Soil Salinization and Ancient Hulled Wheat: A Study on Antioxidant Defense Mechanisms
Abstract
1. Introduction
2. Results
2.1. Effects of Salt Stress on Plant Growth
2.1.1. Effect of Salt Stress on the Fresh Weight of Plant Roots and Shoots
2.1.2. Effect of Salt Stress on the Dry Weight of Plant Roots and Shoots
2.1.3. Effect of Salt Stress on Leaf Length
2.1.4. Effect of Salt Stress on Total Plant Height
2.1.5. Effect of Salt Stress on Chlorophyll (Chl) a, b, and Total Chlorophyll and Carotene Content
2.2. Effect of Salt Stress on Soluble Protein Content
2.3. Effects of Salt Stress on Antioxidant Enzyme Activity in Plant Roots and Shoots
2.3.1. Effect on SOD Activity
2.3.2. Effect on CAT Activity
2.3.3. Effect on GR Activity
2.3.4. Effect on GST Activity
2.3.5. Effect on APX Activity
2.4. Effect on Proline Accumulation
2.5. Effect on Lipid Peroxidation (LPO) (MDA)
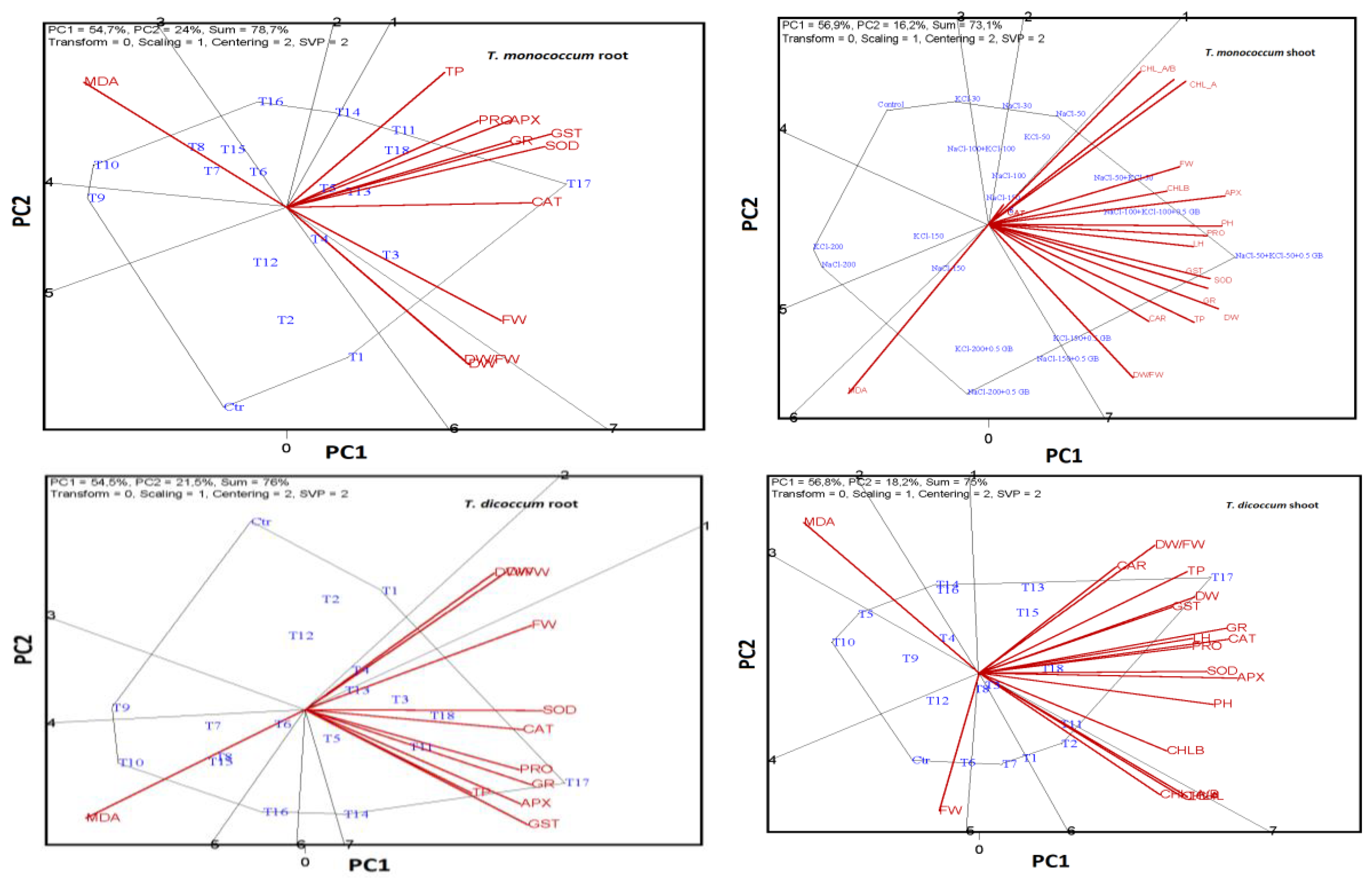
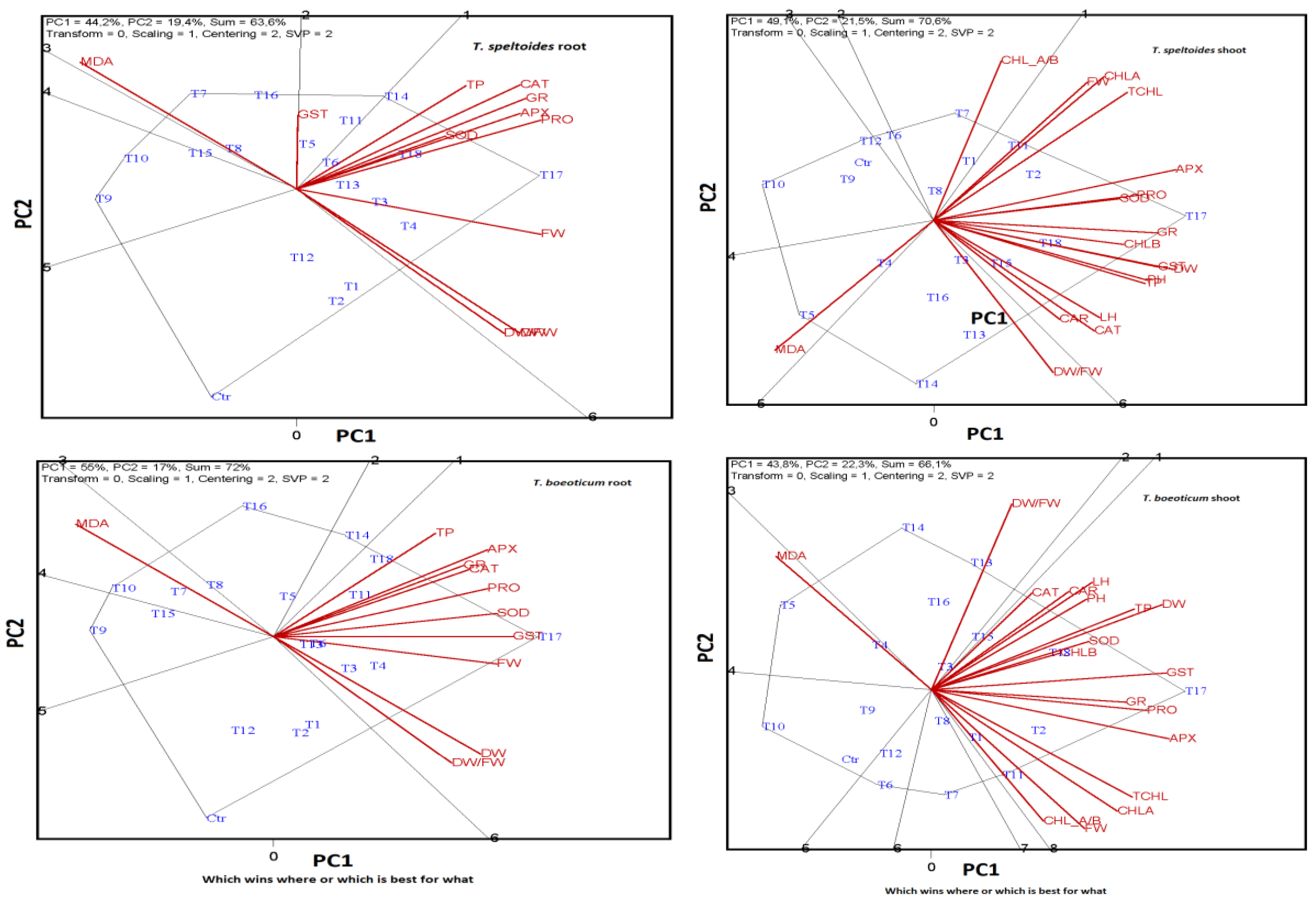
2.6. ANOVA, MANOVA, and Levene Test Results
3. Discussion
3.1. Effects of Salt Stress on Plant Growth
3.1.1. Effect on Plant Biomass
3.1.2. Effect on Chlorophyll a, b, and Total Chlorophyll and Carotene Content
3.2. Effect of Salt Stress on Soluble Protein Concentrations
3.3. Effects of Salt Stress on the Enzymatic and Non-Enzymatic Antioxidant Defense System
3.4. Effects of Salt Stress on Proline Accumulation
3.5. Effects of Salt Stress on Lipid Peroxidation (LPO; MDA)
4. Materials and Methods
4.1. Plant Cultivation and Stress Management Practices
4.2. Measuring the Effects of Salinity on Plant Growth Parameters: Fresh Weight, Dry Weight, Leaf Length, and Height
4.3. Preparation of Crude Enzyme Extracts
4.4. Determination of the Chlorophyll and Carotene Content
4.5. Determination of Enzyme Activity
4.5.1. Catalase (CAT) (EC 1.11.1.6) Activity
4.5.2. Superoxide Dismutase (SOD) (EC 1.15.1.1) Activity
4.5.3. Ascorbate Peroxidase (APX) (EC 1.11.1.11) Activity
4.5.4. Glutathione Reductase (GR) (EC 1.6.4.2) Activity
4.5.5. Glutathione S-Transferase (GST) (EC 2.5.1.18) Activity
4.6. Lipid Peroxidation (LPO, MDA) Assessment
4.7. Determination of Proline Content
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abs | Absorbance |
| APX | Ascorbate peroxidase |
| BSA | Bovine serum albumin |
| CAT | Catalase |
| CDNB | 1-chloro-2,4-dinitrobenzene |
| DMSO | Dimethyl sulfoxide |
| DW | Dry weight |
| Ɛ | molar absorption coefficient |
| FW | Fresh weight |
| Chl | Chlorophyll |
| GB | Glycine betaine |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GSSG | Oxidized glutathione |
| GST | Glutathione S-transferase |
| Inh | Inhibition |
| KCl | Potassium chloride |
| LL | Leaf length |
| LPO | Lipid peroxidation |
| MDA | Malondialdehyde |
| NaCl | Sodium chloride |
| NBT | Nitro blue tetrazolium chloride |
| PH | Plant height |
| PRO | Proline |
| PVP | Polyvinyl pyrrolidone |
| PVPP | Polyvinyl polypyrrolidone |
| ROS | Reactive oxygen species |
| SA | Specific activity |
| SOD | Superoxide dismutase |
| SOS | Salt overlay sensitive |
| TBA | 2-thiobarbituric acid |
| TBARS | Thiobarbituric acid reactive substances |
| TCA | Trichloroacetic acid |
| TP | Total protein |
References
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Zaman, M., Shahid, S.A., Heng, L., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Tanji, K.K. Nature and extent of agricultural salinity. In Agricultural Salinity Assessment and Management, ASCE Manuals and Reports on Engineering Practice; Tanji, K.K., Ed.; ASCE: New York, NY, USA, 1990; No. 71; pp. 1–17. [Google Scholar]
- Hoque, M.N.; Imran, S.; Hannan, A.; Paul, N.C.; Mahamud, M.A.; Chakrobortty, J.; Sarker, P.; Irin, I.J.; Brestic, M.; Rhaman, M.S. Organic Amendments for Mitigation of Salinity Stress in Plants: A Review. Life 2022, 12, 1632. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; El-Sharkawy, I.; Sherif, S. Salt stress signals on demand: Cellular events in the right context. Int. J. Mol. Sci. 2020, 21, 3918. [Google Scholar] [CrossRef]
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.; Mohsin, S.M.; Fujita, M. Quercetin mediated salt tolerance in tomato through the enhancement of plant antioxidant defense and glyoxalase systems. Plants 2019, 8, 247. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Aslam, M.T.; Alhammad, B.A.; Hassan, M.U.; Maqboo, R.; Chattha, M.U.; Khan, I.; Gitari, H.I.; Uslu, O.S.; Roy, R.; et al. Salinity Stress in Wheat: Effects, Mechanisms and Management Strategies. Phyton-Int. J. Exp. Bot. 2022, 91, 667–694. [Google Scholar] [CrossRef]
- Guo, R.; Yang, Z.; Li, F.; Yan, C.; Zhong, X.; Liu, Q.; Zhao, L. Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum L.) to salt and alkali stress. BMC Plant Biol. 2015, 15, 170. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Li, K.; Liu, S.; He, X.; Zhang, X.; Xing, R.; Li, P. Effect of sulfated chitooligosaccharides on wheat seedlings (Triticum aestivum L.) under salt stress. J. Agric. Food Chem. 2016, 64, 2815–2821. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front. Plant Sci. 2016, 7, 609. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.U.; Chattha, M.U.; Mahmood, A.; Sahi, S.T. Performance of sorghum cultivars for biomass quality and biomethane yield grown in semi-arid area of Pakistan. Environ. Sci. Pollut. Res. 2018, 25, 12800–12807. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimova, U.; Zivcak, M.; Gasparovic, K.; Rastogi, A.; Allakhverdiev, S.I.; Yang, X.; Brestic, M. Electron and proton transport in wheat exposed to salt stress: Is the increase of the thylakoid membrane proton conductivity responsible for decreasing the photosynthetic activity in sensitive genotypes? Photosynth. Res. 2021, 150, 195–211. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H.M. Salt stress in maize effects resistance mechanisms and management: A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Dustgeer, Z.; Seleiman, M.F.; Imran, K.; Chattha, M.U.; Alhammad, B.A.; Jalal, R.S.; Hassan, M.U. Glycine-betaine induced salinity tolerance in maize by regulating the physiological attributes, antioxidant defense system and ionic homeostasis. Not. Bot. Horti Agrobo 2021, 49, 12248. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Rao, A.; Ahmad, S.D.; Sabir, S.M.; Awan, S.; Shah, A.H.; Khan, M.F.; Shafique, S.; Arif, S.; Abbas, S.R. Potential biochemical indicators improve salt tolerance in fifteen cultivars of wheat (Triticum aestivum L.) from Pakistan. Int. J. Sci. Eng. Res. 2013, 4, 389–406. Available online: https://api.semanticscholar.org/CorpusID:59036442 (accessed on 12 July 2024).
- United Nations. Department of Economic and Social Affairs, Population Division. In Global Population Growth and Sustainable Development; United Nations: New York, NY, USA, 2021; UN DESA/POP/2021/TR/NO. 2; pp. 103–108. [Google Scholar]
- Dinu, M.; Whittaker, A.; Pagliai, G.; Benedettelli, S.; Sofi, F. Ancient wheat species and human health: Biochemical and clinical implications. J. Nutr. Biochem. 2018, 52, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Temizgul, R.; Ciftci, B.; Kardes, Y.M.; Kara, R.; Temizgul, S.; Yilmaz, S.; Kaplan, M. Comparison of different hulled wheat genotypes in terms of yield, morphological, and nutritional properties. Genet. Resour. Crop Evol. 2024, 72, 475–482. [Google Scholar] [CrossRef]
- Longin, F.; Ziegler, J.; Schweiggert, R.; Koehler, P.; Carle, R.; Würschum, T. Comparative study of hulled (einkorn, emmer, and spelt) and naked wheats (durum and bread wheat): Agronomic performance and quality traits. Crop Sci. 2015, 56, 302–311. [Google Scholar] [CrossRef]
- Arzani, A.; Ashraf, M. Cultivated ancient wheats (Triticum spp.): A potential source of health-beneficial food products. Compr. Rev. Food Sci. Food Saf. 2017, 16, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, J.; Luo, M.; Yang, Z. Genetic evidence on the origin of Triticum aestivum L., The origins of agriculture and crop domestication. In Proceedings of the Harlan Symposium; ICARDA: Aleppo, Syria, 1998; pp. 235–251. Available online: https://hdl.handle.net/10568/104411 (accessed on 12 July 2024).
- Dhanavath, S.; Prasada Rao, U. Nutritional and nutraceutical properties of Triticum dicoccum wheat and its health benefits: An overview. J. Food Sci. 2017, 82, 2243–2250. [Google Scholar] [CrossRef] [PubMed]
- Zaharieva, M.; Ayana, N.G.; Hakimi, A.A.; Misra, S.C.; Monneveux, P. Cultivated emmer wheat (Triticum dicoccon Schrank), an old crop with promising future: A review. Genet. Resour. Crop Evol. 2010, 57, 937–962. [Google Scholar] [CrossRef]
- Konvalina, P.; Moudrý, J. Evaluation of suitability of emmer wheat varieties (Triticum dicoccum Schuebl) for organic farming. Lucr. Ştiinţifice Ser. Agron. 2007, 50, 241–247. [Google Scholar]
- Akcura, M.; Taner, S.; Kaya, Y. Evaluation of bread wheat genotypes under irrigated multi-environment conditions using GGE biplot analyses. Zemdirbyste 2011, 98, 35–40. [Google Scholar]
- Yan, W.; Tinker, N.A. Biplot analysis of multi-environment trial data: Principles and applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef]
- Taha, R.S.; Seleiman, M.F.; Alhammad, B.A.; Alkahtani, J.; Alwahibi, M.S.; Mahdi, A.H. Activated yeast extract enhances growth, anatomical structure, and productivity of Lupinus termis L. plants under actual salinity conditions. Agronomy 2021, 11, 74. [Google Scholar] [CrossRef]
- Hussain, S.; Hussain, S.; Ali, B.; Ren, X.; Chen, X.; Li, Q.; Ahmad, N. Recent progress in understanding salinity tolerance in plants: Story of Na+/K+ balance and beyond. Plant Physiol. Biochem. 2021, 160, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Fortmeier, R.; Schubert, S. Salt tolerance of maize (Zea mays L.): The role of sodium exclusion. Plant Cell Environ. 1995, 18, 1041–1047. [Google Scholar] [CrossRef]
- Neubert, A.B.; Zorb, C.; Schubert, S. Expression of vacuolar Na+/H+ antiporters (ZmNHX) and Na+ exclusion in roots of maize (Zea mays L.) genotypes with improved salt resistance. In Plant Nutrition for Food Security Human Health and Environmental Protection; Li, C.J., Zhang, F.S., Dobermann, A., Hinsinger, P., Lambers, H., Li, X.L., Marschner, P., Maene, L., McGrath, S., Oenema, O., et al., Eds.; Tsinghua University Press: Beijing, China, 2005; pp. 63–89. [Google Scholar]
- Wakeel, A.; Farooq, M.; Qadir, M.; Schubert, S. Potassium substitution by sodium in plants. Crit. Rev. Plant Sci. 2011, 30, 401–413. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int. J. Mol. Sci. 2017, 18, 200. [Google Scholar] [CrossRef]
- Badawy, S.A.; Zayed, B.A.; Bassiouni, S.M.A.; Mahdi, A.H.A.; Majrashi, A.; Ali, E.F.; Seleiman, M.F. Influence of nano silicon and nano selenium on root characters, growth, ion selectivity, yield, and yield components of rice (Oryza sativa L.) under salinity conditions. Plants 2021, 10, 1657. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M.; Ahmad, P.; Chandna, R.; Prasad, M.N.V.; Ozturk, M. Enhancing plant productivity under salt stress: Relevance of poly-omics. In Salt Stress in Plants: Omics, Signaling and Responses; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: Berlin, Germany, 2013; pp. 113–156. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected amaranthus leafy vegetables. J. Sci. Food Agric. 2019, 99, 2275–2284. [Google Scholar] [CrossRef]
- Levy, D.; Coleman, W.K.; Veilleux, R.E. Adaptation of potato to water shortage: Irrigation management and enhancement of tolerance to drought and salinity. Am. J. Potato Res. 2013, 90, 186–206. [Google Scholar] [CrossRef]
- Charfeddine, M.; Charfeddine, S.; Ghazala, I.; Bouaziz, D.; Bouzid, R.G. Investigation of the response to salinity of transgenic potato plants overexpressing the transcription factor StERF94. J. Biosci. 2019, 44, 141. [Google Scholar] [CrossRef]
- Kolomeichuk, L.V.; Efimova, M.V.; Zlobin, I.E.; Kreslavski, V.D.; Murgan, O.K.; Kovtun, I.S.; Khripach, V.A.; Kuznetsov, V.V.; Allakhverdiev, S.I. 24-epibrassinolide alleviates the toxic effects of NaCl on photosynthetic processes in potato plants. Photosynth. Res. 2020, 146, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Luo, F.; Zou, R.; Liu, J.; Yan, Y. Integrated physiological and chloroplast proteome analysis of wheat seedling leaves under salt and osmotic stresses. J. Proteom. 2021, 234, 104097. [Google Scholar] [CrossRef]
- Bose, J.; Munns, R.; Shabala, S.; Gilliham, M.; Pogson, B.; Tyerman, S.D. Chloroplast function and ion regulation in plants growing on saline soils: Lessons from halophytes. J. Exp. Bot. 2017, 68, 3129–3143. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Anee, T.I.; Alam, M.U.; Bhuiyan, T.F.; Oku, H.; Fujita, M. Approaches to Enhance Salt Stress Tolerance in Wheat; IntechOpen Limited 5 Princes Gate Court London: London, UK, 2017; pp. 151–187. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, A.; Yadav, S.; Singh, I.K. Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene 2019, 18, 100173. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Suzuki, T.; Fujita, M. Polyamines-induced aluminum tolerance in mung bean: A study on antioxidant defense and methylglyoxal detoxification systems. Ecotoxicology 2017, 26, 58–73. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Oku, H.; Nahar, K.; Bhuyan, M.B.; Mahmud, J.A.; Baluska, F.; Fujita, M. Nitric oxide-induced salt stress tolerance in plants: ROS metabolism, signaling, and molecular interactions. Plant Biotechnol. Rep. 2018, 12, 77–92. [Google Scholar] [CrossRef]
- El-Sayed, H.E.A. Influence of NaCl and Na2SO4 treatments on growth development of broad bean (Vicia faba L.) plant. J. Life Sci. 2011, 5, 513–523. [Google Scholar]
- Aamer, M.; Muhammad, U.H.; Li, Z.; Abid, A.; Su, Q.; Liu, Y.; Adnan, R.; Muhammad, A.U.K.; Tahir, A.K.; Huang, G. Foliar application of glycinebetaine (GB) alleviates the cadmium (Cd) toxicity in spinach through reducing Cd uptake and improving the activity of antioxidant system. Appl. Ecol. Environ. Res. 2018, 16, 7575–7583. [Google Scholar] [CrossRef]
- Tamaki, S.; Mochida, K.; Suzuki, K. Diverse biosynthetic pathways and protective functions against environmental stress of antioxidants in microalgae. Plants 2021, 10, 1250. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ju, J.; Xia, G. Identification of the flavonoid3-hydroxylase and flavonoid 3,5-hydroxylase genes from Antarctic moss and their regulation during abiotic stress. Gene 2014, 543, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 2019, 9, 3890. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, X.; Tao, M.; Yuan, F.; Liu, L.; Wu, F.; Wu, X.; Xiang, Y.; Niu, Y.; Liu, F.; et al. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nat. Cell Biol. 2019, 572, 341–346. [Google Scholar] [CrossRef]
- Lin, H.; Yang, Y.; Quan, R.; Mendoza, I.; Wu, Y.; Du, W.; Zhao, S.; Schumaker, K.S.; Pardo, J.M.; Guo, Y. Phosphorylation of SOS3 LIKE CALCIUM BINDING PROTEIN8 by SOS2 protein kinase stabilizes their protein complex and regulates salt tolerance in Arabidopsis. Plant Cell 2009, 21, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.S.; Zhu, J.K.; Bressan, R.A.; Hasegawa, P.M.; Shi, H. Reactive oxygen species mediate Na+-induced SOS1 mRNA stability in Arabidopsis. Plant J. 2008, 53, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E.; Batelli, G.; Grillo, S.; Agius, F.; Kim, Y.S.; Zhu, J.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.K. Interaction of SOS2 with nucleoside diphosphate kinase 2 and catalases reveals a point of connection between salt stress and H2O2 signaling in Arabidopsis thaliana. Mol. Cell Biol. 2007, 27, 7771–7780. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, Z.; Hussain, N.; Irshad, F.; Zeng, J.; Tahir, A.; Zhang, G. Physiological and antioxidant responses of cultivated and wild barley under salt stress. Plant Soil. Environ. 2020, 66, 334–344. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Alderfasi, A.; El-Hendawy, S.; Al-Suhaibani, N.; El-Kafafi, S.; Seleiman, M.F. Detecting salt tolerance in doubled haploid wheat lines. Agronomy 2019, 9, 211. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Barbanti, L.; Aamer, M.; Aslam, M.T. Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies—A review. Plant Biosyst. 2020, 155, 211–234. [Google Scholar] [CrossRef]
- Meneguzzo, S.; Navari-Izzo, F.; Izzo, R. Antioxidative responses of shoots and roots of wheat to increasing NaCI concentrations. J. Plant Physiol. 1999, 155, 274–280. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Grimm, B.; Wobus, U.; Weschke, W. Differential response of antioxidant components to salinity stress in salt-tolerant and salt sensitive seedlings of foxtail millet (Setaria italica). Physiol. Plant. 2000, 109, 435–442. [Google Scholar] [CrossRef]
- Athar, H.U.R.; Khan, A.; Ashraf, M. Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environ. Exp. Bot. 2007, 63, 224–231. [Google Scholar] [CrossRef]
- Athar, H.U.R.; Khan, A.; Ashraf, M. Inducing salt tolerance in wheat by exogenously applied ascorbic acid through different modes. J. Plant Nutr. 2009, 32, 1–19. [Google Scholar] [CrossRef]
- Munns, R.; Hare, R.A.; James, R.A.; Rebetzke, G.J. Genetic variation for improving the salt tolerance of durum wheat. Aust. J. Agric. Res. 2000, 51, 69–74. [Google Scholar] [CrossRef]
- Dubcovsky, J.; Maria, G.S.; Epstein, E.; Luo, M.C.; Dvorak, J. Mapping of the K+/Na+ discrimination locus kna1 in wheat. Theor. Appl. Genet. 1996, 92, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, L.; Zhang, H.; Yang, Z.; Wang, H.; Wen, S.; Liu, B. Evolution of physiological responses to salt stress in hexaploid wheat. Proc. Natl. Acad. Sci. USA 2014, 111, 11882–11887. [Google Scholar] [CrossRef] [PubMed]
- Byrt, C.S.; Xu, B.; Krishnan, M.; Lightfoot, D.J. The Na+ transporter, TaHKT1;5-d, limits shoot Na+ accumulation in bread wheat. Plant J. 2014, 80, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dong, W.; Zhang, N.; Ai, X.; Wang, M.; Huang, Z.; Xia, G. Wheat allene oxide cyclase gene enhances salinity tolerance via jasmonate signaling. Plant Physiol. 2014, 164, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, J.; Qin, L.; Shi, W.; Xia, G.; Liu, S. TaCYP81D5, one member in a wheat cytochrome P450 gene cluster, confers salinity tolerance via reactive oxygen species scavenging. Plant Biotechnol. J. 2020, 18, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Lu, M.; Sehar, S.; Holford, P.; Wu, F. Comparison of biochemical, anatomical, morphological, and physiological responses to salinity stress in wheat and barley genotypes deferring in salinity tolerance. Agronomy 2020, 10, 127. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, W.; Hu, G.; Chen, W.; Zhuge, Y.; Wang, Z.L.; He, M.R. Role of exogenous 24-epibrassinolide in enhancing the salt tolerance of wheat seedlings. J. Soil. Sci. Plant Nut 2017, 17, 554–569. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qin, C.; Begum, N.; Qi, M.; Xu, X.D.; El-Esawi, M.; El-Sheikh, M.A.; Alatar, A.A.; Zhang, L. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biol. 2019, 19, 479. [Google Scholar] [CrossRef]
- Mandhania, S.; Madan, S.; Sawhney, V. Antioxidant defense mechanism under salt stress in wheat seedlings. Biol. Plant. 2006, 50, 227–231. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Raza, S.H.; Athar, H.; Ashraf, M. Influence of exogenously applied glycine-betaine on the photosynthetic efficiency of two differently adapted cultivars under salt stress. Pak. J. Bot. 2006, 38, 241–251. [Google Scholar] [CrossRef]
- Akhter, N.; Akram, N.A.; Shahbaz, M. Presowing seed treatments with glycinebetaine and mineral nutrients of wheat (Triticum aestivum L.) under saline conditions. Pak. J. Agric. Sci. 2007, 44, 236–241. [Google Scholar]
- Hendawey, M.H. Biochemical changes associated with induction of salt tolerance in wheat. Glob. Sci. Res. J. 2015, 10, 84–99. [Google Scholar] [CrossRef]
- Duman, F.; Aksoy, A.; Aydin, Z.; Temizgul, R. Effects of Exogenous Glycinebetaine and Trehalose on Cadmium Accumulation and Biological Responses of an Aquatic Plant (Lemna gibba L.). Water Air Soil. Pollut. 2011, 217, 545–556. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Cordovilla, M.P.; Ocana, A.; Ligero, F.; Lluch, C. Salinity effects on growth analysis and nutrient composition in four grain legumes. J. Plant Nutr. 1995, 18, 1595–1609. [Google Scholar] [CrossRef]
- Amtmann, A.; Sanders, D. Mechanisms of Na+ uptake by plant cells. Adv. Bot. Res. 1998, 29, 76–112. [Google Scholar] [CrossRef]
- Blumwald, E. Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 2000, 2, 431–434. [Google Scholar] [CrossRef] [PubMed]
- European Commission. A European Green. Deal. 2019. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 12 July 2024).
- European Commission. The New Common. Agricultural Policy, 2023-27. 2023. Available online: https://agriculture.ec.europa.eu/common-agricultural-policy/cap-overview/new-cap-2023-27_en (accessed on 17 July 2024).
- FAO. SoiLEX FAO Soils Portal. 2023. Available online: https://www.fao.org/soils-portal/soilex/en/ (accessed on 21 July 2024).
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil; University of California, College of Agriculture: Berkeley, CA, USA, 1950; p. 347. [Google Scholar]
- Yilmaz, S.H.; Kaplan, M.; Temizgul, R.; Yilmaz, S. Antioxidant enzyme response of sorghum plant upon exposure to Aluminum, Chromium and Lead heavy metals. Turk. J. Biochem. 2017, 42, 503–512. [Google Scholar] [CrossRef]
- Akbulut, M.; Çakır, S. The effects of Se phytotoxicity on the antioxidant systems of leaf tissues in barley (Hordeum vulgare L.) seedlings. Plant Physiol. Biochem. 2010, 48, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yilmaz, S.; Temizgül, R.; Yürürdurmaz, C.; Kaplan, M. Oxidant and antioxidant enzyme response of redbine sweet sorghum under NaCl salinity stress. Bioagro 2020, 32, 31–38. Available online: https://revistas.uclave.org/index.php/bioagro/article/view/2684 (accessed on 17 July 2024).
- Misra, N.; Gupta, A. Effect of salinity and different nitrogen sources on the activity of antioxidant enzymes and indole alkoloid content in Catarantus roseus seedlings. J. Plant Physiol. 2006, 164, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Jardine, D.; Antolovich, M.; Prenzler, P.D.; Robards, K. Liquid Chromatography−Mass Spectrometry (LC-MS) Investigation of the Thiobarbituric Acid Reactive Substances (TBARS) Reaction. J. Agric. Food Chem. 2002, 50, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Bio Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Madhava, R.K.; Sresty, T.V. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar] [CrossRef]
- Temizgul, R.; Kaplan, M.; Kara, R.; Yilmaz, S. Effects of salt concentrations on antioxidant enzyme activity of grain sorghum. Curr. Trends Nat. Sci. 2016, 5, 171–178. Available online: https://api.semanticscholar.org/CorpusID:89938550 (accessed on 17 July 2024).
- Yan, W. Crop Variety Trials: Data Management and Analysis; John Wiley and Sons: Hoboken, NJ, USA, 2014; p. 349. [Google Scholar] [CrossRef]
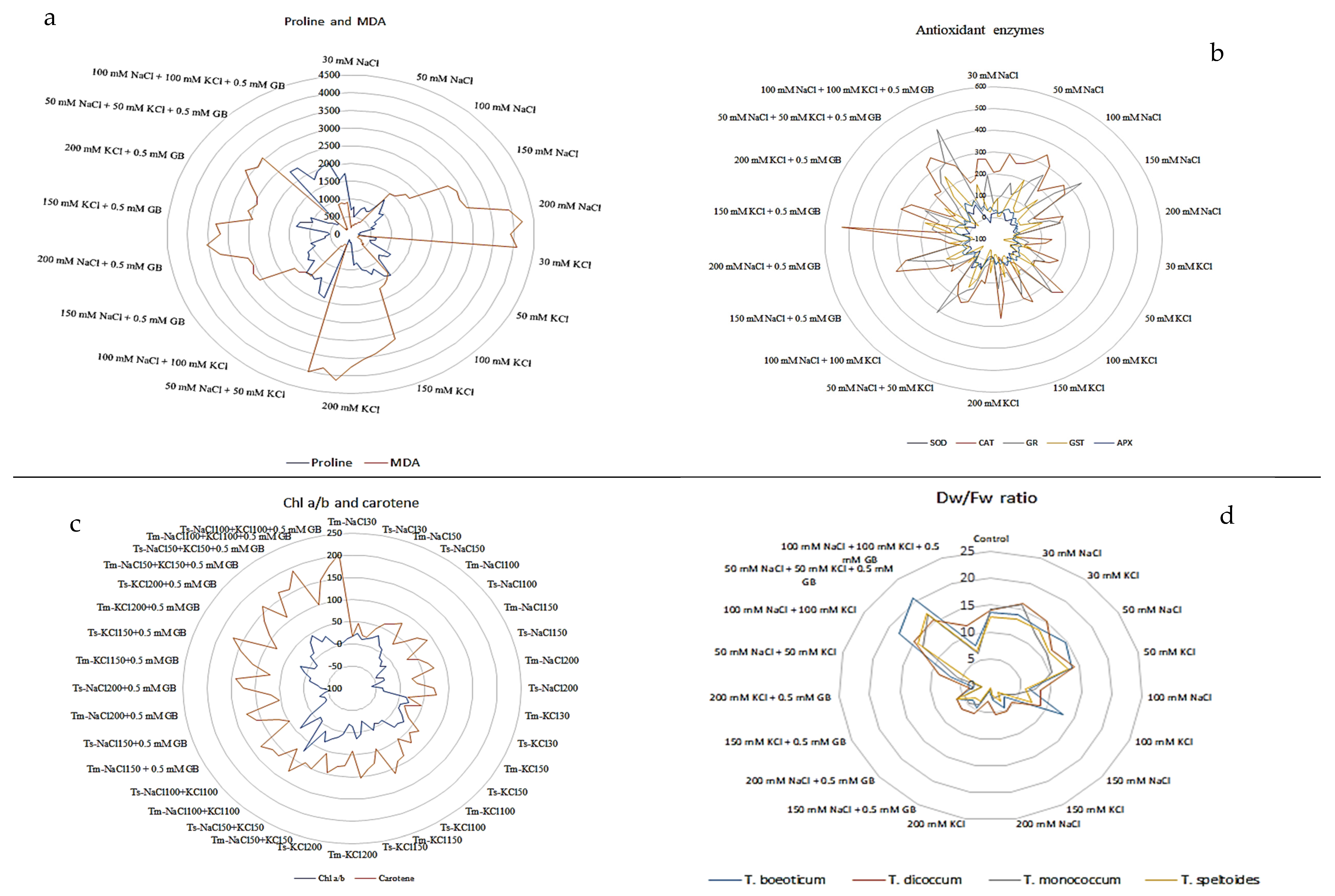
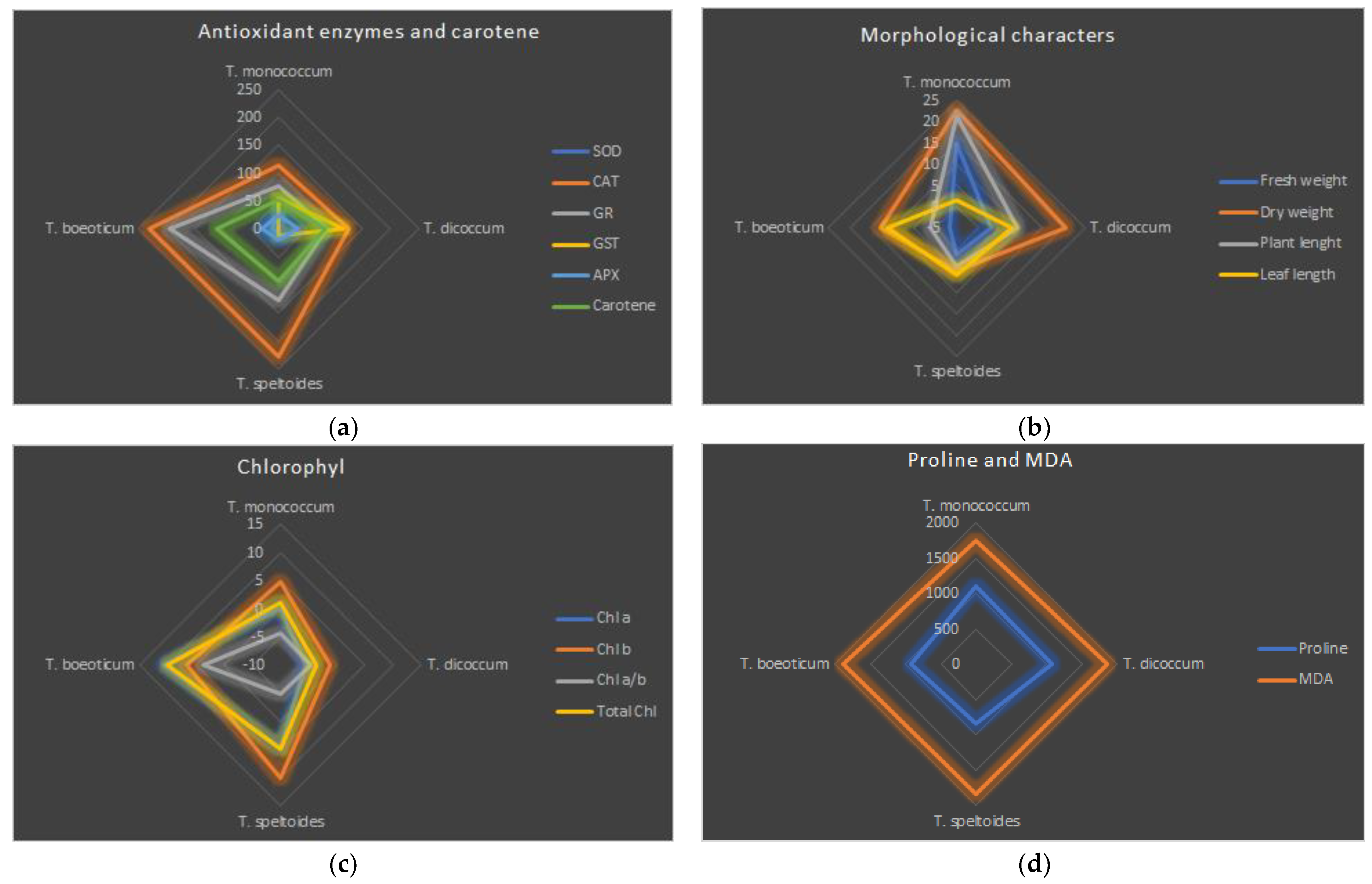

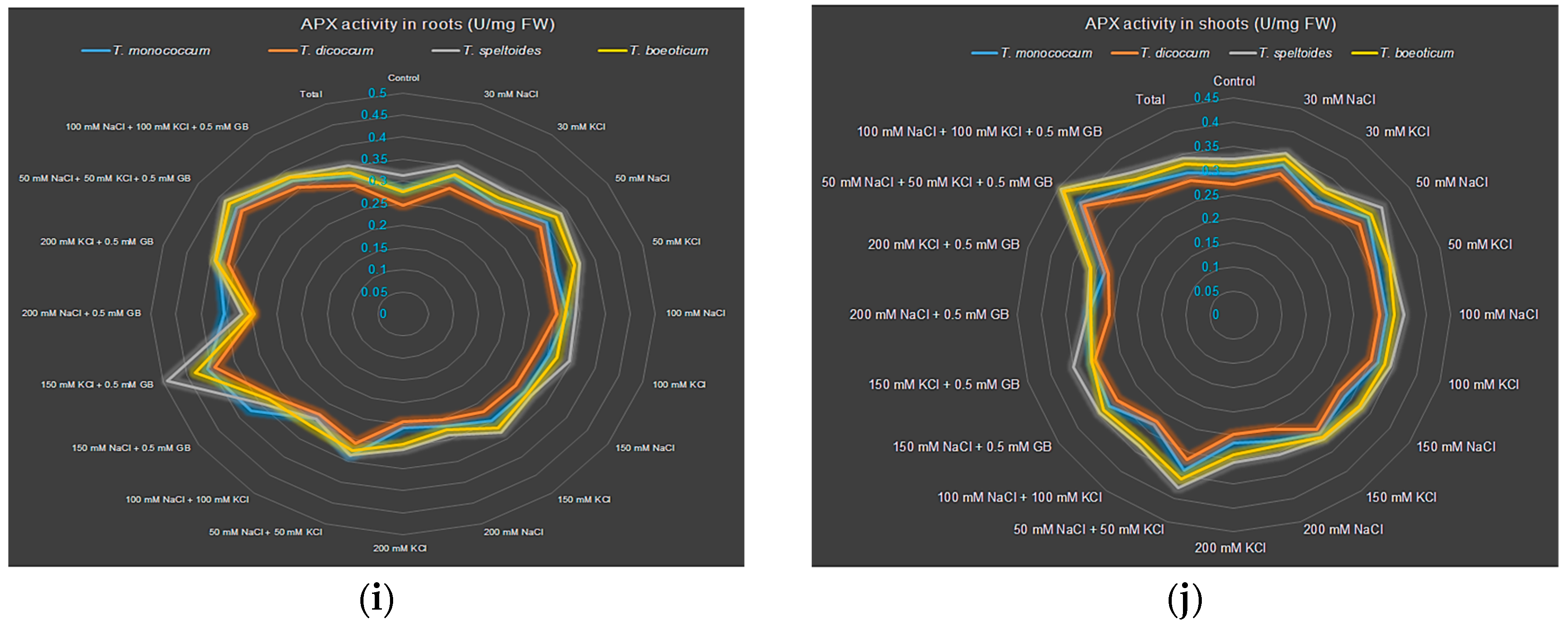

| Salt Treatments | Section | Fresh Weight (g) | Dry Weight (g) | Dw/Fw (%) | Plant Height (cm) | Leaf Height (cm) | Chl a (mg g−1 fw) | Chl b (mg g−1 fw) | Chl a/b | Total Chl (mg g−1 fw) | Carotene (mg g−1 fw) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Shoot | 29.28 ± 0.55 c | 3.68 ± 0.09 b | 12.57 ab | 25.17 ± 0.47 bc | 19.75 ± 0.33 b | 2.18 ± 0.03 bc | 0.89 ± 0.01 b | 2.45 c | 3.08 ± 0.03 bc | 0.29 ± 0.03 a |
| 30 mM NaCl | Shoot | 32.11 ± 0.09 d | 4.32 ± 0.09 c | 13.45 c | 29.75 ± 0.45 e | 20.50 ± 0.33 c | 2.77 ± 0.02 c | 0.97 ± 0.01 c | 2.86 d | 3.74 ± 0.02 d | 0.36 ± 0.03 b |
| 30 mM KCl | Shoot | 32.30 ± 0.19 d | 3.83 ± 0.20 b | 11.86 a | 27.25 ± 0.33 d | 21.42 ± 0.39 d | 2.51 ± 0.01 bc | 0.92 ± 0.01 b | 2.73 cd | 3.43 ± 0.02 cd | 0.35 ± 0.03 ab |
| 50 mM NaCl | Shoot | 33.15 ± 0.22 d | 4.52 ± 0.07 d | 13.64 c | 30.915 ± 0.41 e | 20.42 ± 0.39 c | 2.96 ± 0.01 c | 1.03 ± 0.01 c | 2.87 d | 3.99 ± 0.02 e | 0.46 ± 0.03 bc |
| 50 mM KCl | Shoot | 33.35 ± 0.21 d | 4.20 ± 0.06 c | 12.59 b | 27.67 ± 0.33 d | 21.17 ± 0.33 d | 2.89 ± 0.01 c | 0.96 ± 0.02 c | 3.01 d | 3.86 ± 0.02 de | 0.42 ± 0.05 b |
| 100 mM NaCl | Shoot | 31.28 ± 0.30 cd | 4.28 ± 0.08 c | 13.68 c | 29.25 ± 0.33 e | 18.75 ± 0.33 b | 2.00 ± 0.01 b | 0.98 ± 0.01 c | 2.04 b | 2.98 ± 0.02 bc | 0.47 ± 0.03 bc |
| 100 mM KCl | Shoot | 31.83 ± 0.35 cd | 4.33 ± 0.11 c | 13.60 c | 25.92 ± 0.33 c | 20.09 ± 0.33 c | 2.26 ± 0.02 bc | 0.96 ± 0.01 c | 2.35 bc | 3.21 ± 0.02 c | 0.52 ± 0.03 c |
| 150 mM NaCl | Shoot | 30.27 ± 0.23 c | 3.90 ± 0.10 bc | 12.88 b | 27.50 ± 0.33 d | 20.84 ± 0.33 d | 1.39 ± 0.02 b | 0.94 ± 0.01 b | 1.48 a | 2.41 ± 0.02 ab | 0.45 ± 0.03 bc |
| 150 mM KCl | Shoot | 30.37 ± 0.27 c | 3.76 ± 0.09 b | 12.38 b | 24.09 ± 0.41 b | 17.42 ± 0.25 ab | 1.95 ± 0.01 b | 0.84 ± 0.01 a | 2.32 bc | 2.80 ± 0.01 b | 0.51 ± 0.04 c |
| 200 mM NaCl | Shoot | 24.99 ± 0.34 a | 3.48 ± 0.07 a | 13.93 c | 23.75 ± 0.33 b | 19.75 ± 0.33 b | 1.21 ± 0.02 a | 0.90 ± 0.02 b | 1.34 a | 2.11 ± 0.02 a | 0.44 ± 0.03 b |
| 200 mM KCl | Shoot | 26.34 ± 0.36 b | 3.43 ± 0.09 a | 13.02 b | 21.92 ± 0.39 a | 16.33 ± 0.33 a | 1.76 ± 0.01 b | 0.75 ± 0.02 a | 2.35 bc | 2.51 ± 0.02 ab | 0.50 ± 0.05 c |
| 50 mM NaCl + 50 mM KCl | Shoot | 33.20 ± 0.37 c | 4.38 ± 0.06 b | 13.19 ab | 28.42 ± 0.39 bc | 21.83 ± 0.39 ab | 2.87 ± 0.02 c | 0.88 ± 0.09 b | 3.26 c | 3.75 ± 0.11 c | 0.55 ± 0.02 b |
| 100 mM NaCl + 100 mM KCl | Shoot | 30.77 ± 0.22 bc | 3.81 ± 0.13 a | 12.38 a | 23.49 ± 0.25 a | 19.17 ± 0.33 a | 2.32 ± 0.01 b | 0.90 ± 0.02 b | 2.58 b | 3.22 ± 0.03 b | 0.59 ± 0.02 b |
| 150 mM NaCl + 0.5 mM GB | Shoot | 30.72 ± 0.41 bc | 4.88 ± 0.12 bc | 15.89 b | 30.58 ± 0.45 c | 24.17 ± 0.39 bc | 1.49 ± 0.02 a | 0.97 ± 0.01 bc | 1.54 a | 2.47 ± 0.02 a | 0.55 ± 0.02 b |
| 200 mM NaCl + 0.5 mM GB | Shoot | 25.78 ± 0.45 a | 4.45 ± 0.26 b | 17.26 c | 28.75 ± 0.33 bc | 22.67 ± 0.45 b | 1.38 ± 0.01 a | 0.95 ± 0.01 bc | 1.45 a | 2.33 ± 0.02 a | 0.57 ± 0.02 b |
| 150 mM KCl + 0.5 mM GB | Shoot | 31.13 ± 0.61 c | 4.72 ± 0.30 bc | 15.16 b | 27.67 ± 0.45 b | 23.00 ± 0.39 b | 2.17 ± 0.01 b | 0.89 ± 0.01 b | 2.44 b | 3.06 ± 0.01 b | 0.65 ± 0.02 bc |
| 200 mM KCl + 0.5 mM GB | Shoot | 26.96 ± 0.68 ab | 4.38 ± 0.15 b | 16.25 bc | 26.25 ± 0.45 b | 22.34 ± 0.41 b | 1.96 ± 0.01 b | 0.81 ± 0.01 a | 2.42 b | 2.77 ± 0.01 ab | 0.64 ± 0.02 bc |
| 50 mM NaCl + 50 mM KCl + 0.5 mM GB | Shoot | 28.69 ± 0.55 b | 5.32 ± 0.23 c | 18.54 c | 32.75 ± 0.45 c | 25.42 ± 0.51 c | 3.37 ± 0.01 d | 1.04 ± 0.01 c | 3.24 c | 4.41 ± 0.02 d | 0.73 ± 0.02 c |
| 100 mM NaCl + 100 mM KCl + 0.5 mM GB | Shoot | 31.27 ± 0.76 c | 4.73 ± 0.17 bc | 15.13 b | 28.92 ± 0.45 c | 23.92 ± 0.53 bc | 2.72 ± 0.01 c | 1.03 ± 0.01 c | 2.64 b | 3.77 ± 0.01 c | 0.74 ± 0.02 c |
| Salt Treatments | Section | Fresh Weight (g) | Dry Weight (g) | Dw/Fw (%) |
|---|---|---|---|---|
| Control | Root | 4.17 ± 0.26 c | 0.58 ± 0.07 c | 13.91 c |
| 30 mM NaCl | Root | 4.33 ± 0.11 c | 0.65 ± 0.06 d | 15.01 d |
| 30 mM KCl | Root | 4.41 ± 0.06 c | 0.59 ± 0.06 c | 13.38 c |
| 50 mM NaCl | Root | 3.96 ± 0.12 bc | 0.48 ± 0.03 bc | 12.12 c |
| 50 mM KCl | Root | 4.08 ± 0.09 c | 0.53 ± 0.06 c | 12.99 c |
| 100 mM NaCl | Root | 3.63 ± 0.09 b | 0.26 ± 0.03 b | 7.16 b |
| 100 mM KCl | Root | 3.64 ± 0.05 b | 0.30 ± 0.06 b | 8.24 b |
| 150 mM NaCl | Root | 3.32 ± 0.10 b | 0.10 ± 0.01 a | 3.01 a |
| 150 mM KCl | Root | 3.34 ± 0.09 b | 0.13 ± 0.02 ab | 3.89 a |
| 200 mM NaCl | Root | 2.90 ± 0.05 a | 0.10 ± 0.02 a | 3.45 a |
| 200 mM KCl | Root | 2.91 ± 0.07 a | 0.04 ± 0.01 a | 1.38 a |
| 50 mM NaCl + 50 mM KCl | Root | 3.88 ± 0.09 b | 0.25 ± 0.04 b | 6.44 c |
| 100 mM NaCl + 100 mM KCl | Root | 3.59 ± 0.09 b | 0.54 ± 0.09 cd | 15.04 de |
| 150 mM NaCl + 0.5 mM GB | Root | 3.76 ± 0.13 b | 0.17 ± 0.02 ab | 4.52 b |
| 200 mM NaCl + 0.5 mM GB | Root | 3.21 ± 0.15 a | 0.15 ± 0.02 ab | 4.67 b |
| 150 mM KCl + 0.5 mM GB | Root | 4.18 ± 0.14 c | 0.24 ± 0.03 b | 5.74 bc |
| 200 mM KCl + 0.5 mM GB | Root | 3.61 ± 0.14 b | 0.09 ± 0.02 a | 2.49 a |
| 50 mM NaCl + 50 mM KCl + 0.5 mM GB | Root | 4.74 ± 0.23 d | 0.40 ± 0.02 c | 8.44 c |
| 100 mM NaCl + 100 mM KCl + 0.5 mM GB | Root | 4.53 ± 0.28 d | 0.77 ± 0.03 d | 17.00 e |
| Wheats | N | Soluble Protein (µg mL−1 Protein) | SOD (U mL−1 Protein) | CAT (U mL−1 Protein) | GR (U mL−1 Protein) | GST (U mL−1 Protein) | APX (U mL−1 Protein) | PRO (nmol g−1 fw) | MDA (nmol g−1 fw) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 12 | 383.25 a | 0.89 b | 0.01 a | 0.09 a | 0.10 a | 0.29 a | 66.31 a | 18.35 a |
| T. monococcum | 114 | 508.26 c | 0.873 b | 0.021 b | 0.106 b | 0.097 a | 0.320 b | 332.55 b | 166.19 b |
| T. dicoccum | 114 | 513.75 c | 0.847 a | 0.020 b | 0.104 b | 0.116 b | 0.299 a | 362.71 bc | 157.84 b |
| T. speltoides | 114 | 480.14 b | 1.015 d | 0.037 c | 0.194 c | 0.140 c | 0.348 c | 400.55 c | 194.34 c |
| T. boeoticum | 114 | 482.62 b | 0.971 c | 0.037 c | 0.177 c | 0.121 b | 0.333 b | 385.48 bc | 187.09 c |
| Wheats | FW (g) | DW (g) | PH (cm) | LL (cm) | Chl a (mg g−1 fw) | Chl b (mg g−1 fw) | Chl a/b | Total Chl (mg g−1 fw) | Carotene (mg g−1 fw) |
| Control | 16.72 b | 2.13 b | 25.17 b | 19.75 a | 2.18 a | 0.89 a | 2.45 bc | 3.08 c | 0.29 a |
| T. monococcum | 19.66 c | 2.49 c | 30.44 c | 20.96 b | 2.22 ab | 0.90 a | 2.47 c | 3.12 c | 0.42 c |
| T. dicoccum | 19.98 c | 2.67 d | 32.94 d | 23.28 c | 2.18 a | 0.90 a | 2.42 b | 3.10 c | 0.38 b |
| T. speltoides | 14.16 a | 1.90 a | 22.12 a | 19.34 a | 2.24 ab | 0.94 b | 2.38 ab | 3.18 b | 0.62 d |
| T. boeoticum | 14.72 a | 2.07 b | 23.94 a | 20.26 b | 2.26 b | 0.96 b | 2.35 a | 3.24 a | 0.66 e |
| Salt Treatments | Section | Soluble Protein (µg mL−1 Protein) | SOD (U mL−1 Protein) | CAT (U mL−1 Protein) | GR (U mL−1 Protein) | GST (U mL−1 Protein) | APX (U mL−1 Protein) | PRO (nmol g−1 fw) | MDA (nmol g−1 fw) |
|---|---|---|---|---|---|---|---|---|---|
| Control | Shoot | 450.17 ± 32.23 d | 0.808 ± 0.06 ab | 0.014 ± 0.01 a | 0.092 ± 0.02 a | 0.095 ± 0.02 a | 0.300 ± 0.02 b | 63.63 ± 10.43 a | 17.31 ± 1.14 a |
| 30 mM NaCl | Shoot | 476.17 ± 38.04 de | 0.931 ± 0.10 d | 0.050 ± 0.09 e | 0.122 ± 0.03 b | 0.115 ± 0.01 b | 0.333 ± 0.02 d | 259.58 ± 30.37 c | 34.25 ± 5.21 a |
| 30 mM KCl | Shoot | 467.17 ± 18.10 d | 0.795 ± 0.02 a | 0.016 ± 0.01 ab | 0.110 ± 0.03 ab | 0.093 ± 0.01 a | 0.304 ± 0.02 bc | 251.33 ± 22.55 c | 32.92 ± 1.78 a |
| 50 mM NaCl | Shoot | 502.08 ± 32.38 e | 0.850 ± 0.02 b | 0.029 ± 0.01 bc | 0.190 ± 0.17 cd | 0.150 ± 0.02 c | 0.350 ± 0.02 e | 311.25 ± 41.93 d | 40.25 ± 6.18 a |
| 50 mM KCl | Shoot | 521.92 ± 14.03 e | 0.802 ± 0.22 ab | 0.020 ± 0.01 b | 0.133 ± 0.04 b | 0.106 ± 0.01 ab | 0.325 ± 0.02 cd | 388.50 ± 22.04 e | 45.83 ± 8.87 a |
| 100 mM NaCl | Shoot | 522.08 ± 27.56 e | 0.835 ± 0.05 b | 0.026 ± 0.01 bc | 0.145 ± 0.05 bc | 0.128 ± 0.02 bc | 0.327 ± 0.02 cd | 385.75 ± 33.30 e | 158.00 ± 25.17 bc |
| 100 mM KCl | Shoot | 509.00 ± 20.04 e | 0.904 ± 0.11 cd | 0.018 ± 0.01 ab | 0.119 ± 0.03 b | 0.097 ± 0.01 ab | 0.322 ± 0.02 cd | 486.58 ± 22.94 f | 152.08 ± 18.80 b |
| 150 mM NaCl | Shoot | 526.33 ± 28.24 e | 0.832 ± 0.05 b | 0.020 ± 0.01 b | 0.119 ± 0.03 b | 0.112 ± 0.01 b | 0.302 ± 0.03 bc | 279.75 ± 32.43 cd | 242.50 ± 82.30 c |
| 150 mM KCl | Shoot | 473.08 ± 17.46 d | 0.874 ± 0.10 bc | 0.020 ± 0.02 b | 0.104 ± 0.02 ab | 0.089 ± 0.01 a | 0.307 ± 0.01 bc | 379.17 ± 23.05 de | 355.75 ± 27.34 d |
| 200 mM NaCl | Shoot | 461.00 ± 27.28 d | 0.813 ± 0.06 ab | 0.017 ± 0.01 ab | 0.094 ± 0.02 a | 0.080 ± 0.01 a | 0.279 ± 0.02 a | 117.75 ± 29.58 ab | 394.67 ± 23.06 e |
| 200 mM KCl | Shoot | 404.00 ± 25.08 c | 0.758 ± 0.06 a | 0.012 ± 0.01 a | 0.084 ± 0.01 a | 0.082 ± 0.01 a | 0.277 ± 0.02 a | 117.67 ± 8.27 ab | 369.08 ± 22.36 de |
| 50 mM NaCl + 50 mM KCl | Shoot | 563.42 ± 15.92 f | 0.995 ± 0.11 ef | 0.021 ± 0.01 b | 0.155 ± 0.04 bc | 0.118 ± 0.01 b | 0.347 ± 0.02 de | 606.33 ± 32.26 gh | 47.75 ± 9.03 a |
| 100 mM NaCl + 100 mM KCl | Shoot | 488.83 ± 22.67 de | 0.813 ± 0.10 ab | 0.014 ± 0.01 a | 0.089 ± 0.02 a | 0.087 ± 0.01 a | 0.304 ± 0.03 bc | 451.67 ± 49.49 ef | 157.67 ± 17.31 bc |
| 150 mM NaCl + 0.5 mM GB | Shoot | 553.83 ± 21.20 f | 0.888 ± 0.06 c | 0.028 ± 0.01 bc | 0.153 ± 0.03 bc | 0.128 ± 0.01 bc | 0.326 ± 0.02 cd | 417.83 ± 34.86 e | 250.08 ± 13.07 c |
| 200 mM NaCl + 0.5 mM GB | Shoot | 538.25 ± 15.20 ef | 0.887 ± 0.05 c | 0.023 ± 0.01 b | 0.113 ± 0.03 ab | 0.109 ± 0.01 ab | 0.291 ± 0.02 b | 247.50 ± 26.57 c | 325.42 ± 18.01 d |
| 150 mM KCl + 0.5 mM GB | Shoot | 596.17 ± 6.71 fg | 0.934 ± 0.10 d | 0.024 ± 0.01 b | 0.143 ± 0.02 bc | 0.136 ± 0.03 bc | 0.320 ± 0.03 cd | 437.83 ± 22.51 e | 256.33 ± 11.58 cd |
| 200 mM KCl + 0.5 mM GB | Shoot | 608.42 ± 23.65 fg | 0.822 ± 0.07 b | 0.019 ± 0.01 ab | 0.105 ± 0.02 ab | 0.125 ± 0.02 bc | 0.296 ± 0.02 b | 210.33 ± 7.89 c | 285.00 ± 13.08 cd |
| 50 mM NaCl + 50 mM KCl + 0.5 mM GB | Shoot | 661.33 ± 23.03 g | 1.058 ± 0.10 f | 0.029 ± 0.01 bc | 0.213 ± 0.06 d | 0.149 ± 0.02 c | 0.416 ± 0.03 g | 741.92 ± 45.99 h | 27.83 ± 1.75 a |
| 100 mM NaCl + 100 mM KCl + 0.5 mM GB | Shoot | 604.58 ± 13.10 fg | 0.881 ± 0.09 c | 0.023 ± 0.01 b | 0.116 ± 0.02 ab | 0.138 ± 0.01 bc | 0.339 ± 0.02 d | 569.25 ± 41.12 g | 97.33 ± 7.97 ab |
| Salt Treatments | Section | Soluble Protein (µg mL−1 Protein) | SOD (U mL−1 Protein) | CAT (U mL−1 Protein) | GR (U mL−1 Protein) | GST (U mL−1 Protein) | APX (U mL−1 Protein) | PRO (nmol g−1 fw) | MDA (nmol g−1 fw) |
|---|---|---|---|---|---|---|---|---|---|
| Control | Root | 316.33 ± 27.04 a | 0.972 ± 0.11 e | 0.016 ± 0.01 ab | 0.089 ± 0.03 a | 0.106 ± 0.03 ab | 0.279 ± 0.03 a | 68.99 ± 11.70 a | 19.38 ± 2.16 a |
| 30 mM NaCl | Root | 372.33 ± 36.96 b | 1.047 ± 0.08 f | 0.040 ± 0.01 d | 0.155 ± 0.05 bc | 0.116 ± 0.02 b | 0.328 ± 0.02 cd | 275.75 ± 68.69 cd | 45.67 ± 7.55 a |
| 30 mM KCl | Root | 442.75 ± 25.24 cd | 0.903 ± 0.03 cd | 0.031 ± 0.01 c | 0.141 ± 0.05 bc | 0.108 ± 0.01 ab | 0.318 ± 0.02 c | 261.58 ± 27.28 cd | 40.42 ± 3.82 a |
| 50 mM NaCl | Root | 392.45 ± 24.45 bc | 1.013 ± 0.07 ef | 0.046 ± 0.01 de | 0.207 ± 0.07 d | 0.142 ± 0.02 c | 0.361 ± 0.02 e | 334.64 ± 60.98 d | 62.27 ± 9.87 ab |
| 50 mM KCl | Root | 491.33 ± 18.40 de | 1.012 ± 0.11 ef | 0.038 ± 0.02 cd | 0.185 ± 0.06 cd | 0.127 ± 0.02 bc | 0.336 ± 0.03 d | 418.00 ± 47.61 e | 58.75 ± 8.48 ab |
| 100 mM NaCl | Root | 391.75 ± 31.26 bc | 1.016 ± 0.09 ef | 0.040 ± 0.01 d | 0.221 ± 0.09 d | 0.133 ± 0.02 bc | 0.323 ± 0.01 cd | 442.25 ± 48.18 ef | 179.50 ± 22.73 bc |
| 100 mM KCl | Root | 469.00 ± 10.73 d | 1.028 ± 0.13 f | 0.031 ± 0.02 c | 0.170 ± 0.06 c | 0.120 ± 0.02 b | 0.312 ± 0.03 c | 511.42 ± 44.04 f | 173.50 ± 20.72 bc |
| 150 mM NaCl | Root | 373.75 ± 40.37 b | 0.941 ± 0.06 d | 0.032 ± 0.01 c | 0.174 ± 0.07 c | 0.181 ± 0.09 d | 0.299 ± 0.01 b | 257.00 ± 17.68 c | 283.00 ± 31.27 cd |
| 150 mM KCl | Root | 450.25 ± 12.14 d | 0.965 ± 0.09 de | 0.027 ± 0.01 bc | 0.148 ± 0.04 bc | 0.110 ± 0.02 b | 0.306 ± 0.02 bc | 372.83 ± 20.86 de | 292.00 ± 42.23 cd |
| 200 mM NaCl | Root | 328.75 ± 21.76 a | 0.855 ± 0.05 bc | 0.023 ± 0.01 b | 0.103 ± 0.03 ab | 0.086 ± 0.01 a | 0.271 ± 0.01 a | 144.92 ± 13.40 b | 379.17 ± 23.74 de |
| 200 mM KCl | Root | 410.17 ± 19.66 c | 0.887 ± 0.07 c | 0.025 ± 0.01 bc | 0.109 ± 0.04 ab | 0.097 ± 0.01 ab | 0.276 ± 0.03 a | 141.08 ± 8.21 b | 385.08 ± 26.96 de |
| 50 mM NaCl + 50 mM KCl | Root | 530.17 ± 31.52 ef | 1.179 ± 0.15 g | 0.036 ± 0.01 cd | 0.210 ± 0.07 d | 0.144 ± 0.02 c | 0.326 ± 0.01 cd | 615.67 ± 32.00 gh | 54.58 ± 6.61 ab |
| 100 mM NaCl + 100 mM KCl | Root | 489.67 ± 14.09 de | 0.892 ± 0.07 c | 0.027 ± 0.01 bc | 0.139 ± 0.06 b | 0.094 ± 0.01 a | 0.295 ± 0.03 b | 592.33 ± 27.29 g | 172.17 ± 22.04 bc |
| 150 mM NaCl + 0.5 mM GB | Root | 534.08 ± 22.44 ef | 0.983 ± 0.06 e | 0.039 ± 0.01 cd | 0.193 ± 0.08 cd | 0.115 ± 0.01 b | 0.341 ± 0.02 d | 411.17 ± 28.00 e | 259.00 ± 25.19 cd |
| 200 mM NaCl + 0.5 mM GB | Root | 506.92 ± 12.43 e | 0.904 ± 0.04 cd | 0.061 ± 0.11 f | 0.123 ± 0.03 b | 0.107 ± 0.01 ab | 0.317 ± 0.03 c | 266.33 ± 26.77 c | 317.00 ± 14.35 d |
| 150 mM KCl + 0.5 mM GB | Root | 546.00 ± 12.27 ef | 1.013 ± 0.09 ef | 0.034 ± 0.02 c | 0.187 ± 0.04 cd | 0.141 ± 0.02 c | 0.431 ± 0.05 h | 463.42 ± 31.60 ef | 236.08 ± 21.38 c |
| 200 mM KCl + 0.5 mM GB | Root | 609.17 ± 14.84 fg | 0.964 ± 0.08 de | 0.035 ± 0.01 c | 0.139 ± 0.05 b | 0.121 ± 0.01 b | 0.382 ± 0.01 ef | 245.92 ± 18.69 c | 299.92 ± 28.60 d |
| 50 mM NaCl + 50 mM KCl + 0.5 mM GB | Root | 667.92 ± 15.38 g | 1.252 ± 0.16 h | 0.046 ± 0.01 de | 0.260 ± 0.07 e | 0.179 ± 0.02 d | 0.416 ± 0.02 g | 748.42 ± 54.23 h | 40.58 ± 1.83 a |
| 100 mM NaCl + 100 mM KCl + 0.5 mM GB | Root | 597.25 ± 10.42 fg | 0.897 ± 0.17 c | 0.041 ± 0.01 d | 0.160 ± 0.07 c | 0.128 ± 0.01 bc | 0.373 ± 0.01 e | 772.67 ± 42.89 h | 103.33 ± 7.88 b |
| Wheats | SOD | CAT | GR | GST | APX | PRO | MDA | Carotene | Fw | Dw | PH | LL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T. monococcum | 19.3 b | 112.90 a | 76.20 a | 54.45 b | 24.80 ab | 1099.85 b | 1751.75 a | 51.85 a | 14.74 b | 22.71 c | 21.35 c | 1.52 a |
| T. dicoccum | 0.36 a | 125.68 a | 85.56 a | 115.6 c | 33.96 b | 1079.46 b | 1863.06 b | 82.68 b | 3.19 a | 20.51 c | 9.10 b | 7.95 ab |
| T. speltoides | 4.34 a | 228.59 b | 126.88 b | 12.50 a | 19.70 a | 842.15 a | 1830.44 ab | 93.77 bc | 1.16 a | 4.79 a | 3.91 a | 5.76 a |
| T. boeoticum | 14.02 b | 231.37 b | 195.58 c | 0.97 a | 29.81 ab | 918.78 a | 1885.45 b | 111.28 c | −3.45 a | 12.75 b | 1.25 a | 11.11 b |
| Dependent Variable | (I) Wheat | (J) Wheat | Mean Difference (I − J) | Std. Error | p | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| Soluble Protein Cons. | LSD | T. monococcum | T. dicoccum | −5.4912 * | 1.24804 | 0.000 | −7.9471 | −3.0353 |
| T. speltoides | 28.1228 * | 1.24804 | 0.000 | 25.6669 | 30.5787 | |||
| T. boeoticum | 26.4211 * | 1.24804 | 0.000 | 23.9652 | 28.8769 | |||
| T. dicoccum | T. monococcum | 5.4912 * | 1.24804 | 0.000 | 3.0353 | 7.9471 | ||
| T. speltoides | 33.6140 * | 1.24804 | 0.000 | 31.1582 | 36.0699 | |||
| T. boeoticum | 31.9123 * | 1.24804 | 0.000 | 29.4564 | 34.3682 | |||
| T. speltoides | T. monococcum | −28.1228 * | 1.24804 | 0.000 | −30.5787 | −25.6669 | ||
| T. dicoccum | −33.6140 * | 1.24804 | 0.000 | −36.0699 | −31.1582 | |||
| T. boeoticum | −1.7018 | 1.24804 | 0.174 | −4.1576 | 0.7541 | |||
| T. boeoticum | T. monococcum | −26.4211 * | 1.24804 | 0.000 | −28.8769 | −23.9652 | ||
| T. dicoccum | −31.9123 * | 1.24804 | 0.000 | −34.3682 | −29.4564 | |||
| T. speltoides | 1.7018 | 1.24804 | 0.174 | −0.7541 | 4.1576 | |||
| SOD | LSD | T. monococcum | T. dicoccum | 0.0252 * | 0.00798 | 0.002 | 0.0095 | 0.0410 |
| T. speltoides | −0.1425 * | 0.00798 | 0.000 | −0.1582 | −0.1268 | |||
| T. boeoticum | −0.0981 * | 0.00798 | 0.000 | −0.1138 | −0.0824 | |||
| T. dicoccum | T. monococcum | −0.0252 * | 0.00798 | 0.002 | −0.0410 | −0.0095 | ||
| T. speltoides | −0.1677 * | 0.00798 | 0.000 | −0.1835 | −0.1520 | |||
| T. boeoticum | −0.1233 * | 0.00798 | 0.000 | −0.1390 | −0.1076 | |||
| T. speltoides | T. monococcum | 0.1425 * | 0.00798 | 0.000 | 0.1268 | 0.1582 | ||
| T. dicoccum | 0.1677 * | 0.00798 | 0.000 | 0.1520 | 0.1835 | |||
| T. boeoticum | 0.0444 * | 0.00798 | 0.000 | 0.0287 | 0.0601 | |||
| T. boeoticum | T. monococcum | 0.0981 * | 0.00798 | 0.000 | 0.0824 | 0.1138 | ||
| T. dicoccum | 0.1233 * | 0.00798 | 0.000 | 0.1076 | 0.1390 | |||
| T. speltoides | −0.0444 * | 0.00798 | 0.000 | −0.0601 | −0.0287 | |||
| CAT | LSD | T. monococcum | T. dicoccum | 0.0001 | 0.00288 | 0.971 | −0.0056 | 0.0058 |
| T. speltoides | −0.0169 * | 0.00288 | 0.000 | −0.0225 | −0.0112 | |||
| T. boeoticum | −0.0165 * | 0.00288 | 0.000 | −0.0221 | −0.0108 | |||
| T. dicoccum | T. monococcum | −0.0001 | 0.00288 | 0.971 | −0.0058 | 0.0056 | ||
| T. speltoides | −0.0170 * | 0.00288 | 0.000 | −0.0226 | −0.0113 | |||
| T. boeoticum | −0.0166 * | 0.00288 | 0.000 | −0.0222 | −0.0109 | |||
| T. speltoides | T. monococcum | 0.0169 * | 0.00288 | 0.000 | 0.0112 | 0.0225 | ||
| T. dicoccum | 0.0170 * | 0.00288 | 0.000 | 0.0113 | 0.0226 | |||
| T. boeoticum | 0.0004 | 0.00288 | 0.886 | −0.0053 | 0.0061 | |||
| T. boeoticum | T. monococcum | 0.0165 * | 0.00288 | 0.000 | 0.0108 | 0.0221 | ||
| T. dicoccum | 0.0166 * | 0.00288 | 0.000 | 0.0109 | 0.0222 | |||
| T. speltoides | −0.0004 | 0.00288 | 0.886 | −0.0061 | 0.0053 | |||
| GR | LSD | T. monococcum | T. dicoccum | 0.0014 | 0.00336 | 0.679 | −0.0052 | 0.0080 |
| T. speltoides | −0.0877 * | 0.00336 | 0.000 | −0.0943 | −0.0811 | |||
| T. boeoticum | −0.0715 * | 0.00336 | 0.000 | −0.0781 | −0.0649 | |||
| T. dicoccum | T. monococcum | −0.0014 | 0.00336 | 0.679 | −0.0080 | 0.0052 | ||
| T. speltoides | −0.0891 * | 0.00336 | 0.000 | −0.0957 | −0.0825 | |||
| T. boeoticum | −0.0729 * | 0.00336 | 0.000 | −0.0795 | −0.0663 | |||
| T. speltoides | T. monococcum | 0.0877 * | 0.00336 | 0.000 | 0.0811 | 0.0943 | ||
| T. dicoccum | 0.0891 * | 0.00336 | 0.000 | 0.0825 | 0.0957 | |||
| T. boeoticum | 0.0162 * | 0.00336 | 0.000 | 0.0096 | 0.0228 | |||
| T. boeoticum | T. monococcum | 0.0715 * | 0.00336 | 0.000 | 0.0649 | 0.0781 | ||
| T. dicoccum | 0.0729 * | 0.00336 | 0.000 | 0.0663 | 0.0795 | |||
| T. speltoides | −0.0162 * | 0.00336 | 0.000 | −0.0228 | −0.0096 | |||
| GST | LSD | T. monococcum | T. dicoccum | −0.0189 * | 0.00623 | 0.003 | −0.0312 | −0.0067 |
| T. speltoides | −0.0432 * | 0.00623 | 0.000 | −0.0554 | −0.0309 | |||
| T. boeoticum | −0.0242 * | 0.00623 | 0.000 | −0.0364 | −0.0119 | |||
| T. dicoccum | T. monococcum | 0.0189 * | 0.00623 | 0.003 | 0.0067 | 0.0312 | ||
| T. speltoides | −0.0242 * | 0.00623 | 0.000 | −0.0365 | −0.0120 | |||
| T. boeoticum | −0.0052 | 0.00623 | 0.404 | −0.0175 | 0.0071 | |||
| T. speltoides | T. monococcum | 0.0432 * | 0.00623 | 0.000 | 0.0309 | 0.0554 | ||
| T. dicoccum | 0.0242 * | 0.00623 | 0.000 | 0.0120 | 0.0365 | |||
| T. boeoticum | 0.0190 * | 0.00623 | 0.002 | 0.0067 | 0.0313 | |||
| T. boeoticum | T. monococcum | 0.0242 * | 0.00623 | 0.000 | 0.0119 | 0.0364 | ||
| T. dicoccum | 0.0052 | 0.00623 | 0.404 | −0.0071 | 0.0175 | |||
| T. speltoides | −0.0190 * | 0.00623 | 0.002 | −0.0313 | −0.0067 | |||
| APX | LSD | T. monococcum | T. dicoccum | 0.0207 * | 0.00121 | 0.000 | 0.0183 | 0.0231 |
| T. speltoides | −0.0272 * | 0.00121 | 0.000 | −0.0296 | −0.0248 | |||
| T. boeoticum | −0.0127 * | 0.00121 | 0.000 | −0.0151 | −0.0104 | |||
| T. dicoccum | T. monococcum | −0.0207 * | 0.00121 | 0.000 | −0.0231 | −0.0183 | ||
| T. speltoides | −0.0479 * | 0.00121 | 0.000 | −0.0503 | −0.0455 | |||
| T. boeoticum | −0.0334 * | 0.00121 | 0.000 | −0.0358 | −0.0311 | |||
| T. speltoides | T. monococcum | 0.0272 * | 0.00121 | 0.000 | 0.0248 | 0.0296 | ||
| T. dicoccum | 0.0479 * | 0.00121 | 0.000 | 0.0455 | 0.0503 | |||
| T. boeoticum | 0.0145 * | 0.00121 | 0.000 | 0.0121 | 0.0169 | |||
| T. boeoticum | T. monococcum | 0.0127 * | 0.00121 | 0.000 | 0.0104 | 0.0151 | ||
| T. dicoccum | 0.0334 * | 0.00121 | 0.000 | 0.0311 | 0.0358 | |||
| T. speltoides | −0.0145 * | 0.00121 | 0.000 | −0.0169 | −0.0121 | |||
| Proline | LSD | T. monococcum | T. dicoccum | −30.1632 * | 1.32946 | 0.000 | −32.7793 | −27.5470 |
| T. speltoides | −68.0053 * | 1.32946 | 0.000 | −70.6214 | −65.3892 | |||
| T. boeoticum | −52.9614 * | 1.32946 | 0.000 | −55.5775 | −50.3453 | |||
| T. dicoccum | T. monococcum | 30.1632 * | 1.32946 | 0.000 | 27.5470 | 32.7793 | ||
| T. speltoides | −37.8421 * | 1.32946 | 0.000 | −40.4582 | −35.2260 | |||
| T. boeoticum | −22.7982 * | 1.32946 | 0.000 | −25.4144 | −20.1821 | |||
| T. speltoides | T. monococcum | 68.0053 * | 1.32946 | 0.000 | 65.3892 | 70.6214 | ||
| T. dicoccum | 37.8421 * | 1.32946 | 0.000 | 35.2260 | 40.4582 | |||
| T. boeoticum | 15.0439 * | 1.32946 | 0.000 | 12.4277 | 17.6600 | |||
| T. boeoticum | T. monococcum | 52.9614 * | 1.32946 | 0.000 | 50.3453 | 55.5775 | ||
| T. dicoccum | 22.7982 * | 1.32946 | 0.000 | 20.1821 | 25.4144 | |||
| T. speltoides | −15.0439 * | 1.32946 | 0.000 | −17.6600 | −12.4277 | |||
| MDA | LSD | T. monococcum | T. dicoccum | 8.3553 * | 1.27725 | 0.000 | 5.8419 | 10.8686 |
| T. speltoides | −28.1500 * | 1.27725 | 0.000 | −30.6634 | −25.6366 | |||
| T. boeoticum | −19.8781 * | 1.27725 | 0.000 | −22.3914 | −17.3647 | |||
| T. dicoccum | T. monococcum | −8.3553 * | 1.27725 | 0.000 | −10.8686 | −5.8419 | ||
| T. speltoides | −36.5053 * | 1.27725 | 0.000 | −39.0186 | −33.9919 | |||
| T. boeoticum | −28.2333 * | 1.27725 | 0.000 | −30.7467 | −25.7200 | |||
| T. speltoides | T. monococcum | 28.1500 * | 1.27725 | 0.000 | 25.6366 | 30.6634 | ||
| T. dicoccum | 36.5053 * | 1.27725 | 0.000 | 33.9919 | 39.0186 | |||
| T. boeoticum | 8.2719 * | 1.27725 | 0.000 | 5.7586 | 10.7853 | |||
| T. boeoticum | T. monococcum | 19.8781 * | 1.27725 | 0.000 | 17.3647 | 22.3914 | ||
| T. dicoccum | 28.2333 * | 1.27725 | 0.000 | 25.7200 | 30.7467 | |||
| T. speltoides | −8.2719 * | 1.27725 | 0.000 | −10.7853 | −5.7586 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temizgul, R. Soil Salinization and Ancient Hulled Wheat: A Study on Antioxidant Defense Mechanisms. Plants 2025, 14, 678. https://doi.org/10.3390/plants14050678
Temizgul R. Soil Salinization and Ancient Hulled Wheat: A Study on Antioxidant Defense Mechanisms. Plants. 2025; 14(5):678. https://doi.org/10.3390/plants14050678
Chicago/Turabian StyleTemizgul, Ridvan. 2025. "Soil Salinization and Ancient Hulled Wheat: A Study on Antioxidant Defense Mechanisms" Plants 14, no. 5: 678. https://doi.org/10.3390/plants14050678
APA StyleTemizgul, R. (2025). Soil Salinization and Ancient Hulled Wheat: A Study on Antioxidant Defense Mechanisms. Plants, 14(5), 678. https://doi.org/10.3390/plants14050678







