Effects of Increasing CO2 Concentration on Crop Growth and Soil Ammonia-Oxidizing Microorganisms in a Fababean (Vicia faba L.) and Wheat (Triticum aestivum Yunmai) Intercropping System
Abstract
1. Introduction
2. Results
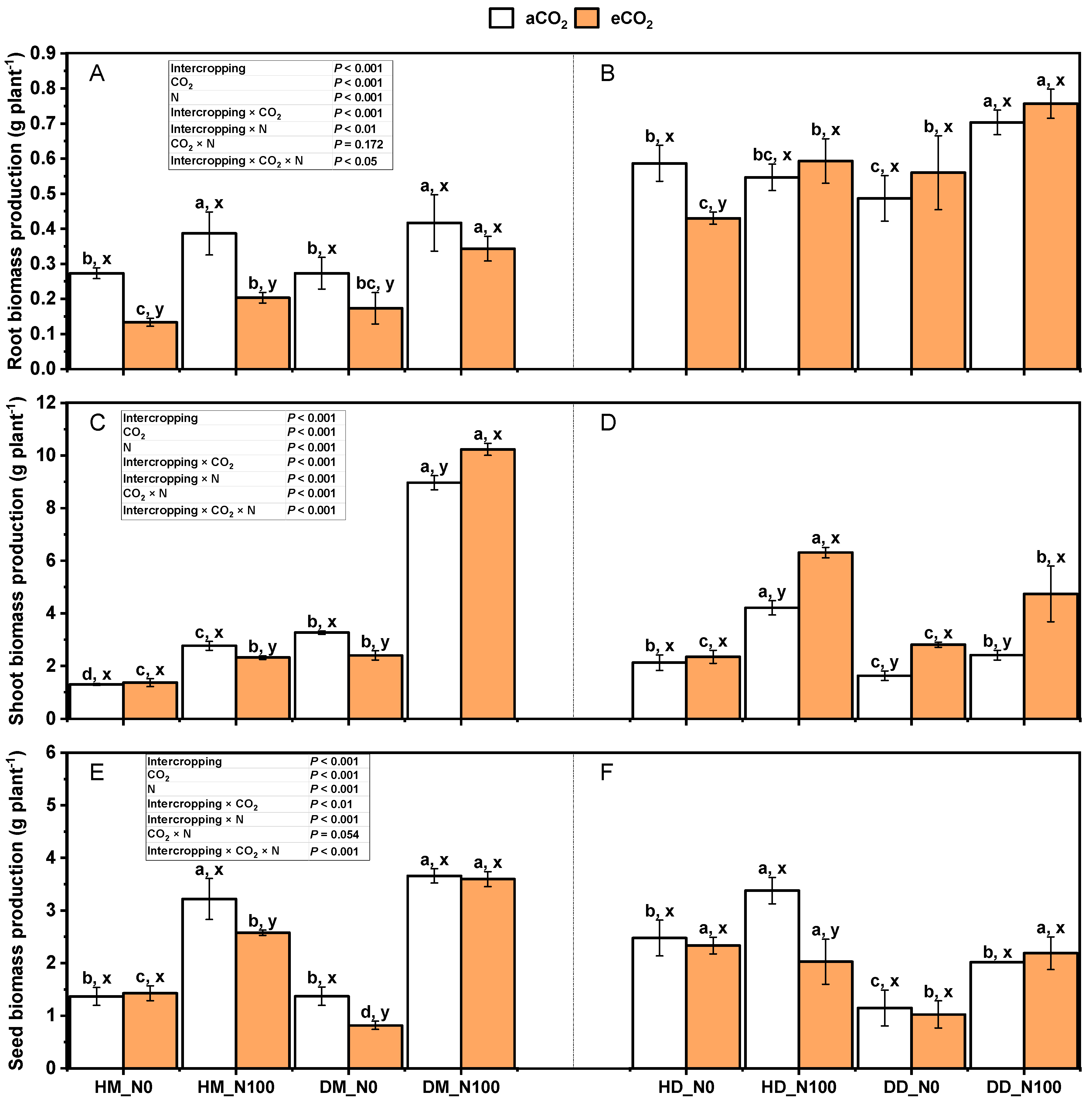
2.1. Effects of Nitrogen and eCO2 on Growth Indexes of Intercropping Fababean and Wheat
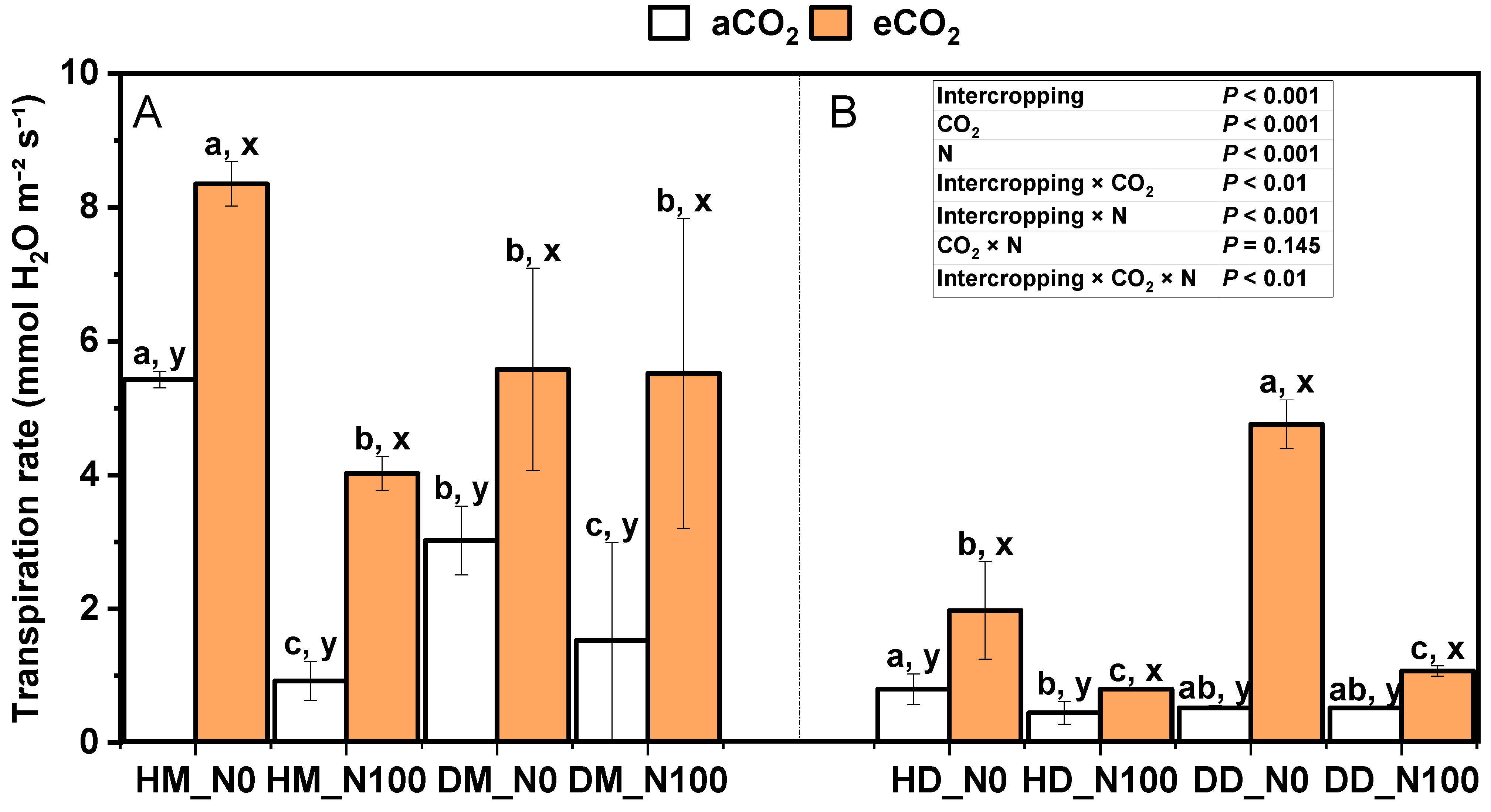
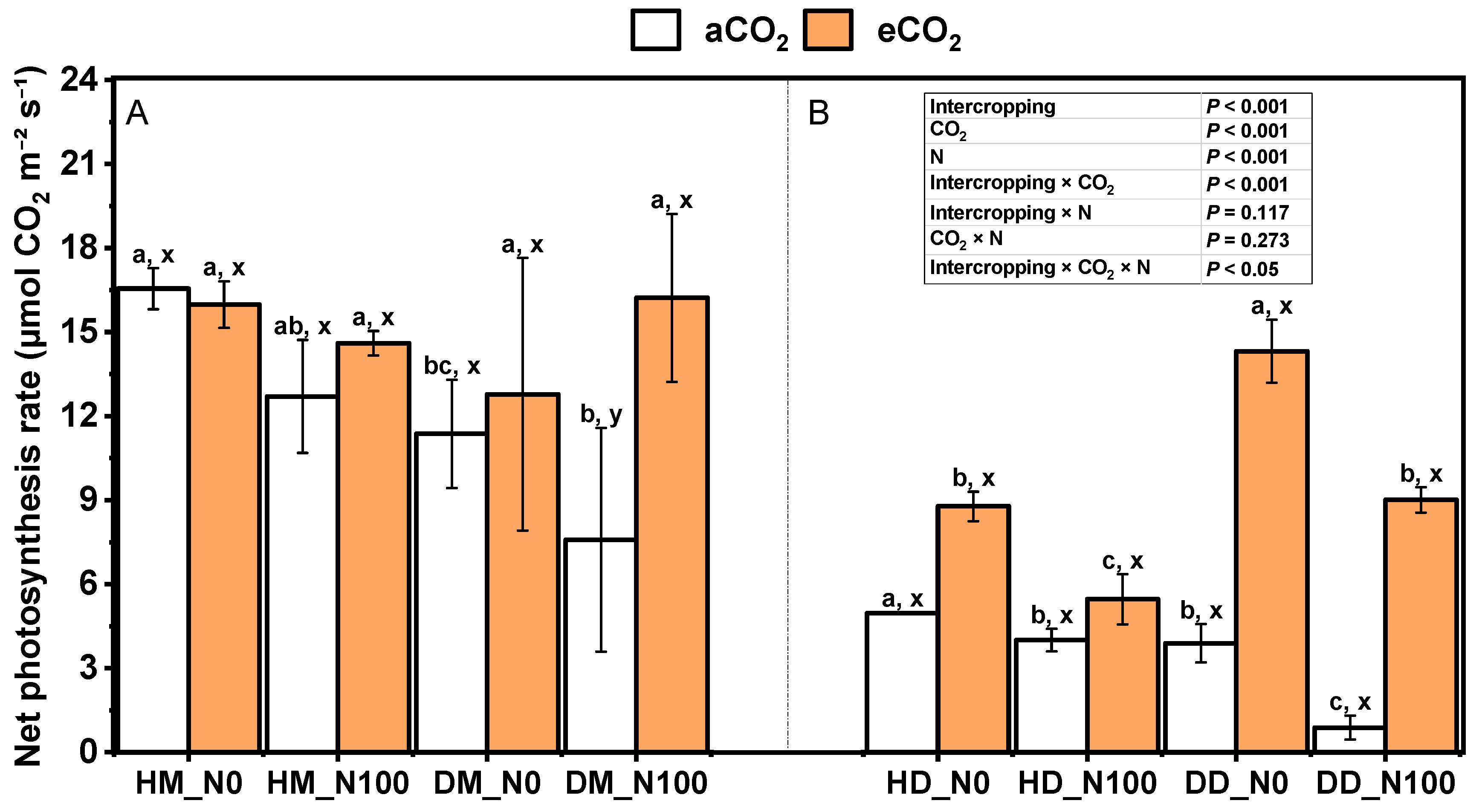
2.2. Effects of Nitrogen and eCO2 on Photosynthetic Parameters of Intercropping Fababean and Wheat Leaves
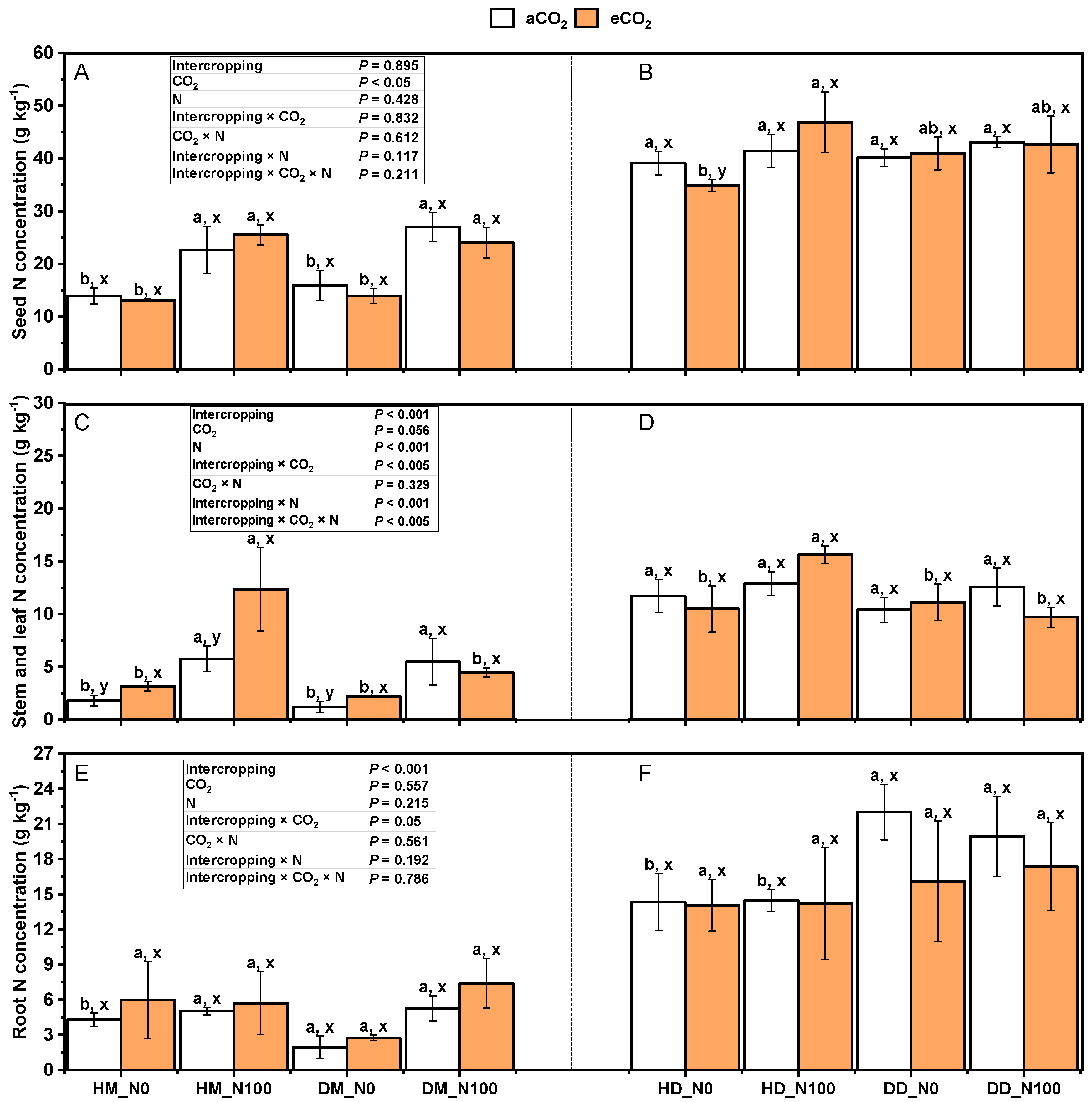
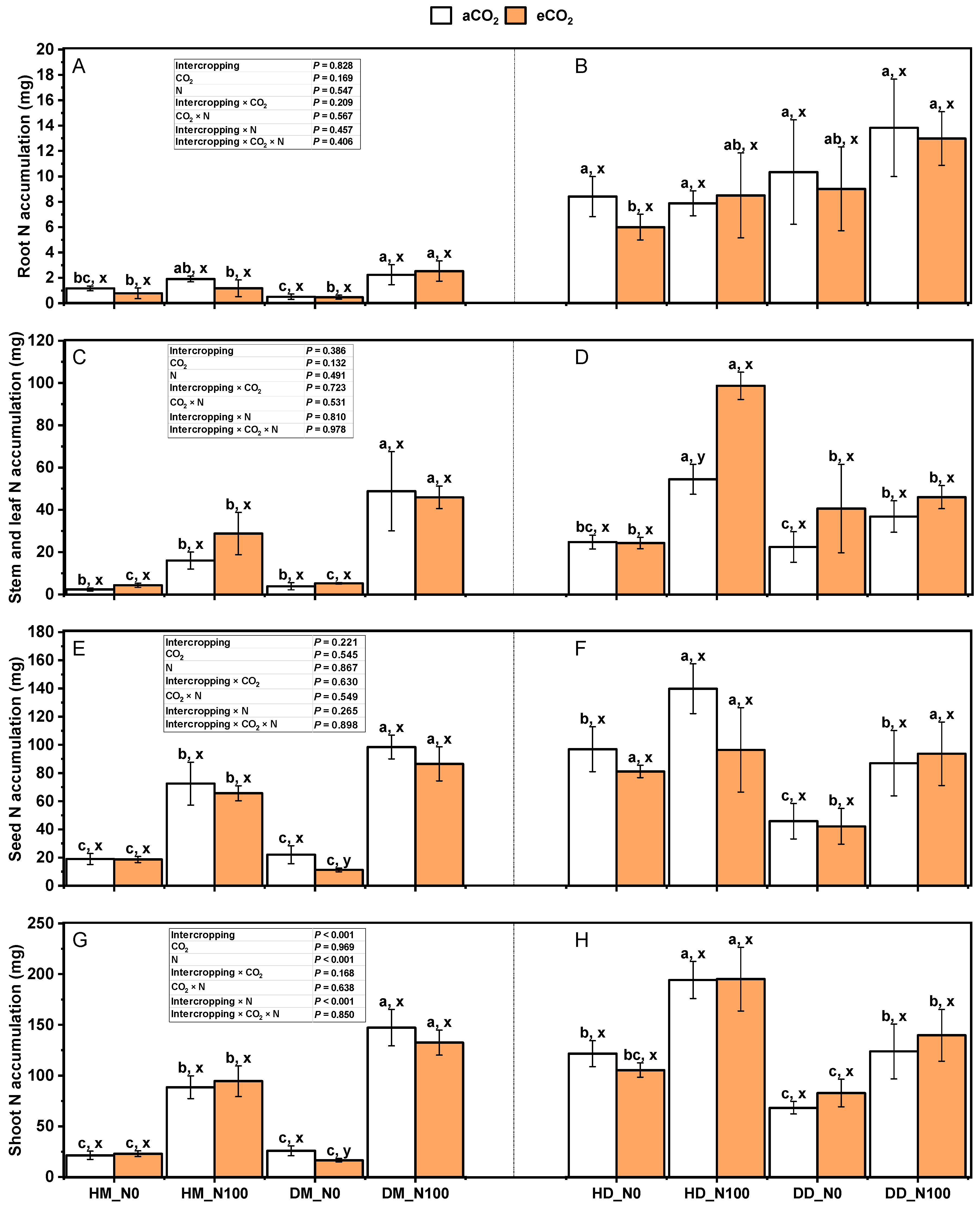
2.3. Effects of Nitrogen and eCO2 on Total Nitrogen in Various Parts of Intercropping Fababean and Wheat
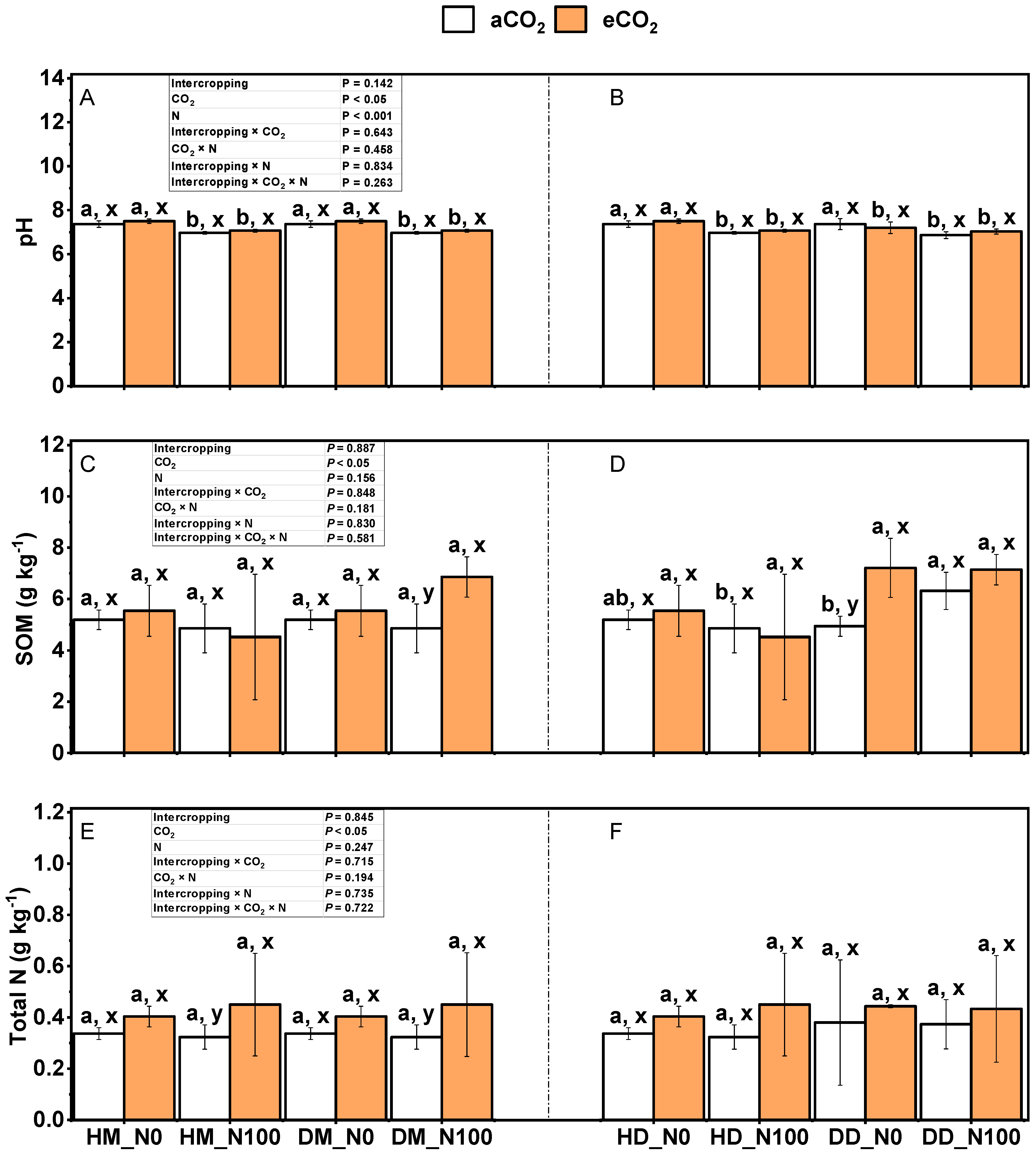
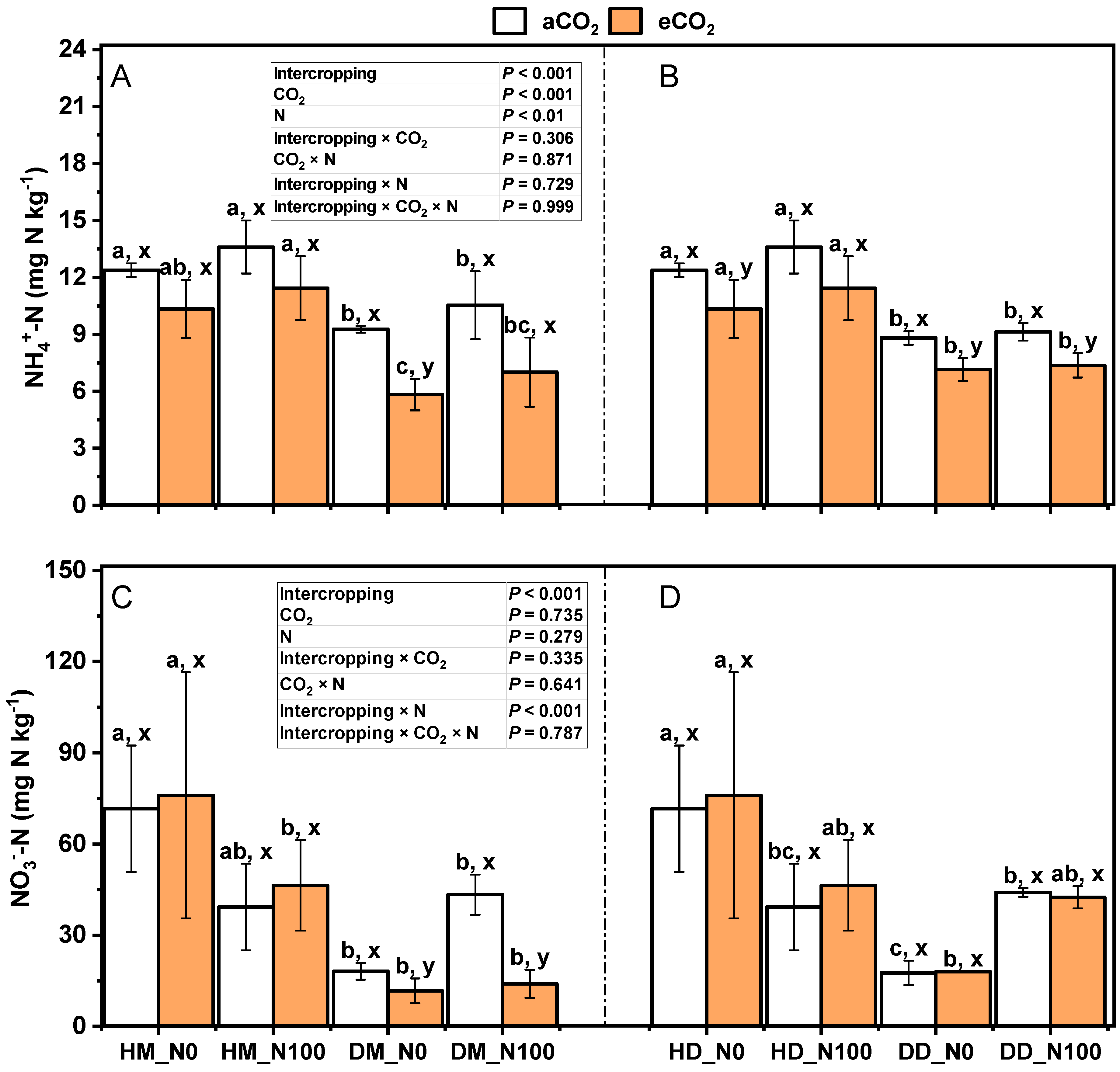
2.4. Effects of Nitrogen and eCO2 Application on pH, Organic Matter, Total Nitrogen, NH4+-N and NO3−-N in Intercropped Soils
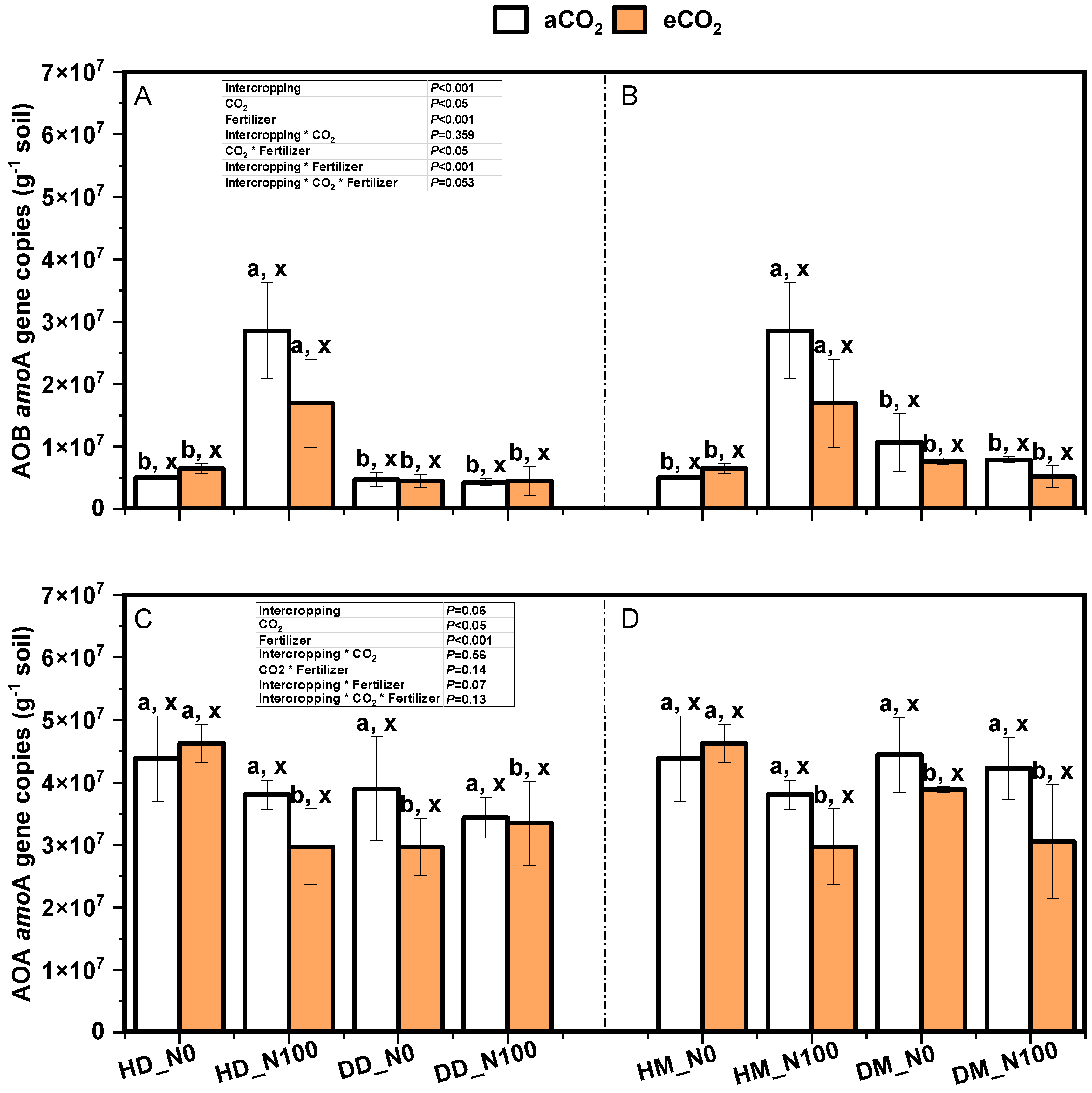
2.5. Effects of Nitrogen and eCO2 on the Gene Copy Number of Ammonia-Oxidizing Bacteria and Ammonia-Oxidizing Archaea in Soil
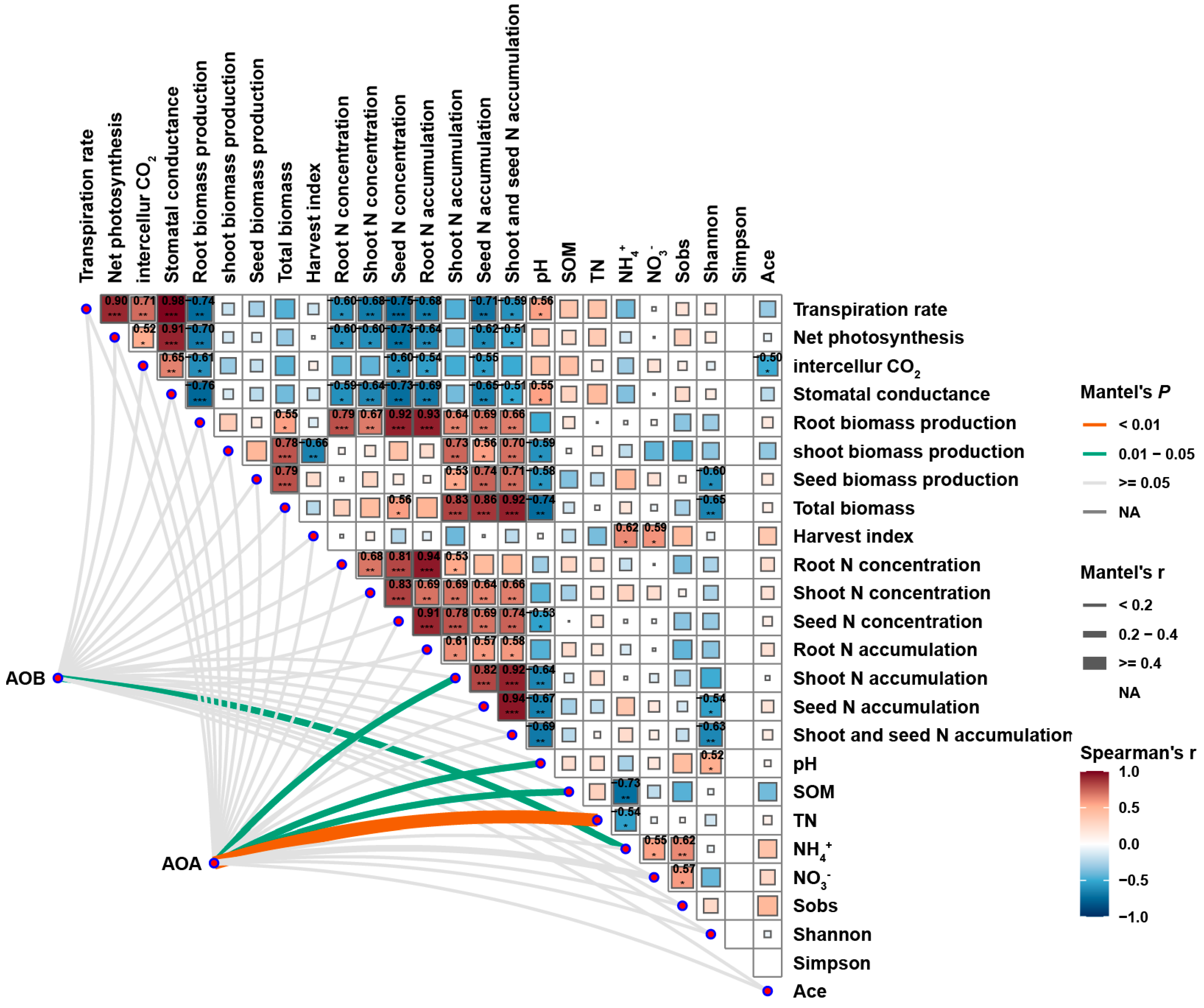
2.6. Pairwise Spearman Correlation Matrix of AOA and AOB Abundance, Environmental Factors, Plant Growth Indexes, and Leaf Photosynthetic Parameters Under Nitrogen Application and eCO2 Treatment
3. Discussion
3.1. Response of Fababean and Wheat Biomass to Nitrogen and eCO2 Application in Intercropping System
3.2. Response of Photosynthetic Parameters of Fababean and Wheat Leaves to Nitrogen and eCO2 Application in Intercropping System
3.3. Response of Nitrogen Concentration in Various Parts of Fababean and Wheat to Nitrogen Application and eCO2 in Intercropping System
3.4. Response of Soil Ammonia-Oxidizing Microorganisms to Nitrogen and eCO2 Application in Intercropping System
4. Methods and Materials
4.1. Plant and Soil Sampling Methods
4.2. Measurement Method of Photosynthetic Parameters
4.3. Methods for Testing Physical and Chemical Properties of Plants and Soils
4.4. Quantitative PCR Method
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC. Changing state of the climate system. In Climate Change 2021—The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change, Ed.; Cambridge University Press: Cambridge, UK, 2023; pp. 287–422. [Google Scholar]
- Ruan, Y.; Kuzyakov, Y.; Liu, X.; Zhang, X.; Xu, Q.; Guo, J.; Guo, S.; Shen, Q.; Yang, Y.; Ling, N. Elevated temperature and CO2 strongly affect the growth strategies of soil bacteria. Nat. Commun. 2023, 14, 391. [Google Scholar] [CrossRef]
- Mardani, A.; Streimikiene, D.; Cavallaro, F.; Loganathan, N.; Khoshnoudi, M. Carbon dioxide (CO2) emissions and economic growth: A systematic review of two decades of research from 1995 to 2017. Sci. Total Environ. 2019, 649, 31–49. [Google Scholar] [CrossRef]
- He, M.; Cui, J.; Zhang, Q.; Li, L.; Huang, L.; Hong, S. Unraveling the role of vegetation CO2 physiological forcing on climate zone shifts in China. Geophys. Res. Lett. 2024, 51, e2023GL107826. [Google Scholar] [CrossRef]
- Houshmandfar, A.; Fitzgerald, G.; O’Leary, G.; Tausz-Posch, S.; Fletcher, A.; Tausz, M. The relationship between transpiration and nutrient uptake in wheat changes under elevated atmospheric CO2. Physiol. Plant. 2017, 163, 516–529. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, G.; Li, P.; Xue, S. Elevated CO2 and nitrogen addition have minimal influence on the rhizospheric effects of Bothriochloa ischaemum. Sci. Rep. 2017, 7, 6527. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, L.; Yang, T.; Liu, R.; Redden, B.; Maalouf, F.; Zong, X. Food legume production in China. Crop J. 2017, 5, 115–126. [Google Scholar] [CrossRef]
- Reich, P.B.; Knops, J.; Tilman, D.; Craine, J.; Ellsworth, D.; Tjoelker, M.; Lee, T.; Wedin, D.; Naeem, S.; Bahauddin, D.; et al. Plant diversity enhances ecosystem responses to elevated CO2 and nitrogen deposition. Nature 2001, 410, 809–812. [Google Scholar] [CrossRef]
- Luscher, A.; Hendrey, G.R.; Nosberger, J. Long-term responsiveness to free air CO2 enrichment of functional types, species and genotypes of plants from fertile permanent grassland. Oecologia 1998, 113, 37–45. [Google Scholar]
- Torres, A.; Avila, C.; Stoddard, F.; Cubero, J. Genetics, Genomics and Breeding of Cool Season Grain Legumes, 1st ed.; Taylor Francis Group: Boca Raton, FL, USA, 2012; pp. 50–97. [Google Scholar]
- Ainsworth, E.A.; Rogers, A.; Nelson, R.; Long, S.P. Testing the “source-sink” hypothesis of down-regulation of photosynthesis in elevated CO2 in the field with single gene substitutions in Glycine max. Agric. For. Meteorol. 2004, 122, 85–94. [Google Scholar] [CrossRef]
- Luo, C.; Zhu, J.; Ma, L.; Guo, Z.; Dong, K.; Dong, Y. Effects of nitrogen regulation and strip intercropping on faba bean biomass, nitrogen accumulation and distribution, and interspecific interactions. Crop Sci. 2021, 61, 4325–4343. [Google Scholar] [CrossRef]
- Ma, G.; Zheng, Y.; Zhang, J.; Guo, Z.; Dong, Y. Changes in canopy microclimate of faba bean under intercropping at controlled nitrogen levels and their correlation with crop yield. J. Sci. Food Agric. 2023, 103, 4489–4502. [Google Scholar] [CrossRef] [PubMed]
- Lukac, M.; Calfapietra, C.; Lagomarsino, A.; Loreto, F. Global climate change and tree nutrition: Effects of elevated CO2 and temperature. Tree Physiol. 2010, 30, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Su, B.; Currie, W.S.; Dukes, J.S.; Finzi, A.C.; Hartwig, U.; Hungate, B.; McMurtrie, R.E.; Oren, R.; Parton, W.J.; et al. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 2004, 54, 731–739. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Broughton, K.; Osanai, Y.; Anderson, I.C.; Bange, M.P.; Tissue, D.T.; Singh, B.K. Effects of elevated temperature and elevated CO2 on soil nitrification and ammonia-oxidizing microbial communities in field-grown crop. Sci. Total Environ. 2019, 675, 81–89. [Google Scholar] [CrossRef]
- Yu, M.; Liu, L.; Yang, S.; Yu, Z.; Li, S.; Yang, Y.; Shi, X. Experimental identification of CO2–oil–brine–rock interactions: Implications for CO2 sequestration after termination of a CO2-EOR project. Appl. Geochem. 2016, 75, 137–151. [Google Scholar] [CrossRef]
- He, Z.; Xiong, J.; Kent, A.D.; Deng, Y.; Xue, K.; Wang, G.; Wu, L.; Van Nostrand, J.D.; Zhou, J. Distinct responses of soil microbial communities to elevated CO2 and O3 in a soybean agro-ecosystem. ISME J. 2014, 8, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Lee, J.-D. Income and CO2 emissions: Evidence from panel unit root and cointegration tests. Energy Policy 2009, 37, 413–423. [Google Scholar] [CrossRef]
- Hu, B.J.; Zheng, Y.R.; Lv, J.X.; Zhang, J.; Dong, Y. Proteomic analysis of the faba bean-wheat intercropping system in controlling the occurrence of faba bean fusarium wilt due to stress caused by Fusarium oxysporum f. sp.and benzoic acid. BMC Plant Biol. 2023, 23, 472. [Google Scholar] [CrossRef] [PubMed]
- De Long, J.R.; van Malland, F.; de Buck, A.; van den Berg, M. Wheat and faba bean intercropping and cultivar impacts on morphology, disease, and yield. Agron. J. 2023, 115, 3010–3024. [Google Scholar] [CrossRef]
- Chapagain, T.; Riseman, A. Intercropping Wheat and Beans: Effects on Agronomic Performance and Land Productivity. Crop Sci. 2014, 54, 2285–2293. [Google Scholar] [CrossRef]
- Zheng, Y.R.; Guo, Y.T.; Li, Y.; Yang, W.H.; Dong, Y. Intercropping of wheat alleviates the adverse effects of phenolic acids on faba bean. Front. Plant Sci. 2022, 13, 997768. [Google Scholar] [CrossRef]
- Zhu, Y.A.; He, J.Y.; Yu, Z.Y.; Zhou, D.; Li, H.Y.; Wu, X.Y.; Dong, Y.; Tang, L.; Zheng, Y.; Xiao, J.X. Wheat and faba bean intercropping together with nitrogen modulation is a good option for balancing the trade-off relationship between grain yield and quality in the southwest of China. Agronomy 2022, 12, 2984. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, B.; Zheng, Y.; Zhang, Z.; Wang, B.; Dong, K.; Dong, Y. Long term intercropping promotes improvement of soil quality and alleviates faba bean wilt disease. Agric. Ecosyst. Environ. 2025, 381, 109443. [Google Scholar] [CrossRef]
- Yang, W.H.; Zhang, Z.Y.; Yuan, T.T.; Li, Y.; Zhao, Q.; Dong, Y. Intercropping improves faba bean photosynthesis and reduces disease caused by and cinnamic acid-induced stress. BMC Plant Biol. 2024, 24, 650. [Google Scholar] [CrossRef]
- Abou Khater, L.; Maalouf, F.; Balech, R.; He, Y.H.; Zong, X.X.; Rubiales, D.; Kumar, S. Improvement of cool-season food legumes for adaptation to intercropping systems: Breeding faba bean for intercropping with durum wheat as a case study. Front. Plant Sci. 2024, 15, 1368509. [Google Scholar] [CrossRef]
- Prior, S.A.; Brett Runion, G.; Rogers, H.H.; Allen Torbert, H.; Wayne Reeves, D. Elevated atmospheric CO2 effects on biomass production and soil carbon in conventional and conservation cropping systems. Glob. Change Biol. 2005, 11, 657–665. [Google Scholar] [CrossRef]
- Rho, H.; Doty, S.L.; Kim, S.-H. Endophytes alleviate the elevated CO2 dependent decrease in photosynthesis in rice, particularly under nitrogen limitation. J. Exp. Bot. 2020, 71, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Gamage, D.; Thompson, M.; Sutherland, M.; Hirotsu, N.; Makino, A.; Seneweera, S. New insights into the cellular mechanisms of plant growth at elevated atmospheric carbon dioxide concentration. Plant Cell Environ. 2018, 41, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Hoffland, E.; Kuyper, T.W.; Yu, Y.; Li, H.G.; Zhang, C.C.; Zhang, F.S.; van der Werf, W. Yield gain, complementarity and competitive dominance in intercropping in China: A meta-analysis of drivers of yield gain using additive partitioning. Eur. J. Agron. 2020, 113, 125987. [Google Scholar] [CrossRef]
- Li, C.; Hoffland, E.; Kuyper, T.W.; Yu, Y.; Werf, W.V.D. Syndromes of production in intercropping impact yield gains. Nat. Plants 2020, 6, 653–660. [Google Scholar] [CrossRef]
- Barnard, R.; Le Roux, X.; Hungate, B.A.; Cleland, E.E.; Blankinship, J.C.; Barthes, L.; Leadley, P.W. Several components of global change alter nitrifying and denitrifying activities in an annual grassland. Funct. Ecol. 2006, 20, 557–564. [Google Scholar] [CrossRef]
- BassiriRad, H.; Griffin, K.L.; Reynolds, J.F.; Strain, B.R. Changes in root NH4+ and NO3− absorption rates of loblolly and ponderosa pine in response to CO2 enrichment. Plant Soil 1997, 190, 1–9. [Google Scholar] [CrossRef]
- BassiriRad, H.; Gutschick, V.P.; Lussenhop, J. Root system adjustments: Regulation of plant nutrient uptake and growth responses to elevated CO2. Oecologia 2001, 126, 305–320. [Google Scholar] [CrossRef]
- Beeckman, F.; Motte, H.; Beeckman, T. Nitrification in agricultural soils: Impact, actors and mitigation. Curr. Opin. Biotechnol. 2018, 50, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.-L.; Zhang, Y.; Qi, J.-H.; Zhang, Y.-J.; Hao, G.-Y.; Finnegan, P.M.; Yan, Q.-S.; Fan, Z.-X. The impact of elevated CO2 concentration on photosynthesis, growth and hydraulics of evergreen and deciduous tree seedlings from a subtropical forest in Southwest China. Agric. For. Meteorol. 2024, 352, 110021. [Google Scholar] [CrossRef]
- López-Arredondo, D.L.; Leyva-González, M.A.; Alatorre-Cobos, F.; Herrera-Estrella, L. Biotechnology of nutrient uptake and assimilation in plants. Int. J. Dev. Biol. 2013, 57, 595–610. [Google Scholar] [CrossRef]
- Tcherkez, G.; Limami, A.M. Net photosynthetic CO2 assimilation: More than just CO2 and O2 reduction cycles. New Phytol. 2019, 223, 520–529. [Google Scholar] [CrossRef]
- Bhattacharya, A. Effect of soil water deficit on growth and development of plants: A review. In Soil Water Deficit and Physiological Issues in Plants; Bhattacharya, A., Ed.; Springer: Singapore, 2021; pp. 393–488. [Google Scholar]
- Bourgault, M.; Webber, H.A.; Chenu, K.; O’Leary, G.J.; Gaiser, T.; Siebert, S.; Dreccer, F.; Huth, N.; Fitzgerald, G.J.; Tausz, M.; et al. Early vigour in wheat: Could it lead to more severe terminal drought stress under elevated atmospheric CO2 and semi-arid conditions? Glob. Change Biol. 2020, 26, 4079–4093. [Google Scholar] [CrossRef] [PubMed]
- Brazel, A.J.; Ó’Maoiléidigh, D.S. Photosynthetic activity of reproductive organs. J. Exp. Bot. 2019, 70, 1737–1754. [Google Scholar] [CrossRef]
- Brito, L.F.; Azenha, M.V.; Janusckiewicz, E.R.; Cardoso, A.S.; Morgado, E.S.; Malheiros, E.B.; La Scala, N., Jr.; Reis, R.A.; Ruggieri, A.C. Seasonal fluctuation of soil carbon dioxide emission in differently managed pastures. Agron. J. 2015, 107, 957–962. [Google Scholar] [CrossRef]
- Broberg, M.C.; Högy, P.; Feng, Z.; Pleijel, H. Effects of elevated CO2 on wheat yield: Non-linear response and relation to site productivity. Agronomy 2019, 9, 243. [Google Scholar] [CrossRef]
- Zheng, G.; Chen, J.; Li, W. Impacts of CO2 elevation on the physiology and seed quality of soybean. Plant Divers. 2020, 42, 44–51. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Osanai, Y.; Anderson, I.C.; Bange, M.P.; Braunack, M.; Tissue, D.T.; Singh, B.K. Impacts of waterlogging on soil nitrification and ammonia-oxidizing communities in farming system. Plant Soil 2018, 426, 299–311. [Google Scholar] [CrossRef]
- Dong, J.; Gruda, N.; Lam, S.K.; Li, X.; Duan, Z. Effects of elevated CO2 on nutritional quality of vegetables: A review. Front. Plant Sci. 2018, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising CO2: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Berghuijs, H.N.C.; Wang, Z.; Stomph, T.J.; Weih, M.; Vico, G. Identification of species traits enhancing yield in wheat-faba bean intercropping: Development and sensitivity analysis of a minimalist mixture model. Plant Soil 2020, 455, 203–226. [Google Scholar] [CrossRef]
- El Masri, B.; Schwalm, C.; Huntzinger, D.N.; Mao, J.F.; Shi, X.Y.; Peng, C.H.; Fisher, J.B.; Jain, A.K.; Tian, H.Q.; Poulter, B.; et al. Carbon and water use efficiencies: A comparative analysis of ten terrestrial ecosystem models under changing climate. Sci. Rep. 2019, 9, 14680. [Google Scholar] [CrossRef]
- Ali, A. Factors affecting on response of broad bean and corn to air quality and soil CO2 flux rates in Egypt. Water Air Soil Pollut. 2008, 195, 311–323. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, F.; Song, Y.; Sun, J.; Bao, X.; Guo, T.; Li, L. Nitrogen fixation of faba bean (Vicia faba L.) interacting with a non-legume in two contrasting intercropping systems. Plant Soil 2006, 283, 275–286. [Google Scholar] [CrossRef]
- Yamori, W.; Ghannoum, O. Adaptation of plants to a high CO2 and high-temperature world. Plant Mol. Biol. 2022, 110, 301–303. [Google Scholar] [CrossRef]
- Chowdhury, F.I.; Arteaga, C.; Alam, M.S.; Alam, I.; Resco de Dios, V. Drivers of nocturnal stomatal conductance in C3 and C4 plants. Sci. Total Environ. 2022, 814, 151952. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, I.; Hussain, S.; Raza, M.A.; Iqbal, N.; Asghar, M.A.; Raza, A.; Fan, Y.-f.; Mumtaz, M.; Shoaib, M.; Ansar, M.; et al. Crop photosynthetic response to light quality and light intensity. J. Integr. Agric. 2021, 20, 4–23. [Google Scholar] [CrossRef]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef]
- Franks, P.J.; Adams, M.A.; Amthor, J.S.; Barbour, M.M.; Berry, J.A.; Ellsworth, D.S.; Farquhar, G.D.; Ghannoum, O.; Lloyd, J.; McDowell, N.; et al. Sensitivity of plants to changing atmospheric CO2 concentration: From the geological past to the next century. New Phytol. 2013, 197, 1077–1094. [Google Scholar] [CrossRef]
- Yamori, W.; Kusumi, K.; Iba, K.; Terashima, I. Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant Cell Environ. 2020, 43, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, C.E.; Posada, J.; Winter, K. Effects of elevated CO2 and defoliation on compensatory growth and photosynthesis of seedlings in a tropical tree, Copaifera aromatica. Biotropica 1999, 31, 279–287. [Google Scholar] [CrossRef]
- Springer, C.J.; Ward, J.K. Flowering time and elevated atmospheric CO2. New Phytol. 2007, 176, 243–255. [Google Scholar] [CrossRef]
- Opoku, E.; Sahu, P.P.; Findurová, H.; Holub, P.; Urban, O.; Klem, K. Differential physiological and production responses of C3 and C4 crops to climate factor interactions. Front. Plant Sci. 2024, 15, 1345462. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Li, H.G.; Hoffland, E.; Zhang, F.S.; Zhang, J.L.; Kuyper, T.W. Common mycorrhizal networks asymmetrically improve chickpea N and P acquisition and cause overyielding by a millet/chickpea mixture. Plant Soil 2022, 472, 279–293. [Google Scholar] [CrossRef]
- Li, C.J.; Stomph, T.J.; Makowski, D.; Li, H.G.; Zhang, C.C.; Zhang, F.S.; van der Werf, W. The productive performance of intercropping. Proc. Natl. Acad. Sci. USA 2023, 120, e2201886120. [Google Scholar] [CrossRef]
- Xiao, J.; Yin, X.; Ren, J.; Zhang, M.; Tang, L.; Zheng, Y. Complementation drives higher growth rate and yield of wheat and saves nitrogen fertilizer in wheat and faba bean intercropping. Field Crops Res. 2018, 221, 119–129. [Google Scholar] [CrossRef]
- Alghamdi, S.S.; Migdadi, H.M.; Ammar, M.H.; Paull, J.G.; Siddique, K.H.M. Faba bean genomics: Current status and future prospects. Euphytica 2012, 186, 609–624. [Google Scholar] [CrossRef]
- Srivastava, A.C.; Tiku, A.K.; Pal, M. Nitrogen and carbon partitioning in soybean under variable nitrogen supplies and acclimation to the prolonged action of elevated CO2. Acta Physiol. Plant. 2006, 28, 181–188. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Horwath, W.R.; Dorodnikov, M.; Blagodatskaya, E. Review and synthesis of the effects of elevated atmospheric CO2 on soil processes: No changes in pools, but increased fluxes and accelerated cycles. Soil Biol. Biochem. 2019, 128, 66–78. [Google Scholar] [CrossRef]
- Lesaulnier, C.; Papamichail, D.; McCorkle, S.; Ollivier, B.; Skiena, S.; Taghavi, S.; Zak, D.; van der Lelie, D. Elevated atmospheric CO2 affects soil microbial diversity associated with trembling aspen. Environ. Microbiol. 2008, 10, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Liu, Y.X.; Rizwan, A.; Shoukat, C.A.; Aftab, Q.; Lu, J.F.; Zhang, Y.X. Impact of elevated CO2 on soil microbiota: A meta-analytical review of carbon and nitrogen metabolism. Sci. Total Environ. 2024, 950, 175354. [Google Scholar] [CrossRef]
- Jin, J.; Krohn, C.; Franks, A.E.; Wang, X.; Wood, J.L.; Petrovski, S.; McCaskill, M.; Batinovic, S.; Xie, Z.; Tang, C. Elevated atmospheric CO2 alters the microbial community composition and metabolic potential to mineralize organic phosphorus in the rhizosphere of wheat. Microbiome 2022, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, K.; Ishimaru, K.; Hirotsu, N.; Nagasaka, S.; Miyakoshi, Y.; Ota, M.; Tokida, T.; Sakai, H.; Usui, Y.; Ono, K.; et al. How elevated CO2 affects our nutrition in rice, and how we can deal with it. PLoS ONE 2019, 14, e0212840. [Google Scholar] [CrossRef] [PubMed]
- Asensio, J.S.; Rachmilevitch, S.; Bloom, A.J. Responses of arabidopsis and wheat to rising CO2 depend on nitrogen source and nighttime CO2 levels. Plant Physiol 2015, 168, 156–163. [Google Scholar] [CrossRef]
- Menzel, C.M. Climate change increases net CO2 assimilation in the leaves of strawberry, but not yield. J. Hortic. Sci. Biotechnol. 2024, 99, 233–266. [Google Scholar] [CrossRef]
- Madan, P.; Jagadish, S.V.K.; Craufurd, P.Q.; Fitzgerald, M.; Lafarge, T.; Wheeler, T.R. Effect of elevated CO2 and high temperature on seed-set and grain quality of rice. J. Exp. Bot. 2012, 63, 3843–3852. [Google Scholar] [CrossRef]
- Reich, P.B.; Hungate, B.A.; Luo, Y. Carbon-nitrogen interactions in terrestrial ecosystems in response to rising atmospheric carbon dioxide. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 611–636. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Kimball, B.A.; Pinter, P.J., Jr. Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat. Clim. Change 2014, 4, 477–480. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Ju, W.; Chen, J.M.; Ciais, P.; Cescatti, A.; Sardans, J.; Janssens, I.A.; Wu, M.; Berry, J.A.; et al. Recent global decline of CO2 fertilization effects on vegetation photosynthesis. Science 2020, 370, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wanasundara, J.P.D.; Shand, P. Short-term germination of faba bean (Vicia faba L.) and the effect on selected chemical constituents. Appl. Food Res. 2022, 2, 100030. [Google Scholar] [CrossRef]
- Yao, H.; Gao, Y.; Nicol, G.W.; Campbell, C.D.; Prosser, J.I.; Zhang, L.; Han, W.; Singh, B.K. Links between ammonia oxidizer community structure, abundance, and nitrification potential in acidic soils. Appl. Environ. Microbiol. 2011, 77, 4618–4625. [Google Scholar] [CrossRef] [PubMed]
- Bao, S. Soil Agrochemical analysis. In Soil Agrochemical Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis; Agronomy Monographs; American Society of Agronomy: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar] [CrossRef]



| Gene Name | Primer Sequence (5′-3′) | Fragment Length |
|---|---|---|
| AOB amoA | Upstream primer amoA-1F GGGGTTTCTACTGGTGGT | 491 bp |
| Downstream primer amoA-2R CCCCTCKGSAAAGCCTTCTTC | ||
| AOA amoA | Upstream primer Arch-amoAF STAATGGTCTGGCTTAGACG | 635 bp |
| Downstream primer Arch-amoAR GCGGCCATCCATCTGTATGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Lin, H.; Wang, F.; Shi, S.; Ma, J.; He, X. Effects of Increasing CO2 Concentration on Crop Growth and Soil Ammonia-Oxidizing Microorganisms in a Fababean (Vicia faba L.) and Wheat (Triticum aestivum Yunmai) Intercropping System. Plants 2025, 14, 516. https://doi.org/10.3390/plants14040516
Dong X, Lin H, Wang F, Shi S, Ma J, He X. Effects of Increasing CO2 Concentration on Crop Growth and Soil Ammonia-Oxidizing Microorganisms in a Fababean (Vicia faba L.) and Wheat (Triticum aestivum Yunmai) Intercropping System. Plants. 2025; 14(4):516. https://doi.org/10.3390/plants14040516
Chicago/Turabian StyleDong, Xingshui, Hui Lin, Feng Wang, Songmei Shi, Junwei Ma, and Xinhua He. 2025. "Effects of Increasing CO2 Concentration on Crop Growth and Soil Ammonia-Oxidizing Microorganisms in a Fababean (Vicia faba L.) and Wheat (Triticum aestivum Yunmai) Intercropping System" Plants 14, no. 4: 516. https://doi.org/10.3390/plants14040516
APA StyleDong, X., Lin, H., Wang, F., Shi, S., Ma, J., & He, X. (2025). Effects of Increasing CO2 Concentration on Crop Growth and Soil Ammonia-Oxidizing Microorganisms in a Fababean (Vicia faba L.) and Wheat (Triticum aestivum Yunmai) Intercropping System. Plants, 14(4), 516. https://doi.org/10.3390/plants14040516







