRef]
- Zuo, X.; Xu, L.; Luo, L.; Zeng, Y.; Ma, Z.; Wu, M.; Chen, B. Physiological responses of Sargassum fusiforme seedlings to high-temperature stress. Reg. Stud. Mar. Sci. 2023, 62, 102900. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 200859, 89–113. [Google Scholar] [CrossRef]
- Schreiber, U. Pulse-Amplitude-Modulation (PAM) Fluorometry and Saturation Pulse Method: An Overview. In Chlorophyll A Fluorescence. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 279–319. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid Light Curves—A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the saturation pulse method. PAM Application Notes 2008, 1, 27–35. [Google Scholar]
- Ito, T.; Borlongan, I.A.; Nishihara, G.N.; Endo, H.; Terada, R. The effects of irradiance, temperature, and desiccation on the photosynthesis of a brown alga, Sargassum muticum (Fucales), from a native distributional range in Japan. J. Appl. Phycol. 2021, 33, 1777–1791. [Google Scholar] [CrossRef]
- Murakami, H.; Serisawa, Y.; Kurashima, A.; Yokohama, Y. Photosynthetic performances of temperate Sargassum and Kelp species growing in the same habitat. Algae 2004, 19, 207–216. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Nishihara, G.N.; Terada, R. Photosynthetic and temperature characteristics of five Sargassum species (Fucales), S. piluliferum, S. patens, S. fusiforme, S. crispifolium, and S. alternato-pinnatum from Kagoshima, Japan, using dissolved oxygen sensor and pulse-amplitude-modulated (PAM) fluorometer. Nippon Suisan Gakkaishi 2012, 78, 189–197. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Q.S.; Tang, Y.Z.; Li, X.M.; Liu, H.L.; Li, L.X. Diurnal changes of photosynthetic quantum yield in the intertidal macroalga Sargassum thunbergii under simulated tidal emersion conditions. J. Sea Res. 2013, 80, 50–57. [Google Scholar] [CrossRef]
- Beer, S.B.; Bjork, M.; Gademann, R.; Ralph, P.J. Measurements of photosynthetic rates in seagrasses. In Global Seagrass Research Methods; Short, F.T., Coles, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 183–198. [Google Scholar] [CrossRef]
- Serôdio, J.; Vieira, S.; Cruz, S.; Coelho, H. Rapid light-response curves of chlorophyll fluorescence in microalgae: Relationship to steady-state light curves and non-photochemical quenching in benthic diatom-dominated assemblages. Photosynth. Res. 2006, 90, 29–43. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B. Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci. 2000, 40, 503–510. [Google Scholar] [CrossRef]
- Liang, M.-H.; Jiang, J.-G.; Wang, L.; Zhu, J. Transcriptomic insights into the heat stress response of Dunaliella bardawil. Enzyme Microb. Technol. 2020, 132, 109436. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K. Safety valves for photosynthesis. Curr. Opin. Plant Biol. 2000, 3, 455–460. [Google Scholar] [CrossRef]
- Gao, G.; Xu, Z.; Shi, Q.; Wu, H. Increased CO2 exacerbates the stress of ultraviolet radiation on photosystem II function in the diatom Thalassiosira weissflogii. Envir. Exp. Bot. 2018, 156, 96–105. [Google Scholar] [CrossRef]
- Schofield, O.; Evens, T.J.; Millie, D.F. Photosystem II quantum yield and xantthophyll-cicle pigments of the macroalga Sargassum natans (PHAEOPHYCEAE): Responses under natural sunlight. J. Phycol. 1998, 34, 104–112. [Google Scholar] [CrossRef]
- Li, X.P.; Gilmore, A.M.; Caffarri, S.; Bassi, R.; Golan, T.; Kramer, D.; Niyogi, K.K. Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J. Bio. Chem. 2004, 279, 22866–22874. [Google Scholar] [CrossRef] [PubMed]
- García-Mendoza, E.; Colombo-Pallotta, M.F. The giant kelp Macrocystis pyriferapresents a different nonphotochemical quenching control than higher plants. New Phytol. 2007, 173, 526–536. [Google Scholar] [CrossRef]
- Garcia-Mendoza, E.; Ocampo-Alvarez, H.; Govindjee. Photoprotection in the brown alga Macrocystis Pyrifera: Evolutionary implications. J. Photochem. Photobiol. B 2011, 104, 377–385. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, Q.S.; Tang, Y.Z.; Yu, Y.Q.; Liu, H.L.; Li, L.X. Highly efficient photoprotective responses to high light stress in Sargassum thunbergii germlings, a representative brown macroalga of intertidal zone. J. Sea Res. 2014, 85, 491–498. [Google Scholar] [CrossRef]
- Das, S.K.; Patra, J.K.; Thatoi, H. Antioxidative response to abiotic and biotic stresses in mangrove plants: A review. Int. Ver. Hydrobiol. 2016, 101, 3–19. [Google Scholar] [CrossRef]
- Wang, L.-J.; Loescher, W.; Duan, W.; Li, W.-D.; Yang, S.-H.; Li, S.-H. Heat acclimation induced acquired heat tolerance and cross adaptation in grape cultivars: Relationships top photosynthetic energy partitioning. Funct. Plant Biol. 2009, 36, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D. Effects of moderate heat stress on photosynthesis: Importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ. 2005, 28, 269–277. [Google Scholar] [CrossRef]
- Nauer, F.; Oliveira, M.C.; Plastino, E.M.; Yokoya, N.S.; Fujii, M.T. Coping with heatwaves: How a key species of seaweed responds to heat stress along its latitudinal gradient. Mar. Environ. Res. 2022, 177, 105620. [Google Scholar] [CrossRef]
- Li, J.-J.; Du, X.-K. Will climate change cause Sargassum beds in temperate waters to expand or contract? Evidence from the range shift pattern of Sargassum. Mar. Environ. Res. 2024, 200, 106659. [Google Scholar] [CrossRef] [PubMed]
- Gouvêa, L.P.; Assis, J.; Gurgel, C.F.D.; Serrão, E.A.; Silveira, T.C.L.; Santos, R.; Peres, L.M.C.; Carvalho, V.F.; Batista, M.; Bastos, E.; et al. Golden carbon of Sargassum forests revealed as an opportunity for climate change mitigation. Sci. Total Environ. 2020, 729, 138745. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Al Lombana, S.A.; Martin, K.D.; et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Wynne, M.J. Checklist of benthic macroalgae of the tropical and subtropical Western Atlantic: Fifth revision. Nova Hedwigia 2022, 153 (Suppl. Sl), 1–180. [Google Scholar]
- Carneiro, I.M.; Diaz, R.S.; Bertocci, I.; Széchy, M.T.M. The Fucales Index: A new tool for monitoring subtidal rocky habitats, and its application to an Atlantic Bay subjected to nuclear power plant’s effluents. Mar. Poll. Bull. 2021, 172, 112804. [Google Scholar] [CrossRef]
- Goss, R.; Lepetit, B. Biodiversity of NPQ. J. Plant Physiol. 2015, 172, 13–32. [Google Scholar] [CrossRef]
- Platt, T.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Labasque, T.; Chaumeryb, C.; Aminotc, A.; Kergoat, G. Spectrophotometric winkler determination of dissolved oxygen: Reexamination of critical factors and reliability. Mar. Chem. 2004, 88, 53–60. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org (accessed on 1 November 2024).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biomed. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
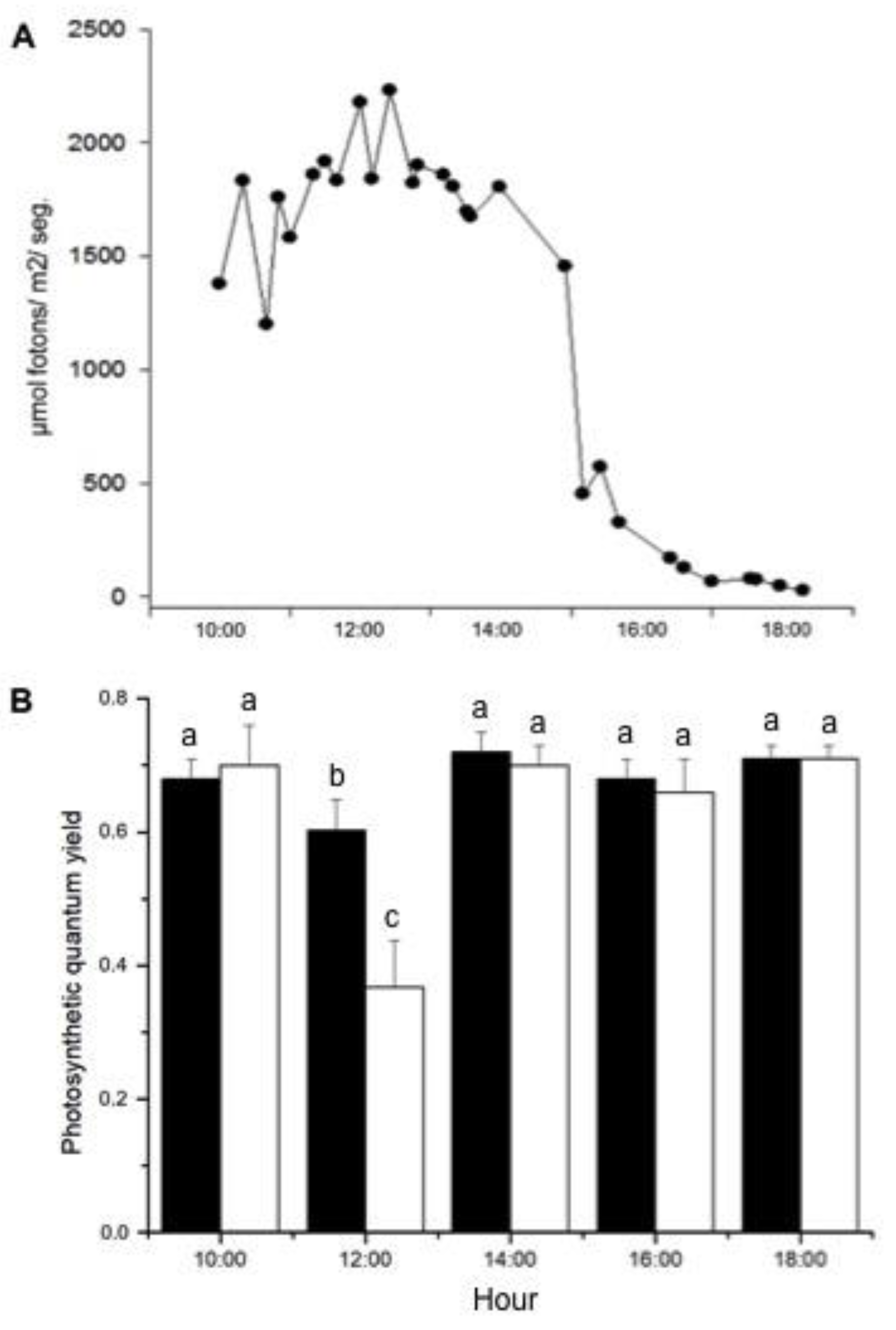
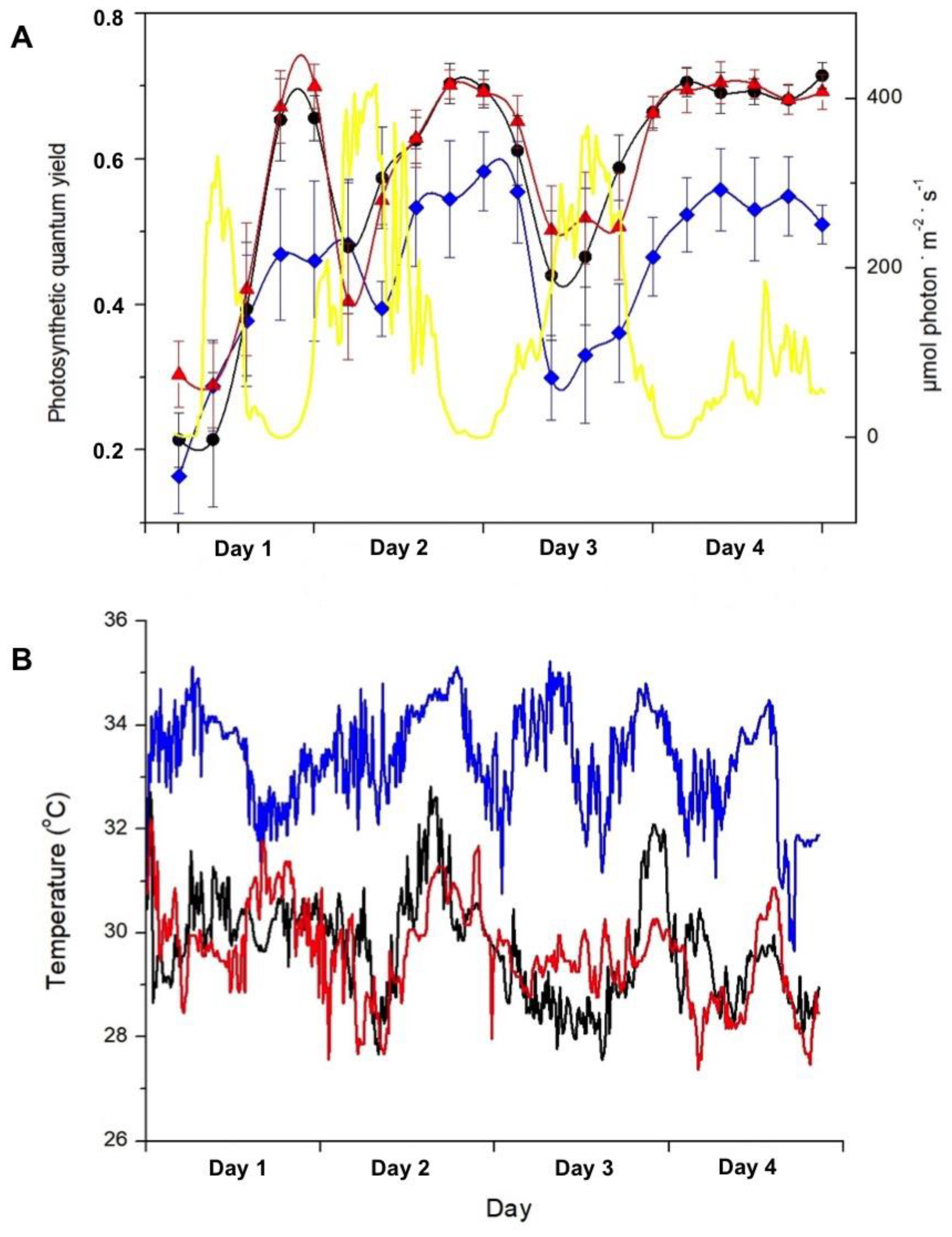
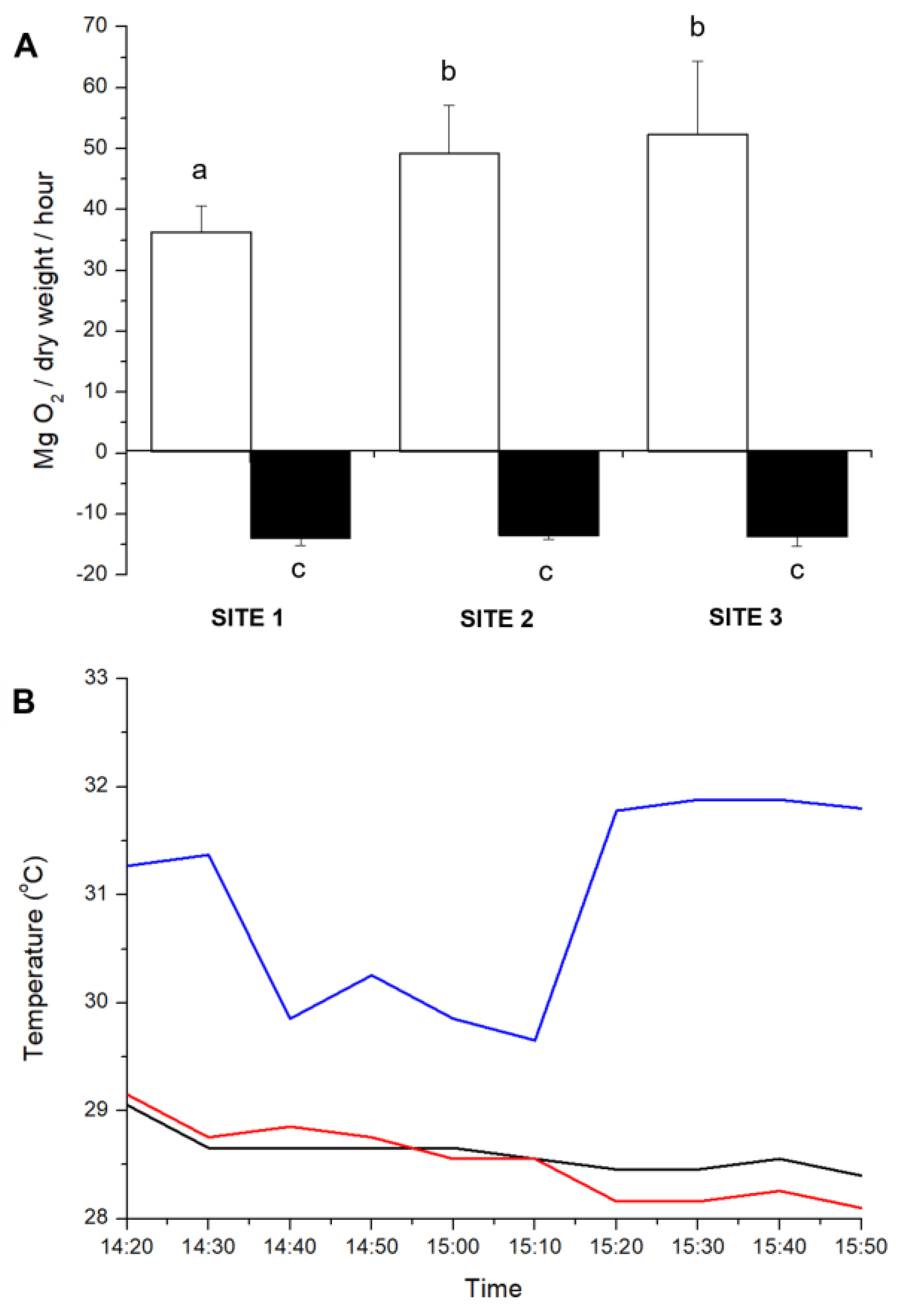
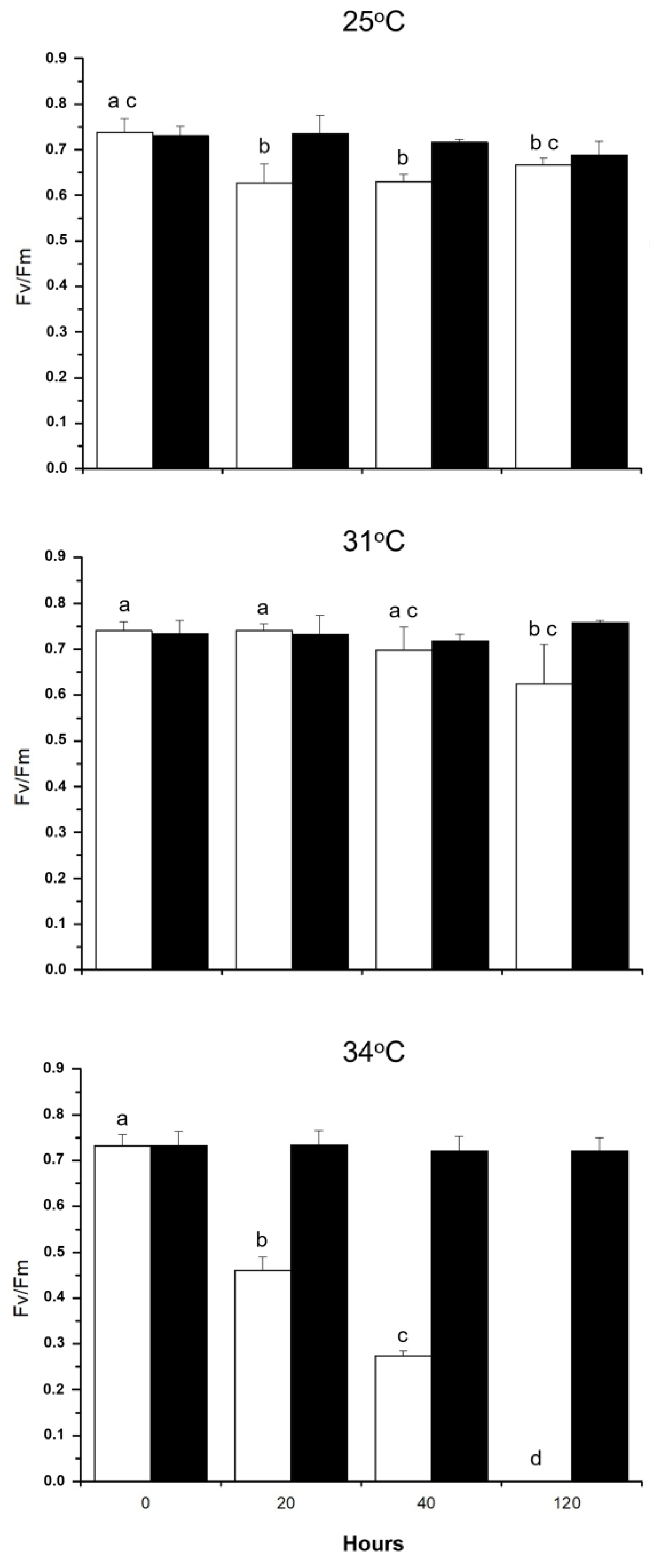
| Time (h) | rETRmax (µmol Electrons·m−2·s−1) | α (µmol Electrons)/(µmol Photons) | Ek (µmol Photons·m−2·s−1) |
|---|---|---|---|
| 10:00 | 19.4 ± 2.0 | 0.384 ± 0.035 | 51 ± 4 |
| 12:00 | 17.8 ± 1.5 | 0.227 ± 0.048 | 62 ± 5 |
| 14:00 | 17.8 ± 1.6 | 0.422 ± 0.012 | 42 ± 3 |
| 16:00 | 16.6 ± 1.4 | 0.411 ± 0.019 | 40 ± 4 |
| 18:00 | 16.1 ± 3.3 | 0.409 ± 0.043 | 32 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaloub, R.M.; da Costa, R.M.V.; Silva, J.; Nassar, C.A.G.; Reinert, F.; Széchy, M.T.M. Response of a Benthic Sargassum Population to Increased Temperatures: Decline in Non-Photochemical Quenching of Chlorophyll a Fluorescence (NPQ) Precedes That of Maximum Quantum Yield of PSII. Plants 2025, 14, 759. https://doi.org/10.3390/plants14050759
Chaloub RM, da Costa RMV, Silva J, Nassar CAG, Reinert F, Széchy MTM. Response of a Benthic Sargassum Population to Increased Temperatures: Decline in Non-Photochemical Quenching of Chlorophyll a Fluorescence (NPQ) Precedes That of Maximum Quantum Yield of PSII. Plants. 2025; 14(5):759. https://doi.org/10.3390/plants14050759
Chicago/Turabian StyleChaloub, Ricardo M., Rodrigo Mariath V. da Costa, João Silva, Cristina A. G. Nassar, Fernanda Reinert, and Maria Teresa M. Széchy. 2025. "Response of a Benthic Sargassum Population to Increased Temperatures: Decline in Non-Photochemical Quenching of Chlorophyll a Fluorescence (NPQ) Precedes That of Maximum Quantum Yield of PSII" Plants 14, no. 5: 759. https://doi.org/10.3390/plants14050759
APA StyleChaloub, R. M., da Costa, R. M. V., Silva, J., Nassar, C. A. G., Reinert, F., & Széchy, M. T. M. (2025). Response of a Benthic Sargassum Population to Increased Temperatures: Decline in Non-Photochemical Quenching of Chlorophyll a Fluorescence (NPQ) Precedes That of Maximum Quantum Yield of PSII. Plants, 14(5), 759. https://doi.org/10.3390/plants14050759








