Chemical Profile and Bioactivities of Three Species of Mentha Growing in the Campania Region, Southern Italy
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Analysis
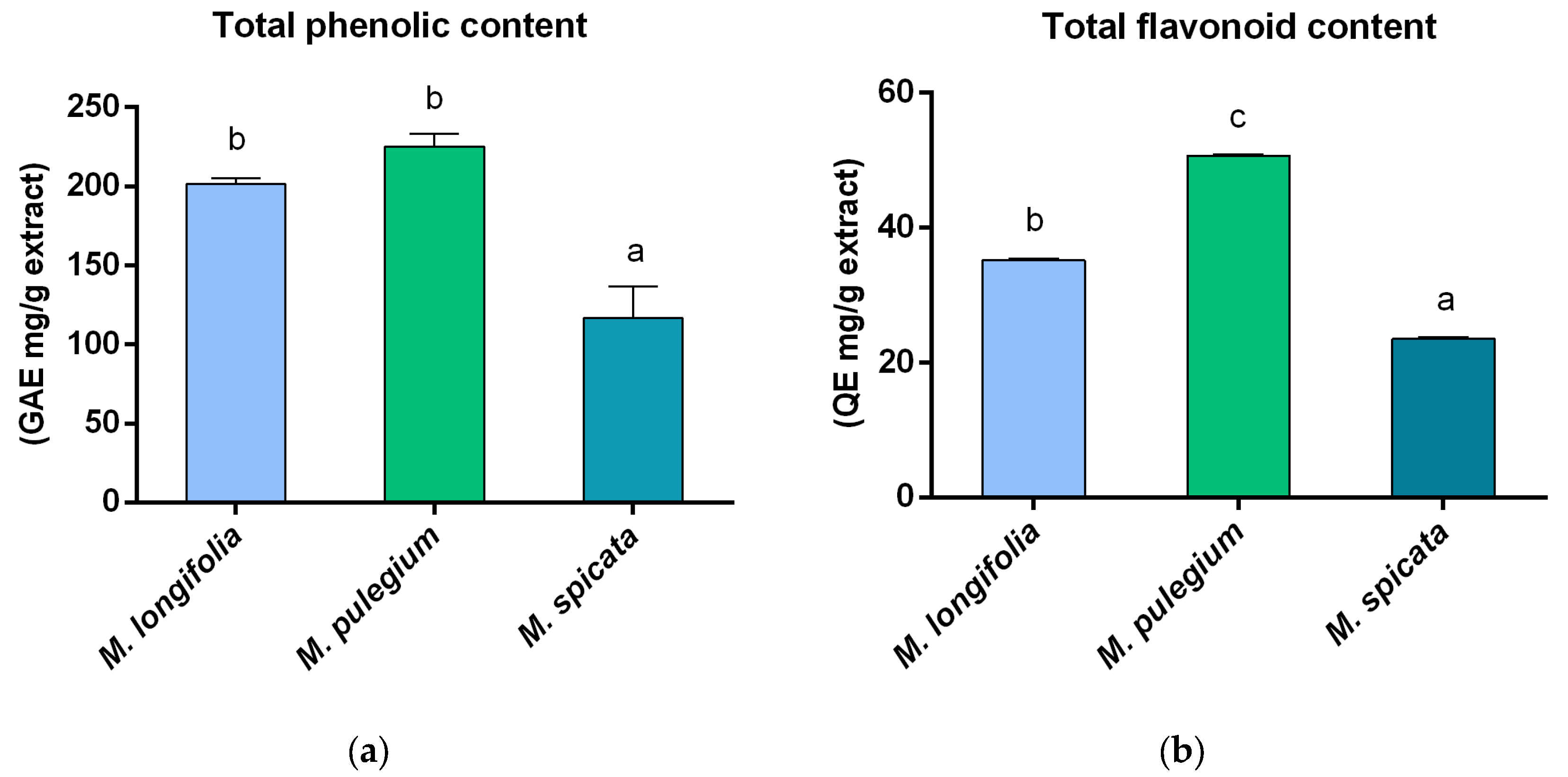
2.2. Determination of Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activity
2.3. Antibiofilm Activity
3. Materials and Methods
3.1. Plant Material and Extract Preparation
3.2. Chemical Analysis: LC-HRMS and LC-HRMS2
3.3. MZmine Data Processing
3.4. Determination of Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activity
3.4.1. Analysis of Total Phenol and Flavonoid Content
3.4.2. Antioxidant Activity
DPPH Assay
FRAP
3.5. Antibiofilm Activity
3.5.1. Bacterial Strains and Minimal Inhibitory Concentration (MIC)
3.5.2. Crystal Violet Test
3.5.3. MTT Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kraza, L.; Mourad, S.M.; Halis, Y. In vitro Investigation of the antioxidant and antimicrobial effects of hydro-alcoholic and aqueous extracts of Globularia alypum L. Acta Sci. Nat. 2020, 7, 46–58. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Batista, I.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. European Pennyroyal (Mentha pulegium) from Portugal: Chemical composition of Essential Oil and antioxidant and antimicrobial properties of extracts and Essential Oil. Ind. Crops Prod. 2012, 36, 81–87. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Shahidi, F. Antioxidant properties of commercial soft and hard winter wheats (Triticum aestivum L.) and their milling fractions. J. Sci. Food Agric. 2006, 86, 477–485. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Raj, X.J.; Bajpai, P.K.; Kumar, G.P.; Murugan, M.P.; Kumar, J.; Chaurasia, O.P.; Singh, S.B. Determination of total phenols, free radical scavenging and antibacterial activities of Mentha longifolia Linn. Hudson from the cold desert, Ladakh, India. Pharmacogn. J. 2010, 2, 470–475. [Google Scholar] [CrossRef]
- Zaidi, S.; Dahiya, P. In vitro antimicrobial activity, phytochemical analysis and total phenolic content of essential oil from Mentha spicata and Mentha Piperita. Int. Food Res. J. 2015, 22, 2440. [Google Scholar]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharopov, F.; Antolak, H.; Kręgiel, D.; Sen, S.; Sharifi-Rad, M.; Acharya, K.; Sharifi-Rad, R.; et al. Plants of genus Mentha: From farm to food factory. Plants 2018, 7, 70. [Google Scholar] [CrossRef]
- İsfendiyaroğlu, H.; Hanoğlu, A.; Yiğit Hanoğlu, D.; Alkaş, F.B.; Başer, K.H.C.; Özkum Yavuz, D. Chemical characterization of the essential oil compositions of Mentha spicata and M. longifolia ssp. cyprica from the Mediterranean basin and multivariate statistical analyses. Molecules 2024, 29, 1970. [Google Scholar] [CrossRef]
- Kivilompolo, M.; Hyötyläinen, T. Comprehensive two-dimensional liquid chromatography in analysis of Lamiaceae herbs: Characterisation and quantification of antioxidant phenolic acids. J. Chromatogr. A 2007, 1145, 155–164. [Google Scholar] [CrossRef]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hamid, A.; Zaidul, I.S.M. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process. 2011, 89, 67–72. [Google Scholar] [CrossRef]
- El Hassani, F.Z. Characterization, activities, and ethnobotanical uses of Mentha species in Morocco. Heliyon 2020, 6, e05480. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Ahmad, T.; Khan, A.; Maryam; Uddin, G.; Ahmad, B.; Mabkhot, Y.N.; Bawazeer, S.; Riaz, N.; Malikovna, B.K.; et al. Green synthesis and biomedicinal applications of silver and gold nanoparticles functionalized with methanolic extract of Mentha longifolia. Artif. Cells Nanomed. Biotechnol. 2021, 49, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Yakoubi, R.; Megateli, S.; Sadok, T.H.; Gali, L. Photoprotective, antioxidant, anticholinesterase activities and phenolic contents of different Algerian Mentha pulegium extracts. Biocatal. Agric. Biotechnol. 2021, 34, 102038. [Google Scholar] [CrossRef]
- Fatiha, B.; Didier, H.; Naima, G.; Khodir, M.; Martin, K.; Léocadie, K.; Pierre, D. Phenolic composition, in vitro antioxidant effects, and tyrosinase inhibitory activity of three Algerian Mentha species: M. spicata (L.), M. pulegium (L.), and M. rotundifolia (L.) Huds, (Lamiaceae). Ind. Crops Prod. 2015, 74, 722–730. [Google Scholar] [CrossRef]
- Mahboubi, M.; Haghi, G. Antimicrobial activity and chemical composition of Mentha pulegium L. essential oil. J. Ethnopharmacol. 2008, 119, 325–327. [Google Scholar] [CrossRef]
- De Sousa Barros, A.; de Morais, S.M.; Ferreira, P.A.T.; Vieira, I.G.P.; Craveiro, A.A.; dos Santos Fontenelle, R.O.; de Menezes, J.E.S.A.; da Silva, F.W.F.; de Sousa, H.A. Chemical composition and functional properties of essential oils from Mentha species. Ind. Crops Prod. 2015, 76, 557–564. [Google Scholar] [CrossRef]
- Zhao, X.; Dai, R.; Wang, J.; Cao, L.; Chen, P.; Yao, W.; Cheng, F.; Bao, B.; Zhang, L. Analysis of the permeable and retainable components of Cayratia japonica ointment through intact or broken skin after topical application by UPLC-Q-TOF-MS/MS combined with in vitro transdermal assay. .J. Pharm. Biomed. Anal. 2024, 238, 115853. [Google Scholar] [CrossRef]
- Henao-Rojas, J.C.; Osorio, E.; Isaza, S.; Madronero-Solarte, I.A.; Sierra, K.; Zapata-Vahos, I.C.; Betancur-Pérez, J.F.; Arboleda-Valencia, J.W.; Gallego, A.M. Towards bioprospection of commercial materials of Mentha spicata L. using a combined strategy of metabolomics and biological activity analyses. Molecules 2022, 27, 3559. [Google Scholar] [CrossRef]
- Taamalli, A.; Arraez-Román, D.; Abaza, L.; Iswaldi, I.; Fernandez-Gutierrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef]
- El-Gazar, A.A.; Emad, A.M.; Ragab, G.M.; Rasheed, D.M. Mentha pulegium L. (Pennyroyal, Lamiaceae) extracts impose abortion or fetal-mediated toxicity in pregnant rats; evidenced by the modulation of pregnancy hormones, MiR-520, MiR-146a, TIMP-1 and MMP-9 protein expressions, inflammatory state, certain related signaling pathways, and metabolite profiling via UPLC-ESI-TOF-MS. Toxins 2022, 14, 347. [Google Scholar] [CrossRef]
- Huang, G.; Liang, J.; Chen, X.; Lin, J.; Wei, J.; Huang, D.; Zhou, Y.; Sun, Z.; Zhao, L. Isolation and identification of chemical constituents from Zhideke granules by Ultra-Performance Liquid Chromatography Coupled with Mass Spectrometry. J. Anal. Methods Chem. 2020, 2020, 8889607. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-L.; Xu, J.-J.; Zhong, K.-R.; Shang, Z.-P.; Wang, F.; Wang, R.-F.; Zhang, L.; Zhang, J.-Y.; Liu, B. Analysis of non-volatile chemical constituents of Mentha haplocalyx Herba by ultra-high performance liquid chromatography-high resolution mass spectrometry. Molecules 2017, 22, 1756. [Google Scholar] [CrossRef] [PubMed]
- March, R.E.; Miao, X.S. A fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int. J. Mass Spectrom. 2004, 231, 157–167. [Google Scholar] [CrossRef]
- Zaidi, F.; Voirin, B.; Jay, M.; Viricel, M.R. Free flavonoid aglycones from leaves of Mentha pulegium and Mentha suaveolens (Labiatae). Phytochemistry 1998, 48, 991–994. [Google Scholar] [CrossRef]
- Wang, J.; Jia, Z.; Zhang, Z.; Wang, Y.; Liu, X.; Wang, L.; Lin, R. Analysis of chemical constituents of Melastoma dodecandrum Lour. by UPLC-ESI-Q-exactive focus-MS/MS. Molecules 2017, 22, 476. [Google Scholar] [CrossRef]
- Xu, L.-L.; Jiao, Q.-S.; Yang, J.-Y.; Wang, F.; Wang, Z.-J.; Jiang, Y.-Y.; Liu, B.; Zhan, J.-Y. Rapid identification of four groups of chemical constituents of aqueous extracts from Mentha haplocalyx Herba by UHPLC-LTQ-Orbitrap HRMS combined with cleavage pathways. J. Chin. Mass. Spectrom. Soc. 2018, 39, 424–432. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.A.; Saleem, M.S.; Al-Hazimi, H.M. Further highly oxygenated flavonoids from Mentha longifolia. J. Saudi Chem. Soc. 2004, 8, 541–546. [Google Scholar]
- Freitas, J.R.L.; Vendramini, P.H.; Melo, J.O.F.; Eberlin, M.N.; Augusti, R. Assessing the spatial distribution of key flavonoids in Mentha × piperita leaves: An application of Desorption Electrospray Ionization Mass Spectrometry Imaging (DESI-MSI). J. Braz. Chem. Soc. 2019, 30, 1437–1446. [Google Scholar] [CrossRef]
- Pavlesic, T.; Poljak, S.; Ostojic, D.M.; Lucin, I.; Reynolds, C.A.; Kalafatovic, D.; Martinovic, L.S. Mint (Mentha spp.) honey: Analysis of the phenolic profile and antioxidant activity. Food Technol. Biotechnol. 2022, 60, 509–519. [Google Scholar] [CrossRef]
- Spring, O.; Pfannstiel, J.; Klaiber, I.; Conrad, J.; Beifuß, U.; Apel, L.; Aschenbrenner, A.K.; Zipper, R. The nonvolatile metabolome of sunflower linear glandular trichomes. Phytochemistry 2015, 119, 83–89. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Maurya, P.; Singh, S.; Gupta, M.M.; Luqman, S. Characterization of bioactive constituents from the gum resin of Gardenia lucida and its pharmacological potential. Biomed. Pharmacother. 2017, 85, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.T.; Proença, C.; Ferreira, A.R.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Araújo, M.E.M. Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices. Food Chem. 2007, 103, 778–786. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Nabavi, S.M.; Nabavi, S.F. Antioxidant and antihemolytic activities of Mentha longifolia. Pharmacologyonline 2010, 2, 464–471. [Google Scholar]
- Conforti, F.; Marrelli, M.; Carmela, C.; Menichini, F.; Valentina, P.; Uzunov, D.; Statti, G.A.; Duez, P.; Menichini, F. Bioactive phytonutrients (omega fatty acids, tocopherols, polyphenols), in vitro inhibition of nitric oxide production and free radical scavenging activity of non-cultivated mediterranean vegetables. Food Chem. 2011, 129, 1413–1419. [Google Scholar] [CrossRef]
- Tacherfiout, M.; Kherbachi, S.; Kheniche, M.; Mattonai, M.; Degano, I.; Ribechini, E.; Khettal, B. HPLC-DAD and HPLC-ESI-MS-MS profiles of hydroalcoholic extracts of Chamaemelum nobile and Mentha pulegium, and study of their antihemolytic activity against AAPH-induced hemolysis. South. Afr. J. Bot. 2022, 150, 678–690. [Google Scholar] [CrossRef]
- Bai, N.; Zhou, Z.; Zhu, N.; Zhang, L.; Quan, Z.; He, K.; Zheng, Q.Y.; Ho, C. Antioxidative flavonoids from the flower of Inula britannica. J. Food Lipids 2005, 12, 141–149. [Google Scholar] [CrossRef]
- Ganapaty, S.; Chandrashekhar, V.M.; Chitme, H.R.; Narsu, M.L. Free radical scavenging activity of gossypin and nevadensin: An in vitro evaluation. Indian. J. Pharmacol. 2007, 39, 281–283. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Lai, C.; Liang, Y.; Gao, L.; Kaliaperumal, K.; Jiang, Y. Nutraceutical potential of navel orange peel in diabetes management: The chemical profile, antioxidant, α-glucosidase inhibitory and antiglycation effects of its flavonoids. Food Biosci. 2022, 49, 101943. [Google Scholar] [CrossRef]
- Mohamadi, S.; Zhao, M.; Amrani, A.; Marchioni, E.; Zama, D.; Benayache, F.; Benayache, S. On-line screening and identification of antioxidant phenolic compounds of Saccocalyx satureioides Coss. et Dur. Ind. Crops Prod. 2015, 76, 910–919. [Google Scholar] [CrossRef]
- Hawas, U.W.; El-Ansari, M.A.; El-Hagrassi, A.M. A new acylated flavone glycoside, in vitro antioxidant and antimicrobial activities from Saudi Diospyros mespiliformis Hochst. ex A.DC (Ebenaceae) Leaves. Z. Naturforsch. C 2022, 77, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, M.B.; Almutairi, H.H.; Andleeb, A.; Jabeen, R.; Mustafa, G.; Habiba, U.-E.; Kazmi, S.A.; Naz, F.; Qammar, N. Mentha longifolia-assisted nanostructures: An approach to obliterate microbial biofilms. PLoS ONE 2024, 19, e0303521. [Google Scholar] [CrossRef] [PubMed]
- Quave, C.L.; Plano, L.R.; Pantuso, T.; Bennett, B.C. Effects of extracts from Italian medicinal plants on Planktonic growth, biofilm formation, and adherence of Methicillin-Resistant Staphylococcus aureus. J. Ethnopharmacol. 2008, 118, 418–428. [Google Scholar] [CrossRef]
- World Health Organization. “WHO Bacterial Priority Pathogens List, 2024.” World Health Organization. 2024. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 25 November 2024).
- Stewart, P.S.; White, B.; Boegli, L.; Hamerly, T.; Williamson, K.S.; Franklin, M.J.; Bothner, B.; James, G.A.; Fisher, S.; Vital-Lopez, F.G.; et al. Conceptual model of biofilm antibiotic tolerance that integrates phenomena of diffusion, metabolism, gene expression, and physiology. J. Bacteriol. 2019, 201, e00307-19. [Google Scholar] [CrossRef]
- Alreshidi, M.; Abdulhakeem, M.A.; Badraoui, R.; Amato, G.; Caputo, L.; De Martino, L.; Nazzaro, F.; Fratianni, F.; Formisano, C.; De Feo, V.; et al. Pulicaria incisa (Lam.) DC. as a Potential Source of Antioxidant, Antibacterial, and Anti-Enzymatic Bioactive Molecules: Phytochemical Constituents, In Vitro and In Silico Pharmacological Analysis. Molecules 2023, 28, 7439. [Google Scholar] [CrossRef]
- Heuckeroth, S.; Damiani, T.; Smirnov, A.; Mokshyna, O.; Brungs, C.; Korf, A.; Smith, J.D.; Stincone, P.; Dreolin, N.; Nothias, L.F.; et al. Reproducible mass spectrometry data processing and compound annotation in MZmine 3. Nat. Protoc. 2024, 19, 2597–2641. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar] [CrossRef]
- Baba, S.A.; Malik, S.A. Determination of Total Phenolic and Flavonoid Content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2015, 9, 449–454. [Google Scholar] [CrossRef]
- Lee, S.K.; Mbwambo, Z.H.; Chung, H.; Luyengi, L.; Gamez, E.J.; Mehta, R.G.; Kinghorn, A.D.; Pezzuto, J.M. Evaluation of the antioxidant potential of Natural Products. Comb. Chem. High Throughput Screen. 1998, 1, 35–46. [Google Scholar] [CrossRef]
- Fratianni, F.; Amato, G.; d’Acierno, A.; Ombra, M.N.; De Feo, V.; Coppola, R.; Nazzaro, F. In vitro prospective healthy and nutritional benefits of different Citrus monofloral honeys. Sci. Rep. 2023, 13, 1088. [Google Scholar] [CrossRef]
| No. | Family | Retention Time (Rt min) | Measured m/z [M + H]+ | Molecular Formula | Δppm | Fragment | Fragment Formula | Fragment Ion (m/z) | Δppm | Identification | M. longifolia | M. pulegium | M. spicata |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Phenolic acid | 7.09 | 355.1021 | C16H18O9 | −0.700 | [M-C7H12O6+H]+ | C9H7O3 | 163.0387 | −1.660 | Chlorogenic acid | 15,085.242 | 13,768.029 | 3206.164 |
| [M-C7H12O6-H2O+H]+ | C9H5O2 | 145.0280 | −2.661 | ||||||||||
| 2 | Oxomonocarboxylic acid | 7.42 | 227.1275 | C12H18O4 | −1.037 | [M-H2O+H]+ | C12H17O3 | 209.1171 | −0.578 | Tuberonic acid | 102,915.09 | 6120.2734 | 36,391.055 |
| [M-2H2O+H]+ | C12H15O2 | 191.1066 | −0.399 | ||||||||||
| [M-C2(H2O)2+H]+ | C10H15O2 | 167.1062 | −2.910 | ||||||||||
| 3 | Flavone-O-glycoside | 8.21 | 595.1647 | C27H30O15 | −1.708 | [M-C6H10O4+H]+ | C21H21O11 | 449.1080 | 0.294 | Luteolin-7-O-Rutinoside | 331,867.03 | 20,862.74 | 73,078.17 |
| [M-C12H20O9+H]+ | C15H11O6 | 287.0552 | 0.576 | ||||||||||
| 4 | Flavone-O-glycoside | 8.46 | 579.1694 | C27H30O14 | −2.421 | [M-C6H10O4+H]+ | C21H21O10 | 433.1116 | −2.986 | Apigenin 7-O-Rutinoside | 4068.215 | 0 | 86,404.055 |
| [M-C6H10O5+H]+ | C21H21O9 | 417.1164 | −3.881 | ||||||||||
| [M-C12H20O9+H]+ | C15H11O5 | 271.0594 | −2.730 | ||||||||||
| 5 | Flavone-O-glycoside | 8.65 | 609.1811 | C28H32O15 | −0.405 | [M-C6H10O4+H]+ | C22H23O11 | 463.1234 | −0.233 | Diosmetin-7-O-rutinoside (Diosmina) | 421,577.75 | 19,798.348 | 8247.895 |
| [M-C12H20O9+H]+ | C16H13O6 | 301.0706 | −0.148 | ||||||||||
| 6 | Flavone-O-glycoside | 9.27 | 593.1859 | C28H32O14 | −0.964 | [M-C6H10O4+H]+ | C22H23O10 | 447.1282 | −0.768 | Acacetin 7-O-rutinoside (Linarin) | 145,369.47 | 0 | 0 |
| [M-C12H20O9+H]+ | C16H13O5 | 285.0754 | −1.087 | ||||||||||
| 7 | Flavone-O-glycoside | 9.38 | 595.2007 | C28H34O14 | −0.894 | [M-C12H20O9+H]+ | C16H15O5 | 287.0917 | 1.184 | Isosakuranetin-O-rutinoside (Didymin) | n.d. | n.d. | n.d. |
| 8 | Caffeic acid dimer | 9.56 | 341.0652 | C18H12O7 | −0.965 | [M-H2O+H]+ | C18H11O6 | 323.0551 | 0.419 | Salvianolic acid G | 9144.336 | 4414.9707 | 0 |
| [M-CO2+H]+ | C17H13O5 | 297.0764 | 2.154 | ||||||||||
| [M-C9H6O4+H]+ | C9H7O3 | 163.0391 | 1.284 | ||||||||||
| [M-C9H6O5+H]+ | C9H7O2 | 147.0439 | −0.993 | ||||||||||
| 9 | Flavonoid | 9.87 | 287.0548 | C15H10O6 | −0.922 | [M-H2O+H]+ | C15H9O5 | 269.0428 | −5.947 | Kaempferol | 159,260.27 | 16,393.633 | 48,949.184 |
| [M-(CH)2O+H]+ | C13H9O5 | 245.0447 | 0.858 | ||||||||||
| [M-C8H6O2+H]+ | C7H5O4 | 153.0436 | −1.275 | ||||||||||
| 10 | Flavonoid | 10.35 | 331.0810 | C17H14O7 | −0.632 | [M-CH3+H]+ | C16H12O7 | 316.0580 | 0.809 | Jaceosidin | 164,605.33 | 0 | 0 |
| [M-CO+H]+ | C16H15O6 | 303.0868 | 1.568 | ||||||||||
| [M-C2H3O+H]+ | C15H12O6 | 288.0623 | −1.908 | ||||||||||
| 11 | Flavonoid | 10.41 | 273.0757 | C15H12O5 | −0.037 | [M-C6H4O2+H]+ | C9H9O3 | 165.0544 | −1.095 | Naringenin | n.d. | n.d. | n.d. |
| [M-C7H11O3+H]+ | C6H2O2 | 130.0039 | −3.083 | ||||||||||
| 12 | Flavonoid | 10.48 | 361.091 | C18H16O8 | −0.509 | [M-CH3+H]+ | C17H14O8 | 346.0681 | −0.689 | Sideritiflavone | 335,510.84 | 4927.666 | 523,152.12 |
| [M-2CH3+H]+ | C16H11O8 | 331.0445 | −1.294 | ||||||||||
| [M-CH5O+H]+ | C17H12O7 | 328.0576 | −0.409 | ||||||||||
| 13 | Flavonoid | 10.59 | 301.0704 | C16H12O6 | −0.779 | [M-CH3+H]+ | C15H10O6 | 286.0471 | −0.348 | Diosmetin | 797,023.06 | 8806.57 | 222,210.06 |
| [M-CH3-CO+H]+ | C14H10O5 | 258.0523 | 0.252 | ||||||||||
| 14 | Flavonoid | 10.87 | 345.0960 | C18H16O7 | −2.519 | [M-CH3+H]+ | C17H14O7 | 330.0727 | −2.073 | Xanthomicrol | 89,237.6 | 83,810.945 | 33,379.188 |
| [M-C2H4O+H]+ | C16H13O6 | 301.0701 | −1.975 | ||||||||||
| 15 | Flavonoid | 10.90 | 361.0912 | C18H16O8 | −1.672 | [M-CH3+H]+ | C17H14O8 | 346.0682 | −0.401 | Thymonin | 640,358.6 | 3886.9512 | 99,089.83 |
| [M-2CH3+H]+ | C16H11O8 | 331.0445 | −0.948 | ||||||||||
| [M-CH5O+H]+ | C17H12O7 | 328.0576 | −0.409 | ||||||||||
| [M-C2H8O+H]+ | C16H9O7 | 313.0340 | −0.796 | ||||||||||
| 16 | Flavonoid | 11.33 | 375.1069 | C19H18O8 | −1.370 | [M-CH3+H]+ | C18H16O8 | 360.0841 | 0.392 | 5,7-Dihydroxy-6,8,3′,4′-tetramethoxyflavone (Hymenoxin) | 513,052.94 | 12,278.242 | 100,773.44 |
| [M-C2H6+H]+ | C17H13O8 | 345.0608 | 0.945 | ||||||||||
| [M-CH5O+H]+ | C18H14O7 | 342.0735 | 0.163 | ||||||||||
| [M-C3H5O4+H]+ | C16H14O4 | 270.0887 | 0.332 | ||||||||||
| [M-C10H10O5+H]+ | C9H9O3 | 165.0548 | 1.329 | ||||||||||
| 17 | Flavonoid | 11.75 | 403.1376 | C21H22O8 | −2.838 | [M-CH3+H]+ | C20H20O8 | 388.1154 | 0.466 | Nobiletin | 1682.9082 | 15,424.356 | 6241.834 |
| [M-C2H6+H]+ | C19H17O8 | 373.0897 | −0.720 | ||||||||||
| 18 | Flavonoid | 11.83 | 287.0916 | C16H14O5 | −0.836 | [M-C(H2O)3+H]+ | C10H9O2 | 161.0596 | −0.100 | Sakuranetin | 16,995.594 | 0 | 1518.3528 |
| [M-(C8H8O)+H]+ | C8H7O4 | 167.0342 | 2.064 | ||||||||||
| 19 | Flavonoid | 11.89 | 345.0963 | C18H16O7 | −1.737 | [M-CH3+H]+ | C17H14O7 | 330.0738 | 1.260 | Nevadensin | 528,516.4 | 306,378.25 | 23,202.852 |
| [M-(CH3)2+H]+ | C16H11O7 | 315.0504 | 1.431 | ||||||||||
| [M-(CH5O)+H]+ | C17H12O6 | 312.0633 | 1.540 | ||||||||||
| [M-C10H10O5+H]+ | C8 H7O2 | 135.0440 | −0.489 | ||||||||||
| 20 | Flavonoid | 12.33 | 359.1122 | C19H18O7 | −0.889 | [M-CH3-H2O+H]+ | C18H14O6 | 326.0788 | 0.860 | 5-Hydroxy-6,7,3′,4′-tetramethoxy flavone | 0 | 131,156.69 | 0 |
| [M-CH3+H]+ | C18H16O7 | 344.0893 | 0.865 | ||||||||||
| [M-H2O+H]+ | C19H17O6 | 341.1017 | −0.923 | ||||||||||
| [M-C2H4O+H]+ | C17H15O6 | 315.0871 | 2.683 | ||||||||||
| [M-C2H5O2+H]+ | C17H14O5 | 298.0840 | 1.359 | ||||||||||
| 21 | Flavonoid | 12.78 | 389.1227 | C20H20O8 | −2.477 | [M-CH3+H]+ | C19H18O8 | 374.0990 | −0.589 | 5-Hydroxyauranetin | 74,619.89 | 17,551.996 | 18,330.71 |
| [M-C2H6+H]+ | C18H15O8 | 359.0758 | −0.846 | ||||||||||
| [M-CH3-H2O+H]+ | C19H16O7 | 356.0883 | −0.764 | ||||||||||
| [M-C2H6-H2O+H]+ | C18H13O7 | 341.0650 | −1.786 | ||||||||||
| [M-C2H3O2+H]+ | C18H16O6 | 328.0930 | −1.120 |
| M. longifolia | M. pulegium | M. spicata | Ascorbic Acid | |
|---|---|---|---|---|
| DPPH 1 IC50(µg/mL) 2 Mean (±SD) | 16.42 (±0.10) b | 16.13 (±2.03) b | 29.26 (±0.35) c | 2.98 (±0.35) a |
| FRAP Emg Fe2+ Equivalents/g Extract 2 Mean (±SD) | 181.89 (±6.88) ab | 272.36 (±46.02) b | 104.45 (±21.99) a | 577.77 (±63.50) c |
| MIC | M. longifolia | M. pulegium | M. spicata | Tetracycline |
|---|---|---|---|---|
| A. baumannii | 38.00 (±1.00) a | 38.00 (±2.00) a | 36.00 (±2.00) a | 30.00 (±1.00) |
| E. coli | >50.00 b | >50.00 b | >50.00 b | 30.00 (±2.00) |
| L. monocytogenes | >50.00 b | 42.00 (±1.00) a | >50.00 b | 28.00 (±1.00) |
| P. aeruginosa | 42.00 (±1.00) a | 46.00 (±2.00) a | 42.00 (±1.00) a | 28.00 (±2.00) |
| S. aureus | >50.00 b | >50.00 b | 36.0 (±2.00) a | 34.00 (±2.00) |
| CV | M. longifolia 10 | M. longifolia 20 | M. pulegium 10 | M. pulegium 20 | M. spicata 10 | M. spicata 20 |
|---|---|---|---|---|---|---|
| A. baumannii | 44.38 (±0.97) b | 47.61 (±0.89) b | 42.95 (±1.09) b | 45.63 (±2.08) b | 46.38 (±0.72) b | 50.78 (±1.92) b |
| E. coli | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) |
| L. monocytogenes | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) | 21.97 (±0.51) a | 0.00 (±0.00) | 0.00 (±0.00) |
| P. aeruginosa | 0.00 (±0.00) | 22.67 (±1.80) a | 0.00 (±0.00) | 5.22 (±0.72) a | 0.00 (±0.00) | 21.54 (±0.18) a |
| S. aureus | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) |
| MTT | M. longifolia 10 | M. longifolia20 | M. pulegium10 | M. pulegium 20 | M. spicata 10 | M. spicata 20 |
| A. baumannii | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) | 17.30 (±1.18) a | 0.00 (±0.00) | 0.00 (±0.00) |
| E. coli | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) |
| L. monocytogenes | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) | 6.02 (±0.21) a | 37.71 (±3.31) b |
| P. aeruginosa | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) | 15.25 (±1.34) a | 0.00 (±0.00) | 28.34 (±2.09) a |
| S. aureus | 0.00 (±0.00) | 0.00 (±0.00) | 0.00 (±0.00) | 9.51 (±0.67) a | 0.00 (±0.00) | 0.00 (±0.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francolino, R.; Martino, M.; Nazzaro, F.; Sirignano, C.; Fratianni, F.; Coppola, F.; De Martino, L.; Formisano, C.; De Feo, V. Chemical Profile and Bioactivities of Three Species of Mentha Growing in the Campania Region, Southern Italy. Plants 2025, 14, 360. https://doi.org/10.3390/plants14030360
Francolino R, Martino M, Nazzaro F, Sirignano C, Fratianni F, Coppola F, De Martino L, Formisano C, De Feo V. Chemical Profile and Bioactivities of Three Species of Mentha Growing in the Campania Region, Southern Italy. Plants. 2025; 14(3):360. https://doi.org/10.3390/plants14030360
Chicago/Turabian StyleFrancolino, Rosaria, Mara Martino, Filomena Nazzaro, Carmina Sirignano, Florinda Fratianni, Francesca Coppola, Laura De Martino, Carmen Formisano, and Vincenzo De Feo. 2025. "Chemical Profile and Bioactivities of Three Species of Mentha Growing in the Campania Region, Southern Italy" Plants 14, no. 3: 360. https://doi.org/10.3390/plants14030360
APA StyleFrancolino, R., Martino, M., Nazzaro, F., Sirignano, C., Fratianni, F., Coppola, F., De Martino, L., Formisano, C., & De Feo, V. (2025). Chemical Profile and Bioactivities of Three Species of Mentha Growing in the Campania Region, Southern Italy. Plants, 14(3), 360. https://doi.org/10.3390/plants14030360










