Exploring the Effects of VgPIP1;2 Overexpression in the Roots of Young Rice Plants: Modifications in Root Architecture, Transcriptomic and Metabolomic Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Transgenic Plant Generation
2.1.1. Vector Construction
2.1.2. Plant Transformation
2.1.3. Transgene Expression Analysis
2.2. Morphology Analysis
2.2.1. Disinfestation and Germination
2.2.2. Growth Conditions and Sampling
2.3. Biochemical and Gene Expression Analyses
2.3.1. Nitrogen Content
2.3.2. Ammonium Content
2.3.3. Free Amino Acid Content
2.3.4. Total Soluble Protein Content
2.3.5. Metabolomics Analysis
2.3.6. Transcriptomics Analysis
2.3.7. Sequence Analyses, Differential Expression and Gene Ontology Enrichment
2.4. Phylogenetic Analysis
2.5. Statistical Analysis
3. Results
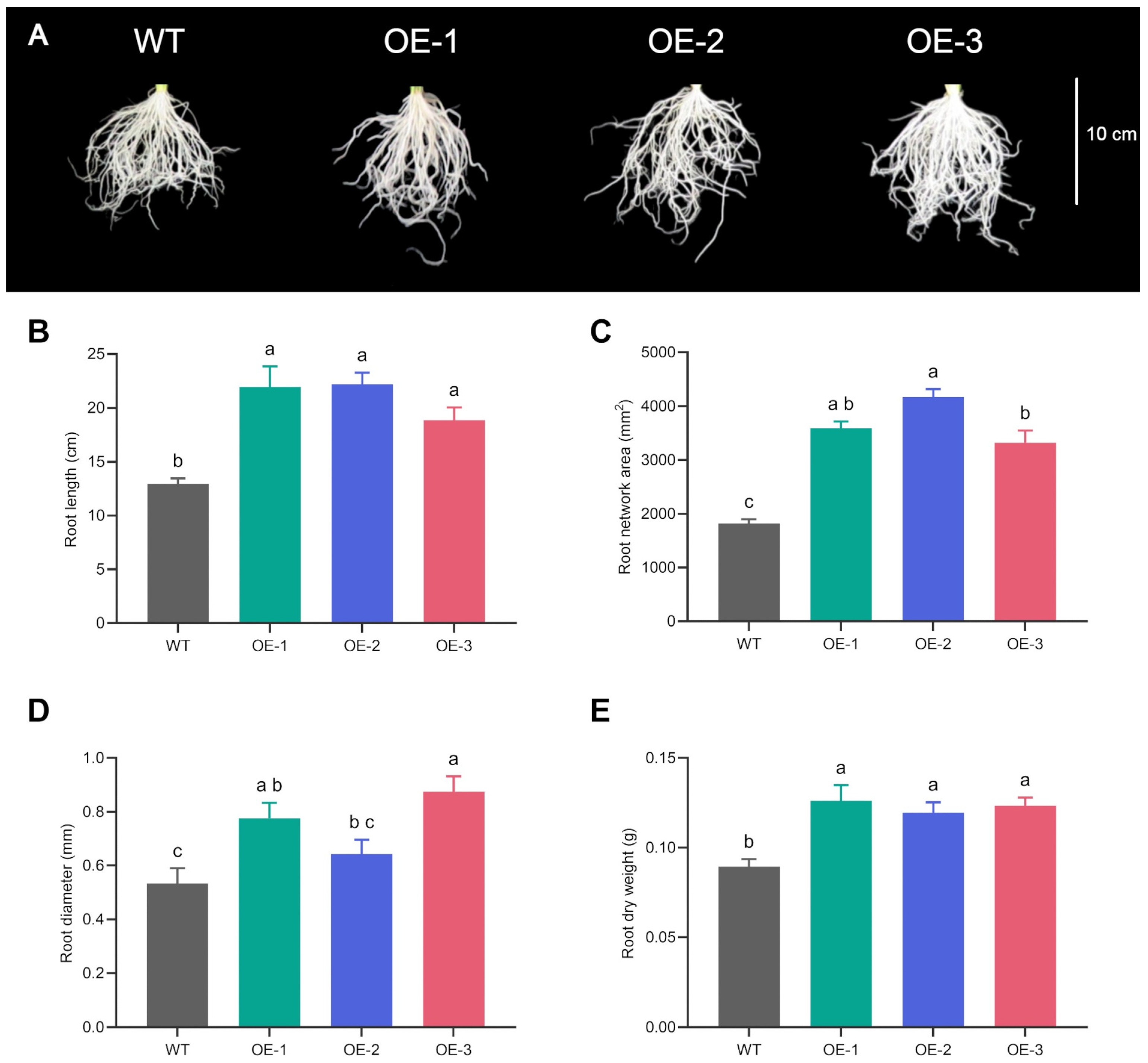
3.1. VgPIP1;2-Overexpressing Lines and Changes in Root Architecture
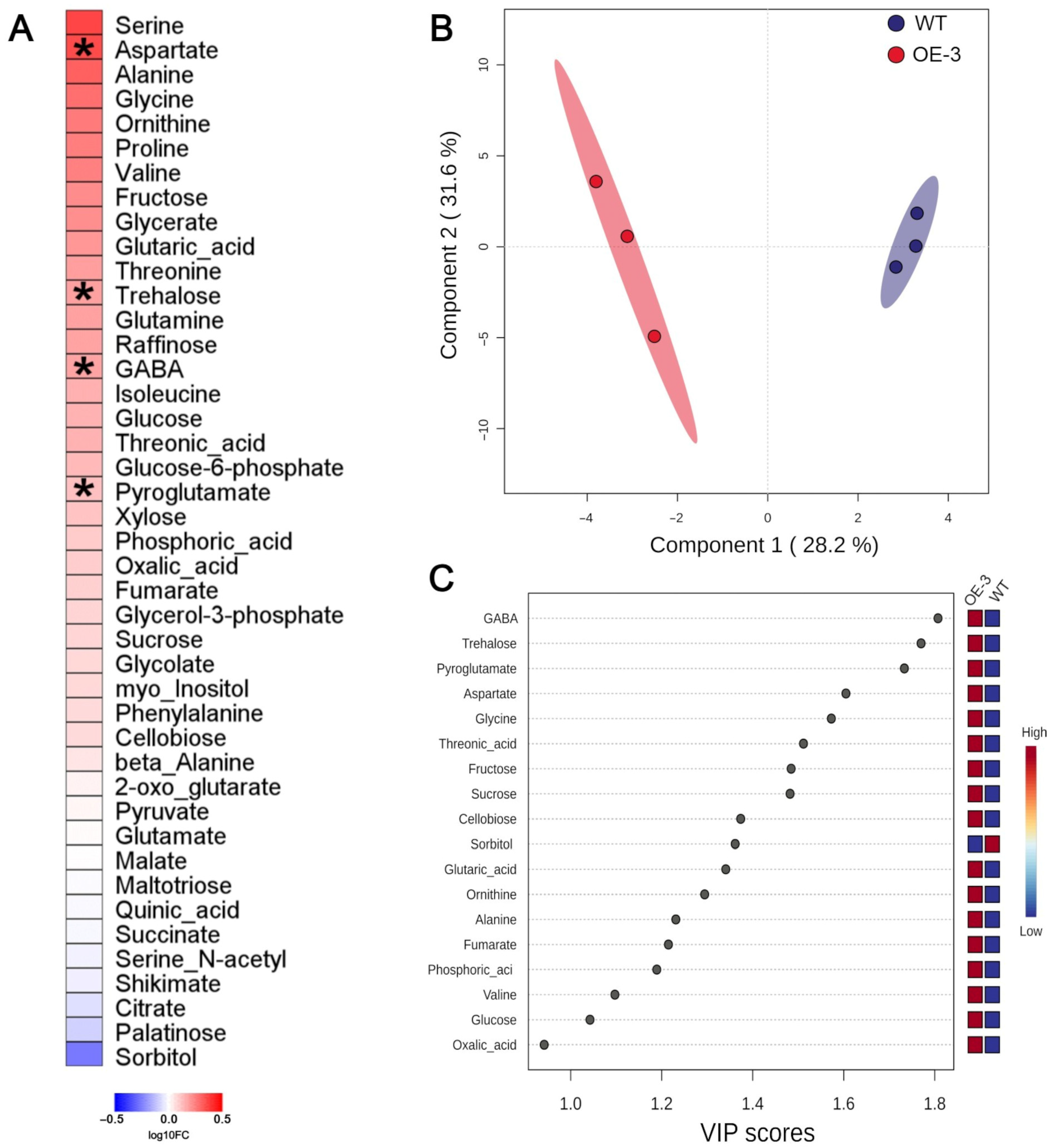
3.2. VgPIP1;2 Overexpression Promotes Changes in the Metabolite Profile
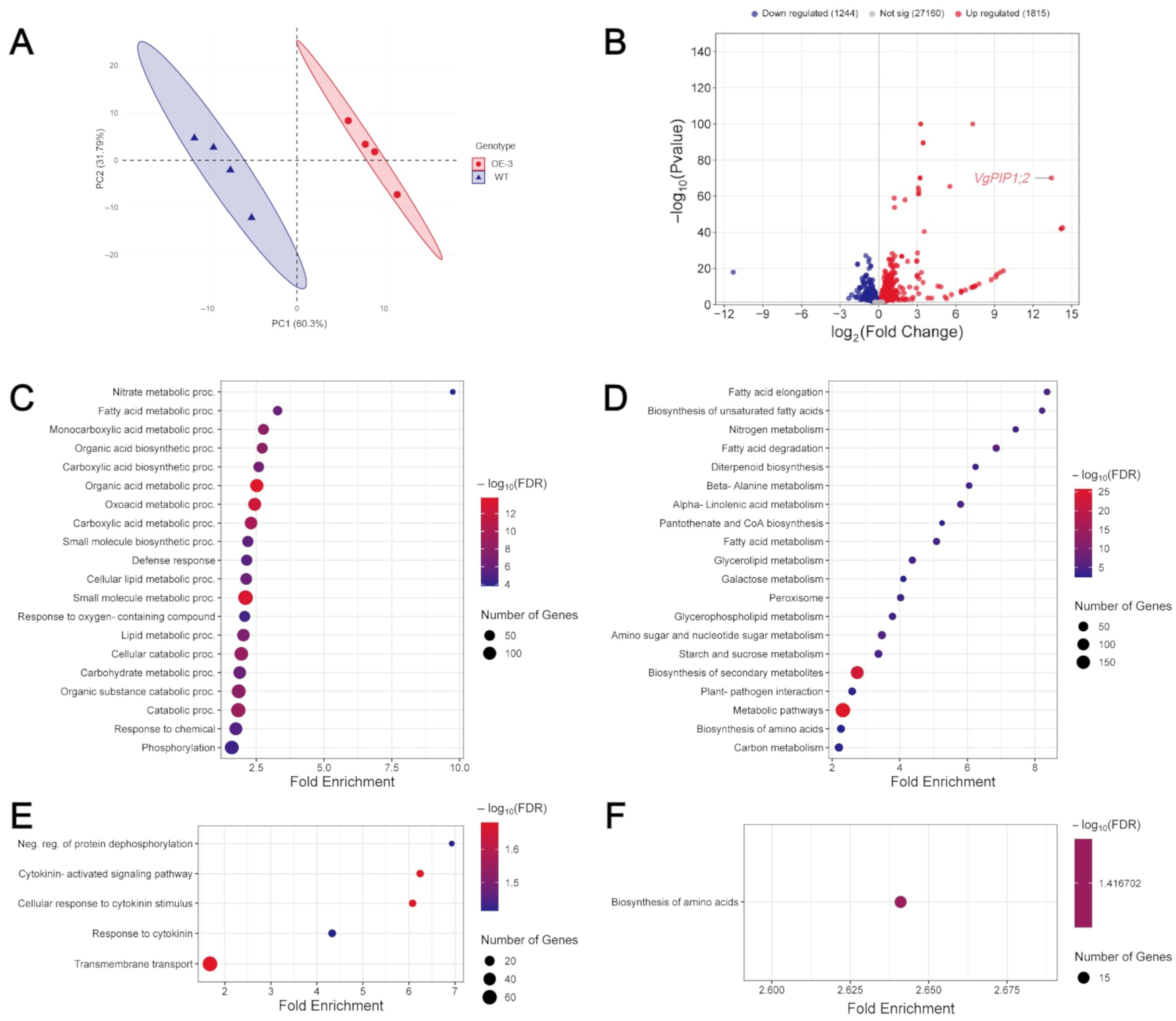
3.3. VgPIP1;2 Overexpression Promotes Changes in Gene Expression Profile
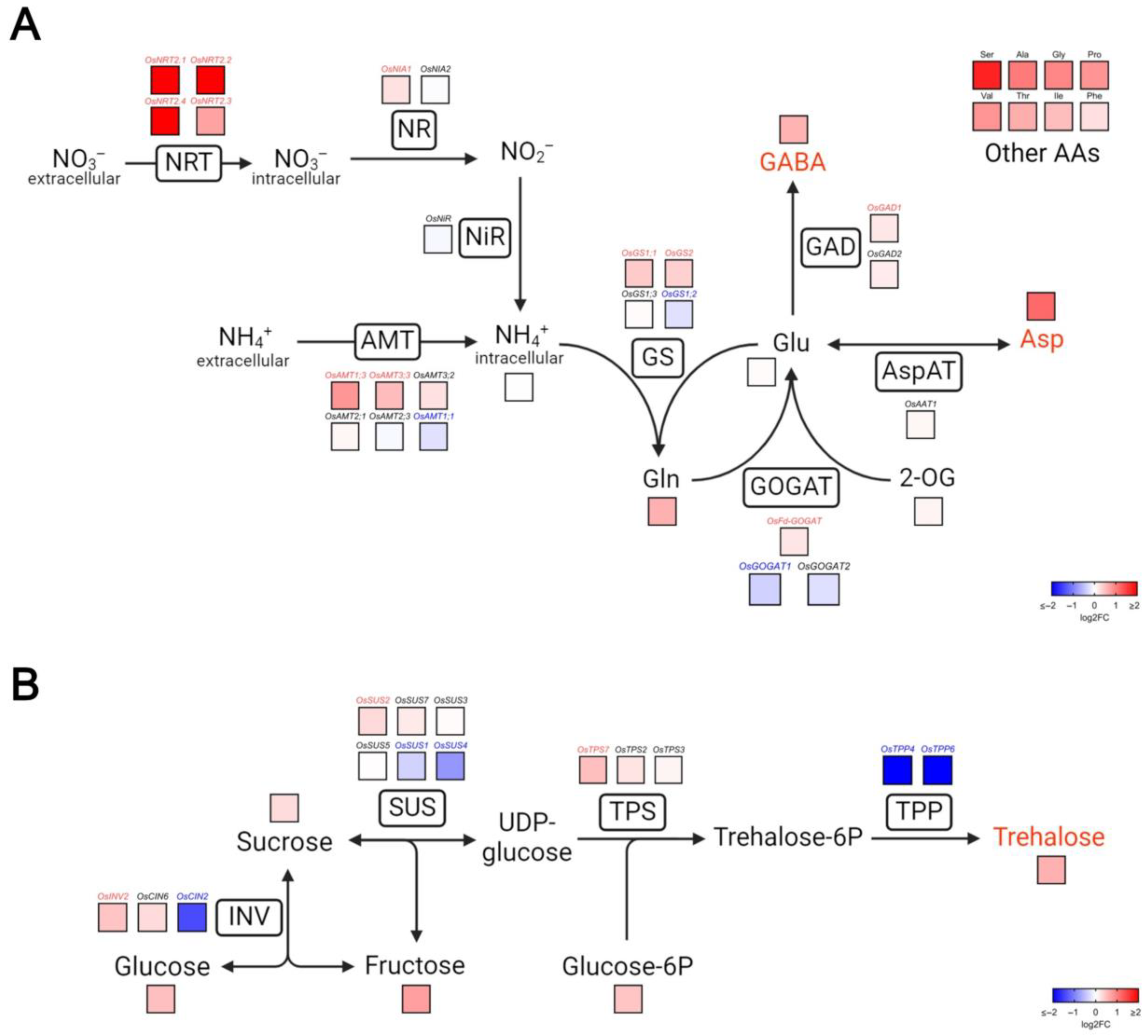
3.4. VgPIP1;2 Overexpression Causes Changes in Nitrogen and Carbon Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elert, E. Rice by the Numbers: A Good Grain. Nature 2014, 514, S50. [Google Scholar] [CrossRef] [PubMed]
- FAO Food and Agricultural Organization of the United States. Available online: https://www.fao.org/faostat/en/#data (accessed on 2 February 2025).
- Jahangir, M.M.R.; Bell, R.W.; Uddin, S.; Ferdous, J.; Nasreen, S.S.; Haque, M.E.; Satter, M.A.; Zaman, M.; Ding, W.; Jahiruddin, M. Conservation Agriculture with Optimum Fertilizer Nitrogen Rate Reduces GWP for Rice Cultivation in Floodplain Soils. Front. Environ. Sci. 2022, 10, 853655. [Google Scholar] [CrossRef]
- Peng, S.-Z.; Yang, S.-H.; Xu, J.-Z.; Luo, Y.-F.; Hou, H.-J. Nitrogen and Phosphorus Leaching Losses from Paddy Fields with Different Water and Nitrogen Managements. Paddy Water Environ. 2011, 9, 333–342. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Lowland Rice Response to Nitrogen Fertilization. Commun. Soil Sci. Plant Anal. 2001, 32, 1405–1429. [Google Scholar] [CrossRef]
- Chivenge, P.; Sharma, S.; Bunquin, M.A.; Hellin, J. Improving Nitrogen Use Efficiency—A Key for Sustainable Rice Production Systems. Front. Sustain. Food Syst. 2021, 5, 737412. [Google Scholar] [CrossRef]
- Hao, S.; Liu, X.; Liu, C.; Liu, W. Nitrogen Loss and Migration in Rice Fields under Different Water and Fertilizer Modes. Plants 2024, 13, 562. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Chen, Y.; Jiang, Y.; Shi, Y.; Zhao, L.; Liao, P.; Wang, W.; Xu, K.; Dai, Q. Excessive Nitrogen Application Leads to Lower Rice Yield and Grain Quality by Inhibiting the Grain Filling of Inferior Grains. Agriculture 2022, 12, 962. [Google Scholar] [CrossRef]
- Heffer, P.; Gruère, A.; Roberts, T. Assessment of Fertilizer Use by Crop at the Global Level; International Fertilizer Industry Association: Paris, France, 2013. [Google Scholar]
- Tyagi, J.; Ahmad, S.; Malik, M. Nitrogenous Fertilizers: Impact on Environment Sustainability, Mitigation Strategies, and Challenges. Int. J. Environ. Sci. Technol. 2022, 19, 11649–11672. [Google Scholar] [CrossRef]
- Guo, S.; Yu, H.; Zeng, X.; Shangguan, Y.; Zhou, Z.; Li, X.; Liu, Z.; He, M.; Luo, X.; Ouyang, Y. Balancing Yield and Environmental Impact: Nitrogen Management and Planting Density for Rice in Southwest China. Agronomy 2024, 14, 1843. [Google Scholar] [CrossRef]
- Liu, C.; Sun, Z.; Wang, X.; Wu, G.; Yuan, M.; Wang, J.; Sun, Y.; Liu, Y.; Wu, L. Optimizing Fertilization Strategies for a Climate-Resilient Rice–Wheat Double Cropping System. Nutr. Cycl. Agroecosyst. 2024, 129, 21–35. [Google Scholar] [CrossRef]
- Linquist, B.A.; Liu, L.; van Kessel, C.; van Groenigen, K.J. Enhanced Efficiency Nitrogen Fertilizers for Rice Systems: Meta-Analysis of Yield and Nitrogen Uptake. Field Crops Res. 2013, 154, 246–254. [Google Scholar] [CrossRef]
- Li, H.; Hu, B.; Chu, C. Nitrogen Use Efficiency in Crops: Lessons from Arabidopsis and Rice. J. Exp. Bot. 2017, 68, 2477–2488. [Google Scholar] [CrossRef]
- Hu, B.; Wang, W.; Chen, J.; Liu, Y.; Chu, C. Genetic Improvement toward Nitrogen-Use Efficiency in Rice: Lessons and Perspectives. Mol. Plant 2023, 16, 64–74. [Google Scholar] [CrossRef]
- Huang, S.; Zhao, C.; Zhang, Y.; Wang, C. Nitrogen Use Efficiency in Rice. In Nitrogen in Agriculture-Updates; IntechOpen: London, UK, 2018; pp. 187–208. [Google Scholar]
- Lee, S. Recent Advances on Nitrogen Use Efficiency in Rice. Agronomy 2021, 11, 753. [Google Scholar] [CrossRef]
- Liu, X.; Hu, B.; Chu, C. Nitrogen Assimilation in Plants: Current Status and Future Prospects. J. Genet. Genom. 2022, 49, 394–404. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, H.; Zhang, J. Root Morphology and Physiology in Relation to the Yield Formation of Rice. J. Integr. Agric. 2012, 11, 920–926. [Google Scholar] [CrossRef]
- Huang, Y.; Ji, Z.; Tao, Y.; Wei, S.; Jiao, W.; Fang, Y.; Jian, P.; Shen, C.; Qin, Y.; Zhang, S. Improving Rice Nitrogen-Use Efficiency by Modulating a Novel Monouniquitination Machinery for Optimal Root Plasticity Response to Nitrogen. Nat. Plants 2023, 9, 1902–1914. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Lu, D.-K.; Wang, H.-Z.; Li, Y. Morphological and Physiological Traits of Rice Roots and Their Relationships to Yield and Nitrogen Utilization as Influenced by Irrigation Regime and Nitrogen Rate. Agric. Water Manag. 2018, 203, 385–394. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.; Lee, J.; Shin, D.; Marmagne, A.; Lim, P.O.; Masclaux-Daubresse, C.; An, G.; Nam, H.G. OsASN1 Overexpression in Rice Increases Grain Protein Content and Yield under Nitrogen-Limiting Conditions. Plant Cell Physiol. 2020, 61, 1309–1320. [Google Scholar] [CrossRef]
- Yoon, D.-K.; Ishiyama, K.; Suganami, M.; Tazoe, Y.; Watanabe, M.; Imaruoka, S.; Ogura, M.; Ishida, H.; Suzuki, Y.; Obara, M. Transgenic Rice Overproducing Rubisco Exhibits Increased Yields with Improved Nitrogen-Use Efficiency in an Experimental Paddy Field. Nat. Food 2020, 1, 134–139. [Google Scholar] [CrossRef]
- Wang, D.; Li, L.; Lian, X. Overexpression of OsMYB305 in Rice Enhances the Nitrogen Uptake under Low-Nitrogen Condition. Front Plant Sci. 2020, 11, 519758. [Google Scholar] [CrossRef]
- Alfatih, A.; Wu, J.; Zhang, Z.-S.; Xia, J.-Q.; Jan, S.U.; Yu, L.-H.; Xiang, C.-B. Rice NIN-LIKE PROTEIN 1 Rapidly Responds to Nitrogen Deficiency and Improves Yield and Nitrogen Use Efficiency. J. Exp. Bot. 2020, 71, 6032–6042. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Wang, L.; Dong, X.; Hu, W.; Jiang, M.; Chen, G.; An, G.; Xiong, F.; Wu, Y. OsDOF11 Affects Nitrogen Metabolism by Sucrose Transport Signaling in Rice (Oryza sativa L.). Front. Plant Sci. 2021, 12, 703034. [Google Scholar] [CrossRef]
- Tiong, J.; Sharma, N.; Sampath, R.; MacKenzie, N.; Watanabe, S.; Metot, C.; Lu, Z.; Skinner, W.; Lu, Y.; Kridl, J. Improving Nitrogen Use Efficiency through Overexpression of Alanine Amino transferase in Rice, Wheat, and Barley. Front. Plant Sci. 2021, 12, 628521. [Google Scholar] [CrossRef]
- Wu, D.; Li, Y.; Cao, Y.; Hu, R.; Wu, X.; Zhang, W.; Tao, W.; Xu, G.; Wang, X.; Zhang, Y. Increased Glutamine Synthetase by Overexpression of TaGS1 Improves Grain Yield and Nitrogen Use Efficiency in Rice. Plant Physiol. Biochem. 2021, 169, 259–268. [Google Scholar] [CrossRef]
- Lal, S.K.; Mehta, S.; Raju, D.; Achary, V.M.M.; Venkatapuram, A.K.; Yadav, S.K.; Parmar, H.; Pandey, R.; Panditi, V.; Sheri, V. Concurrent Overexpression of Rice GS1;1 and GS2 Genes to Enhance the Nitrogen Use Efficiency (NUE) in Transgenic Rice. J. Plant Growth Regul. 2023, 42, 6699–6720. [Google Scholar] [CrossRef]
- Benzing, D.H. Bromeliaceae: Profile of an Adaptive Radiation; Cambridge University Press: Cambridge, UK, 2000; ISBN 0521430313. [Google Scholar]
- Sampaio, J.A.T.; Paggi, G.M.; Zanella, C.M.; Bruxel, M.; Palma-Silva, C.; Goetze, M.; Buettow, M.V.; Bered, F. Inbreeding Depression in Vriesea gigantea, a Perennial Bromeliad from Southern Brazil. Bot. J. Linn. Soc. 2012, 169, 312–319. [Google Scholar] [CrossRef]
- Vanhoutte, B.; Schenkels, L.; Ceusters, J.; De Proft, M.P. Water and Nutrient Uptake in Vriesea Cultivars: Trichomes vs. Roots. Environ. Exp. Bot. 2017, 136, 21–30. [Google Scholar] [CrossRef]
- Inselsbacher, E.; Cambui, C.A.; Richter, A.; Stange, C.F.; Mercier, H.; Wanek, W. Microbial Activities and Foliar Uptake of Nitrogen in the Epiphytic Bromeliad Vriesea gigantea. New Phytol. 2007, 175, 311–320. [Google Scholar] [CrossRef]
- Matiz, A.; Mioto, P.T.; Aidar, M.P.M.; Mercier, H. Utilization of Urea by Leaves of Bromeliad Vriesea gigantea under Water Deficit: Much More than a Nitrogen Source. Biol. Plant 2017, 61, 751–762. [Google Scholar] [CrossRef]
- Matiz, A.; Cambuí, C.A.; Richet, N.; Mioto, P.T.; Gomes, F.; Pikart, F.C.; Chaumont, F.; Gaspar, M.; Mercier, H. Involvement of Aquaporins on Nitrogen-Acquisition Strategies of Juvenile and Adult Plants of an Epiphytic Tank-Forming Bromeliad. Planta 2019, 250, 319–332. [Google Scholar] [CrossRef]
- Mann, D.G.J.; LaFayette, P.R.; Abercrombie, L.L.; King, Z.R.; Mazarei, M.; Halter, M.C.; Poovaiah, C.R.; Baxter, H.; Shen, H.; Dixon, R.A. Gateway-compatible Vectors for High-throughput Gene Functional Analysis in Switchgrass (Panicum virgatum L.) and Other Monocot Species. Plant Biotechnol. J. 2012, 10, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, N.M.; Surin, B.; Ramm, K.; Gaudron, J.; Schünmann, P.H.D.; Taylor, W.; Waterhouse, P.M.; Wang, M.-B. Agrobacterium-Mediated Transformation of Australian Rice Cultivars Jarrah and Amaroo Using Modified Promoters and Selectable Markers. Funct. Plant Biol. 2000, 27, 201–210. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2− ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cock, J.; Yoshida, S.; Forno, D.A. Laboratory Manual for Physiological Studies of Rice; International Rice Research Institute: Laguna, Philippines, 1976; ISBN 9711040352. [Google Scholar]
- Sharma, S.; Borah, P.; Meena, M.K.; Bindraban, P.; Pandey, R. Evaluation of Genotypic Variation for Growth of Rice Seedlings under Optimized Hydroponics Medium. Indian J. Genet. Plant Breed. 2018, 78, 292–301. [Google Scholar]
- Feng, H.; Yan, M.; Fan, X.; Li, B.; Shen, Q.; Miller, A.J.; Xu, G. Spatial Expression and Regulation of Rice High-Affinity Nitrate Transporters by Nitrogen and Carbon Status. J. Exp. Bot. 2011, 62, 2319–2332. [Google Scholar] [CrossRef]
- Hachiya, T.; Sakakibara, H. Interactions between Nitrate and Ammonium in Their Uptake, Allocation, Assimilation, and Signaling in Plants. J. Exp. Bot. 2017, 68, 2501–2512. [Google Scholar] [CrossRef]
- Fu, Y.; Zhong, X.; Lu, C.; Liang, K.; Pan, J.; Hu, X.; Hu, R.; Li, M.; Ye, Q.; Liu, Y. Growth, Nutrient Uptake and Transcriptome Profiling of Rice Seedlings in Response to Mixed Provision of Ammonium-and Nitrate-Nitrogen. J. Plant Physiol. 2023, 284, 153976. [Google Scholar] [CrossRef] [PubMed]
- Moț, A.; Ion, V.A.; Madjar, R.M.; Bădulescu, L. Dynamic Pregl-Dumas Technique Applied in Nitrogen Determination from Inputs Used in Organic Agriculture. Sci. Pap. Ser. A Agron. 2022, 55, 105–110. [Google Scholar]
- Vega-Mas, I.; Sarasketa, A.; Marino, D. High-Throughput Quantification of Ammonium Content in Arabidopsis. Bio Protoc. 2015, 5, e1559. [Google Scholar] [CrossRef]
- López-Hidalgo, C.; Meijón, M.; Lamelas, L.; Valledor, L. The Rainbow Protocol: A Sequential Method for Quantifying Pigments, Sugars, Free Amino Acids, Phenolics, Flavonoids and MDA from a Small Amount of Sample; Wiley Online Library: Hoboken, NJ, USA, 2021. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas Chromatography Mass Spectrometry–Based Metabolite Profiling in Plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Kopka, J.; Schauer, N.; Krueger, S.; Birkemeyer, C.; Usadel, B.; Bergmüller, E.; Dörmann, P.; Weckwerth, W.; Gibon, Y.; Stitt, M. GMD@ CSB. DB: The Golm Metabolome Database. Bioinformatics 2005, 21, 1635–1638. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Varet, H.; Brillet-Guéguen, L.; Coppée, J.-Y.; Dillies, M.-A. SARTools: A DESeq2-and EdgeR-Based R Pipeline for Comprehensive Differential Analysis of RNA-Seq Data. PLoS ONE 2016, 11, e0157022. [Google Scholar] [CrossRef]
- Love, M.; Anders, S.; Huber, W. Differential Analysis of Count Data–the DESeq2 Package. Genome Biol. 2014, 15, 10–1186. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Ge, S.X.; Son, E.W.; Yao, R. IDEP: An Integrated Web Application for Differential Expression and Pathway Analysis of RNA-Seq Data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A Free Online Platform for Data Visualization and Graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The Rapid Generation of Mutation Data Matrices from Protein Sequences. Bioinformatics 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: Oxford, UK, 2000; ISBN 0195350510. [Google Scholar]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis Version 12 for Adaptive and Green Computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards More Transparent and Integrative Metabolomics Analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Web-Based Inference of Biological Patterns, Functions and Pathways from Metabolomic Data Using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Z.; Xia, J.; Alfatih, A.; Song, Y.; Huang, Y.; Wan, G.; Sun, L.; Tang, H.; Liu, Y. Rice NIN-LIKE PROTEIN 4 Plays a Pivotal Role in Nitrogen Use Efficiency. Plant Biotechnol. J. 2021, 19, 448–461. [Google Scholar] [CrossRef]
- Fang, Z.; Wu, B.; Ji, Y. The Amino Acid Transporter OsAAP4 Contributes to Rice Tillering and Grain Yield by Regulating Neutral Amino Acid Allocation through Two Splicing Variants. Rice 2021, 14, 2. [Google Scholar] [CrossRef]
- Gonçalves, A.Z.; Mercier, H. Transcriptomic and Biochemical Analysis Reveal Integrative Pathways Between Carbon and Nitrogen Metabolism in Guzmania monostachia (Bromeliaceae) Under Drought. Front. Plant Sci. 2021, 12, 715289. [Google Scholar] [CrossRef]
- Takahashi, C.A.; Mercier, H. Nitrogen Metabolism in Leaves of a Tank Epiphytic Bromeliad: Characterization of a Spatial and Functional Division. J. Plant Physiol. 2011, 168, 1208–1216. [Google Scholar] [CrossRef]
- Takahashi, C.A.; Coutinho Neto, A.A.; Mercier, H. An Overview of Water and Nutrient Uptake by Epiphytic Bromeliads: New Insights into the Absorptive Capability of Leaf Trichomes and Roots. Prog. Bot. 2022, 83, 345–362. [Google Scholar]
- Gobara, B.N.K.; Alves, F.R.R.; Pikart, F.C.; Gonçalves, A.Z.; Dos Santos, D.Y.A.C.; De Pinna, G.F.D.A.M.; Mercier, H. How Does a C3 Epiphytic Tank Bromeliad Respond to Drought? Bot. J. Linn. Soc. 2020, 192, 855–867. [Google Scholar] [CrossRef]
- Mioto, P.T.; Mercier, H. Abscisic Acid and Nitric Oxide Signaling in Two Different Portions of Detached Leaves of Guzmania monostachia with CAM Up-Regulated by Drought. J. Plant Physiol. 2013, 170, 996–1002. [Google Scholar] [CrossRef]
- Xu, F.; Wang, K.; Yuan, W.; Xu, W.; Liu, S.; Kronzucker, H.J.; Chen, G.; Miao, R.; Zhang, M.; Ding, M. Overexpression of Rice Aquaporin OsPIP1;2 Improves Yield by Enhancing Mesophyll CO2 Conductance and Phloem Sucrose Transport. J. Exp. Bot. 2019, 70, 671–681. [Google Scholar] [CrossRef]
- Heckwolf, M.; Pater, D.; Hanson, D.T.; Kaldenhoff, R. The Arabidopsis thaliana Aquaporin AtPIP1; 2 Is a Physiologically Relevant CO2 Transport Facilitator. Plant J. 2011, 67, 795–804. [Google Scholar] [CrossRef]
- Uehlein, N.; Lovisolo, C.; Siefritz, F.; Kaldenhoff, R. The Tobacco Aquaporin NtAQP1 Is a Membrane CO2 Pore with Physiological Functions. Nature 2003, 425, 734–737. [Google Scholar] [CrossRef]
- Huang, L.; Lu, Y.; Gao, X.; Du, G.; Ma, X.; Liu, M.; Guo, J.; Chen, Y. Ammonium-Induced Oxidative Stress on Plant Growth and Antioxidative Response of Duckweed (Lemna minor L.). Ecol. Eng. 2013, 58, 355–362. [Google Scholar] [CrossRef]
- Bittsánszky, A.; Pilinszky, K.; Gyulai, G.; Komives, T. Overcoming Ammonium Toxicity. Plant Sci. 2015, 231, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Mechanisms of Ammonium Toxicity and the Quest for Tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Yang, J. Nitrogen (N) Transformation in Paddy Rice Field: Its Effect on N Uptake and Relation to Improved N Management. Crop. Environ. 2022, 1, 7–14. [Google Scholar] [CrossRef]
- Valderrama-Martín, J.M.; Ortigosa, F.; Avila, C.; Canovas, F.M.; Hirel, B.; Canton, F.R.; Canas, R.A. A Revised View on the Evolution of Glutamine Synthetase Isoenzymes in Plants. Plant J. 2022, 110, 946–960. [Google Scholar] [CrossRef]
- Fortunato, S.; Nigro, D.; Lasorella, C.; Marcotuli, I.; Gadaleta, A.; de Pinto, M.C. The Role of Glutamine Synthetase (GS) and Glutamate Synthase (GOGAT) in the Improvement of Nitrogen Use Efficiency in Cereals. Biomolecules 2023, 13, 1771. [Google Scholar] [CrossRef]
- Liang, T.; Yuan, Z.; Fu, L.; Zhu, M.; Luo, X.; Xu, W.; Yuan, H.; Zhu, R.; Hu, Z.; Wu, X. Integrative Transcriptomic and Proteomic Analysis Reveals an Alternative Molecular Network of Glutamine Synthetase 2 Corresponding to Nitrogen Deficiency in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2021, 22, 7674. [Google Scholar] [CrossRef]
- Kosegarten, H.; Grolig, F.; Wieneke, J.; Wilson, G.; Hoffmann, B. Differential Ammonia-Elicited Changes of Cytosolic PH in Root Hair Cells of Rice and Maize as Monitored by 2 [Prime], 7 [Prime]-Bis-(2-Carboxyethyl)-5 (and-6)-Carboxyfluorescein-Fluorescence Ratio. Plant Physiol. 1997, 113, 451–461. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. Nitrogen Acquisition, PEP Carboxylase, and Cellular PH Homeostasis: New Views on Old Paradigms. Plant Cell Environ. 2005, 28, 1396–1409. [Google Scholar] [CrossRef]
- Martinière, A.; Desbrosses, G.; Sentenac, H.; Paris, N. Development and Properties of Genetically Encoded PH Sensors in Plants. Front. Plant Sci. 2013, 4, 523. [Google Scholar] [CrossRef]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and Regulators of Intracellular PH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef]
- Fan, X.; Tang, Z.; Tan, Y.; Zhang, Y.; Luo, B.; Yang, M.; Lian, X.; Shen, Q.; Miller, A.J.; Xu, G. Overexpression of a PH-Sensitive Nitrate Transporter in Rice Increases Crop Yields. Proc. Natl. Acad. Sci. USA 2016, 113, 7118–7123. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhang, C.; Suglo, P.; Sun, S.; Wang, M.; Su, T. L-Aspartate: An Essential Metabolite for Plant Growth and Stress Acclimation. Molecules 2021, 26, 1887. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cai, H.; Xiao, J.; Li, X.; Zhang, Q.; Lian, X. Over-Expression of Aspartate Aminotransferase Genes in Rice Resulted in Altered Nitrogen Metabolism and Increased Amino Acid Content in Seeds. Theor. Appl. Genet. 2009, 118, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The Versatile GABA in Plants. Plant Signal Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef]
- Ansari, M.I.; Jalil, S.U.; Ansari, S.A.; Hasanuzzaman, M. GABA Shunt: A Key-Player in Mitigation of ROS during Stress. Plant Growth Regul. 2021, 94, 131–149. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Jalil, S.U.; Chopra, P.; Chhillar, H.; Ferrante, A.; Khan, N.A.; Ansari, M.I. Role of GABA in Plant Growth, Development and Senescence. Plant Gene 2021, 26, 100283. [Google Scholar] [CrossRef]
- Wu, Y.; Fang, W.; Peng, W.; Jiang, M.; Chen, G.; Xiong, F. Sucrose Transporter in Rice. Plant Signal Behav. 2021, 16, 1952373. [Google Scholar] [CrossRef]
- Sun, L.; Deng, R.; Liu, J.; Lai, M.; Wu, J.; Liu, X.; Shahid, M.Q. An Overview of Sucrose Transporter (SUT) Genes Family in Rice. Mol. Biol. Rep. 2022, 49, 5685–5695. [Google Scholar] [CrossRef]
- Wu, Y.; Lee, S.-K.; Yoo, Y.; Wei, J.; Kwon, S.-Y.; Lee, S.-W.; Jeon, J.-S.; An, G. Rice Transcription Factor OsDOF11 Modulates Sugar Transport by Promoting Expression of Sucrose Transporter and SWEET Genes. Mol. Plant 2018, 11, 833–845. [Google Scholar] [CrossRef]
- Ponnu, J.; Wahl, V.; Schmid, M. Trehalose-6-Phosphate: Connecting Plant Metabolism and Development. Front. Plant Sci. 2011, 2, 70. [Google Scholar] [CrossRef]
- Yadav, U.P.; Ivakov, A.; Feil, R.; Duan, G.Y.; Walther, D.; Giavalisco, P.; Piques, M.; Carillo, P.; Hubberten, H.-M.; Stitt, M. The Sucrose–Trehalose 6-Phosphate (Tre6P) Nexus: Specificity and Mechanisms of Sucrose Signalling by Tre6P. J. Exp. Bot. 2014, 65, 1051–1068. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, F.; Lunn, J.E. The Role of Trehalose 6-Phosphate (Tre6P) in Plant Metabolism and Development. Annu. Rev. Plant Biol. 2021, 72, 737–760. [Google Scholar] [CrossRef] [PubMed]
- Kerbler, S.M.; Armijos-Jaramillo, V.; Lunn, J.E.; Vicente, R. The Trehalose 6-phosphate Phosphatase Family in Plants. Physiol. Plant 2023, 175, e14096. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Wang, S.; Dao, Y.; Wang, J.; Wang, K. Arabidopsis thaliana Trehalose-6-Phosphate Phosphatase Gene TPPI Enhances Drought Tolerance by Regulating Stomatal Apertures. J. Exp. Bot. 2020, 71, 4285–4297. [Google Scholar] [CrossRef] [PubMed]
- Blasing, O.E.; Gibon, Y.; Gunther, M.; Hohne, M.; Morcuende, R.; Osuna, D.; Thimm, O.; Usadel, B.; Scheible, W.-R.; Stitt, M. Sugars and Circadian Regulation Make Major Contributions to the Global Regulation of Diurnal Gene Expression in Arabidopsis. Plant Cell 2005, 17, 3257–3281. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, Í.V.C.; Menguer, P.K.; Lira, B.S.; Balbinott, N.; Ricachenevsky, F.K.; de Menezes Daloso, D.; Rossi, M.; Margis-Pinheiro, M.; Margis, R.; Mercier, H. Exploring the Effects of VgPIP1;2 Overexpression in the Roots of Young Rice Plants: Modifications in Root Architecture, Transcriptomic and Metabolomic Profiles. Plants 2025, 14, 3628. https://doi.org/10.3390/plants14233628
Santos ÍVC, Menguer PK, Lira BS, Balbinott N, Ricachenevsky FK, de Menezes Daloso D, Rossi M, Margis-Pinheiro M, Margis R, Mercier H. Exploring the Effects of VgPIP1;2 Overexpression in the Roots of Young Rice Plants: Modifications in Root Architecture, Transcriptomic and Metabolomic Profiles. Plants. 2025; 14(23):3628. https://doi.org/10.3390/plants14233628
Chicago/Turabian StyleSantos, Ítalo Vinícius Cantanhêde, Paloma Koprovski Menguer, Bruno Silvestre Lira, Natalia Balbinott, Felipe Klein Ricachenevsky, Danilo de Menezes Daloso, Magdalena Rossi, Marcia Margis-Pinheiro, Rogério Margis, and Helenice Mercier. 2025. "Exploring the Effects of VgPIP1;2 Overexpression in the Roots of Young Rice Plants: Modifications in Root Architecture, Transcriptomic and Metabolomic Profiles" Plants 14, no. 23: 3628. https://doi.org/10.3390/plants14233628
APA StyleSantos, Í. V. C., Menguer, P. K., Lira, B. S., Balbinott, N., Ricachenevsky, F. K., de Menezes Daloso, D., Rossi, M., Margis-Pinheiro, M., Margis, R., & Mercier, H. (2025). Exploring the Effects of VgPIP1;2 Overexpression in the Roots of Young Rice Plants: Modifications in Root Architecture, Transcriptomic and Metabolomic Profiles. Plants, 14(23), 3628. https://doi.org/10.3390/plants14233628







