Rice Cinnamoyl CoA Reductase-like Gene OsCCR14 Involved in Heat Stress via Regulation Lignin and Flavonoid Accumulation
Abstract
1. Introduction
2. Results
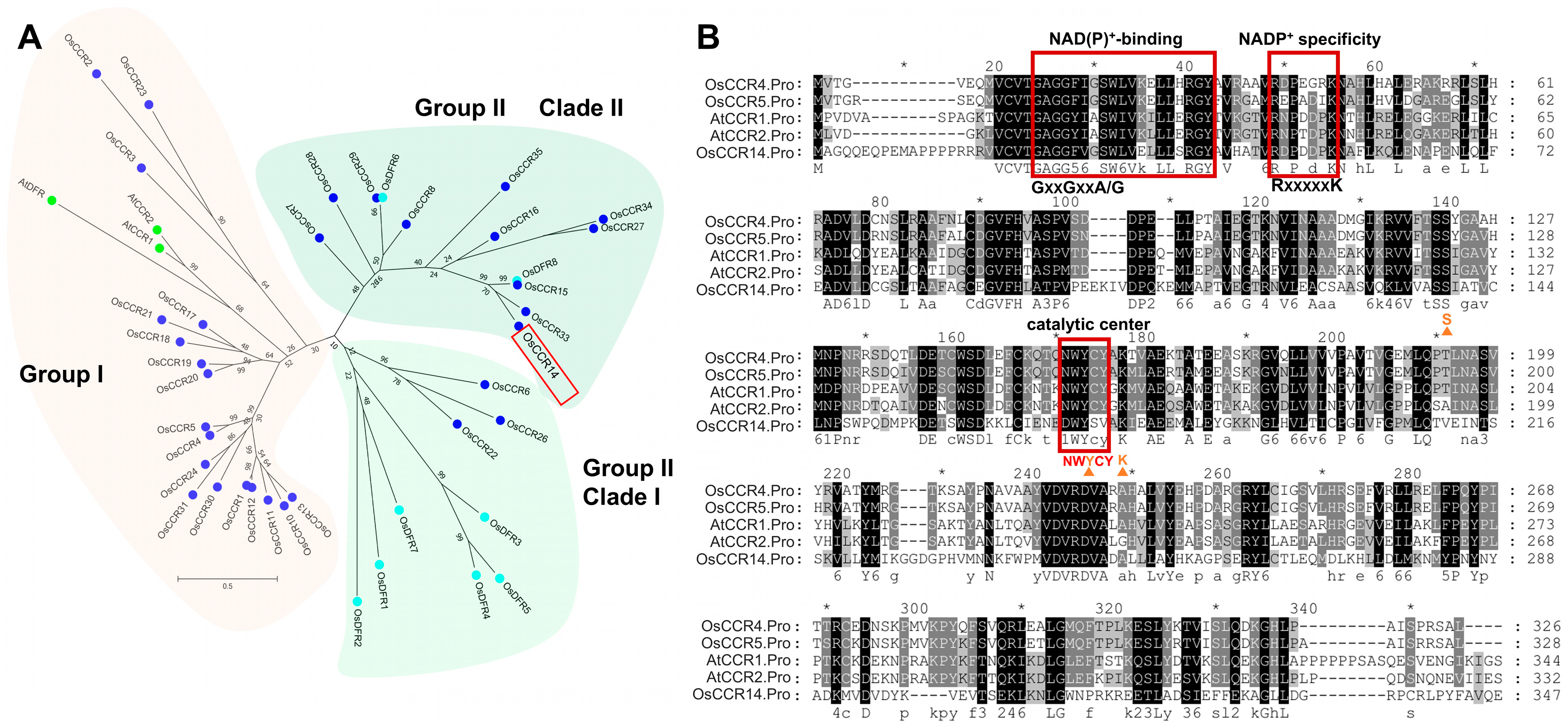
2.1. Sequence and Phylogenetic Analysis of OsCCR14
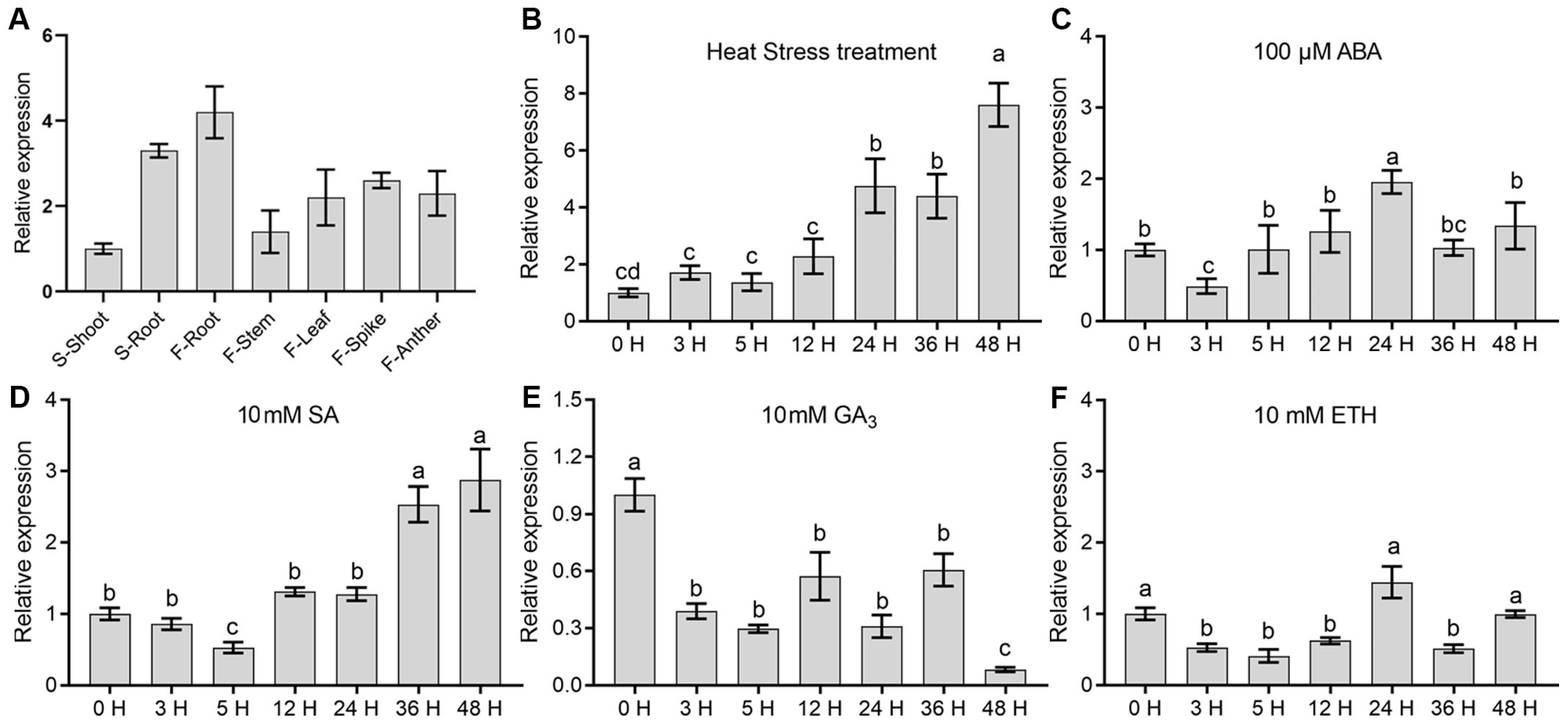
2.2. Expression Analysis of OsCCR14
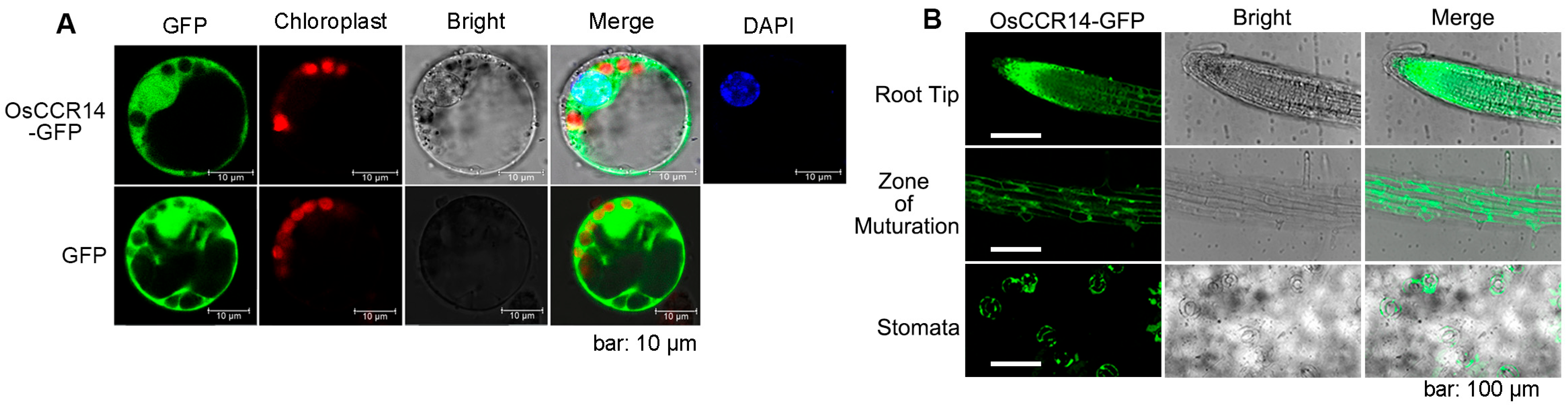
2.3. Subcellular Localization of OsCCR14
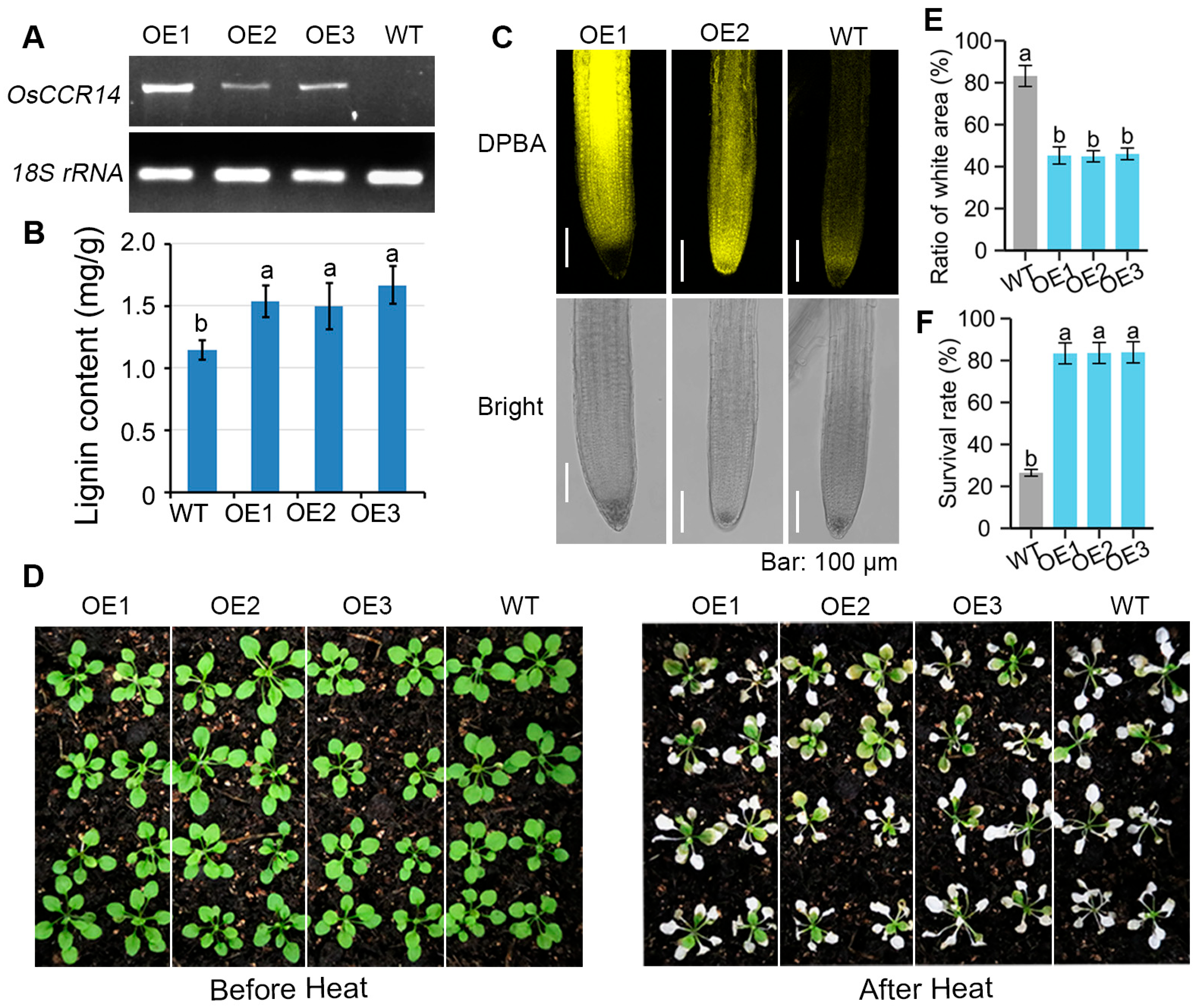
2.4. Impact of OsCCR14-Overexpression on Lignin Content, Flavonol Fluorescence, and Heat Tolerance in Arabidopsis
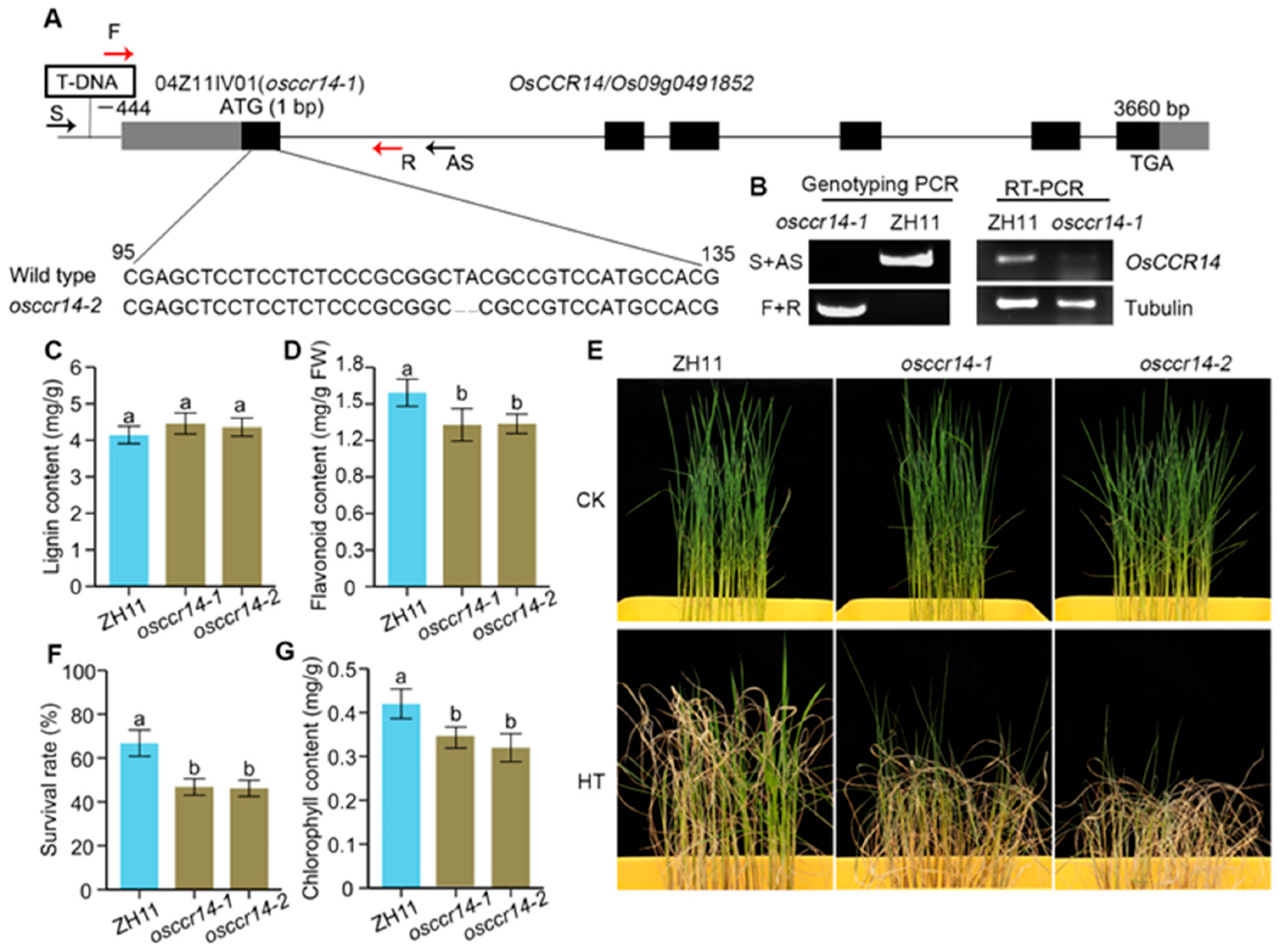
2.5. osccr14 Mutants Decrease Heat Tolerance at Seedling Stage
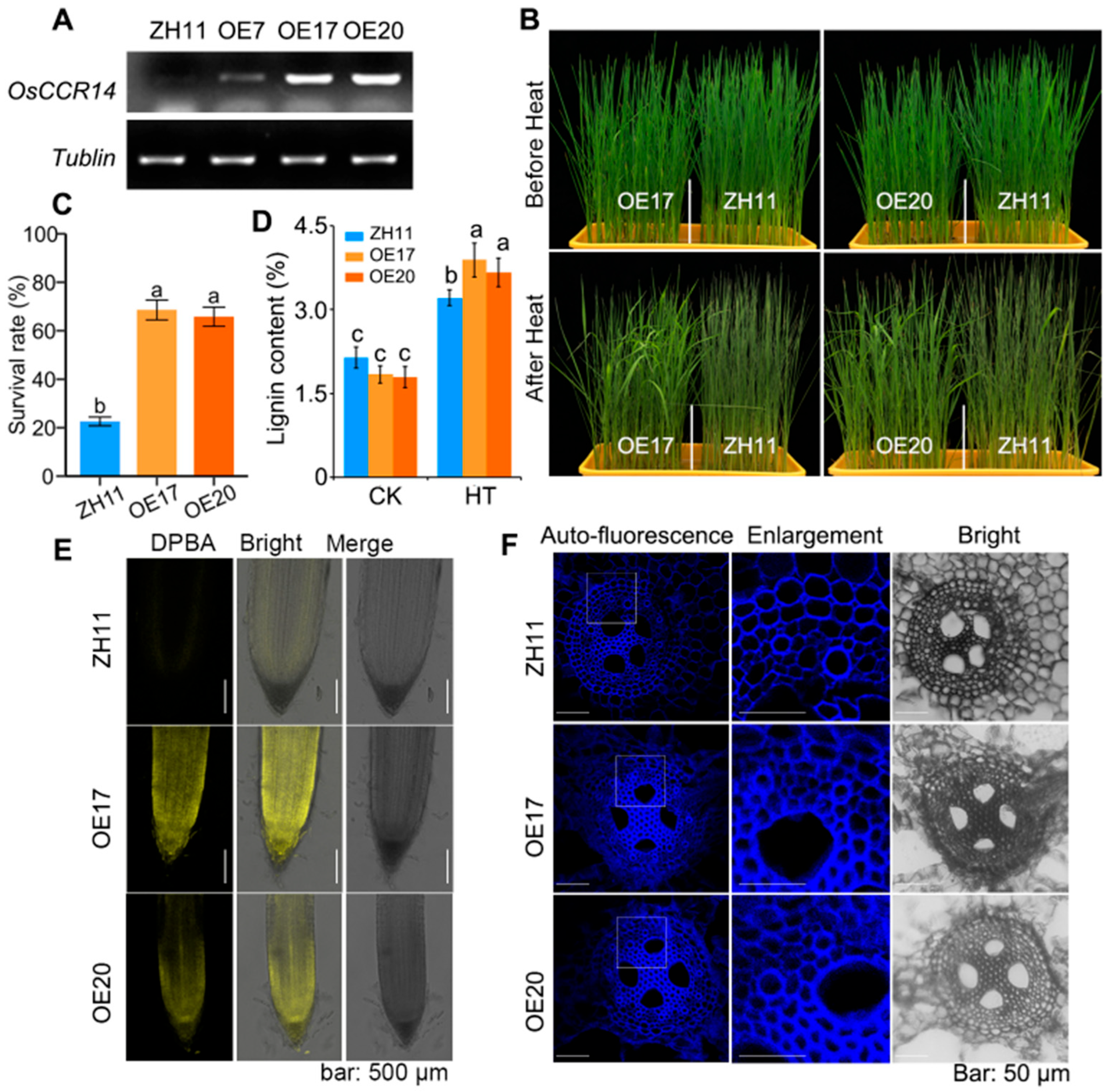
2.6. Overexpression OsCCR14-Enhanced Plant Thermotolerance
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Stress Treatments
4.3. RNA Extraction, RT-PCR and qRT-PCR Analysis
4.4. Subcellular Localization
4.5. Seedling Survival Rate Assessment
4.6. Ratio of Leaf White Area Assessment
4.7. Determination of Lignin and Flavonoid Level
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, W.; Yin, X.; Struik, P.C.; Solis, C.; Xie, F.; Schmidt, R.C.; Huang, M.; Zou, Y.; Ye, C.; Jagadish, S.V.K. High day- and night-time temperatures affect grain growth dynamics in contrasting rice genotypes. J. Exp. Bot. 2017, 68, 5233–5245. [Google Scholar] [CrossRef]
- Jagadish, S.V.; Murty, M.V.; Quick, W.P. Rice responses to rising temperatures--challenges, perspectives and future directions. Plant Cell Environ. 2015, 38, 1686–1698. [Google Scholar] [CrossRef]
- Mahmood, A.; Wang, W.; Ali, I.; Zhen, F.; Osman, R.; Liu, B.; Liu, L.; Zhu, Y.; Cao, W.; Tang, L. Individual and combined effects of booting and flowering high-temperature stress on rice biomass accumulation. Plants 2021, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed]
- Samat, A.T.; Soltabayeva, A.; Bekturova, A.; Zhanassova, K.; Auganova, D.; Masalimov, Z.; Srivastava, S.; Satkanov, M.; Kurmanbayeva, A. Plant responses to heat stress and advances in mitigation strategies. Front. Plant Sci. 2025, 16, 1638213. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Bao, J.; Gao, Z.; Sun, D.; Zheng, S.; Bai, J. How rice adapts to high temperatures. Front. Plant Sci. 2023, 14, 1137923. [Google Scholar] [CrossRef]
- Wang, P.; Liu, W.C.; Han, C.; Wang, S.; Bai, M.Y.; Song, C.P. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Yin, Q.; Qin, W.; Zhou, Z.; Wu, A.M.; Deng, W.; Li, Z.; Shan, W.; Chen, J.Y.; Kuang, J.F.; Lu, W.J. Banana MaNAC1 activates secondary cell wall cellulose biosynthesis to enhance chilling resistance in fruit. Plant Biotechnol. J. 2024, 22, 413–426. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Ji, Y.; Wang, C.; Wang, S.; Shi, Y.; Le, J.; Zhang, M. The heat shock factor 20-HSF4-cellulose synthase A2 module regulates heat stress tolerance in maize. Plant Cell 2024, 36, 2652–2667. [Google Scholar] [CrossRef]
- Lu, C.; Li, W.; Feng, X.; Chen, J.; Hu, S.; Tan, Y.; Wu, L. The dynamic remodeling of plant cell wall in response to heat stress. Genes 2025, 16, 628. [Google Scholar] [CrossRef]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.; Wang, L. The phenylpropanoid pathway and plant defence-a genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Choi, H.; An, G. Roles of lignin biosynthesis and regulatory genes in plant development. J. Integr. Plant Biol. 2015, 57, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Ninkuu, V.; Aluko, O.O.; Yan, J.; Zeng, H.; Liu, G.; Zhao, J.; Li, H.; Chen, S.; Dakora, F.D. Phenylpropanoids metabolism: Recent insight into stress tolerance and plant development cues. Front. Plant Sci. 2025, 16, 1571825. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, S.; Dard, A.; Meyer, A.J.; Reichheld, J.P. Redox-mediated responses to high temperature in plants. J. Exp. Bot. 2023, 74, 2489–2507. [Google Scholar] [CrossRef]
- Daryanavard, H.; Postiglione, A.E.; Mühlemann, J.K.; Muday, G.K. Flavonols modulate plant development, signaling, and stress responses. Curr. Opin. Plant Biol. 2023, 72, 102350. [Google Scholar] [CrossRef]
- Zhao, S.; Ma, Y.; Ding, Y.; Dong, G.; Liu, C.; Ma, X.; Hou, B. Rice glycosyltransferase UGT706F1 functions in heat tolerance through glycosylating flavonoids under the regulation of transcription factor MYB61. Plant J. 2025, 121, e17252. [Google Scholar] [CrossRef]
- Yang, W.; Yao, D.; Duan, H.; Zhang, J.; Cai, Y.; Lan, C.; Zhao, B.; Mei, Y.; Zheng, Y.; Yang, E.; et al. VAMP726 from maize and Arabidopsis confers pollen resistance to heat and UV radiation by influencing lignin content of sporopollenin. Plant Commun. 2023, 4, 100682. [Google Scholar] [CrossRef]
- Han, J.; Wang, Z.; Wu, X.; Xia, J.; Wang, L.; Wang, Z.; Zhang, Y. Deciphering high-temperature-induced lignin biosynthesis in wheat through comprehensive transcriptome analysis. Plants 2024, 13, 1832. [Google Scholar] [CrossRef]
- Giordano, A.; Liu, Z.; Panter, S.N.; Dimech, A.M.; Shang, Y.; Wijesinghe, H.; Fulgueras, K.; Ran, Y.; Mouradov, A.; Rochfort, S.; et al. Reduced lignin content and altered lignin composition in the warm season forage grass Paspalum dilatatum by down-regulation of a Cinnamoyl CoA reductase gene. Transgenic Res. 2014, 23, 503–517. [Google Scholar] [CrossRef]
- Pan, H.; Zhou, R.; Louie, G.V.; Mühlemann, J.K.; Bomati, E.K.; Bowman, M.E.; Dudareva, N.; Dixon, R.A.; Noel, J.P.; Wang, X. Structural studies of cinnamoyl-CoA reductase and cinnamyl-alcohol dehydrogenase, key enzymes of monolignol biosynthesis. Plant Cell 2014, 26, 3709–3727. [Google Scholar] [CrossRef]
- Costa, M.A.; Collins, R.E.; Anterola, A.M.; Cochrane, F.C.; Davin, L.B.; Lewis, N.G. An in silico assessment of gene function and organization of the phenylpropanoid pathway metabolic networks in Arabidopsis thaliana and limitations thereof. Phytochemistry 2003, 64, 1097–1112. [Google Scholar] [CrossRef]
- Shi, R.; Sun, Y.H.; Li, Q.; Heber, S.; Sederoff, R.; Chiang, V.L. Towards a systems approach for lignin biosynthesis in Populus trichocarpa: Transcript abundance and specificity of the monolignol biosynthetic genes. Plant Cell Physiol. 2010, 51, 144–163. [Google Scholar] [CrossRef] [PubMed]
- Carocha, V.; Soler, M.; Hefer, C.; Cassan-Wang, H.; Fevereiro, P.; Myburg, A.A.; Paiva, J.A.; Grima-Pettenati, J. Genome-wide analysis of the lignin toolbox of Eucalyptus grandis. New Phytol. 2015, 206, 1297–1313. [Google Scholar] [CrossRef] [PubMed]
- Park, H.L.; Bhoo, S.H.; Kwon, M.; Lee, S.W.; Cho, M.H. Biochemical and expression analyses of the rice cinnamoyl-CoA reductase gene family. Front. Plant Sci. 2017, 8, 2099. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Cui, L.; Song, N.; Liu, X.; Guo, G.; Zhang, Y. Comprehensive analysis of cinnamoyl-CoA reductase (CCR) gene family in wheat: Implications for lignin biosynthesis and stress responses. BMC Plant Biol. 2025, 25, 567. [Google Scholar] [CrossRef]
- Song, X.; Liu, D.; Yao, Y.; Tang, L.; Cheng, L.; Yang, L.; Jiang, Z.; Kang, Q.; Chen, S.; Ru, J.; et al. Genome-wide identification and expression pattern analysis of the cinnamoyl-CoA reductase gene family in flax (Linum usitatissimum L.). BMC Genom. 2025, 26, 315. [Google Scholar] [CrossRef]
- Kawasaki, T.; Koita, H.; Nakatsubo, T.; Hasegawa, K.; Wakabayashi, K.; Takahashi, H.; Umemura, K.; Umezawa, T.; Shimamoto, K. Cinnamoyl-CoA reductase, a key enzyme in lignin biosynthesis, is an effector of small GTPase Rac in defense signaling in rice. Proc. Natl. Acad. Sci. USA 2006, 103, 230–235. [Google Scholar] [CrossRef]
- Bang, S.W.; Choi, S.; Jin, X.; Jung, S.E.; Choi, J.W.; Seo, J.S.; Kim, J.K. Transcriptional activation of rice CINNAMOYL-CoA REDUCTASE 10 by OsNAC5, contributes to drought tolerance by modulating lignin accumulation in roots. Plant Biotechnol. J. 2022, 20, 736–747. [Google Scholar] [CrossRef]
- Chao, N.; Jiang, W.T.; Wang, X.C.; Jiang, X.N.; Gai, Y. Novel motif is capable of determining CCR and CCR-like proteins based on the divergence of CCRs in plants. Tree Physiol. 2019, 39, 2019–2026. [Google Scholar] [CrossRef]
- Cai, Z.; He, F.; Feng, X.; Liang, T.; Wang, H.; Ding, S.; Tian, X. Transcriptomic analysis reveals important roles of lignin and flavonoid biosynthetic pathways in rice thermotolerance during reproductive stage. Front. Genet. 2020, 11, 562937. [Google Scholar] [CrossRef] [PubMed]
- Borah, P.; Khurana, J.P. The OsFBK1 E3 ligase subunit affects anther and root secondary cell wall thickenings by mediating turnover of a cinnamoyl-CoA reductase. Plant Physiol. 2018, 176, 2148–2165. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, C.; Li, Y. Genome-wide identification and expression profiles of 13 key structural gene families involved in the biosynthesis of rice flavonoid scaffolds. Genes 2022, 13, 410. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Kim, E.; Kwag, Y.; An, H.; Kim, H.S.; Shah, S.; Lee, J.H.; Ha, E. Heat wave exposure and increased heat-related hospitalizations in young children in South Korea: A time-series study. Environ. Res. 2024, 241, 117561. [Google Scholar] [CrossRef]
- Pascual, L.S.; Segarra-Medina, C.; Gómez-Cadenas, A.; López-Climent, M.F.; Vives-Peris, V.; Zandalinas, S.I. Climate change-associated multifactorial stress combination: A present challenge for our ecosystems. J. Plant Physiol. 2022, 276, 153764. [Google Scholar] [CrossRef]
- Wagner, A.; Tobimatsu, Y.; Goeminne, G.; Phillips, L.; Flint, H.; Steward, D.; Torr, K.; Donaldson, L.; Boerjan, W.; Ralph, J. Suppression of CCR impacts metabolite profile and cell wall composition in Pinus radiata tracheary elements. Plant Mol. Biol. 2013, 81, 105–117. [Google Scholar] [CrossRef]
- Tamasloukht, B.; Wong, Q.; Lam, M.S.; Martinez, Y.; Tozo, K.; Barbier, O.; Jourda, C.; Jauneau, A.; Borderies, G.; Balzergue, S.; et al. Characterization of a cinnamoyl-CoA reductase 1 (CCR1) mutant in maize: Effects on lignification, fibre development, and global gene expression. J. Exp. Bot. 2011, 62, 3837–3848. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, J.; Luo, W.; Niu, S.; Qu, L.; Li, J.; Chen, Y.; Li, G.; Yang, H.; Lu, D. Salicylic acid cooperates with lignin and sucrose signals to alleviate waxy maize leaf senescence under heat stress. Plant Cell Environ. 2025, 48, 4341–4355. [Google Scholar] [CrossRef]
- Gallego-Giraldo, L.; Escamilla-Trevino, L.; Jackson, L.A.; Dixon, R.A. Salicylic acid mediates the reduced growth of lignin down-regulated plants. Proc. Natl. Acad. Sci. USA 2011, 108, 20814–20819. [Google Scholar] [CrossRef]
- Chao, N.; Li, S.; Li, N.; Qi, Q.; Jiang, W.T.; Jiang, X.N.; Gai, Y. Two distinct cinnamoyl-CoA reductases in Selaginella moellendorffii offer insight into the divergence of CCRs in plants. Planta 2017, 246, 33–43. [Google Scholar] [CrossRef]
- Bart, R.S.; Chern, M.; Vega-Sánchez, M.E.; Canlas, P.; Ronald, P.C. Rice Snl6, a cinnamoyl-CoA reductase-like gene family member, is required for NH1-mediated immunity to Xanthomonas oryzae pv. oryzae. PLoS Genet. 2010, 6, e1001123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, L.; Wu, J.; Liu, Y.; Liu, W.; He, G.; Wang, N. OsCCRL1 is essential for phenylpropanoid metabolism in rice anthers. Rice 2023, 16, 10. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Kamble, N.U. Decoding the role of flavonoids in ROS management during heat stress in tomato pollen. Plant Cell 2024, 36, 4285–4286. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Barakat, A.; Yassin, N.B.; Park, J.S.; Choi, A.; Herr, J.; Carlson, J.E. Comparative and phylogenomic analyses of cinnamoyl-CoA reductase and cinnamoyl-CoA-reductase-like gene family in land plants. Plant Sci. 2011, 181, 249–257. [Google Scholar] [CrossRef]
- Ding, S.; Gao, Z.; Chen, Y.; Liu, J.; Xue, Y.; Teng, Y.; Qin, S.; Tian, X.; Wang, H. OsLAC12, a laccase-encoding gene, contributes to rice heat tolerance through regulation of antioxidant defense and secondary metabolism. Plants 2025, 14, 2846. [Google Scholar] [CrossRef]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Tian, W.; Teng, Y.; Xue, Y.; Qin, S.; Liu, J.; Ding, S. Rice Cinnamoyl CoA Reductase-like Gene OsCCR14 Involved in Heat Stress via Regulation Lignin and Flavonoid Accumulation. Plants 2025, 14, 3626. https://doi.org/10.3390/plants14233626
Wang H, Tian W, Teng Y, Xue Y, Qin S, Liu J, Ding S. Rice Cinnamoyl CoA Reductase-like Gene OsCCR14 Involved in Heat Stress via Regulation Lignin and Flavonoid Accumulation. Plants. 2025; 14(23):3626. https://doi.org/10.3390/plants14233626
Chicago/Turabian StyleWang, Hongwei, Wei Tian, Yulu Teng, Yuxin Xue, Simin Qin, Jiaxin Liu, and Shuangcheng Ding. 2025. "Rice Cinnamoyl CoA Reductase-like Gene OsCCR14 Involved in Heat Stress via Regulation Lignin and Flavonoid Accumulation" Plants 14, no. 23: 3626. https://doi.org/10.3390/plants14233626
APA StyleWang, H., Tian, W., Teng, Y., Xue, Y., Qin, S., Liu, J., & Ding, S. (2025). Rice Cinnamoyl CoA Reductase-like Gene OsCCR14 Involved in Heat Stress via Regulation Lignin and Flavonoid Accumulation. Plants, 14(23), 3626. https://doi.org/10.3390/plants14233626





