Drought-Induced Changes in Morphology and Phenology of Olive Trees (Olea europaea L.)
Abstract
1. Introduction
2. Effect of Water Stress on Plant Morphology
2.1. Leaf Response to Water Stress
2.2. Stem Response to Water Stress
2.3. Root Response to Water Stress
| Relative Cultivar Tolerance | Morpho-Anatomical Traits Studied | Reference |
|---|---|---|
| Manzanilla, Negrinha, Cobrançosa > Arbequina, Blanqueta | Leaf area, leaf tissue thickness, stomatal density, density of leaf tissue (high palisade/spongy parenchyma ratio), cuticle thickness, epidermis thickness, and trichome layer thickness | [3] |
| Chemlali > Chétoui | Leaf area, density, density of leaf tissue, stomatal density, trichome density, thickness of epidermis, and cuticle | [34] |
| Chemlali > Meski | Leaf area, leaf tissue thickness, stomatal density, density of leaf tissue, epidermis thickness, stomatal density, trichome density | [19] |
| Coratina > Biancolilla | Root morpho-anatomical parameters: cell wall suberization degree, root section circularity index, intercellular spaces area, cell number per unit area, cell size | [94] |
| Koroneiki > Manzanillo | Leaf area, stomatal density, and dimensions (length and width) and trichome density and diameter, trunk cross-sectional area, plant height | [17] |
| Empeltre > Arbequina | Leaf area, leaf appearance rate, total number of leaves per plant, growth after water stress | [33] |
| Dezful, Konservolia > Zard Aliabad, Roughani, Shengeh, Manzanilla, Sevillana, Mission | Shoot and fruit length and diameter | [61] |
| Lechín de Sevilla, Azeradj, Picholine Marocaine > Leccino > Chetoui > Arbequina, Blanqueta, Maurino > Madural > Sevillenca, Coratina > Moraiolo, Vernina, Frantoio | Leaf area, leafing intensity, fruit weight, petiole elasticity, stomatal density, stomatal length, trichomes density, trichome width, trichomes per stoma, trichome area index | [25] |
| Erlik > Hamdi > BARI-2, HP Olive, QR Olive, FS-17, Nabali, Gemlik, Souri, Manzanilla | Plant traits evaluated included plant height, trunk circumference, number of leaves and fruits, and fruit length, diameter, and weight. Root: thickness of the thinnest cortical region, cortical cell area, collenchymatous and sclerenchymatous layers, phloem and epidermal thickness, metaxylem area, and total cross-sectional area. Stem: cross-sectional area, epidermal and cortical region thickness, collenchymatous and sclerenchymatous layer, phloem thickness, cortical cell area, and metaxylem vessel diameter. Leaf: length, width and area, lamina, spongy and palisade parenchyma thickness, cuticle and epidermal thickness, and metaxylem vessel and phloem thickness | [26] |
| Maurino > Leccino > Degli | Leaf area, epicuticular waxes thickness, cuticle thickness, longitudinal diameter of epidermis cells, density of leaf tissue, shoot and fruit length | [32] |
| Leccio Corno > Arbequina > Maurino | Leaf area, leaf tissue thickness, cuticle thickness, density of leaf tissue, epidermis thickness, shoot length, stem diameter, vulnerability to xylem embolism formation | [23] |
2.4. Response of Flowering and Fruit Formation to Water Stress
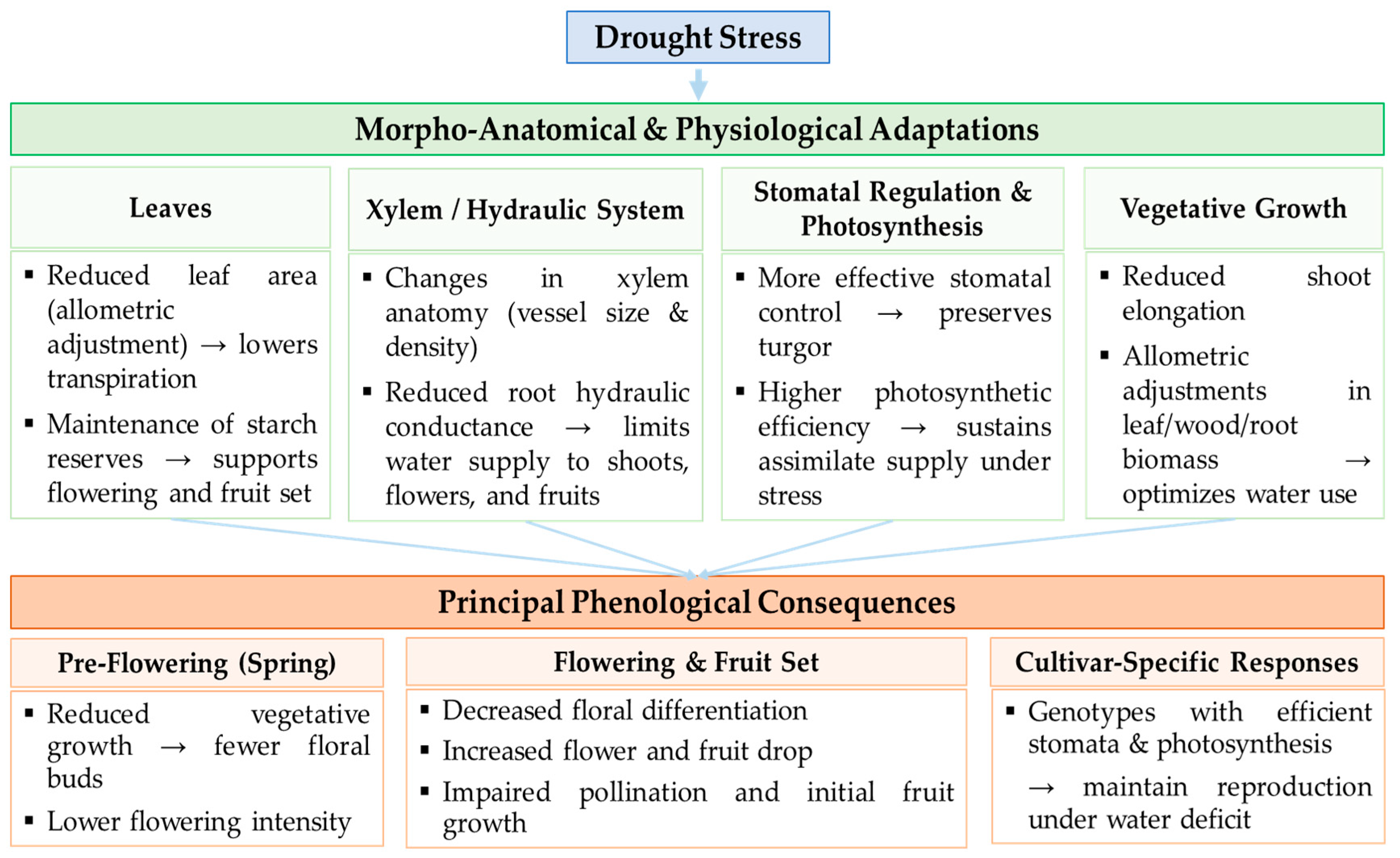
3. Linking Morphological and Anatomical Adjustments to Phenological Responses of Olive Trees Under Drought Stress
4. Phenological Adaptations of Olive Trees to Drought Stress and Agronomic Implications
4.1. Key Phenological Stages of the Olive Tree
4.2. Impact of Drought Stress on Olive Tree Phenology
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abbruzzese, G.; Beritognolo, I.; Muleo, R.; Piazzai, M.; Sabatti, M.; Mugnozza, G.S.; Kuzminsky, E. Leaf morphological plasticity and stomatal conductance in three Populus alba L. genotypes subjected to salt stress. Environ. Exp. Bot. 2009, 66, 381–388. [Google Scholar] [CrossRef]
- Rico, E.I.; de la Fuente, G.C.M.; Morillas, A.O.; Ocaña, A.M.F. Physiological and biochemical study of the drought tolerance of 14 main olive cultivars in the Mediterranean Basin. Photosynth. Res. 2023, 159, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bacelar, E.A.; Santos, D.L.; Moutinho-Pereira, J.M.; Lopes, J.I.; Goncalves, B.C.; Ferreira, T.C.; Correia, C.M. Physiological behaviour, oxidative damage and antioxidative protection of olive trees grown under different irrigation regimes. Plant Soil 2007, 292, 1–12. [Google Scholar] [CrossRef]
- Diaz-Rueda, P.; Franco-Navarro, J.D.; Messora, R.; Espartero, J.; Rivero-Núñez, C.M.; Aleza, P.; Capote, N.; Cantos, M.; García-Fernández, J.L.; De Cires, A. SILVOLIVE, a germplasm collection of wild subspecies with high genetic variability as a source of rootstocks and resistance genes for olive breeding. Front. Plant Sci. 2020, 11, 629. [Google Scholar] [CrossRef]
- Rapoport, H.F.; Hammami, S.B.; Martins, P.; Pérez-Priego, O.; Orgaz, F. Influence of water deficits at different times during olive tree inflorescence and flower development. Environ. Exp. Bot. 2012, 77, 227–233. [Google Scholar] [CrossRef]
- Didevarasl, A.; Esmaeilzadeh, M.; Sadeghi, M. Impacts of water deficit on olive (Olea europaea L.) fruit development and oil yield under Mediterranean conditions. Agric. Water Manag. 2025, 307, 108880. [Google Scholar]
- Baraldi, R.; Przybysz, A.; Facini, O.; Pierdona, L.; Carriero, G.; Bertazza, G.; Neri, L. Impact of drought and salinity on sweetgum tree (Liquidambar styraciflua L.): Understanding tree ecophysiological responses in the urban context. Forests 2019, 10, 1032. [Google Scholar] [CrossRef]
- Petruccelli, R.; Bartolini, G.; Ganino, T.; Zelasco, S.; Lombardo, L.; Perri, E.; Durante, M.; Bernardi, R. Cold stress, freezing adaptation, varietal susceptibility of Olea europaea L.: A review. Plants 2022, 11, 1367. [Google Scholar] [CrossRef] [PubMed]
- Muluneh, M.G. Impact of climate change on biodiversity and food security: A global perspective. Agric. Food Secur. 2021, 10, 36. [Google Scholar] [CrossRef]
- Rosecrance, R.C.; Krueger, W.H.; Milliron, L.; Bloese, J.; Garcia, C.; Mori, B. Moderate regulated deficit irrigation can increase olive oil yields and decrease tree growth in super high density ‘Arbequina’ olive orchards. Sci. Hortic. 2015, 190, 75–78. [Google Scholar] [CrossRef]
- Gholami, R.; Zahedi, S.M. Identifying superior drought-tolerant olive genotypes and their biochemical and some physiological responses to various irrigation levels. J. Plant Nutr. 2019, 42, 2057–2069. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Correia, C.M.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Lopes, J.I.; Torres-Pereira, J.M. Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree Physiol. 2004, 24, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Hammami, S.B.; Ben Laya, M.; Baazaoui, N.; Sghaier-Hammami, B. Vegetative Growth Dynamic and Its Impact on the Flowering Intensity of the Following Season Depend on Water Availability and Bearing Status of the Olive Tree. Sustainability 2022, 14, 15614. [Google Scholar] [CrossRef]
- Massenti, R.; Scalisi, A.; Marra, F.P.; Caruso, T.; Marino, G.; Lo Bianco, R. Physiological and structural responses to prolonged water deficit in young trees of two olive cultivars. Plants 2022, 11, 1695. [Google Scholar] [CrossRef]
- Boussadia, O.; Bchir, A.; Steppe, K.; Van Labeke, M.C.; Lemeur, R.; Braham, M. Active and passive osmotic adjustment in olive tree leaves during drought stress. Eur. Sci. J. 2013, 9, 423–439. [Google Scholar]
- Ben Ahmed, C.; Ben Rouina, B.; Sensoy, S.; Boukhris, M.; Ben Abdallah, F. Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environ. Exp. Bot. 2009, 67, 345–352. [Google Scholar] [CrossRef]
- Khalil, H.A.; El-Ansary, D.O. Morphological, physiological and anatomical responses of two olive cultivars to deficit irrigation and mycorrhizal inoculation. Eur. J. Hortic. Sci. 2020, 85, 51–62. [Google Scholar] [CrossRef]
- Didevarasl, A.; Costa-Saura, J.M.; Spano, D.; Deiana, P.; Snyder, R.L.; Rechid, D.; Bülow, B.; Mulas, M.; Nieddu, G.; Trabucco, A. The phenological phases of early and mid-late budbreak olive cultivars in a changing future climate over the Euro-Mediterranean region. Eur. J. Agron. 2025, 168, 127658. [Google Scholar] [CrossRef]
- Ennajeh, M.; Vadel, A.M.; Cochard, H.; Khemira, H. Comparative impacts of water stress on the leaf anatomy of a drought-resistant and a drought-sensitive olive cultivar. J. Hortic. Sci. Biotechnol. 2010, 85, 289–294. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Ruiz-Lozano, J.M.; Azcon, R.; Beuzon, C.R.; Garcia, J.L.; Cantos, M.; Aroca, R. Phenotypic and molecular traits determine the tolerance of olive trees to drought stress. Plant Physiol. Biochem. 2019, 139, 521–527. [Google Scholar] [CrossRef]
- Hernandez-Santana, V.; Fernandez, J.E.; Cuevas, M.V.; Perez-Martin, A.; Diaz-Espejo, A. Photosynthetic limitations by water deficit: Effect on fruit and olive oil yield, leaf area and trunk diameter and its potential use to control vegetative growth of super-high density olive orchards. Agric. Water Manag. 2017, 184, 9–18. [Google Scholar] [CrossRef]
- Parri, S.; Cai, G.; Romi, M.; Cantini, C.; Pinto, D.C.G.A.; Silva, A.M.S.; Dias, M.C.P. Comparative metabolomics of leaves and stems of three Italian olive cultivars under drought stress. Front. Plant Sci. 2024, 15, 1408731. [Google Scholar] [CrossRef] [PubMed]
- Alderotti, F.; Lo Piccolo, E.; Brunetti, C.; Stefano, G.; Ugolini, T.; Cecchi, L.; Beccaluva, M.; Renna, D.; Detti, C.; Ferrini, F.; et al. Cultivar-specific responses of young olive trees to water deficit: Impacts on physiology, leaf anatomy, and fruit quality in ’Arbequina’, ’Leccio del Corno’ and ‘Maurino’. Plant Physiol. Biochem. 2025, 229, 110331. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Castañeda, T.; Brown, K.M.; Lynch, J.P. Reduced root cortical burden improves growth and grain yield under low phosphorus availability in maize. Plant Cell Environ. 2018, 41, 1579–1592. [Google Scholar] [CrossRef]
- Razouk, R.; Hssaini, L.; Alghoum, M.; Adiba, A.; Hamdani, A. Phenotyping olive cultivars for drought tolerance using leaf macro-characteristics. Horticulturae 2022, 8, 939. [Google Scholar] [CrossRef]
- Ahmad, I.; Sohail, M.; Hameed, M.; Fatima, S.; Ahmad, M.S.A.; Ahmad, F.; Mehmood, A.; Basharat, S.; Asghar, A.; Shah, S.M.R.; et al. Morphoanatomical determinants of yield potential in Olea europaea L. cultivars belonging to diversified origin grown in semi-arid environments. PLoS ONE 2023, 18, e0286736. [Google Scholar] [CrossRef] [PubMed]
- Gholami, R.; Hajiamiri, A. Effects of regulated deficit irrigation regime on vegetative and pomological characteristics and yield of oil ‘Amphisis’ cultivar. J. Plant Prod. 2018, 25, 63–72. [Google Scholar]
- Fischlin, A.G.F.; Midgley, J.T.; Price, R.; Leemans, B.; Gopal, C.; Turley, M.D.A.; Rounsevell, O.P.; Dube, J.; Tarazona, A.A.; Velichko, A. Ecosystems, their properties, goods, and services. In Climate Change: Impacts, Adaptation and Vulnerability; Parry, M.L., Canziani, O., Palutikof, J.P., van der Linden, P.J., Hanson, C., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 211–272. [Google Scholar]
- Edziri, H.; Chehab, H.; Aissaoui, F.; Boujnah, D.; Mastouri, M. Photosynthetic, anatomical and biochemical responses of olive tree (Olea europaea) cultivars under water stress. Plant Biosyst. 2021, 155, 740–746. [Google Scholar] [CrossRef]
- Ahmad, K.S.; Wazarat, A.; Mehmood, A.; Ahmad, M.S.A.; Tahir, M.M.; Nawaz, F.; Ahmed, H.; Zafar, M.; Ulfat, A. Adaptations in Imperata cylindrica (L.) Raeusch. and Cenchrus ciliaris L. for altitude tolerance. Biologia 2020, 75, 183–198. [Google Scholar] [CrossRef]
- Nevo, E.; Bolshakova, M.A.; Martyn, G.I.; Musatenko, L.I.; Sytnik, K.; Pavlíček, T.; Beharav, A. Drought and light anatomical adaptive leaf strategies in three woody species caused by microclimatic selection at ‘‘Evolution Canyon’’, Israel. Isr. J. Plant Sci. 2000, 48, 33–46. [Google Scholar]
- Marchioni, I.; Rodolfi, M.; Massa, D.; Cacini, S.; Ughini, V.; Bartolini, G.; Fabbri, A.; Petruccelli, R.; Ganino, T. Comparative effects of drought stress on three olive cultivars focusing on older leaves. Sci. Hortic. 2024, 332, 113234. [Google Scholar] [CrossRef]
- Melaouhi, A.; Baraza, E.; Escalona, J.M.; El-AouOuad, H.; Mahjoub, I.; Bchir, A.; Braham, M.; Bota, J. Physiological and biochemical responses to water deficit and recovery of two olive cultivars (Olea europaea L., Arbequina and Empeltre Cvs.) under Mediterranean conditions. Theor. Exp. Plant Physiol. 2021, 33, 369–383. [Google Scholar] [CrossRef]
- Guerfel, M.; Baccouri, O.; Boujnah, D.; Chaïbi, W.; Zarrouk, M. Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Sci. Hortic. 2009, 119, 257–263. [Google Scholar] [CrossRef]
- M’Barki, N.; Chehab, H.; Alssaoul, F.; Dabbaghl, O.; Attla, F.; Mahjoub, Z.; Laamarl, S.; Chihaoul, B.; Del Gludlce, T.; Jemal, A.; et al. Effects of mycorrhizal fungi inoculation and soil amendment with hydrogel on leaf anatomy, growth and physiology performance of olive plantlets under two contrasting water regimes. Acta Physiol. Plant. 2018, 40, 116. [Google Scholar] [CrossRef]
- Hernandez-Santana, V.; Diaz-Rueda, P.; Díaz-Espejo, A.; Raya-Sereno, M.D.; Gutierrez-Gordillo, S.; Montero, A.; Perez-Martin, A.; Colmenero-Flores, J.M.; Rodriguez-Dominguez, C.M. Hydraulic traits emerge as relevant determinants of growth patterns in wild olive genotypes under water stress. Front. Plant Sci. 2019, 10, 291. [Google Scholar] [CrossRef]
- Baccari, S.; Elloumi, O.; Chaari-Rkhis, A.; Fenollosa, E.; Morales, M.; Drira, N.; Ben Abdallah, F.; Fki, L.; Munné-Bosch, S. Linking leaf water potential, photosynthesis and chlorophyll loss with mechanisms of photo- and antioxidant protection in juvenile olive trees subjected to severe drought. Front. Plant Sci. 2020, 11, 614144. [Google Scholar] [CrossRef]
- Osuagwu, G.G.E.; Edeoga, H.O.; Osuagwu, A.N. The influence of water stress (drought) on the mineral and vitamin potential of the leaves of Ocimum gratissimum (L.). Recent Res. Sci. Technol. 2010, 2, 27–33. [Google Scholar]
- Toscano, S.; Scuderi, D.; Giuffrida, F.; Romano, D. Responses of Mediterranean ornamental shrubs to drought stress and recovery. Sci. Hortic. 2014, 178, 145–153. [Google Scholar] [CrossRef]
- Sack, L.; Holbrook, N.M. Leaf hydraulics. Annu. Rev. Plant Biol. 2006, 57, 361–381. [Google Scholar] [CrossRef]
- Wang, D.; Pan, Y.; Zhao, X.; Zhu, L.; Fu, B.; Li, Z. Genome-wide temporal-spatial gene expression profiling of drought responsiveness in rice. BMC Genom. 2011, 12, 149. [Google Scholar] [CrossRef]
- Escribano-Rocafort, A.G.; Ventre-Lespiaucq, A.B.; Granado-Yela, C.; Rubio de Casas, R.; Delgado, J.A.; Escudero, A.; Balaguer, L. Intra-individual variation in light-related functional traits: Magnitude and structure of leaf trait variability across global scales in Olea europaea trees. Trees 2017, 31, 1505–1517. [Google Scholar] [CrossRef]
- Sebastiani, L.; Gucci, R.; Kerem, Z.; Fernández, J.E. Physiological responses to abiotic stresses. In The Olive Tree Genome. Compendium of Plant Genomes; Rugini, E., Baldoni, L., Muleo, R., Sebastiani, L., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Ehrlich, Y.; Regev, L.; Kerem, Z.; Boaretto, E. Radiocarbon dating of an olive tree cross-section: New insights on growth patterns and implications for age estimation of olive trees. Front. Plant Sci. 2017, 8, 1918. [Google Scholar] [CrossRef]
- Bosabalidis, A.M.; Kofidis, G. Comparative effects of drought stress on leaf anatomy of two olive cultivars. Plant Sci. 2002, 163, 375–379. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Moutinho-Pereira, J.; Correia, C.M. Olive tree response to drought stress: Physiological and molecular mechanisms. Tree Physiol. 2019, 39, 1902–1916. [Google Scholar]
- Rowland, L.; Ramírez-Valiente, J.A.; Hartley, I.P.; Mencuccini, M. How woody plants adjust above- and below-ground traits in response to sustained drought. New Phytol. 2023, 239, 1173–1189. [Google Scholar] [CrossRef] [PubMed]
- Fayek, M.A.; Fayed, T.A.; Emtithal, H.; El-Sayed, E.; Abd El-Hameed, E. Comparative impacts of salt stress on survival and leaf anatomy traits in olive genotypes. Biosci. Res. 2018, 15, 565–574. [Google Scholar]
- Naija, D.S.; Gueddes, S.B.M.; Braham, M. Effects of water scarcity and salinity on the anatomy of the Tunisian table olive cultivar ‘Meski’. Not. Bot. Horti Agrobot. 2021, 49, 12157. [Google Scholar] [CrossRef]
- Colombi, T.; Walter, A. Root responses of triticale and soybean to soil compaction in the field are reproducible under controlled conditions. Funct. Plant Biol. 2016, 43, 114–128. [Google Scholar] [CrossRef]
- Luković, J.; Maksimović, I.; Zorić, L.; Nagl, N.; Perčić, M.; Polić, D.; Putnik-Delic, M. Histological characteristics of sugar beet leaves potentially linked to drought tolerance. Ind. Crops Prod. 2009, 30, 281–286. [Google Scholar] [CrossRef]
- Chen, K.M.; Wang, F.; Wang, Y.H.; Chen, T.; Hu, Y.X.; Lin, J.X. Anatomical and chemical characteristics of foliar vascular bundles in four reed ecotypes adapted to different habitats. Flora 2006, 201, 555–569. [Google Scholar] [CrossRef]
- Jacobsen, A.L.; Pratt, R.B.; Davis, S.D.; Ewers, F.W. Cavitation resistance and seasonal hydraulics differ among three arid Californian plant communities. Plant Cell Environ. 2007, 30, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Guerfel, M.; Baccouri, O.; Boujnah, D.; Chaïbi, W.; Zarrouk, M. Effects of water stress on leaf structure of two main Tunisian olive cultivars (Olea europaea L.). In Proceedings of the 7th Edition of Tunisia Japan Symposium on Science Society and Technology, Sousse, Tunisia, 4–6 December 2006; Elsevier: Amsterdam, The Netherlands; p. 9. [Google Scholar]
- Schuster, A.C.; Burghardt, M.; Riederer, M. The ecophysiology of leaf cuticular transpiration: Are cuticular water permeabilities adapted to ecological conditions? J. Exp. Bot. 2017, 68, 5271–5279. [Google Scholar] [CrossRef]
- Sofo, A.; Manfreda, S.; Fiorentino, M.; Dichio, B.; Xiloyannis, C. The olive tree: A paradigm for drought tolerance in Mediterranean climates. Hydrol. Earth Syst. Sci. 2008, 12, 293–301. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought stress tolerance in plants: Interplay of molecular, biochemical and physiological responses in important development stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology; Springer: Berlin, Germany, 1995; 506p. [Google Scholar]
- Save, R.; Biel, C.; de Herralde, F. Leaf pubescence, water relations and chlorophyll fluorescence in two subspecies of Lotus creticus L. Biol. Plant. 2000, 43, 239–244. [Google Scholar]
- Baldini, E.; Facini, O.; Nerozzi, F.; Rosso, F.; Rotondi, A. Leaf characteristics and optical properties of different woody species. Trees 1997, 12, 73–81. [Google Scholar] [CrossRef]
- Gholami, R.; Hoveizeh, N.F.; Zahedi, S.M.; Gholami, H.; Carillos, P. Effect of three water-regimes on morpho-physiological, biochemical and yield responses of local and foreign olive cultivars under field conditions. BMC Plant Biol. 2022, 22, 477. [Google Scholar] [CrossRef]
- Lima, T.R.A.; Carvalho, E.C.D.; Martins, F.R.; Oliveira, R.S.; Miranda, R.S.; Müller, C.S.; Pereira, L.; Bittencourt, P.R.L.; Sobczak, J.C.M.S.M.; Gomes-Filho, E.; et al. Lignin composition is related to xylem embolism resistance and leaf life span in trees in a tropical semiarid climate. New Phytol. 2018, 219, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Elkelish, A.; Qari, S.H.; Mazrou, Y.S.A.; Abdelaal, K.A.A.; Hafez, Y.M.; Abu-Elsaoud, A.M.; Batiha, G.E.S.; El-Esawi, M.A.; El Nahhas, N. Exogenous ascorbic acid induced chilling tolerance in tomato plants through modulating metabolism, osmolytes, antioxidants, and transcriptional regulation of catalase and heat shock proteins. Plants 2020, 9, 431. [Google Scholar] [CrossRef]
- Moriana, A.; Orgaz, F.; Pastor, M.; Fereres, E. Yield responses of a mature olive orchard to water deficits. J. Am. Soc. Hortic. Sci. 2003, 128, 425–431. [Google Scholar] [CrossRef]
- Abboud, S.; Dbara, S.; Abidi, W.; Braham, M. Differential agro-physiological responses induced by partial root-zone drying irrigation in olive cultivars grown in semi-arid conditions. Environ. Exp. Bot. 2019, 167, 103863. [Google Scholar] [CrossRef]
- McDowell, N.G.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.E. Understanding olive adaptation to abiotic stresses as a tool to increase crop performance. Environ. Exp. Bot. 2014, 103, 158–179. [Google Scholar] [CrossRef]
- Nadezhdina, N.; Nadezhdin, V.; Ferreira, M.I.; Pitacco, A. Variability with xylem depth in sap flow in trunks and branches of mature olive trees. Tree Physiol. 2007, 27, 105–113. [Google Scholar] [CrossRef]
- Sevanto, S.; McDowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef]
- Venturas, M.D.; Sperry, J.S.; Hacke, U.G. Plant xylem hydraulics: What we understand, current research, and future challenges. J. Integr. Plant Biol. 2017, 59, 356–389. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, Z.; Kim, S.; Jeong, E.; Shim, J.S. Modulation of lignin biosynthesis for drought tolerance in plants. Front. Plant Sci. 2023, 14, 1116426. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.A.; Marchin, R.M.; Abit, P.; Lau, O.L. Hydraulic failure and tree dieback are associated with high wood density in a temperate forest under extreme drought. Glob. Change Biol. 2011, 17, 2731–2742. [Google Scholar] [CrossRef]
- Sabella, E.; Aprile, A.; Genga, A.; Siciliano, T.; Nutricati, E.; Nicol, F.; Vergine, M.; Negro, C.; De Bellis, L.; Luvisi, A. Xylem cavitation susceptibility and refilling mechanisms in olive trees infected by Xylella fastidiosa. Sci. Rep. 2019, 9, 9602. [Google Scholar] [CrossRef]
- Petit, G.; Bleve, G.; Gallo, A.; Mita, G.; Montanaro, G.; Nuzzo, V.; Zambonini, D.; Pitacco, A. Susceptibility to Xylella fastidiosa and functional xylem anatomy in Olea europaea: Revisiting a tale of plant–pathogen interaction. AoB Plants 2021, 13, plab027. [Google Scholar] [CrossRef]
- Burridge, J.D.; Grondin, A.; Vadez, V. Optimizing crop water use for drought and climate change adaptation requires a multi-scale approach. Front. Plant Sci. 2022, 13, 824720. [Google Scholar] [CrossRef]
- Rossi, L.; Sebastiani, L.; Tognetti, R.; d’Andria, R.; Morelli, G.; Cherubini, P. Tree-ring wood anatomy and stable isotopes show structural and functional adjustments in olive trees under different water availability. Plant Soil 2013, 372, 567–579. [Google Scholar] [CrossRef]
- Grill, E.; Tausz, M.; Pötsch, M. Structural and ecophysiological traits of plants under drought stress. In Ecophysiology of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef]
- Perez-Martin, A.; Michelazzo, C.; Torres-Ruiz, J.M.; Flexas, J.; Fernandez, J.E.; Sebastiani, L.; Diaz-Espejo, A. Regulation of photosynthesis and stomatal and mesophyll conductance under water deficit stress and recovery in olive trees: Correlation with gene expression of carbonic anhydrase and aquaporins. J. Exp. Bot. 2014, 65, 3143–3156. [Google Scholar] [CrossRef]
- Dichio, B.; Xiloyannis, C.; Sofo, A.; Montanaro, G. Osmotic regulation in leaves and roots of olive trees during a water deficit and rewatering. Tree Physiol. 2006, 26, 179–185. [Google Scholar] [CrossRef]
- Celano, G.; Dichio, B.; Montanaro, G.; Nuzzo, V.; Palese, A.M.; Xiloyannis, C. Distribution of dry matter and amount of mineral elements in irrigated and non-irrigated olive trees. Acta Hortic. 1999, 474, 381–384. [Google Scholar] [CrossRef]
- Fernandez, J.E.; Moreno, F.; Cabrera, F.; Arrue, J.L.; Martin-Aranda, J. Drip irrigation, soil characteristics and the root distribution and root activity of olive trees. Plant Soil 1991, 133, 239–251. [Google Scholar] [CrossRef]
- Fernandez, J.E.; Moreno, F.; Martin-Aranda, J.; Fereres, E. Olive-tree root dynamics under different soil water regimes. Agric. Mediterr. 1992, 122, 225–235. [Google Scholar]
- Ben-Gal, A.; Dag, A.; Yermiyahu, U.; Birger, R.; Morira, Y.; Abu Touma, M.; ElHaady, F. Introducing Irrigation to Traditional Rain-Fed Olive (Oil) Orchards; Final Report; Agricultural Research Organization of Israel: Rishon LeZion, Israel, 2010. (In Hebrew) [Google Scholar]
- Kardiman, R.; Raebild, A. Relationship between stomatal density, size and speed of opening in Sumatran rainforest species. Tree Physiol. 2018, 38, 696–705. [Google Scholar] [CrossRef]
- Polverigiani, S.; McCormack, M.L.; Mueller, C.W.; Eissenstat, D.M. Growth and physiology of olive pioneer and fibrous roots exposed to soil moisture deficits. Tree Physiol. 2011, 31, 1228–1237. [Google Scholar] [CrossRef]
- Aslam, M.M.; Idris, A.L.; Zhang, Q.; Xu, W.; Karanja, J.K.; Wei, Y. Rhizosphere microbiomes can regulate plant drought tolerance. Pedosphere 2022, 32, 61–74. [Google Scholar] [CrossRef]
- García-Tejera, O.; López-Bernal, A.; Orgaz, F.; Testi, L.; Villalobos, F.J. Are olive root systems optimal for deficit irrigation? Eur. J. Agron. 2018, 99, 72–79. [Google Scholar] [CrossRef]
- Huang, B.; Gao, H. Root physiological characteristics associated with drought resistance in tall fescue cultivars. Crop Sci. 2000, 40, 196–203. [Google Scholar] [CrossRef]
- Steudle, E. Water uptake by roots: Effects of water deficit. J. Exp. Bot. 2000, 51, 1531–1542. [Google Scholar] [CrossRef]
- Hose, E.; Clarkson, D.T.; Steudle, E.; Schreiber, L.; Hartung, W. The exodermis: A variable apoplastic barrier. J. Exp. Bot. 2001, 52, 2245–2264. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.M.; Diaz-Espejo, A.; Perez-Martin, A.; Hernandez-Santana, V. Role of hydraulic and chemical signals in leaves, stems and roots in the stomatal behaviour of olive trees under water stress and recovery conditions. Tree Physiol. 2015, 35, 415–424. [Google Scholar] [CrossRef]
- Verdoucq, L.; Maurel, C. Plant aquaporins. In Advances in Botanical Research: Membrane Transport in Plants; Maurel, C., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2018; Volume 87, pp. 26–56. [Google Scholar]
- Tataranni, G.; Santarcangelo, M.; Sofo, A.; Xiloyannis, C.; Tyerman, S.D.; Dichio, B. Correlations between morpho-anatomical changes and radial hydraulic conductivity in roots of olive trees under water deficit and rewatering. Tree Physiol. 2015, 35, 1356–1365. [Google Scholar] [CrossRef][Green Version]
- Yentur, S. Bitki Anatomisi; İstanbul Universitesi, Fen Fakultesi, Biyoloji Bolumu: İstanbul, Turkey, 2003; No. 227. [Google Scholar]
- Dichio, B.; Xiloyannis, C.; Angelopoulos, K.; Nuzzo, V.; Bufo, S.A.; Celano, G. Drought-induced variations of water relations parameters in Olea europaea. Plant Soil 2003, 257, 381–389. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Zhou, M.; Shabala, S. Physiological and morphological mechanisms mediating plant tolerance to osmotic stress: Balancing tolerance and productivity. In Climate Change and Crop Production; Benkeblia, N., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 35–57. [Google Scholar] [CrossRef]
- Schneider, H.M.; Strock, C.F.; Hanlon, M.T.; Vanhees, D.J.; Perkins, A.C.; Ajmera, I.B.; Sidhu, J.S.; Mooney, S.J.; Brown, K.M.; Lynch, J.P. Multiseriate cortical sclerenchyma enhance root penetration in compacted soils. Proc. Natl. Acad. Sci. USA 2021, 118, e2012087118. [Google Scholar] [CrossRef]
- Schneider, H.M. Functional implications of multiseriate cortical sclerenchyma for soil resource capture and crop improvement. AoB Plants 2022, 6, plac050. [Google Scholar] [CrossRef]
- Hueso, A.; Camacho, G.; Gómez-del-Campo, M. Water stress during early olive fruit development affects fruit growth and metabolism. Agric. Water Manag. 2021, 244, 106547. [Google Scholar]
- Beyá-Marshall, V.; Herrera, J.; Fichet, T.; Trentacoste, E.R.; Kremer, C. The effect of water status on productive and flowering variables in young ‘Arbequina’ olive trees under limited irrigation water availability in a semiarid region of Chile. Hortic. Environ. Biotech. 2018, 59, 815–826. [Google Scholar] [CrossRef]
- Gucci, R.; Caruso, G.; Servili, M. Sustainable irrigation management in olive orchards to balance yield and oil quality. Agric. Water Manag. 2019, 222, 59–67. [Google Scholar]
- Moriana, A.; Perez-López, D.; Prieto, M.H.; Ramírez-Santa-Pau, M.; Pérez-Rodriguez, J.M. Midday stem water potential as a useful tool for estimating irrigation requirements in olive trees. Agric. Water Manag. 2012, 112, 43–54. [Google Scholar] [CrossRef]
- Trentacoste, E.R.; Calderón, F.J.; Contreras-Zanessi, O.; Galarza, W.; Banco, A.P.; Puertas, C.M. Effect of regulated deficit irrigation during the vegetative growth period on shoot elongation and oil yield components in olive hedgerows (cv. Arbosana) pruned annually on alternate sides in San Juan, Argentina. Irrig. Sci. 2019, 37, 533–546. [Google Scholar] [CrossRef]
- Khoyerdi, F.F.; Shamshiri, M.H.; Estaji, A. Changes in some physiological and osmotic parameters of several pistachio genotypes under drought stress. Sci. Hortic. 2016, 198, 44–51. [Google Scholar] [CrossRef]
- Tognetti, R.; d’Andria, R.; Lavini, A.; Morelli, G. The effect of deficit irrigation on crop yield and vegetative development of Olea europaea L. (cvs. Frantoio and Leccino). Eur. J. Agron. 2006, 25, 356–364. [Google Scholar] [CrossRef]
- Iniesta, F.; Testi, L.; Orgaz, F.; Villalobos, F.J. The effects of regulated and continuous deficit irrigation on the water use, growth and yield of olive trees. Eur. J. Agron. 2009, 30, 258–265. [Google Scholar] [CrossRef]
- Sanchez-Pinero, M.; Corell, M.; de Sosa, L.L.; Moriana, A.; Medina-Zurita, N.; Madejón, E.; Girón, I.; Castro-Valdecantos, P.; Martin-Palomo, M.J.; Perez-Lopez, D. Assessment of water stress impact on olive trees using an accurate determination of the endocarp development. Irrig. Sci. 2024, 42, 461–476. [Google Scholar] [CrossRef]
- Mezghani, M.A.; Charfi, C.M.; Gouiaa, M.; Labidi, F. Vegetative and reproductive behaviour of some olive tree varieties (Olea europaea L.) under deficit irrigation regimes in semi-arid conditions of Central Tunisia. Sci. Hortic. 2012, 146, 143–152. [Google Scholar] [CrossRef]
- Garrido, A.; Fernández-González, M.; Vazquez-Ruiz, R.A.; Rodríguez-Rajo, F.J.; Aira, M.J. Reproductive biology of olive trees (Arbequina cultivar) at the northern limit of their distribution areas. Forests 2021, 12, 204. [Google Scholar] [CrossRef]
- Hillel, D.; Rosenzweig, C. Climate Change and the Global Harvest: Potential Impacts of the Greenhouse Effect on Agriculture; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- IPCC. Climate Change 2007: Impacts, Adaptation and Vulnerability; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; 976p. [Google Scholar]
- Benlloch-González, M.; Sánchez-Lucas, R.; Benlloch, M. Effects of water stress on flower development and pollen viability in olive. Environ. Exp. Bot. 2018, 155, 110–116. [Google Scholar]
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean olive orchards under climate change: A review of future impacts and adaptation strategies. Agronomy 2021, 11, 56. [Google Scholar] [CrossRef]
- Medda, S.; Fadda, A.; Mulas, M. Influence of climate change on metabolism and biological characteristics in perennial woody fruit crops in the Mediterranean environment. Horticulturae 2022, 8, 273. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Moutinho-Pereira, J.M.; Correia, C.M. Drought stress effects and olive tree acclimation under a changing climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef]
- Garcia-Mozo, H.; Oteros, J.; Galan, C. Phenological response of Olea europaea L. to climate variability in southern Spain. Agric. For. Meteorol. 2015, 203, 423–433. [Google Scholar]
- Fraga, H.; Pinto, J.G.; Santos, J.A. Climate change projections for chilling and heat forcing conditions in European vineyards and olive orchards: A multi-model assessment. Clim. Change 2019, 152, 179–193. [Google Scholar] [CrossRef]
- Rallo, L.; Barranco, D.; Díez, C.M.; Rallo, P.; Suárez, M.P.; Trapero, C.; Pliego-Alfaro, F. Strategies for olive (Olea europaea L.) breeding: Cultivated genetic resources and crossbreeding. In Advances in Plant Breeding Strategies: Fruits; Springer: Cham, Switzerland, 2018; Volume 3, pp. 535–600. [Google Scholar]
- Colbrant, P.; Fabre, P. Stades Repères de L’olivier; L’Olivier; Invuflec: Paris, France, 1975; pp. 24–25. [Google Scholar]
- De Andrés, F. Estados tipo fenológicos del olivo. In Comunicaciones del Servicio de Defensa contra Plagas. Estudios y Experiencias; Ministerio de Agricultura: Madrid, Spain, 1974; pp. 33–74. [Google Scholar]
- Sanz-Cortés, F.; Martinez-Calvo, J.; Badenes, M.L.; Bleiholder, H.; Hack, H.; Llácer, G.; Meier, U. Phenological growth stages of olive trees (Olea europaea). Ann. Appl. Biol. 2002, 140, 151–157. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Rapoport, H.F.; Hammami, S.; Costagli, S.; Gucci, R. Influence of water stress on reproductive development in olive (Olea europaea L.). J. Hortic. Sci. Biotechnol. 2012, 87, 227–233. [Google Scholar]
- Didevarasl, A.; Costa Saura, J.M.; Spano, D.; Deiana, P.; Snyder, R.L.; Mulas, M.; Trabucco, A. Modeling phenological phases across olive cultivars in the Mediterranean. Plants 2023, 12, 3181. [Google Scholar] [CrossRef] [PubMed]
- Connor, D.J.; Centeno, A.; Gómez-del-Campo, M. Yield determination in olive hedgerow orchards. II. Analysis of radiation and water use efficiency. Crop Pasture Sci. 2012, 63, 480–498. [Google Scholar]
- Iniesta, F.; Testi, L.; Orgaz, F.; Villalobos, F.J. Seasonal patterns of crop development and water use in olive orchards under different irrigation regimes. Agric. Water Manag. 2021, 243, 106459. [Google Scholar]
- Vanderborght, J.; Huisman, J.A.; Van der Kruk, J.; Vereecken, H. Influence of water deficit on the seasonal patterns of shoot and root growth in olive. Tree Physiol. 2010, 30, 1027–1037. [Google Scholar]
- Oteros, J.; García-Mozo, H.; Vázquez, L.; Mestre, A.; Domínguez-Vilches, E.; Galán, C. Modelling olive phenological response to weather and topography. Agric. Ecosyst. Environ. 2013, 179, 62–68. [Google Scholar] [CrossRef]
- Podgornik, M.; Fantinič, J.; Pogacar, T.; Zupanc, V. Climate Change Induced Olive Trees Phenological Changes, Frosts and Droughts–Analysis for the Northern Mediterranean Region. 2024. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5018094 (accessed on 12 September 2025).
- Siakou, M.; Bruggeman, A.; Eliades, M.; Djuma, H.; Kyriacou, M.C.; Moriana, A. Phenology, morphology and physiology responses of deficit irrigated ‘Koroneiki’ olive trees as affected by environmental conditions and alternate bearing. Agronomy 2022, 12, 879. [Google Scholar] [CrossRef]
- Rosetti, V.; Hammami, S.B.M.; Rapoport, H.F. Hormonal regulation of olive fruit development under water deficit conditions. Plant Physiol. Biochem. 2019, 141, 377–385. [Google Scholar]
- Hueso, A.; Camacho, G.; Gómez-del-Campo, M. Spring deficit irrigation promotes significant reduction on vegetative growth, flowering, fruit growth and production in hedgerow olive orchards (cv Arbequina). Agric. Water Manag. 2021, 248, 106695. [Google Scholar] [CrossRef]
- Siakou, M.; Bruggeman, A.; Eliades, M.; Zoumides, C.; Djuma, H.; Kyriacou, M.C.; Moriana, A. Effects of deficit irrigation on ‘Koroneiki’ olive tree growth, physiology and olive oil quality at different harvest dates. Agric. Water Manag. 2021, 258, 107200. [Google Scholar] [CrossRef]
- Hammami, S.B.M.; Manrique, T.; Rapoport, H.F. Cultivar-based fruit growth patterns in olive trees under different irrigation regimes. Sci. Hortic. 2013, 150, 435–441. [Google Scholar]
- Gucci, R.; Caruso, G.; Gennai, C.; Esposto, S.; Urbani, S.; Servili, M. Fruit growth, yield and oil quality changes induced by deficit irrigation at different stages of olive fruit development. Agric. Water Manag. 2019, 212, 88–98. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Selvaggini, R. Influence of moderate water deficit on olive oil quality: Phenolic compounds and oxidative stability. Food Chem. 2019, 288, 160–168. [Google Scholar]
- Ben-Ari, T.; Lecerf, R.; Manceau, L.; Ollivier, T. Impact of drought severity on olive oil yield and composition under Mediterranean conditions. Agric. Water Manag. 2021, 252, 106888. [Google Scholar]
- Sofo, A.; Dichio, B.; Xiloyannis, C.; Masia, A. Effects of different irrigation regimes on olive tree (Olea europaea L.) photosynthesis, water relations and carboxylating enzymes. Plant Soil 2004, 257, 381–394. [Google Scholar]
- Hamze, L.M.; Trentacoste, E.R.; Searles, P.S.; Rousseaux, M.C. Spring reproductive and vegetative phenology of olive (Olea europaea L.) cultivars at different air temperatures along a latitudinal-altitudinal gradient in Argentina. Sci. Hortic. 2022, 304, 111327. [Google Scholar] [CrossRef]
| Cultivar | Origin | Cultivar | Origin |
|---|---|---|---|
| Arbequina | Spain | Lechín de Sevilla | Spain |
| Azeradj | Algeria | Madural | Portugal |
| BARI-2 | Pakistan | Manzanilla | Spain |
| Biancolilla | Italy | Manzanillo | Spain |
| Blanqueta | Spain | Maurino | Italy |
| Chemlali | Tunisia | Meski | Tunisia |
| Chétoui | Tunisia | Mission | United States |
| Cobrançosa | Portugal | Moraiolo | Italy |
| Coratina | Italy | Morisca | Spain |
| Degli | Italy | Nabali | Palestine |
| Dezful | Iran | Negrinha | Portugal |
| Empeltre | Spain | Nocellara del Belice | Italy |
| Erlik | Israel | Picholine Marocaine | Morocco |
| Frantoio | Italy | QR Olive | Pakistan |
| FS-17 | Italy | Roughani | Iran |
| Gemlik | Turkey | Sevillana | Spain |
| Hamdi | Tunisia | Sevillenca | Spain |
| HP Olive | Pakistan | Shengeh | Iran |
| Konservolia | Greece | Souri | Lebanon |
| Koroneiki | Greece | Vernina | Italy |
| Leccino | Italy | Zard Aliabad | Iran |
| Leccio del Corno | Italy |
| BBCH Code | Description | |
|---|---|---|
| Principal Stage 0: | Bud development | |
| Secondary Stages (Sec. Stgs) | 00 | Foliar buds at the apex of shoots grown the previous crop-year are completely closed, sharp-pointed, stemless and ochre-coloured. |
| 01 | Foliar buds start to swell and open, showing the new foliar primordia. | |
| 03 | Foliar buds lengthen and separate from the base. | |
| 07 | External small leaves open, not totally separated, remaining joined at apices. | |
| 09 | External small leaves opening further with their tips inter-crossing. | |
| Principal Stage 1: | Leaf development | |
| Sec. Stgs | 11 | First leaves are fully separated and exhibit a greenish-grey colour |
| 15 | Leaves are longer without attaining final length. First leaves turn greenish on the upper side. | |
| 19 | Leaves achieve typical cultivar length and shape. | |
| 31 | Shoots reach 10% of final length. | |
| Principal Stage 3: | Shoot development | |
| Sec. Stgs | 33 | Shoots achieve 30% of final length. |
| 37 | Shoots achieve 70% of final length. | |
| Principal Stage 5: | Inflorescence emergence | |
| Sec. Stgs | 50 | Flower buds in the leaf axils are fully closed, sharp-pointed, stemless, and ochre- coloured. |
| 51 | Inflorescence buds begin to swell. | |
| 52 | Inflorescence buds open and development of flower clusters begins. | |
| 54 | Flower cluster growing. | |
| 55 | Flower cluster completely expanded. Floral buds start to open. | |
| 57 | Corolla green-coloured, longer than calyx. | |
| 59 | Corolla changes from green to white. | |
| Principal Stage 6: | Flowering | |
| Sec. Stgs | 60 | First flowers open. |
| 61 | Start of flowering: 10% of flowers open. | |
| 65 | Full flowering: at least 50% of flowers open. | |
| 67 | First petals falling. | |
| 68 | Majority of petals fallen or faded. | |
| 69 | End of flowering, fruit set, unfertilised ovaries fallen. | |
| Principal Stage 7: | Fruit development | |
| Sec. Stgs | 71 | Fruit reaches ~10% of its final size. |
| 75 | Fruit reaches ~50% of its final size. Stone (endocarp) becomes lignified (resistant to cutting). | |
| 79 | Fruit ~90% of final size and is suitable for green picking. | |
| Principal Stage 8: | Maturity of fruit | |
| Sec. Stgs | 80 | Fruit colour changes from deep green to light green or yellowish. |
| 81 | Fruit begins to color | |
| 85 | Specific fruit coloring increases. | |
| 89 | Harvest maturity: fruits achieve the typical cultivar colour, remain turgid and are suitable for oil extraction. | |
| Principal Stage 9: | Senescence | |
| Sec. Stg | 92 | Overripe: Fruits lose turgidity and begin to drop. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordovilla, M.d.P.; Rharrabti, Y.; El Yamani, M. Drought-Induced Changes in Morphology and Phenology of Olive Trees (Olea europaea L.). Plants 2025, 14, 3624. https://doi.org/10.3390/plants14233624
Cordovilla MdP, Rharrabti Y, El Yamani M. Drought-Induced Changes in Morphology and Phenology of Olive Trees (Olea europaea L.). Plants. 2025; 14(23):3624. https://doi.org/10.3390/plants14233624
Chicago/Turabian StyleCordovilla, María del Pilar, Yahia Rharrabti, and Mohamed El Yamani. 2025. "Drought-Induced Changes in Morphology and Phenology of Olive Trees (Olea europaea L.)" Plants 14, no. 23: 3624. https://doi.org/10.3390/plants14233624
APA StyleCordovilla, M. d. P., Rharrabti, Y., & El Yamani, M. (2025). Drought-Induced Changes in Morphology and Phenology of Olive Trees (Olea europaea L.). Plants, 14(23), 3624. https://doi.org/10.3390/plants14233624








