Optimal Stubble Management Strategies of Caragana tibetica for Enhancing Stress Resistance and Vegetation Restoration
Abstract
1. Introduction
2. Results
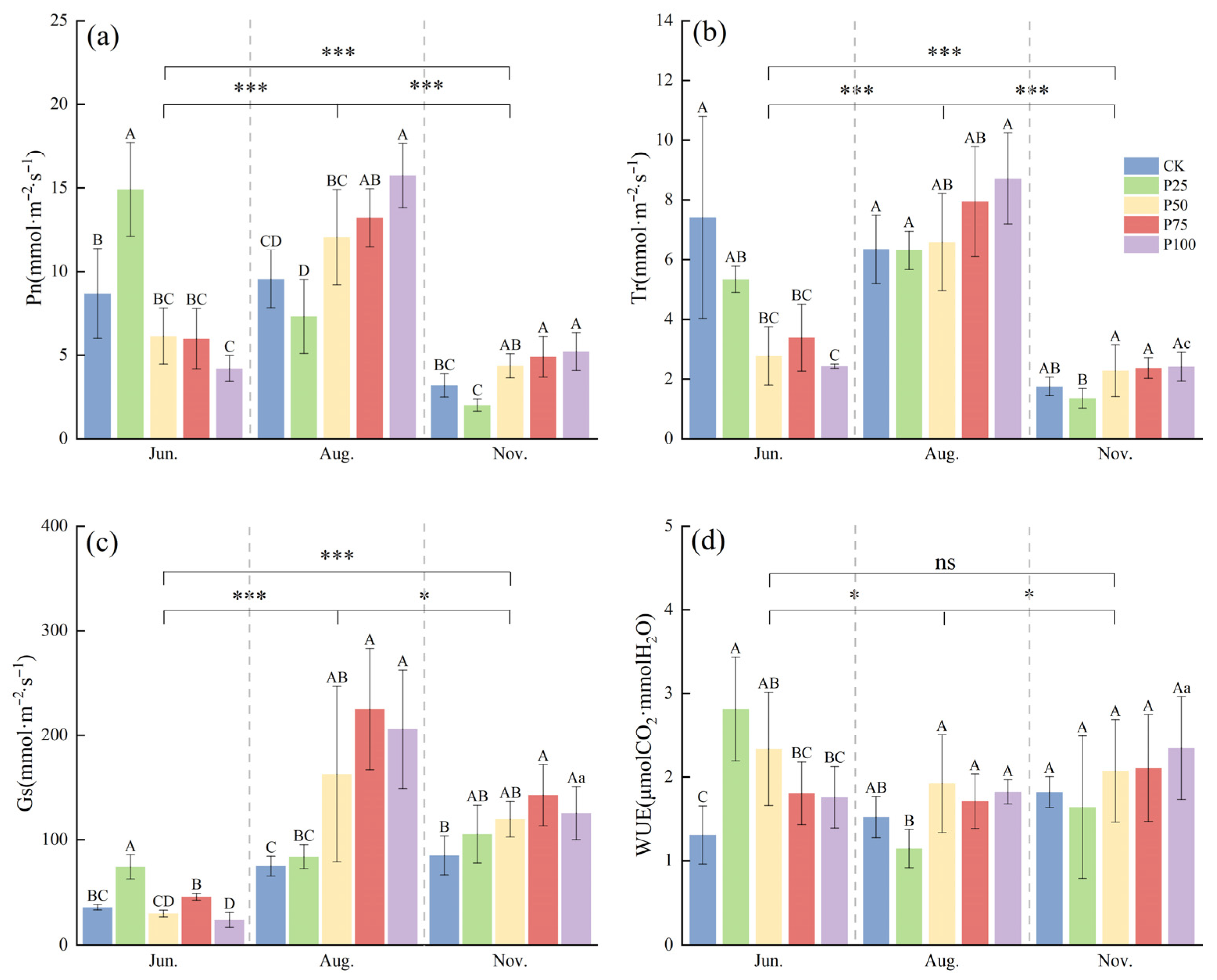
2.1. Analysis of Photosynthetic Physiological Characteristics of Caragana tibetica
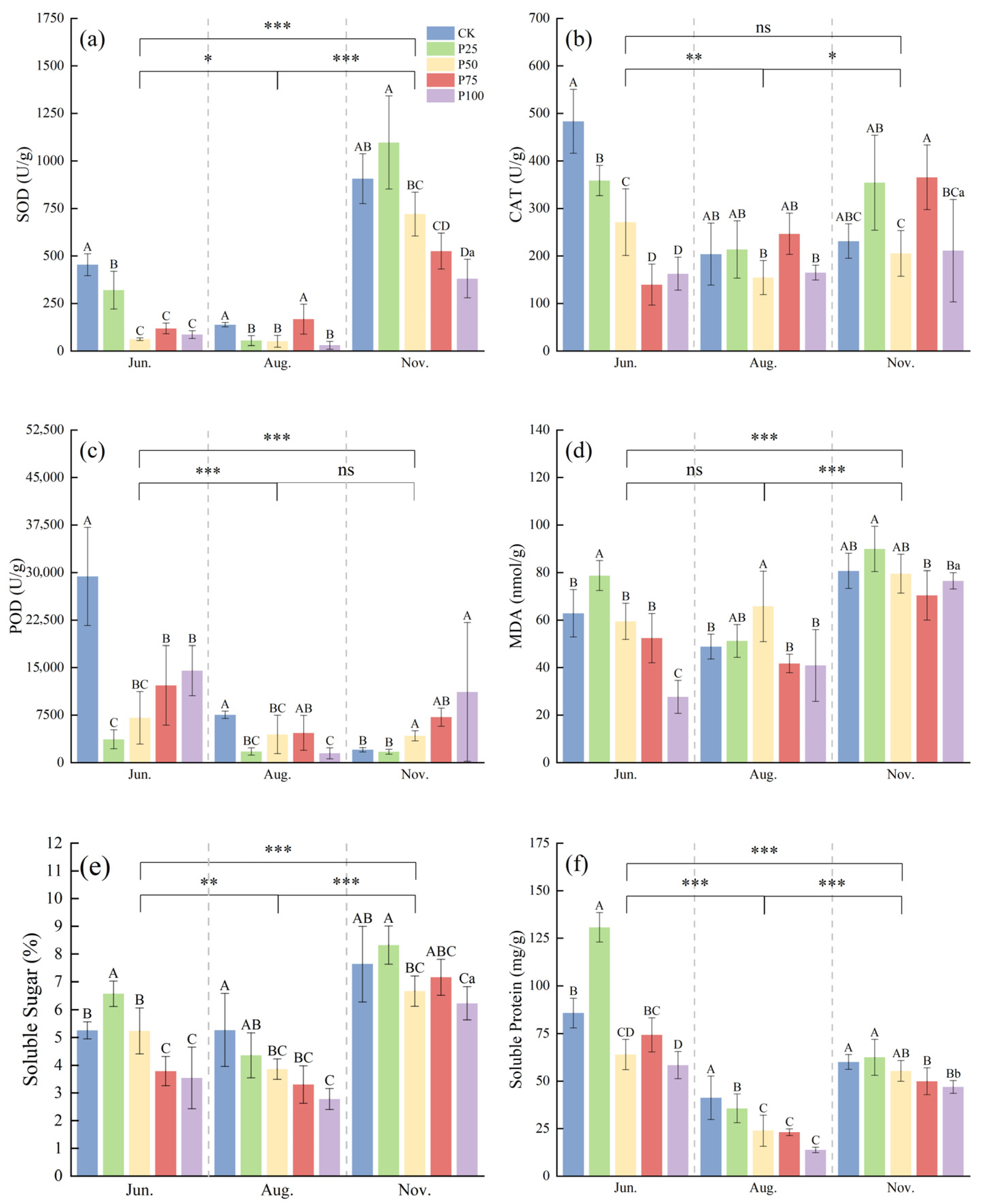
2.2. Analysis of Antioxidant Characteristics of Caragana tibetica
2.3. Correlation Analysis of Photosynthetic Physiological Characteristics, Antioxidant Enzyme Activities, and Soluble Substances in Caragana tibetica
2.4. Principal Component Analysis of Physiological Indicators and Their Comprehensive Performance in Caragana tibetica
2.5. Comprehensive Evaluation of Stress Resistance in Caragana tibetica Based on Principal Component Analysis and Optimal Stubble Height Analysis Using the Generalized Additive Model
3. Discussion
4. Materials and Methods
4.1. Overview of the Study Area
4.2. Experimental Design
4.3. Index Measurements
4.3.1. Photosynthetic Index Measurement
4.3.2. Measurement of Physiological Indices
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
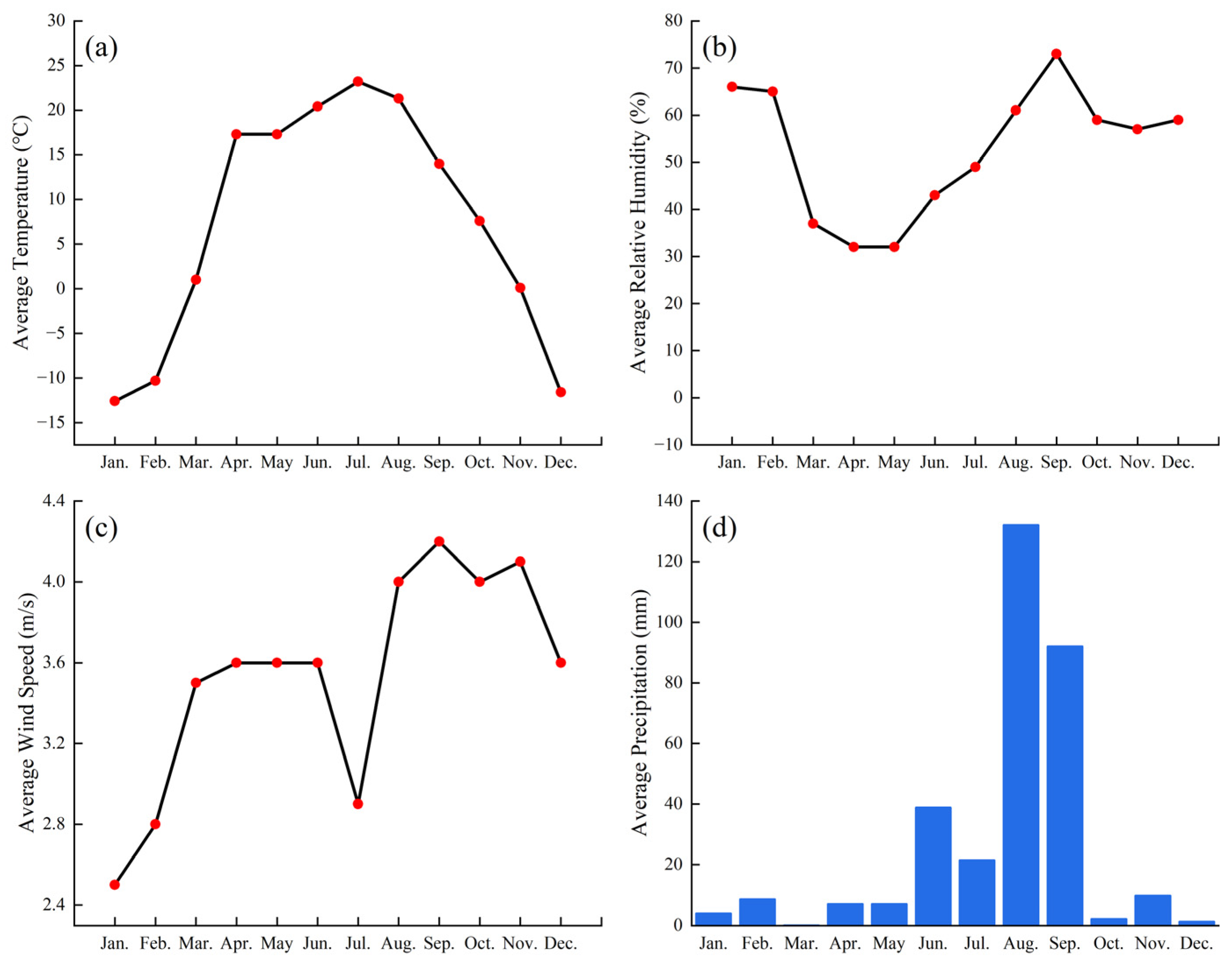
Appendix A. Meteorological Conditions
Basic Meteorological Data
References
- Zhang, P.-J.; Yang, J.; Song, B.-Y.; Zhao, L.-Q.; Qing, H. Spatial heterogeneity of soil resources of Caragana tibetica community. Chin. J. Plant Ecol. 2009, 33, 338. [Google Scholar]
- Fang, X.-W.; Zhang, J.-J.; Xu, D.-H.; Pang, J.; Gao, T.-P.; Zhang, C.-H.; Li, F.-M.; Turner, N.C. Seed germination of Caragana species from different regions is strongly driven by environmental cues and not phylogenetic signals. Sci. Rep. 2017, 7, 11248. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wu, L.; Yang, G.; Gao, Y.; Li, H. Simulation and prediction of the geographical distribution of five Caragana species in the north temperate zone. Environ. Monit. Assess. 2023, 195, 1427. [Google Scholar] [CrossRef] [PubMed]
- Sala, O.E.; Chapin, F.S., 3rd; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, J.; Li, X.; Li, J.; Brierley, G. Effects of disturbances on aboveground biomass of alpine meadow in the Yellow River Source Zone, Western China. Ecol. Evol. 2022, 12, e8640. [Google Scholar] [CrossRef]
- Wang, R.; Mattox, C.M.; Phillips, C.L.; Kowalewski, A.R. Carbon Sequestration in Turfgrass-Soil Systems. Plants 2022, 11, 2478. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef]
- Huhta, A.-P.; Hellström, K.; Rautio, P.; Tuomi, J. Grazing tolerance of Gentianella amarella and other monocarpic herbs: Why is tolerance highest at low damage levels? Plant Ecol. 2003, 166, 49–61. [Google Scholar] [CrossRef]
- Wu, T.; Xia, L.; Pan, B.; Zou, Y.; Li, F.; Xie, Y.; Wang, S.; Li, Z. Mowing of Carex brevicuspis (Cyperaceae) improves food quality for herbivorous geese in Dongting Lake: The potential mechanisms. Front. Plant Sci. 2025, 16, 1566808. [Google Scholar] [CrossRef]
- Wani, S.H.; Singh, N.B.; Haribhushan, A.; Mir, J.I. Compatible solute engineering in plants for abiotic stress tolerance—Role of glycine betaine. Curr. Genom. 2013, 14, 157–165. [Google Scholar] [CrossRef]
- Gao, J. Guidance for Plant Physiology Experiments; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Fan, J.; Zhang, Y.; Sun, H.; Duan, R.; Jiang, Y.; Wang, X.; Sun, Y.; Luo, Z.; Wang, P.; Guan, S.; et al. Overexpression of soybean GmDHN9 gene enhances drought resistance of transgenic Arabidopsis. GM Crops Food 2024, 15, 118–129. [Google Scholar] [CrossRef]
- Ou, C.; Dong, Z.; Zheng, X.; Cheng, W.; Chang, E.; Yao, X. Functional Characterization of the PoWHY1 Gene from Platycladus orientalis and Its Role in Abiotic Stress Tolerance in Transgenic Arabidopsis thaliana. Plants 2025, 14, 218. [Google Scholar] [CrossRef]
- Haq, I.U.; Ullah, S.; Amin, F.; Nafees, M.; Shah, W.; Ali, B.; Iqbal, R.; Kaplan, A.; Ali, M.A.; Elshikh, M.S.; et al. Physiological and Germination Responses of Muskmelon (Cucumis melo L.) Seeds to Varying Osmotic Potentials and Cardinal Temperatures via a Hydrothermal Time Model. ACS Omega 2023, 8, 33266–33279. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Han, Y.; Gao, Y.; Han, M.; Duan, L. Decoding the metabolomic responses of Caragana tibetica to livestock grazing in fragile ecosystems. Front. Plant Sci. 2024, 15, 1339424. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yang, H.; Shi, J.; Duan, Y.; Wu, W.; Lyu, L.; Li, W. Effects of Different Light Wavelengths on Fruit Quality and Gene Expression of Anthocyanin Biosynthesis in Blueberry (Vaccinium corymbosm). Cells 2023, 12, 1225. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.; Shah, F.; Ullah, S.; Shah, W.; Ahmed, I.; Ali, B.; Khan, A.A.; Malik, T.; Mustafa, A.E.Z.M. The germination response of Zea mays L. to osmotic potentials across optimal temperatures via halo-thermal time model. Sci. Rep. 2024, 14, 3225. [Google Scholar] [CrossRef]
- Cazzaniga, S.; Ballottari, M. Photosynthesis 2.0. Int. J. Mol. Sci. 2023, 24, 4355. [Google Scholar] [CrossRef]
- Zahra, N.; Hafeez, M.B.; Ghaffar, A.; Kausar, A.; Al Zeidi, M.; Siddique, K.H.M.; Farooq, M. Plant photosynthesis under heat stress: Effects and management. Environ. Exp. Bot. 2023, 206, 105178. [Google Scholar] [CrossRef]
- Erb, T.J. Photosynthesis 2.0: Realizing new-to-nature CO2-fixation to overcome the limits of natural metabolism. Cold Spring Harb. Perspect. Biol. 2024, 16, a041669. [Google Scholar] [CrossRef]
- Nowak, R.S.; Caldwell, M.M. Test of compensatory photosynthesis in the field: Implications for herbivory tolerance. Oecologia 1984, 61, 311–318. [Google Scholar] [CrossRef]
- Alem, H.; Ojeda, H.; Rigou, P.; Schneider, R.; Torregrosa, L. The reduction of plant sink/source does not systematically improve the metabolic composition of Vitis vinifera white fruit. Food Chem. 2021, 345, 128825. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kolb, T.E.; Clancy, K.M. Effects of artificial and western spruce budworm (Lepidoptera: Tortricidae) defoliation on growth and biomass allocation of Douglas-fir seedlings. J. Econ. Entomol. 2002, 95, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Y.; Qi, W.; Zhen, L.; Yao, Y.; Qin, F. Compensatory growth and understory soil stoichiometric features of Hippophae rhamnoides at different stubble heights. PeerJ 2022, 10, e13363. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Mao, K.; Wang, P.; Wang, Y.; Jia, X.; Huo, L.; Sun, X.; Che, R.; Gong, X.; Ma, F. Overexpression of MdATG8i improves water use efficiency in transgenic apple by modulating photosynthesis, osmotic balance, and autophagic activity under moderate water deficit. Hortic. Res. 2021, 8, 81. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. The Application of Arbuscular Mycorrhizal Fungi as Microbial Biostimulant, Sustainable Approaches in Modern Agriculture. Plants 2023, 12, 3101. [Google Scholar] [CrossRef]
- Liu, M.; Gong, J.; Yang, B.; Ding, Y.; Zhang, Z.; Wang, B.; Zhu, C.; Hou, X. Differences in the photosynthetic and physiological responses of Leymus chinensis to different levels of grazing intensity. BMC Plant Biol. 2019, 19, 558. [Google Scholar] [CrossRef]
- Sobczak, A.; Sujkowska-Rybkowska, M.; Gajc-Wolska, J.; Kowalczyk, W.; Borucki, W.; Kalaji, H.M.; Kowalczyk, K. Photosynthetic Efficiency and Anatomical Structure of Pepper Leaf (Capsicum annuum L.) Transplants Grown under High-Pressure Sodium (HPS) and Light-Emitting Diode (LED) Supplementary Lighting Systems. Plants 2021, 10, 1975. [Google Scholar] [CrossRef]
- Bielczynski, L.W.; Łącki, M.K.; Hoefnagels, I.; Gambin, A.; Croce, R. Leaf and Plant Age Affects Photosynthetic Performance and Photoprotective Capacity. Plant Physiol. 2017, 175, 1634–1648. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Franco-Navarro, J.D.; Padilla, Y.G.; Álvarez, S.; Calatayud, Á.; Colmenero-Flores, J.M.; Gómez-Bellot, M.J.; Hernández, J.A.; Martínez-Alcalá, I.; Penella, C.; Pérez-Pérez, J.G.; et al. Advancements in Water-Saving Strategies and Crop Adaptation to Drought: A Comprehensive Review. Physiol. Plant. 2025, 177, e70332. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8, e23681. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Moon, J.-C.; Kim, C.; Manoharan, K.; Kim, W. Reactive Oxygen Species in Plants: Their Generation, Signal Transduction, and Scavenging Mechanisms. Aust. J. Crop Sci. 2011, 5, 709–725. [Google Scholar]
- Xiang, M.; Yu, S.; Gopinath, L.; Salahi, H.; Moss, J.; Wu, Y. Raising Mowing Height Improves Freeze Tolerance of Putting Green–type Bermudagrass. HortScience 2023, 58, 1277–1281. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, A.; Sedlářová, M.; Pospíšil, P. Malondialdehyde enhances PsbP protein release during heat stress in Arabidopsis. Plant Physiol. Biochem. 2023, 202, 107984. [Google Scholar] [CrossRef] [PubMed]
- Yee, T.W.; Mitchell, N.D. Generalized additive models in plant ecology. J. Veg. Sci. 1991, 2, 587–602. [Google Scholar] [CrossRef]
- Siddique, A.B.; Parveen, S.; Rahman, M.Z.; Rahman, J. Revisiting plant stress memory: Mechanisms and contribution to stress adaptation. Physiol. Mol. Biol. Plants 2024, 30, 349–367. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Considine, M.J.; Foyer, C.H. Oxygen and reactive oxygen species-dependent regulation of plant growth and development. Plant Physiol. 2020, 186, 79–92. [Google Scholar] [CrossRef]
- Liebelt, D.J.; Jordan, J.T.; Doherty, C.J. Only a matter of time: The impact of daily and seasonal rhythms on phytochemicals. Phytochem. Rev. 2019, 18, 1409–1433. [Google Scholar] [CrossRef]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef]
- Yoshida, K.; Kaothien, P.; Matsui, T.; Kawaoka, A.; Shinmyo, A. Molecular biology and application of plant peroxidase genes. Appl. Microbiol. Biotechnol. 2003, 60, 665–670. [Google Scholar] [CrossRef]
- Gao, Y.; Jia, Z.; Wu, R.; He, L.; Liu, T.; Li, Q.; Dai, J.; Zhang, J.; Wang, L. Difference in Response of Caragana intermedia Photosynthesis to Soil Water Content in Different Afforestation Years and Related Threshold Effects in Alpine Sandy Lands. Forests 2023, 14, 701. [Google Scholar] [CrossRef]
- Davey, M.W.; Stals, E.; Panis, B.; Keulemans, J.; Swennen, R.L. High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem. 2005, 347, 201–207. [Google Scholar] [CrossRef]
- Somani, B.L.; Khanade, J.; Sinha, R. A modified anthrone-sulfuric acid method for the determination of fructose in the presence of certain proteins. Anal. Biochem. 1987, 167, 327–330. [Google Scholar] [CrossRef]
- Liu, S.; Qiang, X.; Liu, H.; Han, Q.; Yi, P.; Ning, H.; Li, H.; Wang, C.; Zhang, X. Effects of Nutrient Solution Application Rates on Yield, Quality, and Water-Fertilizer Use Efficiency on Greenhouse Tomatoes Using Grown-in Coir. Plants 2024, 13, 893. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Gao, Y.; Liang, Y. Optimal Stubble Management Strategies of Caragana tibetica for Enhancing Stress Resistance and Vegetation Restoration. Plants 2025, 14, 3867. https://doi.org/10.3390/plants14243867
Yuan X, Gao Y, Liang Y. Optimal Stubble Management Strategies of Caragana tibetica for Enhancing Stress Resistance and Vegetation Restoration. Plants. 2025; 14(24):3867. https://doi.org/10.3390/plants14243867
Chicago/Turabian StyleYuan, Xiaoman, Yong Gao, and Yumei Liang. 2025. "Optimal Stubble Management Strategies of Caragana tibetica for Enhancing Stress Resistance and Vegetation Restoration" Plants 14, no. 24: 3867. https://doi.org/10.3390/plants14243867
APA StyleYuan, X., Gao, Y., & Liang, Y. (2025). Optimal Stubble Management Strategies of Caragana tibetica for Enhancing Stress Resistance and Vegetation Restoration. Plants, 14(24), 3867. https://doi.org/10.3390/plants14243867


.png)


