Exploring the Biochemical Profile of Beta vulgaris L.: A Comparative Study of Beetroots and Swiss Chard
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Extract Preparation
2.3. Proximal Composition
2.4. Chemical Profile of Hydrophilic Compounds
2.4.1. Free Sugars
2.4.2. Organic Acids
2.5. Chemical Profile of Lipophilic Compounds
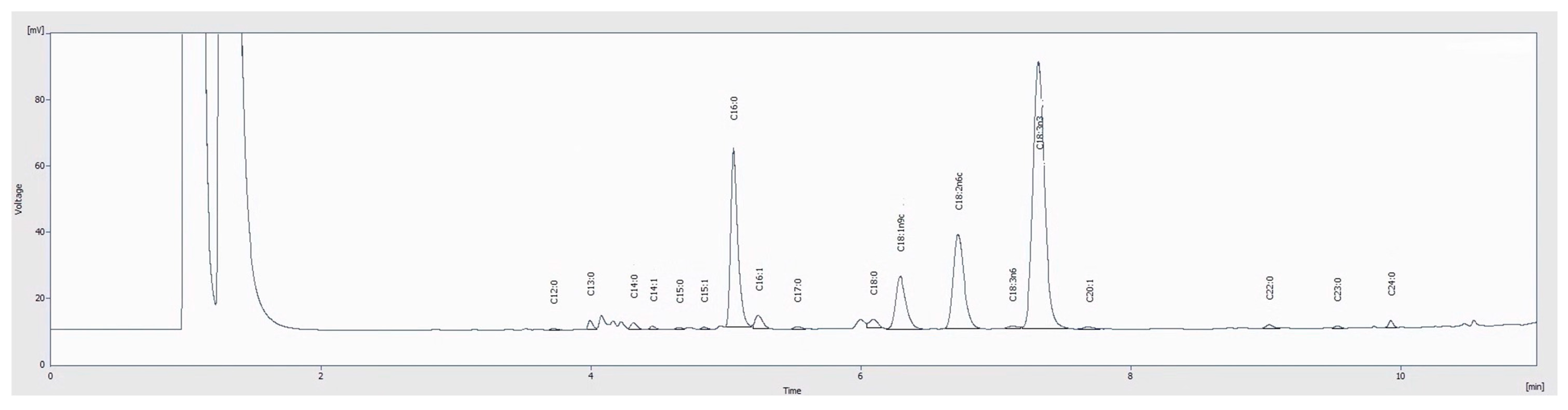
2.5.1. Fatty Acids
2.5.2. Tocopherols
2.6. Betalains
2.7. Phenolic Compounds
2.8. Bioactive Properties
2.8.1. Antioxidant Activity
2.8.2. Antimicrobial Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Proximal Composition
3.2. Chemical Composition Regarding Hydrophilic Compounds
3.2.1. Free Sugars
3.2.2. Organic Acids
3.3. Chemical Composition of Lipophilic Compounds
3.3.1. Fatty Acids
3.3.2. Tocopherols
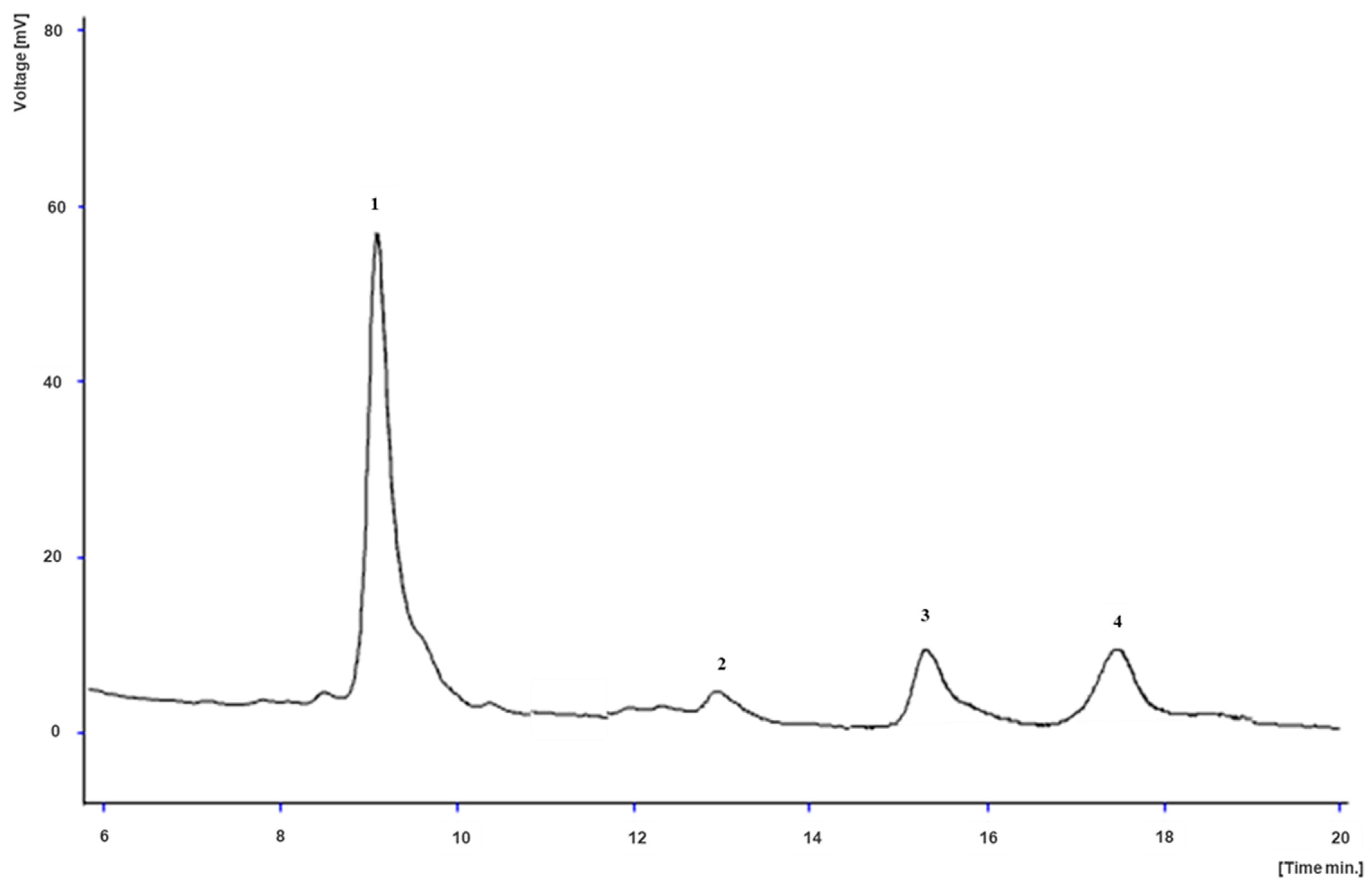
3.4. Betalains
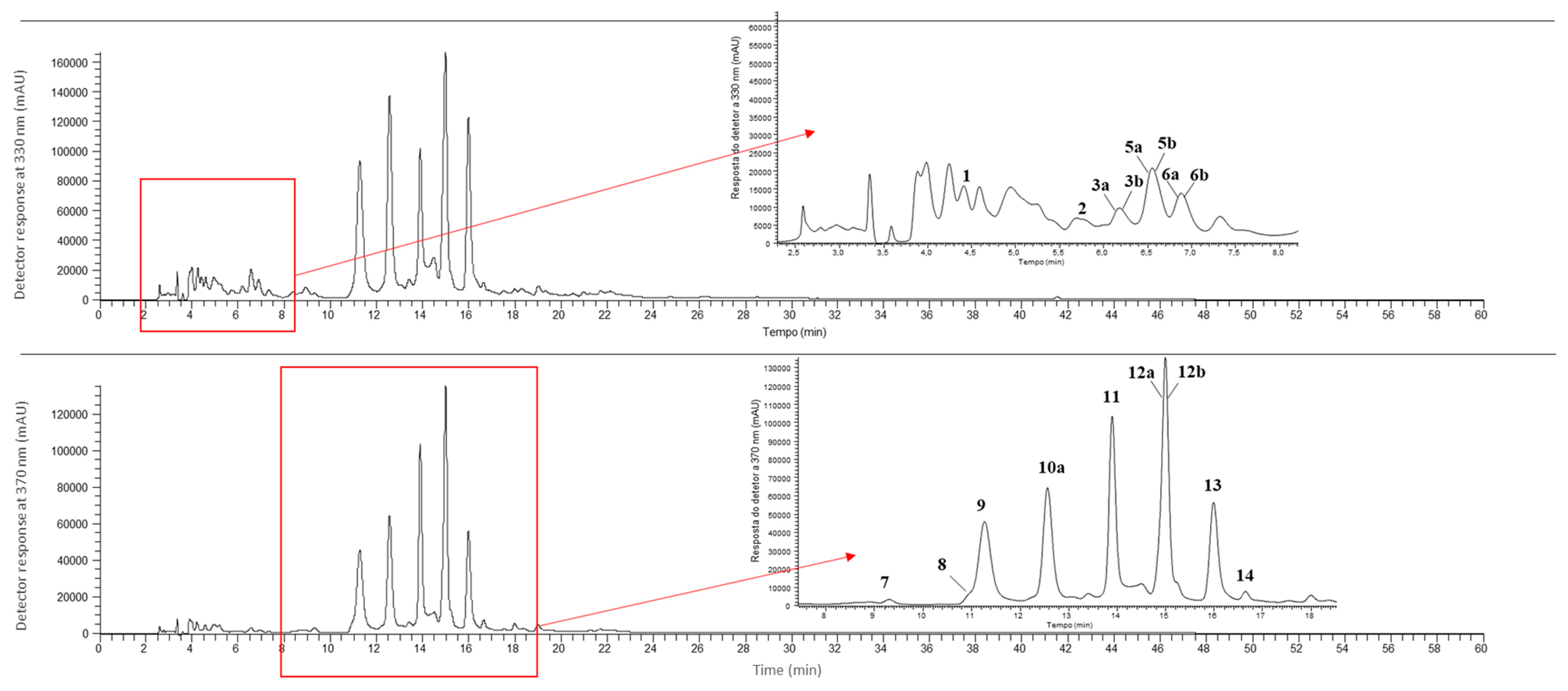
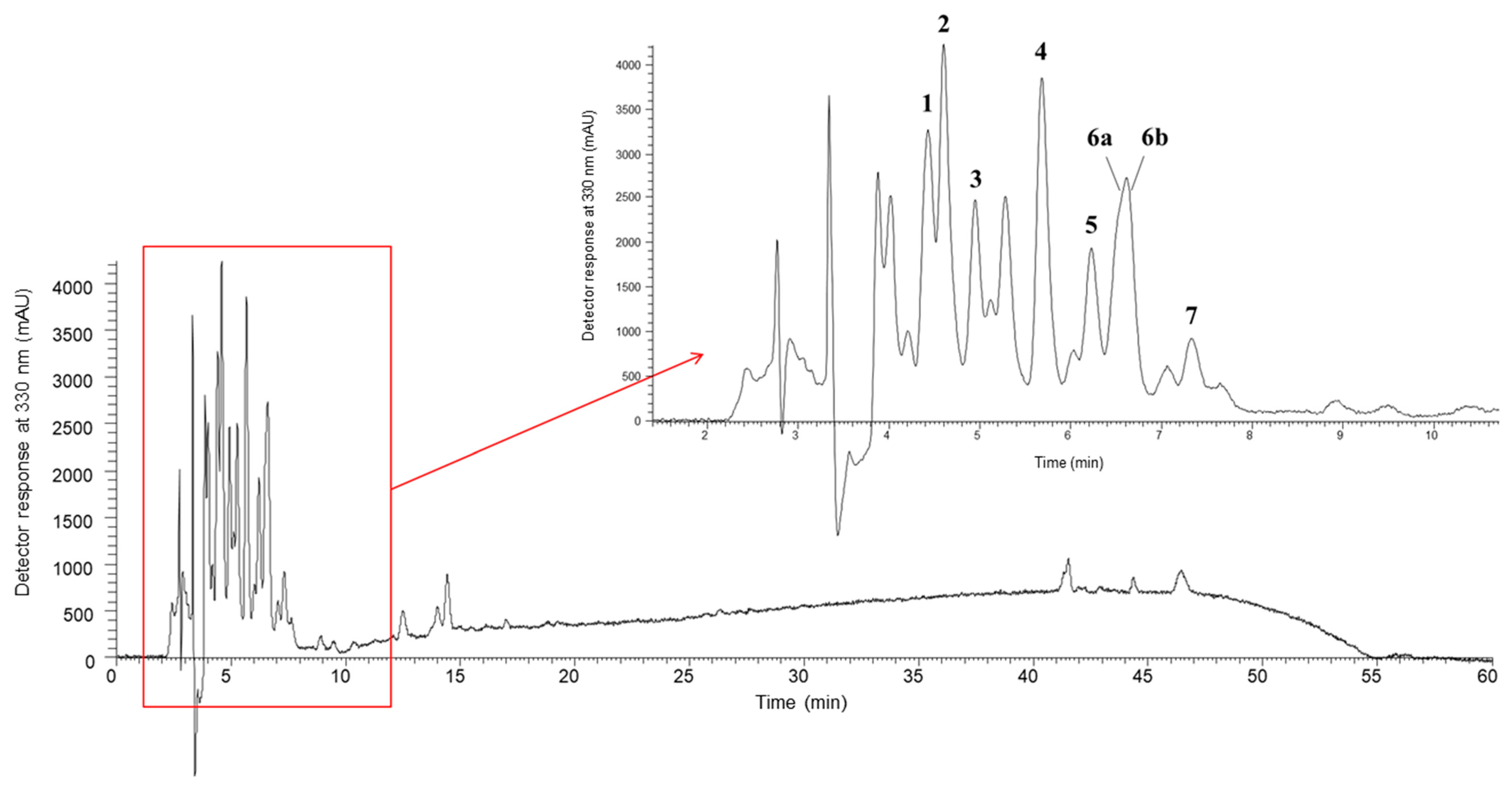
3.5. Phenolic Compounds
| Leaves | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak n.° | Rt (min) | λmax (nm) | [M–H]− (m/z) | MS2 Fragments (m/z) | Tentative Identification | Quantification (mg/g of Extract) | |||
| Beta vulgaris cv. Albina Vereduna | Beta vulgaris cv. Burpee’s Golden | Beta vulgaris cv. Pablo F1 | Beta vulgaris subsp. cicla var. Flavescens | ||||||
| 1 | 4.42 | 315 | 355 | MS2 (355): 209 (100), 191 (90), 193 (87), 147 (56), 163 (29), 178 (25), 173 (10), 202 (10), 119 (8) | p-Coumaroyl hexaric acid [65,67,68] | tr | nd | nd | nd |
| 2 | 5.69 | - | 369 | MS2 (369): 129 (100), 223 (84), 205 (72), 163 (41), 119 (18), 202 (7) | Sinapic acid rhamnoside 1 [76] | 0.050 ± 0.001 a | nd | nd | nd |
| 3a * | 6.18 | - | 355 | MS2 (355): 193 (100), 178 (36), 134 (30), 149 (28), 194 (4), 175 (1) | Ferulic acid hexoside 1 [77,78,79,80] | tr | 0.050 ± 0.002 a | 0.030 ± 0.002 b | tr |
| 3b * | 6.18 | - | 387 | MS2 (387): 163 (100), 387 (68), 119 (8), 388 (5), 164 (4), 207 (2), 369 (2) | Medioresinol 1 [69,70,72,73] | 0.520 ± 0.002 c | 0.540 ± 0.006 b | 0.550 ± 0.001 a | 0.490 ± 0.004 d |
| 4 | 6.53 | 330 | 355 | MS2 (355): 175 (100), 160 (21), 193 (9), 134 (6), 191 (3) | Ferulic acid hexoside 2 [77,78,79,80] | nd | nd | nd | 0.39 ± 0.03 a |
| 5a * | 6.60 | 230 | 387 | MS2 (387): 163 (100), 387 (68), 119 (8), 388 (5), 164 (4), 207 (2), 369 (2) | Medioresinol 2 [69,70,72,73] | 0.530 ± 0.002 c | 0.88 ± 0.02 a | 0.74 ± 0.01 b | 0.890 ± 0.004 a |
| 5b * | 6.60 | 326 | 517 | MS2 (517): 175 (100), 193(98), 235 (32), 134 (26), 149 (9), 337 (8) | Ferulic acid dihexoside [66,75] | tr | 0.46 ± 0.01 a | 0.040 ± 0.002 c | 0.100 ± 0.003 b |
| 6a * | 6.88 | 319 | 459 | MS2 (459): 175 (100), 193 (46), 160 (12), 134 (10) | Ferulic acid derivative | 0.14 ± 0.01 c | 0.35 ± 0.01 a | 0.25 ± 0.01 b | 0.020 ± 0.001 d |
| 6b * | 6.88 | 319 | 385 | MS2 (385): 223 (100), 208 (70), 164 (38), 179 (27), 149 (7), 205 (3), 193 (3) | Sinapic acid hexoside [82,83] | 0.240 ± 0.008 a | nd | nd | 0.110 ± 0.002 b |
| 7 | 9.31 | 257/340/351 | 625 | MS2 (625): 300 (100), 301 (61), 625 (31) | Quercetin dihexoside [67] | 0.170 ± 0.003 c | 0.170 ± 0.003 c | 0.37 ± 0.01 a | 0.250 ± 0.005 b |
| 8 | 11.04 | 267/302/342 | 595 | MS2 (595): 300 (100), 301 (54), 595 (34) | Quercetin pentosyl-hexoside [65] | 0.28 ± 0.01 b | 0.230 ± 0.002 c | 0.38 ± 0.02 a | 0.090 ± 0.003 d |
| 9 | 11.27 | 214/269/336 | 593 | MS2 (593): 293 (100), 413 (38), 311 (9), 294 (5), 414 (2), 473 (1) | Vitexin hexoside [66] | 2.81 ± 0.02 b | 0.55 ± 0.04 d | 3.2 ± 0.1 a | 0.89 ± 0.04 c |
| 10a * | 12.57 | 215/269/334 | 563 | MS2 (563): 293 (100), 413 (36), 311 (11), 294 (6), 201 (4), 414 (3), 341 (1) | Vitexin pentoside [64,66] | 2.87 ± 0.09 c | 8.3 ± 0.4 a | 8.5 ± 0.5 a | 4.87 ± 0.03 b |
| 10b * | 12.57 | 214/269/337 | 577 | MS2 (577): 293 (100), 413 (43), 311 (16), 294 (4), 457 (3), 341 (3) | Vitexin deoxyhexoside [73] | nd | nd | nd | 5.0 ± 0.2a |
| 11 | 13.9 | 204/256/352 | 639 | MS2 (639): 315 (100), 314 (30), 300 (7), 639 (6), 316 (4), 299 (3), 357 (1) | Isorhamnetin dihexoside [66] | 3.29 ± 0.05 c | 3.5 ± 0.2 b | 5.2 ± 0.3 a | 1.8 ± 0.1 d |
| 12a * | 14.99 | 258/266/347 | 679 | MS2 (679): 293 (100), 455 (44), 311 (11), 203 (6), 575 (6), 635 (1), 593 (1) | Vitexin malonyl-hexoside [66] | 4.8 ± 0.2 a | 2.32 ± 0.06 b | 4.7 ± 0.2a | 1.24 ± 0.02 c |
| 12b * | 14.99 | 258/266/347 | 609 | MS2 (609): 315 (100), 314 (36), 609 (8), 300 (6) | Isorhamnetin hexosyl-pentoside [65] | 4.84 ± 0.05 a | 2.34 ± 0.07 b | 4.9 ± 0.2 a | nd |
| 13 | 15.99 | 216/269/334 | 649 | MS2 (649): 293 (100), 455 (38), 311 (13), 545 (7), 294 (5), 341 (2), 605 (1), 563 (1) | Vitexin malonyl-pentoside [66] | 2.37 ± 0.04 d | 7.0± 0.3 a | 5.9 ± 0.3 b | 4.0 ± 0.3 c |
| 14 | 16.64 | 267/338/367 | 623 | MS2 (623): 315 (100), 314 (45), 623 (12), 300 (7) | Isorhamnetin rhamnoside-hexoside [65] | 0.280 ± 0.003 b | nd | 0.41 ± 0.02 a | nd |
| Total phenolic acids | 0.92 ± 0.01 a | 0.86 ± 0.005 b | 0.32 ± 0.01 d | 0.62 ± 0.02c | |||||
| Total flavonoids | 21.7 ± 0.4 c | 25 ± 1 b | 34 ± 2 a | 18.2 ± 0.6 d | |||||
| Total phenolic compounds | 23.2 ± 0.4 c | 26.8 ± 0.9 b | 35 ± 2 a | 20.1 ± 0.7 d | |||||
| Roots | ||||||||
|---|---|---|---|---|---|---|---|---|
| Peak n.° | Rt (min) | λmax (nm) | [M–H]– (m/z) | MS2 Fragments (m/z) | Tentative Identification | Quantification (mg/g of Extract) | ||
| Beta vulgaris cv. Albina Vereduna | Beta vulgaris cv. Burpee’s Golden | Beta vulgaris cv. Pablo F1 | ||||||
| 1 | 4.42 | 315 | 355 | MS2 (355): 209 (100), 191 (90), 193 (87), 147 (56), 163 (29), 178 (25), 173 (10), 119 (8) | p-Coumaroyl hexaric acid [65,67,68] | tr | tr | nd |
| 2 | 4.60 | 296 | 341 | MS2 (341): 119 (100), 179 (47), 143 (29), 161 (24), 341 (24), 149 (20), 135 (3) | Caffeic acid-hexoside 1 [85,87] | 0.060 ± 0.002 b | 0.060 ± 0.002 a | nd |
| 3 | 4.95 | 300 | 341 | MS2 (341): 153 (100), 119 (19), 179 (19), 143 (7), 161 (6), 341 (5), 149 (3), 135 (1) | Caffeic acid-hexoside 2 [85,87] | 0.050 ± 0.001 b | 0.060 ± 0.001 a | nd |
| 4 | 5.68 | 313 | 369 | MS2 (369): 129 (100), 223 (77), 205 (69), 163 (42), 119 (39), 161 (8) | Sinapic acid rhamnoside 1 [76] | 0.020 ± 0.002 b | tr | 0.040 ± 0.003 a |
| 5 | 6.23 | 300 | 369 | MS2 (369): 129 (100), 223 (80), 205 (70), 163 (47), 119 (19), 161 (10) | Sinapic acid rhaminoside 2 [76] | tr | tr | nd |
| 6a * | 6.60 | 326 | 399 | MS2 (399): 129 (100), 205 (48), 223 (44), 193 (37), 134 (14), 161 (6), 175 (6) | Feruloyl sinapic acid 1 [86] | 0.020 ± 0.001 c | 0.030 ± 0.001 b | 0.060 ± 0.002 a |
| 6b * | 6.60 | 326 | 517 | MS2 (517): 175 (100), 193(83), 235 (27), 134 (21), 149 (9), 337 (8) | Ferulic acid dihexoside [66,75] | tr | tr | tr |
| 7 | 7.34 | 323 | 399 | MS2 (399): 129 (100), 205 (48), 193 (48), 223 (37), 134 (17), 161 (8), 175 (3) | Feruloyl sinapic acid 2 [86] | tr | tr | 0.020 ± 0.002 a |
| Total phenolic acids | 0.150 ± 0.001 a | 0.150 ± 0.002 a | 0.130 ± 0.003 b | |||||
| Total phenolic compounds | 0.150 ± 0.001 a | 0.150 ± 0.002 a | 0.130 ± 0.003 b | |||||
3.6. Bioactive Properties
3.6.1. Antioxidant Activity
3.6.2. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lange, W.; Brandenburg, W.A.; De Bock, T.S.M. Taxonomy and Cultonomy of Beet (Beta vulgaris L.). Bot. J. Linn. Soc. 1999, 130, 81–96. [Google Scholar] [CrossRef]
- Nikan, M.; Manayi, A. Beta vulgaris L. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 153–158. ISBN 9780128124918. [Google Scholar]
- Galewski, P.; McGrath, J.M. Genetic Diversity among Cultivated Beets (Beta vulgaris) Assessed via Population-Based Whole Genome Sequences. BMC Genom. 2020, 21, 189. [Google Scholar] [CrossRef] [PubMed]
- Babarykin, D.; Smirnova, G.; Pundinsh, I.; Vasiljeva, S.; Krumina, G.; Agejchenko, V. Red Beet (Beta vulgaris) Impact on Human Health. J. Biosci. Med. 2019, 07, 61–79. [Google Scholar] [CrossRef]
- Ninfali, P.; Angelino, D. Nutritional and Functional Potential of Beta vulgaris cicla and rubra. Fitoterapia 2013, 89, 188–199. [Google Scholar] [CrossRef]
- Pandita, D.; Pandita, A.; Pamuru, R.; Nayik, G. Beetroot. In Antioxidants in Vegetables and Nuts—Properties and Health Benefits; Nayik, G.A., Gull, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 45–74. ISBN 9789811574702. [Google Scholar]
- Goldman, I.L.; Janick, J. Evolution of Root Morphology in Table Beet: Historical and Iconographic. Front. Plant Sci. 2021, 12, 689926. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Melkis, K.; Janda-Milczarek, K.; Skonieczna-Żydecka, K. Phenolic Compounds and Antioxidant Properties of Fermented Beetroot Juices Enriched with Different Additives. Foods 2023, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, M.; Rosellini, I.; Malorgio, F.; Pardossi, A.; Pezzarossa, B. Iodine Biofortification of Swiss Chard (Beta vulgaris Ssp. vulgaris Var. Cicla) and Its Wild Ancestor Sea Beet (Beta vulgaris ssp. maritima) Grown Hydroponically as Baby Leaves: Effects on Leaf Production and Quality. J. Sci. Food Agric. 2023, 103, 7888–7895. [Google Scholar] [CrossRef] [PubMed]
- Camelo-Silva, C.; Mota e Souza, B.; Vicente, R.; Arend, G.D.; Sanches, M.A.R.; Barreto, P.L.M.; Ambrosi, A.; Verruck, S.; Di Luccio, M. Polyfunctional Sugar-Free White Chocolate Fortified with Lacticaseibacillus rhamnosus GG Co-Encapsulated with Beet Residue Extract (Beta vulgaris L.). Food Res. Int. 2024, 179, 114016. [Google Scholar] [CrossRef] [PubMed]
- Halladj, F.; Amellal-Chibane, H.; Aitfella-Lahlou, R.; Bourai, M.A.; Tigrine, A. Effect of Red Beet Cooking Water on Yoghurt’s Physico-Chemical, Textural and Antioxidant Characteristics. Food Sci. Technol. Int. 2024, 30, 85–93. [Google Scholar] [CrossRef]
- Aphurvika, M.; Hemalatha, M.; Mohanasrinivasan, V.; Devi, S.C. Metabolite Profiling and Bioactivity Assessment of Beet Juice Fermented by Lactobacillus spp. Res. J. Biotechnol. 2023, 18, 69–76. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Chung, I.M.; Samynathan, R.; Chander, S.R.H.; Venkidasamy, B.; Sarkar, T.; Rebezov, M.; Gorelik, O.; Shariati, M.A.; Simal-Gandara, J. A Comprehensive Review of Beetroot (Beta vulgaris L.) Bioactive Components in the Food and Pharmaceutical Industries. Crit. Rev. Food Sci. Nutr. 2022, 64, 708–739. [Google Scholar] [CrossRef] [PubMed]
- Paviani, B.; Masarweh, C.; Bhattacharya, M.; Ozturk, G.; Castillo, J.; Couture, G.; Lebrilla, C.B.; Mills, D.A.; Barile, D. Eat Your Beets: Conversion of Polysaccharides into Oligosaccharides for Enhanced Bioactivity. Int. J. Biol. Macromol. 2024, 256, 128472. [Google Scholar] [CrossRef] [PubMed]
- Sakihama, Y.; Kato, T.; Sawatdee, S.; Yakushi, Y.; Asano, J.; Hayashi, H.; Goto, Y.; Hashimoto, M.; Hashidoko, Y. Isolation of High-Purity Betanin from Red Beet and Elucidation of Its Antioxidant Activity against Peroxynitrite: An in Vitro Study. Int. J. Mol. Sci. 2023, 24, 15411. [Google Scholar] [CrossRef] [PubMed]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. Betalains: Natural Plant Pigments with Potential Application in Functional Foods. LWT 2015, 64, 645–649. [Google Scholar] [CrossRef]
- Fu, Y.; Shi, J.; Xie, S.Y.; Zhang, T.Y.; Soladoye, O.P.; Aluko, R.E. Red Beetroot Betalains: Perspectives on Extraction, Processing, and Potential Health Benefits. J. Agric. Food Chem. 2020, 68, 11595–11611. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, P.T.; Meramo, S.; Ninivaggi, L.; Pasutto, E.; Babaei, M.; Avila-Neto, P.M.; Pastor, M.C.; Sabri, P.; Rago, D.; Parekh, T.U.; et al. Beet Red Food Colourant Can Be Produced More Sustainably with Engineered Yarrowia lipolytica. Nat. Microbiol. 2023, 8, 2290–2303. [Google Scholar] [CrossRef] [PubMed]
- Mullick, H. Influence of Betalain Natural Dye from Red Beet in Gum Acacia Biopolymer: Optical and Electrical Perspective. J. Polym. Eng. 2023, 43, 783–790. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Dziki, L.; Anisiewicz, J.; Habza-Kowalska, E.; Sikora, M.; Dziki, D. Leaves of White Beetroot as a New Source of Antioxidant and Anti-Inflammatory Compounds. Plants 2020, 9, 944. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Houshialsadat, Z.; Gaeini, Z.; Bahadoran, Z.; Azizi, F. Functional Properties of Beetroot (Beta vulgaris) in Management of Cardio-Metabolic Diseases. Nutr. Metab. 2020, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Daab, W.; Zghal, F.; Nassis, G.P.; Rebai, H.; Moalla, W.; Bouzid, M.A. Chronic Beetroot Juice Supplementation Attenuates Neuromuscular Fatigue Etiology during Simulated Soccer Match Play. Appl. Physiol. Nutr. Metab. 2023, 49, 105–113. [Google Scholar] [CrossRef]
- Lee, H.S.; Choi, S.M.; Lim, S.H.; Choi, C.I. Betanin from Beetroot (Beta vulgaris L.) Regulates Lipid Metabolism and Promotes Fat Browning in 3T3-L1 Adipocytes. Pharmaceuticals 2023, 16, 1727. [Google Scholar] [CrossRef] [PubMed]
- Sagar, P.S.; Munt, A.; Saravanabavan, S.; Vahedi, F.A.; Elhindi, J.; Nguyen, B.; Chau, K.; Harris, D.C.; Lee, V.; Sud, K.; et al. Efficacy of Beetroot Juice on Reducing Blood Pressure in Hypertensive Adults with Autosomal Dominant Polycystic Kidney Disease (BEET-PKD): Study Protocol for a Double-Blind, Randomised, Placebo-Controlled Trial. Trials 2023, 24, 482. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists; Horwitz, W., Latimer, G., Eds.; AOAC Inter.: Gaithersburg, MD, USA, 2019; ISBN 0935584773. [Google Scholar]
- Harumi Iyda, J.; Fernandes, Â.; Calhelha, R.C.; Alves, M.J.; Ferreira, F.D.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Nutritional Composition and Bioactivity of Umbilicus rupestris (Salisb.) Dandy: An Underexploited Edible Wild Plant. Food Chem. 2019, 295, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Spréa, R.M.; Fernandes, Â.; Calhelha, R.C.; Pereira, C.; Pires, T.C.S.P.; Alves, M.J.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical and Bioactive Characterization of the Aromatic Plant Levisticum officinale W.D.J. Koch: A Comprehensive Study. Food Funct. 2020, 11, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized Analysis of Organic Acids in Edible Mushrooms from Portugal by Ultra Fast Liquid Chromatography and Photodiode Array Detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Obodai, M.; Mensah, D.L.N.; Fernandes, Â.; Kortei, N.K.; Dzomeku, M.; Teegarden, M.; Schwartz, S.J.; Barros, L.; Prempeh, J.; Takli, R.K.; et al. Chemical Characterization and Antioxidant Potential of Wild Ganoderma Species from Ghana. Molecules 2017, 22, 196. [Google Scholar] [CrossRef]
- Lazăr, S.; Constantin, O.E.; Stănciuc, N.; Aprodu, I.; Croitoru, C.; Râpeanu, G. Optimization of Betalain Pigments Extraction Using Beetroot By-Products as a Valuable Source. Inventions 2021, 6, 50. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic Profile and Antioxidant Activity of Coleostephus myconis (L.) Rchb.f.: An Underexploited and Highly Disseminated Species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Sarmento, A.; Barros, L.; Fernandes, Â.; Carvalho, A.M.; Ferreira, I.C.F.R. Valorization of Traditional Foods: Nutritional and Bioactive Properties of Cicer arietinum L. and Lathyrus sativus L. Pulses. J. Sci. Food Agric. 2015, 95, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.; Santos-Buelga, C.; Ferreira, I.C. Antioxidant and antimicrobial properties of dried Portuguese apple variety (Malus domestica Borkh. cv Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Ferreira, I.C.F.R.; Esteves, A.P.; Ćirić, A.; Glamočlija, J.; Martins, A.; Soković, M.; Queiroz, M.J.R.P. Antimicrobial and Demelanizing Activity of Ganoderma lucidum Extract, p-Hydroxybenzoic and Cinnamic Acids and Their Synthetic Acetylated Glucuronide Methyl Esters. Food Chem. Toxicol. 2013, 58, 95–100. [Google Scholar] [CrossRef]
- Lucky, A.R.; Al-Mamun, A.; Hosen, A.; Toma, M.A.; Mazumder, M.A.R. Nutritional and Sensory Quality Assessment of Plain Cake Enriched with Beetroot Powder. Food Res. 2020, 4, 2049–2053. [Google Scholar] [CrossRef]
- Kohajdová, Z.; Karovičová, J.; Kuchtová, V.; Lauková, M. Utilisation of Beetroot Powder for Bakery Applications. Chem. Pap. 2018, 72, 1507–1515. [Google Scholar] [CrossRef]
- Asadi, S.Z.; Khan, M.A. The Effect of Beetroot (Beta vulgaris L.) Leaves Powder on Nutritional, Textural, Sensorial and Antioxidant Properties of Cookies. J. Culin. Sci. Technol. 2021, 19, 424–438. [Google Scholar] [CrossRef]
- Abdi, F.A.; Gemede, H.F.; Olika Keyata, E. Nutritional Composition, Antinutrient Contents, and Polyphenol Compounds of Selected Underutilized and Some Commonly Consumed Vegetables in East Wollega, West Ethiopia. J. Food Qual. 2022, 2022, 6942039. [Google Scholar] [CrossRef]
- Vaitkevičienė, N.; Sapronaitė, A.; Kulaitienė, J. Evaluation of Proximate Composition, Mineral Elements and Bioactive Compounds in Skin and Flesh of Beetroot Grown in Lithuania. Agriculture 2022, 12, 1833. [Google Scholar] [CrossRef]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U.; Höglinger, O.; Weghuber, J. Compositional Characteristics of Commercial Beetroot Products and Beetroot Juice Prepared from Seven Beetroot Varieties Grown in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef]
- Gruska, R.M.; Baryga, A.; Kunicka-Styczyńska, A.; Brzeziński, S.; Rosicka-Kaczmarek, J.; Miśkiewicz, K.; Sumińska, T. Fresh and Stored Sugar Beet Roots as a Source of Various Types of Mono- and Oligosaccharides. Molecules 2022, 27, 5125. [Google Scholar] [CrossRef] [PubMed]
- Mzoughi, Z.; Chahdoura, H.; Chakroun, Y.; Cámara, M.; Fernández-Ruiz, V.; Morales, P.; Mosbah, H.; Flamini, G.; Snoussi, M.; Majdoub, H. Wild Edible Swiss Chard Leaves (Beta vulgaris L. var. cicla): Nutritional, Phytochemical Composition and Biological Activities. Food Res. Int. 2019, 119, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Bavec, M.; Turinek, M.; Grobelnik-Mlakar, S.; Slatnar, A.; Bavec, F. Influence of Industrial and Alternative Farming Systems on Contents of Sugars, Organic Acids, Total Phenolic Content, and the Antioxidant Activity of Red Beet (Beta vulgaris L. ssp. vulgaris Rote Kugel). J. Agric. Food Chem. 2010, 58, 11825–11831. [Google Scholar] [CrossRef]
- Sagardoy, R.; Morales, F.; Rellán-Álvarez, R.; Abadía, A.; Abadía, J.; López-Millán, A.F. Carboxylate Metabolism in Sugar Beet Plants Grown with Excess Zn. J. Plant Physiol. 2011, 168, 730–733. [Google Scholar] [CrossRef]
- Freidig, A.K.; Goldman, I.L. Variation in Oxalic Acid Content among Commercial Table Beet Cultivars and Related Crops. J. Am. Soc. Hortic. Sci. 2011, 136, 54–60. [Google Scholar] [CrossRef]
- Stuiver, C.E.E.; Kuiper, P.J.C.; Marschner, H. Lipids from Bean, Barley and Sugar Beet in Relation to Salt Resistance. Physiol. Plant 1978, 42, 124–128. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Mihaylova, D.; Marangon, C.M.; Grigoletto, L.; Lante, A. Impact of Sample Pretreatment and Extraction Methods on the Bioactive Compounds of Sugar Beet (Beta vulgaris L.) Leaves. Molecules 2022, 27, 8110. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Sakalauskienė, S.; Vaštakaitė, V.; Novičkovas, A.; Viškelienė, A.; Sasnauskas, A.; Duchovskis, P. Blue Light Dosage Affects Carotenoids and Tocopherols in Microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef]
- Santos, J.; Mendiola, J.A.; Oliveira, M.B.P.P.; Ibáñez, E.; Herrero, M. Sequential Determination of Fat- and Water-Soluble Vitamins in Green Leafy Vegetables during Storage. J. Chromatogr. A 2012, 1261, 179–188. [Google Scholar] [CrossRef]
- Roriz, C.L.; Heleno, S.A.; Carocho, M.; Rodrigues, P.; Pinela, J.; Dias, M.I.; Fernandes, I.P.; Barreiro, M.F.; Morales, P.; Barros, L.; et al. Betacyanins from Gomphrena globosa L. Flowers: Incorporation in Cookies as Natural Colouring Agents. Food Chem. 2020, 329, 121178. [Google Scholar] [CrossRef] [PubMed]
- Escribano, J.; Cabanes, J.; Jiménez-Atiénzar, M.; Ibañez-Tremolada, M.; Gómez-Pando, L.R.; García-Carmona, F.; Gandía-Herrero, F. Characterization of Betalains, Saponins and Antioxidant Power in Differently Colored Quinoa (Chenopodium quinoa) Varieties. Food Chem. 2017, 234, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Herbach, K.; Stintzing, F.; Carle, R. Impact of Thermal Treatment on Color and Pigment Pattern of Red Beet (Beta vulgaris L.) Preparations. J. Food Sci. 2004, 69, 419–498. [Google Scholar] [CrossRef]
- Slatnar, A.; Stampar, F.; Veberic, R.; Jakopic, J. HPLC-MSn Identification of Betalain Profile of Different Beetroot (Beta vulgaris L. Sssp. vulgaris) Parts and Cultivars. J. Food Sci. 2015, 80, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Schliemann, W.; Kobayashi, N.; Strack, D. The Decisive Step in Betaxanthin Biosynthesis Is a Spontaneous Reaction. Plant Physiol. 1999, 119, 1217–1232. [Google Scholar] [CrossRef] [PubMed]
- Kugler, F.; Graneis, S.; Stintzing, F.C.; Carle, R. Studies on Betaxanthin Profiles of Vegetables and Fruits from the Chenopodiaceae and Cactaceae. Z. Naturforschung-Sect. C J. Biosci. 2007, 62, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Bączek, N.; Wiczkowski, W. Betalain Profile, Content and Antioxidant Capacity of Red Beetroot Dependent on the Genotype and Root Part. J. Funct. Foods 2016, 27, 249–261. [Google Scholar] [CrossRef]
- Ferreres, F.; Andrade, P.B.; Valentão, P.; Gil-Izquierdo, A. Further Knowledge on Barley (Hordeum vulgare L.) Leaves O-Glycosyl-C-Glycosyl Flavones by Liquid Chromatography-UV Diode-Array Detection-Electrospray Ionisation Mass Spectrometry. J. Chromatogr. A 2008, 1182, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, N.M.; Radwan, R.A.; Bakry, S.M.; Saad, H.H. Molecular Networking Aided Metabolomic Profiling of Beet Leaves Using Three Extraction Solvents and in Relation to Its Anti-Obesity Effects. J. Adv. Res. 2020, 24, 545–555. [Google Scholar] [CrossRef]
- Gennari, L.; Felletti, M.; Blasa, M.; Angelino, D.; Celeghini, C.; Corallini, A.; Ninfali, P. Total Extract of Beta vulgaris var. cicla Seeds versus Its Purified Phenolic Components: Antioxidant Activities and Antiproliferative Effects against Colon Cancer Cells. Phytochem. Anal. 2011, 22, 272–279. [Google Scholar] [CrossRef]
- Pereira, O.R.; Silva, A.M.S.; Domingues, M.R.M.; Cardoso, S.M. Identification of Phenolic Constituents of Cytisus multiflorus. Food Chem. 2012, 131, 652–659. [Google Scholar] [CrossRef]
- Zeb, A.; Imran, M. Carotenoids, Pigments, Phenolic Composition and Antioxidant Activity of Oxalis corniculata Leaves. Food Biosci. 2019, 32, 100472. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Gouveia, S.; Castilho, P.C. Analysis of Phenolic Compounds in Leaves from Endemic Trees from Madeira Island. A Contribution to the Chemotaxonomy of Laurisilva Forest Species. Ind. Crops Prod. 2015, 64, 135–151. [Google Scholar] [CrossRef]
- Sakalem, M.E.; Negri, G.; Tabach, R. Chemical Composition of Hydroethanolic Extracts from Five Species of the Passiflora Genus. Rev. Bras. Farmacogn. 2012, 22, 1219–1232. [Google Scholar] [CrossRef]
- Cruz, T.M.; Santos, J.S.; do Carmo, M.A.V.; Hellström, J.; Pihlava, J.M.; Azevedo, L.; Granato, D.; Marques, M.B. Extraction Optimization of Bioactive Compounds from Ora-pro-Nobis (Pereskia aculeata Miller) Leaves and Their in Vitro Antioxidant and Antihemolytic Activities. Food Chem. 2021, 361, 130078. [Google Scholar] [CrossRef]
- Vissers, A.; Kiskini, A.; Hilgers, R.; Marinea, M.; Wierenga, P.A.; Gruppen, H.; Vincken, J.P. Enzymatic Browning in Sugar Beet Leaves (Beta vulgaris L.): Influence of Caffeic Acid Derivatives, Oxidative Coupling, and Coupled Oxidation. J. Agric. Food Chem. 2017, 65, 4911–4920. [Google Scholar] [CrossRef] [PubMed]
- Apea-Bah, F.B.; Li, X.; Beta, T. Phenolic Composition and Antioxidant Properties of Cooked Rice Dyed with Sorghum-Leaf Bio-Colorants. Foods 2021, 10, 2058. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.O.; Jamali, A.; Grand, E.; Morreel, K.; Marcelo, P.; Gontier, E.; Dauwe, R. Phenylpropanoid Profiling Reveals a Class of Hydroxycinnamoyl Glucaric Acid Conjugates in Isatis tinctoria Leaves. Phytochemistry 2017, 144, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Adebiyi, J.A.; Njobeh, P.B.; Kayitesi, E. Assessment of Nutritional and Phytochemical Quality of Dawadawa (an African Fermented Condiment) Produced from Bambara Groundnut (Vigna subterranea). Microchem. J. 2019, 149, 104034. [Google Scholar] [CrossRef]
- Di Gioia, F.; Petropoulos, S.A. Phytoestrogens, Phytosteroids and Saponins in Vegetables: Biosynthesis, Functions, Health Effects and Practical Applications. Adv. Food Nutr. Res. 2019, 90, 351–421. [Google Scholar] [CrossRef] [PubMed]
- Nyau, V.; Prakash, S.; Rodrigues, J.; Farrant, J. Idenfication of Nutraceutical Phenolic Compounds in Bambara Groundnuts (Vigna subterranea L. Verdc) by HPLC-PDA-ESI-MS. Br. J. Appl. Sci. Technol. 2015, 6, 77–85. [Google Scholar] [CrossRef]
- Ozarowski, M.; Mikolajczak, P.L.; Bogacz, A.; Gryszczynska, A.; Kujawska, M.; Jodynis-Liebert, J.; Piasecka, A.; Napieczynska, H.; Szulc, M.; Kujawski, R.; et al. Rosmarinus officinalis L. Leaf Extract Improves Memory Impairment and Affects Acetylcholinesterase and Butyrylcholinesterase Activities in Rat Brain. Fitoterapia 2013, 91, 261–271. [Google Scholar] [CrossRef]
- Llorent-Martinez, E.J.; Spinola, V.; Gouveia, S.; Castilho, P.C. HPLC-ESI-MSn Characterization of Phenolic Compounds, Terpenoid Saponins, and Other Minor Compounds in Bituminaria bituminosa. Ind. Crops Prod. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Tsamo, A.T.; Ndibewu, P.P.; Dakora, F.D. Phytochemical Profile of Seeds from 21 Bambara Groundnut Landraces via UPLC-QTOF-MS. Food Res. Int. 2018, 112, 160–168. [Google Scholar] [CrossRef]
- Chahdoura, H.; Barreira, J.C.M.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Achour, L. Seeds of Opuntia spp. as a Novel High Potential by-Product: Phytochemical Characterization and Antioxidant Activity. Ind. Crops Prod. 2015, 65, 383–389. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, B.; Lin, Z.; Tong, L.; Wang, H.; Chen, S. An Analysis Method for Simultaneous Screening of Deoxyribonucleic Acid-Binding Active Compounds and Investigating Their Mechanisms by Ultra-Fast Liquid Chromatography Tandem Mass Spectrometry Coupled with Fluorescence Detection Technology. J. Chromatogr. A 2015, 1381, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Dueňas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C. Phenolic Profiles of in Vivo and in Vitro Grown Coriandrum sativum L. Food Chem. 2012, 132, 841–848. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Yang, T.; Slovin, J.; Chen, P. Profiling Polyphenols of Two Diploid Strawberry (Fragaria vesca) Inbred Lines Using UHPLC-HRMSn. Food Chem. 2014, 146, 289–298. [Google Scholar] [CrossRef] [PubMed]
- M’rabet, Y.; Rokbeni, N.; Cluzet, S.; Boulila, A.; Richard, T.; Krisa, S.; Marzouki, L.; Casabianca, H.; Hosni, K. Profiling of Phenolic Compounds and Antioxidant Activity of Melia azedarach L. Leaves and Fruits at Two Stages of Maturity. Ind. Crops Prod. 2017, 107, 232–243. [Google Scholar] [CrossRef]
- Mata, A.; Ferreira, J.P.; Semedo, C.; Serra, T.; Duarte, C.M.M.; Bronze, M.R. Contribution to the Characterization of Opuntia spp. Juices by LC-DAD-ESI-MS/MS. Food Chem. 2016, 210, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.W.; Yoon, K.D.; Ahn, M.J.; Kim, J. Two New Phenylpropanoid Glycosides from the Aerial Parts of Paederia scandens. Bull. Korean Chem. Soc. 2010, 31, 1070–1072. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Caligari, P.D.S.; Schmeda-Hirschmann, G. Identification of Phenolic Compounds from the Fruits of the Mountain Papaya Vasconcellea Ppubescens A. DC. Grown in Chile by Liquid Chromatography-UV Detection-Mass Spectrometry. Food Chem. 2009, 115, 775–784. [Google Scholar] [CrossRef]
- Spínola, V.; Pinto, J.; Castilho, P.C. Identification and Quantification of Phenolic Compounds of Selected Fruits from Madeira Island by HPLC-DAD-ESI-MSn and Screening for Their Antioxidant Activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Barreira, J.C.M.; Dias, M.I.; Živković, J.; Stojkovic, D.; Soković, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic Profiling of Veronica Spp. Grown in Mountain, Urban and Sandy Soil Environments. Food Chem. 2014, 163, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Ben Said, R.; Hamed, A.I.; Mahalel, U.A.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative Characterization of Polyphenolic Compounds in the Male Flowers of Phoenix dactylifera by Liquid Chromatography Coupled with Mass Spectrometry and DFT. Int. J. Mol. Sci. 2017, 18, 512. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez-Cuenca, C.E.; Vincken, J.P.; Gruppen, H. Polyphenolic Composition and Antioxidant Activity of Açai (Euterpe oleracea Mart.) from Colombia. Food Chem. 2017, 217, 364–372. [Google Scholar] [CrossRef]
- Gouveia, S.; Castilho, P.C. Characterisation of Phenolic Acid Derivatives and Flavonoids from Different Morphological Parts of Helichrysum obconicum by a RP-HPLC–DAD-(−)–ESI-MSn Method. Food Chem. 2011, 129, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of Phenolic Composition in Lamiaceae Spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef]
- Koolen, H.H.F.; Da Silva, F.M.A.; Gozzo, F.C.; De Souza, A.Q.L.; De Souza, A.D.L. Antioxidant, Antimicrobial Activities and Characterization of Phenolic Compounds from Buriti (Mauritia flexuosa L. f.) by UPLC-ESI-MS/MS. Food Res. Int. 2013, 51, 467–473. [Google Scholar] [CrossRef]
- Płatosz, N.; Sawicki, T.; Wiczkowski, W. Profile of Phenolic Acids and Flavonoids of Red Beet and Its Fermentation Products. Does Long-Term Consumption of Fermented Beetroot Juice Affect Phenolics Profile in Human Blood Plasma and Urine? Pol. J. Food Nutr. Sci. 2020, 70, 55–65. [Google Scholar] [CrossRef]
- Rey, A.I.; Hopia, A.; Kivikari, R.; Kahkonen, M. Use of Natural Food/Plant Extracts: Cloudberry (Rubus chamaemorus), Beetroot (Beta vulgaris “Vulgaris”) or Willow Herb (Epilobium angustifolium) to Reduce Lipid Oxidation of Cooked Pork Patties. LWT-Food Sci. Technol. 2005, 38, 363–370. [Google Scholar] [CrossRef]
- Salamatullah, A.M.; Hayat, K.; Alkaltham, M.S.; Ahmed, M.A.; Arzoo, S.; Husain, F.M.; Al-Dossari, A.M.; Shamlan, G.; Al-Harbi, L.N. Bioactive and Antimicrobial Properties of Oven-Dried Beetroot (Pulp and Peel) Using Different Solvents. Processes 2021, 9, 588. [Google Scholar] [CrossRef]
- Vulić, J.J.; Ćebović, T.N.; Čanadanović, V.M.; Ćetković, G.S.; Djilas, S.M.; Čanadanović-Brunet, J.M.; Velićanski, A.S.; Cvetković, D.D.; Tumbas, V.T. Antiradical, Antimicrobial and Cytotoxic Activities of Commercial Beetroot Pomace. Food Funct. 2013, 4, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Chaari, M.; Elhadef, K.; Akermi, S.; Ben Hlima, H.; Fourati, M.; Chakchouk Mtibaa, A.; Sarkar, T.; Shariati, M.A.; Rebezov, M.; D’Amore, T.; et al. Multiobjective Response and Chemometric Approaches to Enhance the Phytochemicals and Biological Activities of Beetroot Leaves: An Unexploited Organic Waste. Biomass Convers. Biorefin 2023, 13, 15067–15081. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Soleimanian-Zad, S.; Zaeim, D. Antibacterial and Antifungal Activity of the Aqueous and Methanolic Extracts and Essential Oils of Red Beets Beta vulgaris Leaves. Zahedan J. Res. Med. Sci. 2020, 22, e83725. [Google Scholar] [CrossRef]
- Yerubay, A.; Akhmetova, S.; Konkabayeva, A.; Rakhimova, B.; Baiguzhina, S.; Baiduissenova, A.; Kalymanov, I. Chemical Composition and Antibacterial Activity of Subcritical CO2 Extract of Beta vulgaris L. Online J. Biol. Sci. 2024, 24, 189–194. [Google Scholar] [CrossRef]

| Beta vulgaris cv. Albina Vereduna | Beta vulgaris cv. Burpee’s Golden | Beta vulgaris cv. Pablo F1 | Beta vulgaris subsp. cicla var. Flavescens | ||||
|---|---|---|---|---|---|---|---|
| Leaves | Roots | Leaves | Roots | Leaves | Roots | Leaves | |
| Proximal composition | (g/100 g dw) | ||||||
| Fat | 3.30 ± 0.06 b | 1.12 ± 0.03 d | 3.28 ± 0.02 b | 0.48 ± 0.06 f | 4.01 ± 0.04 a | 0.55 ± 0.05 e | 2.18 ± 0.10 c |
| Proteins | 11.84 ± 0.06 c | 5.46 ± 0.04 g | 12.23 ± 0.06 a | 6.22 ± 0.07 f | 12.18 ± 0.04 b | 7.32 ± 0.06 e | 9.94 ± 0.01 d |
| Ash | 13.46 ± 0.01 c | 2.35 ± 0.05 g | 17.77 ± 0.07 b | 3.21 ± 0.03 f | 20.99 ± 0.04 a | 4.0 ± 0.1 e | 11.91 ± 0.09 d |
| Dietary fibers (%) | 34.2 ± 0.2 b | 20.9 ± 0.2 d | 33.9 ± 0.4 b | 19.5 ± 0.6 e | 37.3 ± 0.7 a | 19.9 ± 0.1 e | 33.1 ± 0.9 c |
| Carbohydrates | 37.18 ± 0.05 d | 70.2 ± 0.2 a | 32.8 ± 0.3 e | 70.6 ± 0.4 a | 25.5 ± 0.5 f | 68.3 ± 0.1 b | 42.9 ± 0.6 c |
| Energy (kcal/100 g dw) | 294.24 ± 0.5 e | 354.4 ± 0.5 a | 277.5 ± 0.7 f | 350.5 ± 0.7 b | 261.5 ± 0.8 g | 347.0 ± 0.3 c | 297.1 ± 0.8 d |
| Free sugars | (g/100 g dw) | ||||||
| Frutose | 2.8 ± 0.02 a | nd | 0.97 ± 0.07 c | nd | 0.45 ± 0.04 d | nd | 1.57 ± 0.02 b |
| Glucose | 5.65 ± 0.08 a | nd | 3.84 ± 0.06 b | nd | 2.24 ± 0.04 c | nd | 3.5 ± 0.06 d |
| Sucrose | 5.08 ± 0.04 e | 35.93 ± 0.03 b | 6.21 ± 0.04 d | 33.94 ± 0.03 c | 3.24 ± 0.04 g | 40.79 ± 0.67 a | 4.04 ± 0.08 f |
| Trehalose | 0.69 ± 0.03 b | 0.38 ± 0.01 d | 0.82 ± 0.01 a | 0.46 ± 0.03 c | 0.7 ± 0.05 b | 0.7 ± 0.03 b | 0.41 ± 0.06 d |
| Total | 11.4 ± 0.2 d | 36.31 ± 0.03 b | 10.87 ± 0.03 e | 34.4 ± 0.06 c | 6.2 ± 0.1 g | 41.5 ± 0.6 a | 7.96 ± 0.2 f |
| Organic acids | (g/100 g dw) | ||||||
| Oxalic acid | 2.13 ± 0.02 d | 0.89 ± 0.02 f | 2.89 ± 0.03 b | 0.85 ± 0.03 f | 3.78 ± 0.21 a | 1.33 ± 0.01 e | 2.37 ± 0.03 c |
| Quinic acid | 1.61 ± 0.03 d | 0.49 ± 0.04 g | 3.15 ± 0.07 b | 0.64 ± 0.04 f | 3.97 ± 0.09 a | 1.36 ± 0.01 e | 2.54 ± 0.01 c |
| Malic acid | 1.44 ± 0.01 c | 0.34 ± 0.01 g | 1.86 ± 0.04 b | 0.76 ± 0.02 f | 2.8 ± 0.04 a | 0.95 ± 0.01 e | 1.40 ± 0.01 d |
| Citric acid | 1.42 ± 0.02 d | 0.35 ± 0.02 g | 2.02 ± 0.03 c | 0.81 ± 0.03 f | 2.67 ± 0.05 a | 1.11 ± 0.02 e | 2.2 ± 0.2 b |
| Succinic acid | 5.2 ± 0.3 f | 6.0 ± 0.2 e | 6.7 ± 0.1 d | 4.29 ± 0.01 g | 9.7 ± 0.2 c | 15 ± 0.3 a | 12.4 ± 0.07 b |
| Fumaric acid | tr | tr | tr | tr | tr | tr | tr |
| Total | 11.8 ± 0.4 e | 8.1 ± 0.3 f | 16.6 ± 0.3 d | 7.4 ± 0.1 g | 22.9 ± 0.2 a | 19.7 ± 0.3 c | 20.9 ± 0.3 b |
| Beta vulgaris cv. Albina Vereduna | Beta vulgaris cv. Burpee’s Golden | Beta vulgaris cv. Pablo F1 | Beta vulgaris subsp. cicla var. Flavescens | ||||
|---|---|---|---|---|---|---|---|
| Leaves | Roots | Leaves | Roots | Leaves | Roots | Leaves | |
| Fatty Acids | Relative Percentage (%) | ||||||
| C11:0 | nd | 3.57 ± 0.02 | nd | 0.55 ± 0.01 | 0.11 ± 0.01 | 0.47 ± 0.01 | nd |
| C12:0 | 0.61 ± 0.01 | 0.49 ± 0.01 | 0.21 ± 0.01 | 0.75 ± 0.01 | 0.21 ± 0.01 | 0.41 ± 0.01 | 0.10 ± 0.01 |
| C13:0 | 0.60 ± 0.01 | nd | 0.61 ± 0.04 | nd | 0.64 ± 0.04 | 0.054 ± 0.002 | 0.56 ± 0.02 |
| C14:0 | 0.91 ± 0.02 | 0.83 ± 0.01 | 0.64 ± 0.01 | 0.90 ± 0.01 | 0.75 ± 0.02 | 0.69 ± 0.01 | 0.52 ± 0.01 |
| C14:1 | nd | nd | 0.26 ± 0.01 | nd | nd | nd | nd |
| C15:0 | 0.22 ± 0.01 | 1.05 ± 0.01 | 0.19 ± 0.01 | 1.4 ± 0.04 | 0.21 ± 0.01 | 0.76 ± 0.03 | 0.19 ± 0.01 |
| C15:1 | 0.17 ± 0.02 | nd | 0.19 ± 0.01 | nd | nd | nd | 0.19 ± 0.01 |
| C16:0 | 19.17 ± 0.05 | 42.6 ± 0.1 | 19.6 ± 0.3 | 57.7 ± 0.1 | 20 ± 0.2 | 29.3 ± 0.4 | 19.6 ± 0.5 |
| C16:1 | 1.59 ± 0.02 | nd | 1.64 ± 0.08 | nd | 1.78 ± 0.04 | 0.61 ± 0.02 | 1.43 ± 0.03 |
| C17:0 | 0.55 ± 0.01 | 1.99 ± 0.03 | 0.48 ± 0.02 | 7.14 ± 0.09 | 0.48 ± 0.03 | 1.87 ± 0.03 | 0.36 ± 0.03 |
| C18:0 | 2.07 ± 0.02 | 4.9 ± 0.01 | 1.4 ± 0.08 | 2.74 ± 0.03 | 1.66 ± 0.01 | 2.55 ± 0.1 | 1.36 ± 0.03 |
| C18:1n9c | 7.5 ± 0.01 | 9.53 ± 0.03 | 7 ± 0.2 | 17.4 ± 0.1 | 7.5 ± 0.2 | 16.03 ± 0.04 | 7.67 ± 0.09 |
| C18:2n6c | 13.82 ± 0.01 | 21.37 ± 0.06 | 12.61 ± 0.07 | 2.95 ± 0.02 | 13.1 ± 0.1 | 37.2 ± 0.4 | 17.6 ± 0.1 |
| C18:3n6 | nd | 1.99 ± 0.01 | 0.34 ± 0.01 | 1.03 ± 0.01 | nd | nd | 0.37 ± 0.02 |
| C18:3n3 | 49.85 ± 0.03 | 2.7 ± 0.01 | 52.5 ± 0.5 | 0.31 ± 0.01 | 50.3 ± 0.7 | 5.53 ± 0.03 | 47.8 ± 0.1 |
| C20:0 | nd | nd | nd | 0.82 ± 0.02 | nd | nd | nd |
| C20:1 | 0.45 ± 0.01 | nd | 0.31 ± 0.01 | 0.78 ± 0.01 | 0.41 ± 0.01 | 0.61 ± 0.02 | 0.45 ± 0.02 |
| C22:0 | 0.85 ± 0.02 | 2.39 ± 0.07 | 0.67 ± 0.05 | 1.15 ± 0.01 | 0.96 ± 0.02 | 1.08 ± 0.02 | 0.51 ± 0.01 |
| C22:1 | nd | nd | nd | 0.32 ± 0.01 | nd | nd | nd |
| C23:0 | 0.53 ± 0.01 | 1.71 ± 0.05 | 0.36 ± 0.01 | 1.23 ± 0.01 | 0.45 ± 0.01 | 1.00 ± 0.01 | 0.25 ± 0.01 |
| C24:0 | 1.11 ± 0.01 | 4.89 ± 0.01 | 0.97 ± 0.06 | 2.92 ± 0.02 | 1.31 ± 0.01 | 1.8 ± 0.03 | 0.74 ± 0.03 |
| SFA | 26.62 ± 0.01 d | 64.4 ± 0.1 b | 25.1 ± 0.6 e | 77.3 ± 0.2 a | 26.8 ± 0.3 d | 40 ± 0.5 c | 24.2 ± 0.4 f |
| MUFA | 9.71 ± 0.04 d | 9.53 ± 0.03 e | 9.4 ± 0.08 f | 18.4 ± 0.2 a | 9.7 ± 0.2 d | 17.25 ± 0.01 b | 9.9 ± 0.1 c |
| PUFA | 63.67 ± 0.04 c | 26.06 ± 0.08 e | 65.5 ± 0.5 b | 4.29 ± 0.03 f | 63.5 ± 0.5 c | 42.8 ± 0.5 d | 65.8 ± 0.2 a |
| Tocopherols | (mg/100 g dw) | ||||||
| α-Tocopherol | 26.02 ± 0.05 c | 0.22 ± 0.01 f | 35.68 ± 0.03 a | 0.09 ± 0.01 g | 26.06 ± 0.01 b | 0.31 ± 0.01 e | 17.23 ± 0.09 d |
| β-Tocopherol | 0.393 ± 0.003 a | nd | 0.37 ± 0.02 b | nd | 0.40 ± 0.01 a | nd | 0.40 ± 0.01 a |
| γ-Tocopherol | nd | nd | 3.10 ± 0.05 a | nd | 0.42 ± 0.03 b | nd | 0.42 ± 0.01 b |
| Total | 26.41 ± 0.04 c | 0.22 ± 0.01 f | 39.15 ± 0.06 a | 0.09 ± 0.01 g | 26.88 ± 0.01 b | 0.31 ± 0.01 e | 18.05 ± 0.08 d |
| Leaves | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Quantification (mg/g of dw) | |||||||||
| Peak n.° | Rt (min) | λmax (nm) | [M-H]+ (m/z) | MS2 Fragments (m/z) | Tentative Identification | Leaves | Roots | ||
| Beta vulgaris cv. Pablo F1 | Beta vulgaris subsp. cicla var. Flavescens | Beta vulgaris cv. Pablo F1 | Beta vulgaris cv. Burpee’s Golden | ||||||
| 1 | 18.00 | 534 | 551 | MS2 (551): 389 (100), 390 (11), 302 (2) | Betanin [51,52,53,54] | 0.820 ± 0.001 c | 3.86 ± 0.02 a | 0.90 ± 0.02 b | nd |
| 2 | 19.40 | 532 | 551 | MS2 (551): 389 (100), 390 (19) | Isobetanin [51,52,53] | 0.800 ± 0.002 c | 2.35 ± 0.04 a | 1.48 ± 0.07 b | nd |
| 3a * | 20.65 | 468 | 347 | MS2 (347): 303 (100), 137 (93), 259 (57), 106 (44), 194 (14), 164 (12) | Miraxanthin-V [51,55] | nd | 0.350 ± 0.007 a | nd | 0.14 ± 0.002 b |
| 3b * | 20.65 | 501 | 507 | MS2 (507): 345 (100), 346 (20), 257 (3), 417 (3), 357 (2) | Decarboxy-betanin [54] | 0.210 ± 0.001 c | 0.350 ± 0.007 b | 0.69 ± 0.05 a | nd |
| 4 | 22.50 | 533 | 507 | MS2 (507): 345 (100), 346 (12), 479 (1) | Decarboxy-isobetanin [54] | 1.29 ± 0.02 c | 2.54 ± 0.02 b | 18.18 ± 0.03 a | nd |
| 5 | 23.30 | 535 | 549 | MS2 (549): 387 (100), 388 (20), 343 (1) | Neobetanin [53,54] | nd | 0.300 ± 0.008 b | 0.63 ± 0.06 a | nd |
| 6 | 26.10 | 481 | 505 | MS2 (505): 343 (100), 344 (11), 296 (3) | Decarboxy-neobetanin [53,54] | nd | 0.170 ± 0.001 b | 1.64 ± 0.11 a | nd |
| 7 | 28.80 | 535 | 889 | MS2 (889): 389 (100), 390 (14), 713 (3), 727 (3) | Feruloyl-hexosyl betanin [53] | nd | 0.47 ± 0.04 a | nd | nd |
| Total betacyanins | 3.12 ± 0.02 c | 10.0 ± 0.1 b | 23 ± 1 a | - | |||||
| Total betaxanthins | - | 0.350 ± 0.007 a | - | 0.140 ± 0.002 b | |||||
| Total betalains | 3.12 ± 0.02 c | 10.4 ± 0.1 b | 23 ± 1 a | 0.140 ± 0.002 d | |||||
| Beta vulgaris cv. Albina Vereduna | Beta vulgaris cv. Burpee’s Golden | Beta vulgaris cv. Pablo F1 | Beta vulgaris subsp. cicla var. Flavescens | |||||
|---|---|---|---|---|---|---|---|---|
| Leaves | Roots | Leaves | Roots | Leaves | Roots | Leaves | Trolox | |
| TBARS (EC50, mg/mL) | 0.39 ± 0.06 f | 3.06 ± 0.05 b | 0.65 ± 0.02 e | 12.3 ± 0.6 a | 0.35 ± 0.02 g | 1.13 ± 0.04 c | 0.88 ± 0.02 d | 0.054 ± 0.003 |
| Beta vulgaris cv. Albina Vereduna | Beta vulgaris cv. Burpee’s Golden | Beta vulgaris cv. Pablo F1 | Beta vulgaris subsp. cicla var. Flavescens | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive Controls | ||||||||||||||||||||
| Antibacterial Activity | Leaves | Roots | Leaves | Roots | Leaves | Roots | Leaves | Streptomicin 1 mg/mL | Methicilin 1 mg/mL | Ampicillin 10 mg/mL | ||||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Gram-negative bacteria | ||||||||||||||||||||
| Enterobacter cloacae | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 0.007 | 0.007 | nt * | nt | 0.15 | 0.15 |
| Escherichia coli | 5 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 5 | >10 | 10 | >10 | 5 | >10 | 0.01 | 0.01 | nt | nt | 0.15 | 0.15 |
| Pseudomonas aeruginosa | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 0.06 | 0.06 | nt | nt | 0.63 | 0.63 |
| Salmonella enterocolitica | 5 | >10 | 5 | >10 | 5 | >10 | >10 | >10 | 10 | >10 | 10 | >10 | 5 | >10 | 0.007 | 0.007 | nt | nt | 0.15 | 0.15 |
| Yersinia enterocolitica | 1.25 | >10 | 1.25 | >10 | 2.5 | >10 | >10 | >10 | 5 | >10 | 5 | >10 | 2.5 | >10 | 0.007 | 0.007 | nt | nt | 0.15 | 0.15 |
| Gram-positive bacteria | ||||||||||||||||||||
| Bacillus cereus | 10 | >10 | >10 | >10 | 5 | >10 | >10 | >10 | 10 | >10 | 2.5 | >10 | >10 | >10 | 0.007 | 0.007 | nt | nt | nt | nt |
| Listeria monocytogenes | 10 | >10 | >10 | >10 | 2.5 | >10 | 5 | >10 | 2.5 | >10 | 5 | >10 | 5 | >10 | 0.007 | 0.007 | nt | nt | 0.15 | 0.15 |
| Staphylococcus aureus | 0.6 | >10 | 1.25 | >10 | 2.5 | >10 | 5 | >10 | 5 | >10 | 1.25 | >10 | 0.6 | >10 | 0.007 | 0.007 | 0.007 | 0.007 | 0.15 | 0.15 |
| Antifungal activity | Ketoconazole | |||||||||||||||||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | - | - | - | - | |
| A. fumigatus | >10 | >10 | 5 | >10 | >10 | >10 | 10 | 10 | >10 | 10 | >10 | 10 | 10 | 10 | 0.5 | 0.06 | - | - | - | - |
| A. brasiliensis | 10 | >10 | 5 | >10 | 10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 1 | 0.125 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, D.; Petropoulos, S.A.; da Silveira, T.F.F.; Pires, T.C.S.P.; Ferreira, I.C.F.R.; Fernandes, Â.; Barros, L. Exploring the Biochemical Profile of Beta vulgaris L.: A Comparative Study of Beetroots and Swiss Chard. Plants 2025, 14, 591. https://doi.org/10.3390/plants14040591
Almeida D, Petropoulos SA, da Silveira TFF, Pires TCSP, Ferreira ICFR, Fernandes Â, Barros L. Exploring the Biochemical Profile of Beta vulgaris L.: A Comparative Study of Beetroots and Swiss Chard. Plants. 2025; 14(4):591. https://doi.org/10.3390/plants14040591
Chicago/Turabian StyleAlmeida, Daiana, Spyridon A. Petropoulos, Tayse F. F. da Silveira, Tânia C. S. P. Pires, Isabel C. F. R. Ferreira, Ângela Fernandes, and Lillian Barros. 2025. "Exploring the Biochemical Profile of Beta vulgaris L.: A Comparative Study of Beetroots and Swiss Chard" Plants 14, no. 4: 591. https://doi.org/10.3390/plants14040591
APA StyleAlmeida, D., Petropoulos, S. A., da Silveira, T. F. F., Pires, T. C. S. P., Ferreira, I. C. F. R., Fernandes, Â., & Barros, L. (2025). Exploring the Biochemical Profile of Beta vulgaris L.: A Comparative Study of Beetroots and Swiss Chard. Plants, 14(4), 591. https://doi.org/10.3390/plants14040591











