Phosphatidic Acid Homeostasis and Membrane Lipid Remodeling Confer Salt Tolerance in Zoysia japonica by Stabilizing Metabolic Networks and a Putative SOS Signaling Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Treatments
2.2. Sample Preparation and LC-MS Analysis
2.3. MS Data Acquisition and Processing
2.4. Statistical Analysis and Metabolite Identification
3. Results
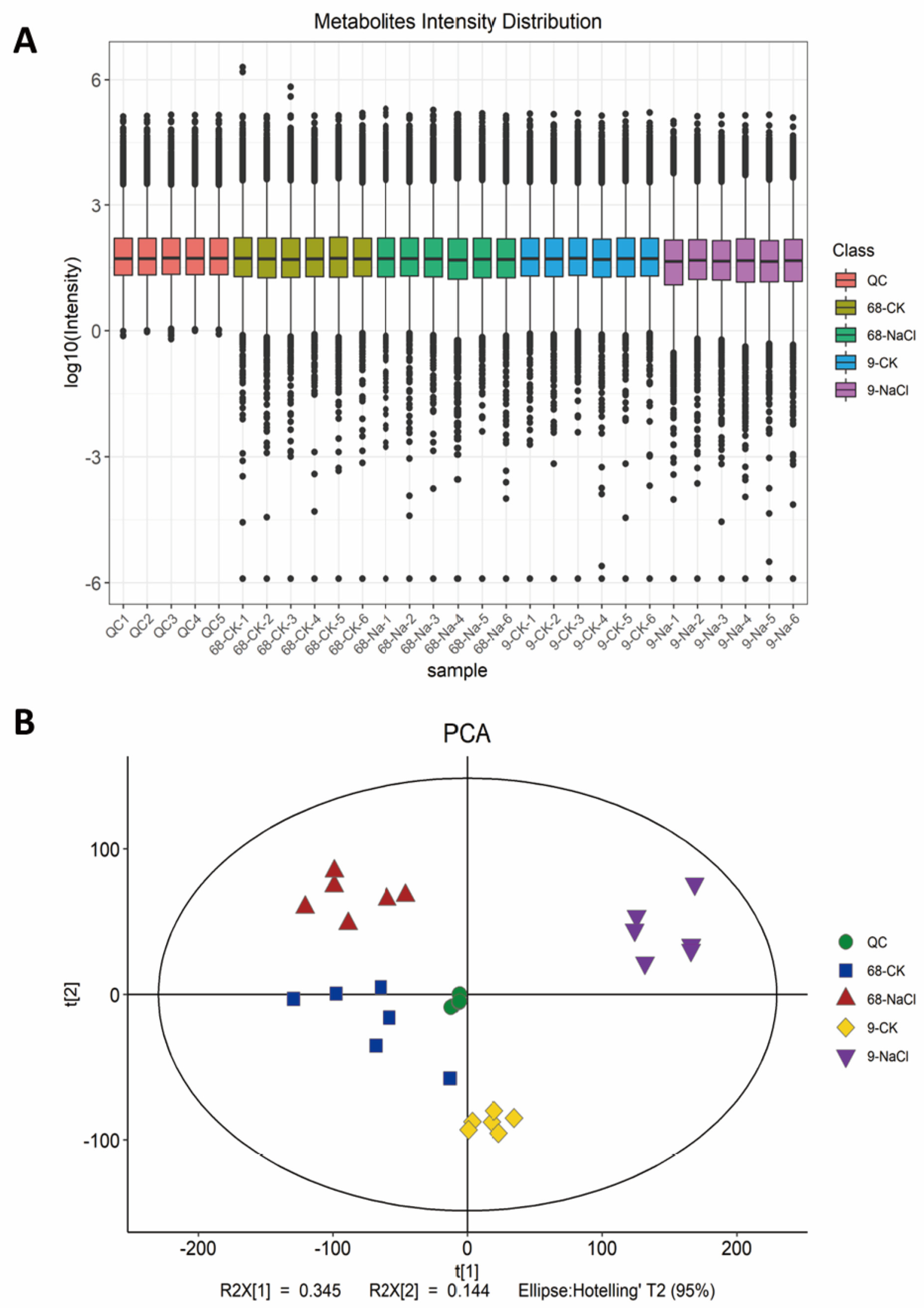
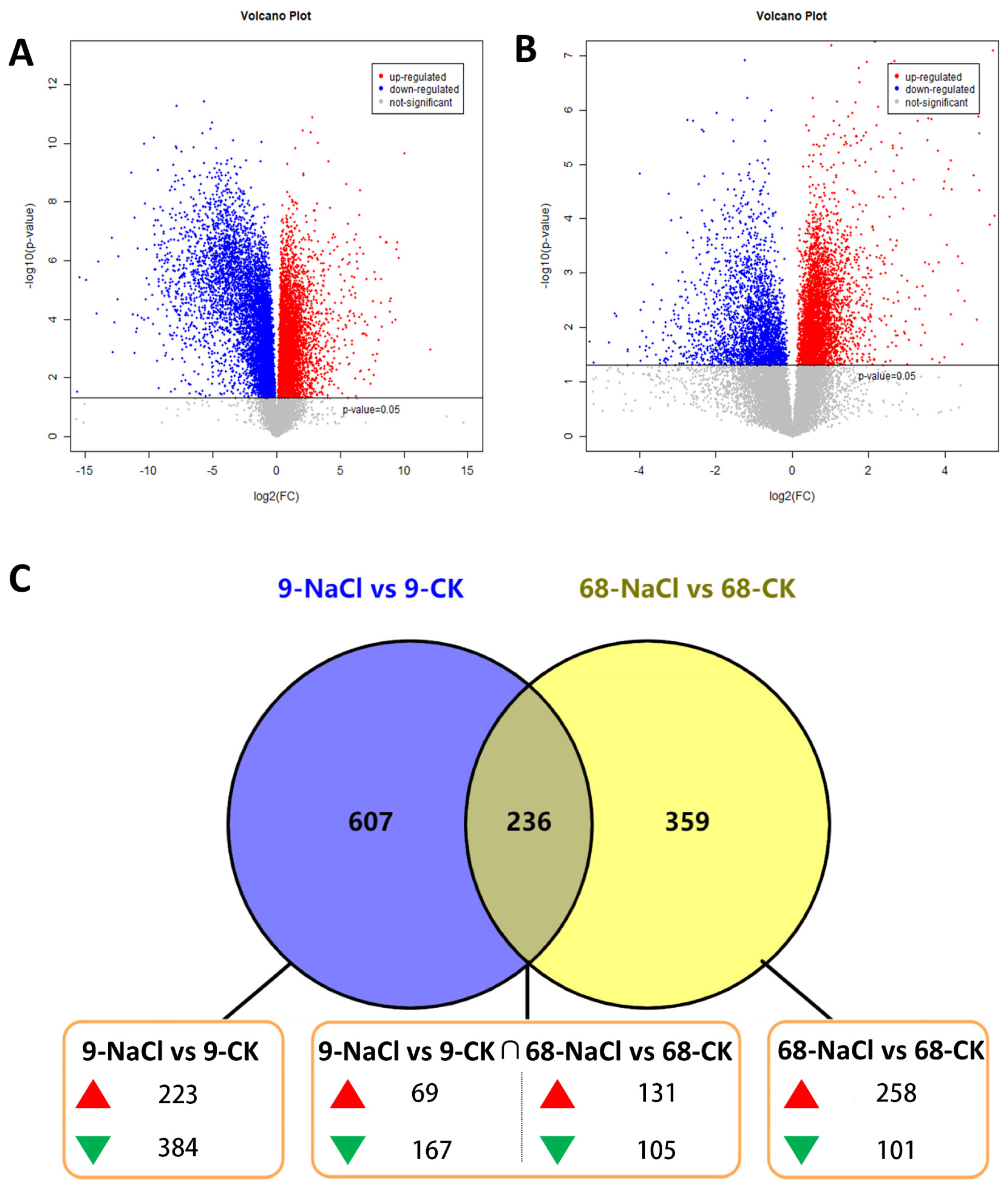
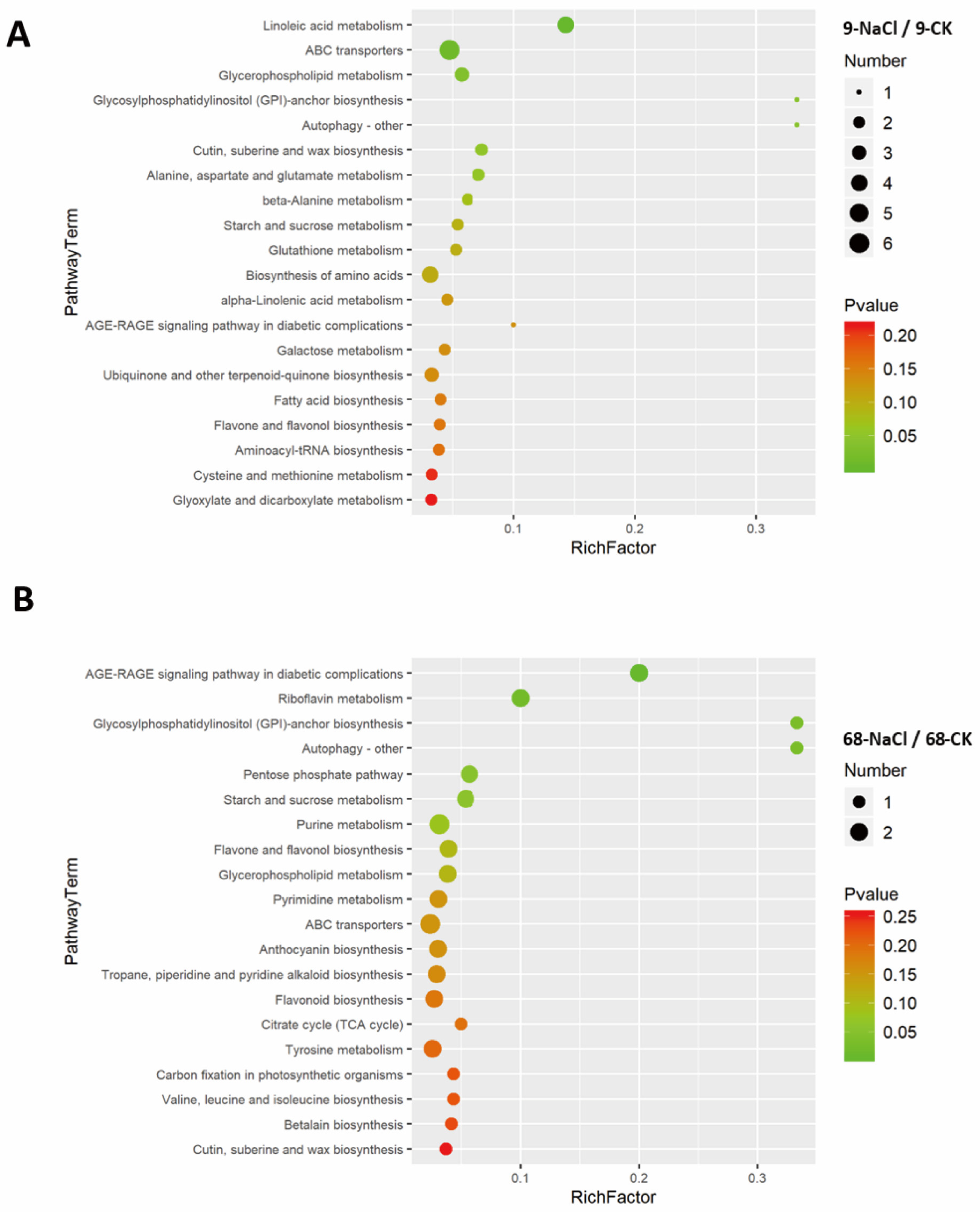
3.1. Metabolomic Profiling Reveals Greater Global Perturbation in the Sensitive Genotype
3.2. Predominant Metabolite Up-Regulation Underpins Metabolic Stability in Tolerant Genotype
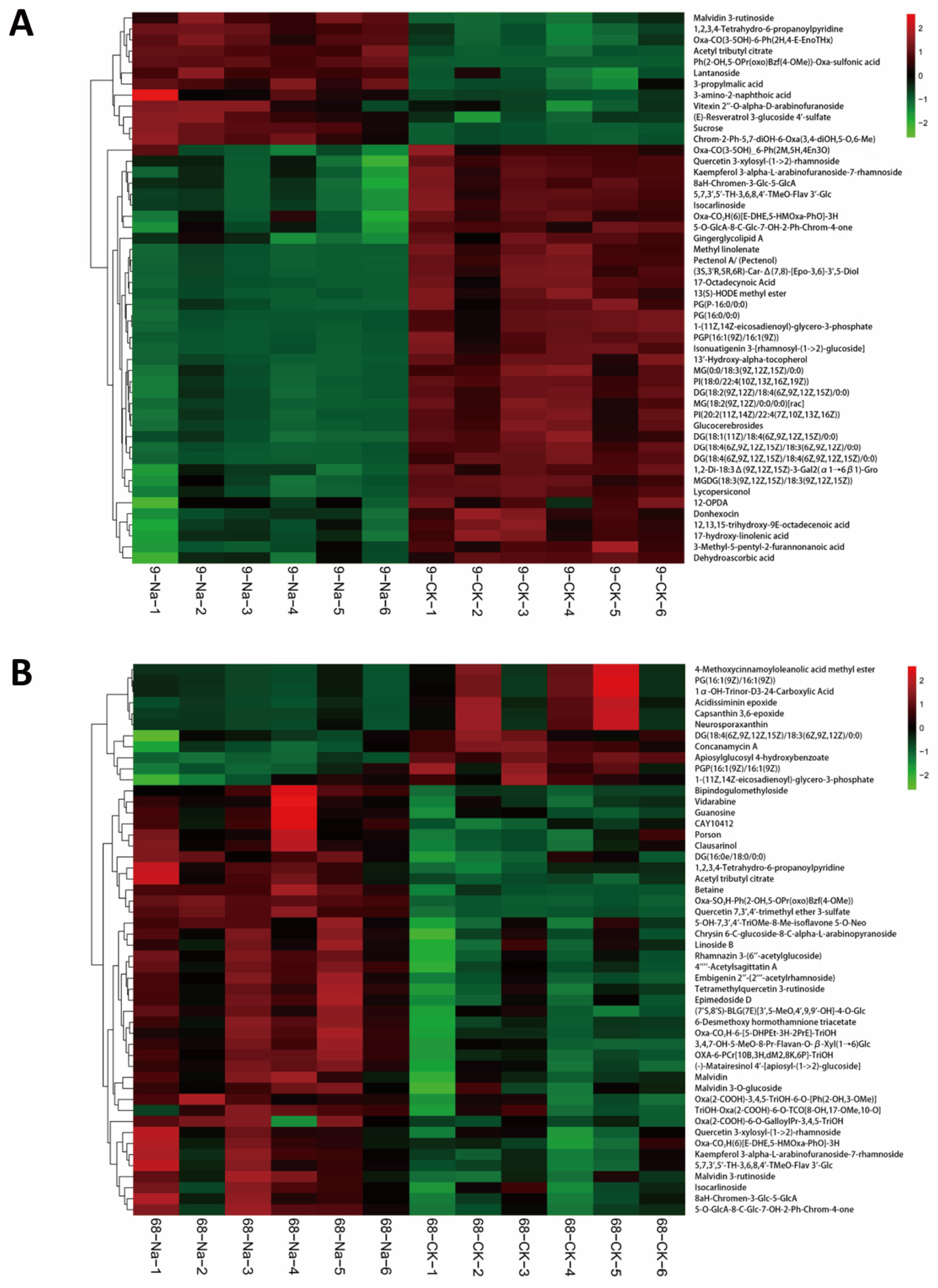
3.3. Cluster Analysis Identifies Lipid Remodeling as a Central Response, with PA Playing a Pivotal Role
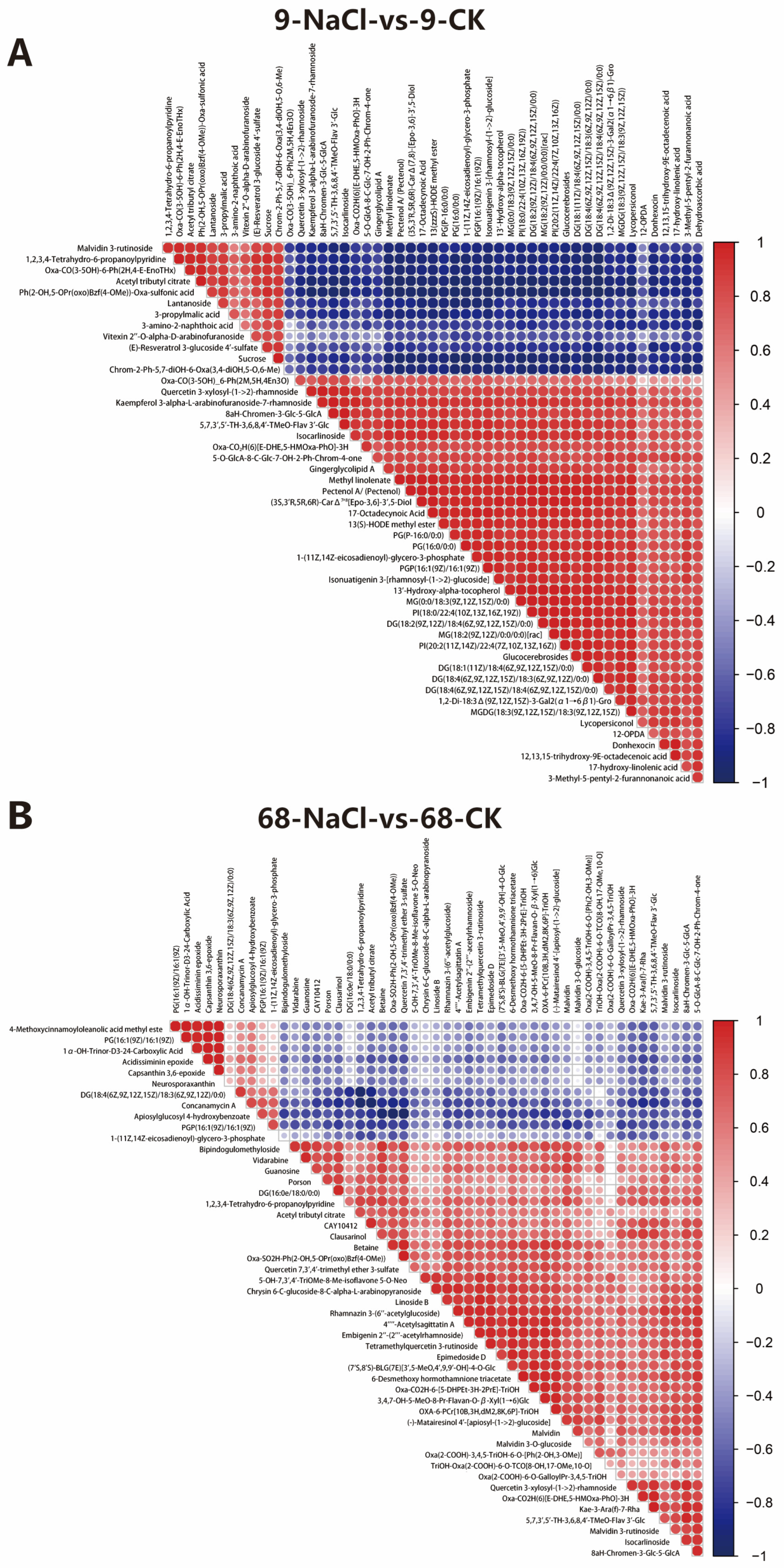
3.4. Correlation Analysis Uncovers a More Coordinated Metabolic Response in the Sensitive Genotype
3.5. Tolerant Genotype Couples Lipid with Energy Metabolism to Sustain Salt Stress Response
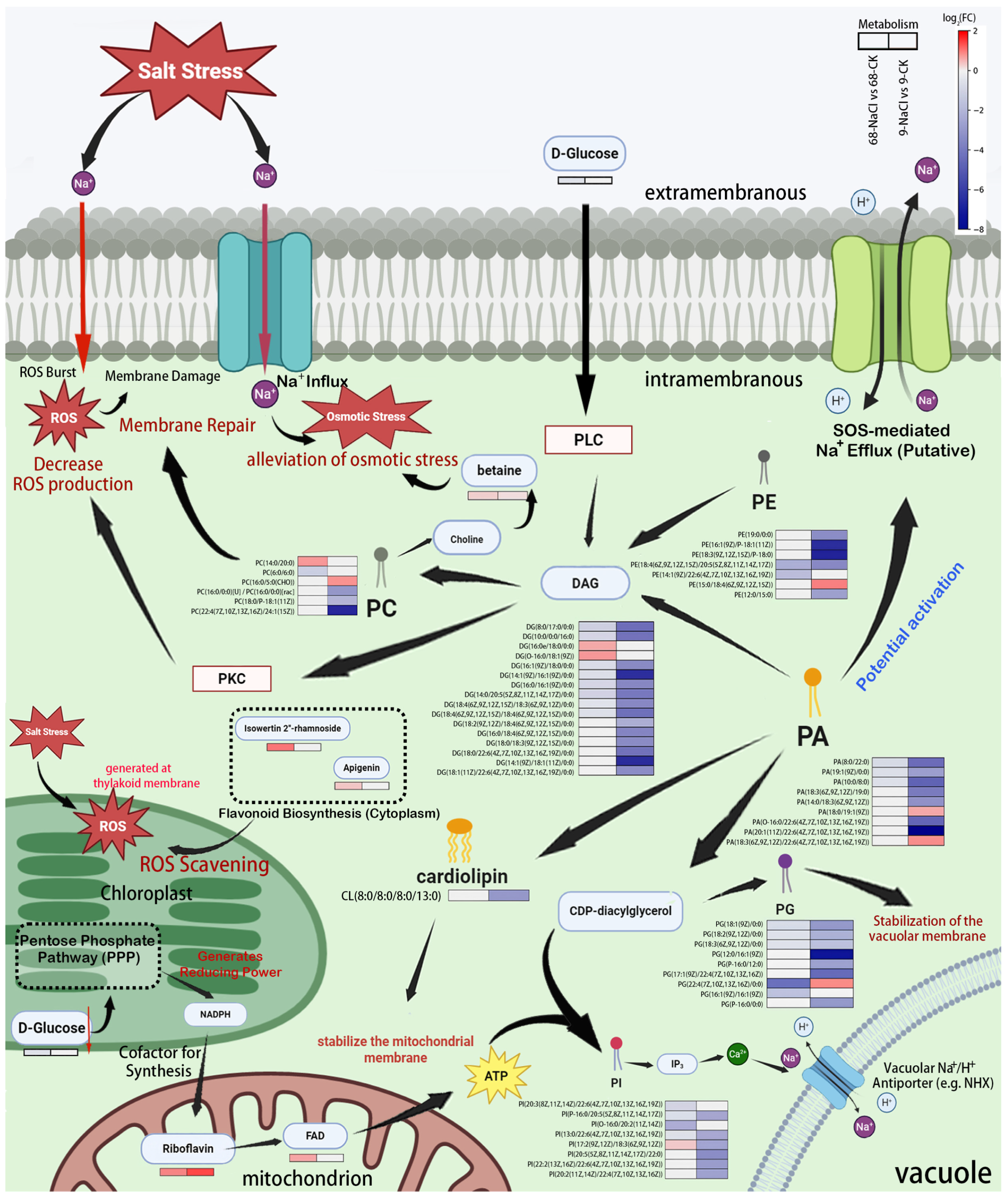
3.6. Proposed Model of a PA-Mediated Metabolic Network for Salt Tolerance
4. Discussion
4.1. Remodeling of Glycerophospholipid Metabolic Network Centered on Phosphatidic Acid
4.2. DAG-PKC Signaling Axis in Oxidative Stress Regulation
4.3. Cardiolipin and Riboflavin Metabolism Maintain Mitochondrial Energy Supply
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, K.; Chang, L.; Li, G.; Li, Y. Advances and future research in ecological stoichiometry under saline-alkali stress. Environ. Sci. Pollut. Res. 2023, 30, 5475–5486. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, L.; Zhao, F.; Li, J.; Zhang, X.; Kong, X.; Wu, H.; Zhang, Z. Plant Salinity Stress Response and Nano-Enabled Plant Salt Tolerance. Front. Plant Sci. 2022, 13, 843994. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef]
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U.; et al. Using Exogenous Melatonin, Glutathione, Proline, and Glycine Betaine Treatments to Combat Abiotic Stresses in Crops. Int. J. Mol. Sci. 2022, 23, 12913. [Google Scholar] [CrossRef]
- Kesawat, M.S.; Satheesh, N.; Kherawat, B.S.; Kumar, A.; Kim, H.U.; Chung, S.M.; Kumar, M. Regulation of Reactive Oxygen Species during Salt Stress in Plants and Their Crosstalk with Other Signaling Molecules-Current Perspectives and Future Directions. Plants 2023, 12, 864. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense Against Abiotic and Biotic Stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Cao, Y.-H.; Lü, Z.-L.; Li, Y.-H.; Jiang, Y.; Zhang, J.-L. Integrated metabolomic and transcriptomic analysis reveals the role of root phenylpropanoid biosynthesis pathway in the salt tolerance of perennial ryegrass. BMC Plant Biol. 2024, 24, 1225. [Google Scholar] [CrossRef]
- Singh, P.; Choudhary, K.K.; Chaudhary, N.; Gupta, S.; Sahu, M.; Tejaswini, B.; Sarkar, S. Salt stress resilience in plants mediated through osmolyte accumulation and its crosstalk mechanism with phytohormones. Front. Plant Sci. 2022, 13, 1006617. [Google Scholar] [CrossRef]
- Song, T.; Xu, H.; Sun, N.; Jiang, L.; Tian, P.; Yong, Y.; Yang, W.; Cai, H.; Cui, G. Metabolomic Analysis of Alfalfa (Medicago sativa L.) Root-Symbiotic Rhizobia Responses under Alkali Stress. Front. Plant Sci. 2017, 8, 1208. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, L.; Barkla, B.J. Membrane Lipid Remodeling in Response to Salinity. Int. J. Mol. Sci. 2019, 20, 4264. [Google Scholar] [CrossRef]
- Sun, M.; Liu, X.; Gao, H.; Zhang, B.; Peng, F.; Xiao, Y. Phosphatidylcholine Enhances Homeostasis in Peach Seedling Cell Membrane and Increases Its Salt Stress Tolerance by Phosphatidic Acid. Int. J. Mol. Sci. 2022, 23, 2585. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhou, C.; Feng, N.; Zheng, D.; Shen, X.; Rao, G.; Huang, Y.; Cai, W.; Liu, Y.; Zhang, R. Transcriptomic and Lipidomic Analysis Reveals Complex Regulation Mechanisms Underlying Rice Roots’ Response to Salt Stress. Metabolites 2024, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Vaziriyeganeh, M.; Khan, S.; Zwiazek, J.J. Transcriptome and Metabolome Analyses Reveal Potential Salt Tolerance Mechanisms Contributing to Maintenance of Water Balance by the Halophytic Grass Puccinellia nuttalliana. Front. Plant Sci. 2021, 12, 760863. [Google Scholar] [CrossRef] [PubMed]
- Al-Huqail, A.A.; Aref, N.M.A.; Khan, F.; Sobhy, S.E.; Hafez, E.E.; Khalifa, A.M.; Saad-Allah, K.M. Azolla filiculoides extract improved salt tolerance in wheat (Triticum aestivum L.) is associated with prompting osmostasis, antioxidant potential and stress-interrelated genes. Sci. Rep. 2024, 14, 11100. [Google Scholar] [CrossRef]
- Wu, X.; Jia, Q.; Ji, S.; Gong, B.; Li, J.; Lü, G.; Gao, H. Gamma-aminobutyric acid (GABA) alleviates salt damage in tomato by modulating Na+ uptake, the GAD gene, amino acid synthesis and reactive oxygen species metabolism. BMC Plant Biol. 2020, 20, 465. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Dong, G.; Zhu, G.; Zhou, G. Progress of Research on the Physiology and Molecular Regulation of Sorghum Growth under Salt Stress by Gibberellin. Int. J. Mol. Sci. 2023, 24, 6777. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, L.; Wei, Z.; Bai, Q.; Zhao, C.; Zhang, S.; Pan, J.; Yu, J.; Zhang, S.; Wei, J. Gamma-aminobutyric acid (GABA) improves salinity stress tolerance in soybean seedlings by modulating their mineral nutrition, osmolyte contents, and ascorbate-glutathione cycle. BMC Plant Biol. 2024, 24, 365. [Google Scholar] [CrossRef]
- Huang, X.; Yang, L.; Jin, Y.; Lin, J.; Liu, F. Generation, Annotation, and Analysis of a Large-Scale Expressed Sequence Tag Library from Arabidopsis pumila to Explore Salt-Responsive Genes. Front. Plant Sci. 2017, 8, 955. [Google Scholar] [CrossRef]
- Wang, J.; An, C.; Guo, H.; Yang, X.; Chen, J.; Zong, J.; Li, J.; Liu, J. Physiological and transcriptomic analyses reveal the mechanisms underlying the salt tolerance of Zoysia japonica Steud. BMC Plant Biol. 2020, 20, 114. [Google Scholar] [CrossRef]
- Uddin, K.; Juraimi, A.S.; Ismail, M.R.; Hossain, A.; Othman, R.; Abdul Rahim, A. Physiological and growth responses of six turfgrass species relative to salinity tolerance. Sci. World J. 2012, 2012, 905468. [Google Scholar] [CrossRef]
- Yamamoto, A.; Hashiguchi, M.; Akune, R.; Masumoto, T.; Muguerza, M.; Saeki, Y.; Akashi, R. The relationship between salt gland density and sodium accumulation/secretion in a wide selection from three Zoysia species. Aust. J. Bot. 2016, 64, 277–284. [Google Scholar] [CrossRef]
- Xie, Q.; Niu, J.; Xu, X.; Xu, L.; Zhang, Y.; Fan, B.; Liang, X.; Zhang, L.; Yin, S.; Han, L. De novo assembly of the Japanese lawngrass (Zoysia japonica Steud.) root transcriptome and identification of candidate unigenes related to early responses under salt stress. Front. Plant Sci. 2015, 6, 610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Z.; Liu, W.; Li, L.; Han, L.; Xu, L.; Zhao, Y. Transcriptome Analysis Revealed a Positive Role of Ethephon on Chlorophyll Metabolism of Zoysia japonica under Cold Stress. Plants 2022, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Himabindu, Y.; Chakradhar, T.; Reddy, M.C.; Kanygin, A.; Redding, K.E.; Chandrasekhar, T. Salt-tolerant genes from halophytes are potential key players of salt tolerance in glycophytes. Environ. Exp. Bot. 2016, 124, 39–63. [Google Scholar] [CrossRef]
- Teng, K.; Yue, Y.; Zhang, H.; Li, H.; Xu, L.; Han, C.; Fan, X.; Wu, J. Functional Characterization of the Pheophytinase Gene, ZjPPH, From Zoysia japonica in Regulating Chlorophyll Degradation and Photosynthesis. Front. Plant Sci. 2021, 12, 786570. [Google Scholar] [CrossRef]
- Guan, J.; Teng, K.; Yue, Y.; Guo, Y.; Liu, L.; Yin, S.; Han, L. Zoysia japonica Chlorophyll b Reductase Gene NOL Participates in Chlorophyll Degradation and Photosynthesis. Front. Plant Sci. 2022, 13, 906018. [Google Scholar] [CrossRef]
- Zuo, Z.F.; Sun, H.J.; Lee, H.Y.; Kang, H.G. Identification of bHLH genes through genome-wide association study and antisense expression of ZjbHLH076/ZjICE1 influence tolerance to low temperature and salinity in Zoysia japonica. Plant Sci. 2021, 313, 111088. [Google Scholar] [CrossRef]
- Riechmann, J.L.; Meyerowitz, E.M. The AP2/EREBP family of plant transcription factors. Biol. Chem. 1998, 379, 633–646. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, S.; Xu, X.; Sun, J.; Li, M.; Sun, Y.; Wang, K.; Mao, P.; Ma, X. Comparative physiological and transcriptomic analyses of zoysiagrass (Zoysia japonica) species reveal key metabolic pathways for salt tolerance. Grass Res. 2025, 5, e013. [Google Scholar] [CrossRef]
- Spijker, P.; van Hoof, B.; Debertrand, M.; Markvoort, A.J.; Vaidehi, N.; Hilbers, P.A. Coarse grained molecular dynamics simulations of transmembrane protein-lipid systems. Int. J. Mol. Sci. 2010, 11, 2393–2420. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Mostofa, M.G.; Keya, S.S.; Siddiqui, M.N.; Ansary, M.M.U.; Das, A.K.; Rahman, M.A.; Tran, L.S. Adaptive Mechanisms of Halophytes and Their Potential in Improving Salinity Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 10733. [Google Scholar] [CrossRef] [PubMed]
- Baczewska, A.H.; Dmuchowski, W.; Jozwiak, A.; Gozdowski, D.; Brągoszewska, P.; Dąbrowski, P.; Swiezewska, E. Effect of salt stress on prenol lipids in the leaves of Tilia ‘Euchlora’. Dendrobiology 2014, 72, 177–186. [Google Scholar] [CrossRef]
- Guo, R.; Yang, Z.; Li, F.; Yan, C.; Zhong, X.; Liu, Q.; Xia, X.; Li, H.; Zhao, L. Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biol. 2015, 15, 170. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.; Yan, C.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Ionomic and metabolic responses to neutral salt or alkaline salt stresses in maize (Zea mays L.) seedlings. BMC Plant Biol. 2017, 17, 41. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Huang, B. Lipidomic reprogramming associated with drought stress priming-enhanced heat tolerance in tall fescue (Festuca arundinacea). Plant Cell Environ. 2019, 42, 947–958. [Google Scholar] [CrossRef]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Zhu, Y.; Fu, Q.; Zhu, C.; Li, Y.; Yuan, F.; Sun, X.; Fu, J. Review on physiological and molecular mechanisms for enhancing salt tolerance in turfgrass. Grass Res. 2024, 4, e024. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, C.; Wang, Y.; Yun, C.; Zou, X.; Cheng, N.; Zhang, W.; Jing, Y.; Li, H. Understanding of Plant Salt Tolerance Mechanisms and Application to Molecular Breeding. Int. J. Mol. Sci. 2024, 25, 10940. [Google Scholar] [CrossRef]
- Flis, V.V.; Daum, G. Lipid transport between the endoplasmic reticulum and mitochondria. Cold Spring Harb. Perspect. Biol. 2013, 5, a013235. [Google Scholar] [CrossRef]
- Sui, N.; Tian, S.; Wang, W.; Wang, M.; Fan, H. Overexpression of Glycerol-3-Phosphate Acyltransferase from Suaeda salsa Improves Salt Tolerance in Arabidopsis. Front. Plant Sci. 2017, 8, 1337. [Google Scholar] [CrossRef] [PubMed]
- Carraro, E.; Di Iorio, A. Eligible strategies of drought response to improve drought resistance in woody crops: A mini-review. Plant Biotechnol. Rep. 2022, 16, 265–282. [Google Scholar] [CrossRef]
- Kaushal, M. Insights Into Microbially Induced Salt Tolerance and Endurance Mechanisms (STEM) in Plants. Front. Microbiol. 2020, 11, 1518. [Google Scholar] [CrossRef]
- Luo, Q.; Xie, H.; Chen, Z.; Ma, Y.; Yang, H.; Yang, B.; Ma, Y. Morphology, photosynthetic physiology and biochemistry of nine herbaceous plants under water stress. Front. Plant Sci. 2023, 14, 1147208. [Google Scholar] [CrossRef]
- Lee, B.-R.; Muneer, S.; Park, S.-H.; Zhang, Q.; Kim, T.-H. Ammonium-induced proline and sucrose accumulation, and their significance in antioxidative activity and osmotic adjustment. Acta Physiol. Plant. 2013, 35, 2655–2664. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Alam, M.R.; Kamal, M.A.; Seo, K.J.; Singh, L.R. AGE-RAGE axis culminates into multiple pathogenic processes: A central road to neurodegeneration. Front. Mol. Neurosci. 2023, 16, 1155175. [Google Scholar] [CrossRef]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.G.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef]
- Feng, S.; Yao, Y.-T.; Wang, B.-B.; Li, Y.-M.; Li, L.; Bao, A.-K. Flavonoids are involved in salt tolerance through ROS scavenging in the halophyte Atriplex canescens. Plant Cell Rep. 2023, 43, 5. [Google Scholar] [CrossRef]
- Tian, P.; Feng, Y.-X.; Li, Y.-H. SOS! Hydrogen Sulfide Enhances the Flavonoid Early Warning System in Rice Plants to Cope with Thiocyanate Pollution. Toxics 2024, 12, 591. [Google Scholar] [CrossRef]
- Huan, L.; Xie, X.; Zheng, Z.; Sun, F.; Wu, S.; Li, M.; Gao, S.; Gu, W.; Wang, G. Positive Correlation Between PSI Response and Oxidative Pentose Phosphate Pathway Activity During Salt Stress in an Intertidal Macroalga. Plant Cell Physiol. 2014, 55, 1395–1403. [Google Scholar] [CrossRef]
- Yang, B.; Sun, Y.; Fu, S.; Xia, M.; Su, Y.; Liu, C.; Zhang, C.; Zhang, D. Improving the Production of Riboflavin by Introducing a Mutant Ribulose 5-Phosphate 3-Epimerase Gene in Bacillus subtilis. Front. Bioeng. Biotechnol. 2021, 9, 704650. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
| Metabolite Pathway | Metabolite | Log2(FC) | |
|---|---|---|---|
| SS9(NaCl/CK) | ST68(NaCl/CK) | ||
| Membrane Lipid Remodeling and Stability | PC (14:0/20:0) | – | 0.721 |
| PC (22:4/24:1) | −6.700 | – | |
| PE (18:4/20:5) | −3.430 | −2.213 | |
| PE (14:1/22:6) | – | −1.788 | |
| PE (15:0/18:4) | 0.936 | – | |
| CL (8:0/8:0/8:0/13:0) | −2.958 | – | |
| Lipid Signaling Molecules | PA (8:0/22:0) | −4.448 | −0.799 |
| PA (19:1/0:0) | −2.743 | −0.640 | |
| PA (10:0/8:0) | −4.702 | −0.595 | |
| PA (20:1/22:6) | −8.580 | – | |
| DG (8:0/17:0/0:0) | −4.354 | −1.089 | |
| DG (10:0/0:0/16:0) | −4.202 | −0.908 | |
| Antioxidant Metabolites | Apigenin | – | 0.358 |
| Isowertin 2′′-rhamnoside | – | 1.020 | |
| Osmoprotectants | Betaine | 0.159 | 0.277 |
| L-Proline | 3.688 | – | |
| Energy and Cofactor Metabolism | Riboflavin | 1.402 | 0.894 |
| FAD | – | 0.609 | |
| D-Glucose | – | −0.514 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Zeng, X.; Liu, Z.; Liu, Z.; Hu, Q.; Li, M. Phosphatidic Acid Homeostasis and Membrane Lipid Remodeling Confer Salt Tolerance in Zoysia japonica by Stabilizing Metabolic Networks and a Putative SOS Signaling Activation. Plants 2025, 14, 3630. https://doi.org/10.3390/plants14233630
Yang Q, Zeng X, Liu Z, Liu Z, Hu Q, Li M. Phosphatidic Acid Homeostasis and Membrane Lipid Remodeling Confer Salt Tolerance in Zoysia japonica by Stabilizing Metabolic Networks and a Putative SOS Signaling Activation. Plants. 2025; 14(23):3630. https://doi.org/10.3390/plants14233630
Chicago/Turabian StyleYang, Qinhao, Xiangcui Zeng, Zhenzhen Liu, Zhongkuan Liu, Qiannan Hu, and Mingna Li. 2025. "Phosphatidic Acid Homeostasis and Membrane Lipid Remodeling Confer Salt Tolerance in Zoysia japonica by Stabilizing Metabolic Networks and a Putative SOS Signaling Activation" Plants 14, no. 23: 3630. https://doi.org/10.3390/plants14233630
APA StyleYang, Q., Zeng, X., Liu, Z., Liu, Z., Hu, Q., & Li, M. (2025). Phosphatidic Acid Homeostasis and Membrane Lipid Remodeling Confer Salt Tolerance in Zoysia japonica by Stabilizing Metabolic Networks and a Putative SOS Signaling Activation. Plants, 14(23), 3630. https://doi.org/10.3390/plants14233630






