Effects of Biochar- and Hydrochar-Amended Organic Fertilizer on Crop Production, NH3 Loss, and Fertility of Coastal Saline–Alkali Soil
Abstract
1. Introduction
2. Results
2.1. Yield and Agronomic Traits of Rice and Wheat
2.2. N Utilization Efficiency in Rice and Wheat
2.3. NH3 Volatilization Analysis
2.4. Floodwater pH, NH4+-N, and NO3−-N Concentration in the Rice Season
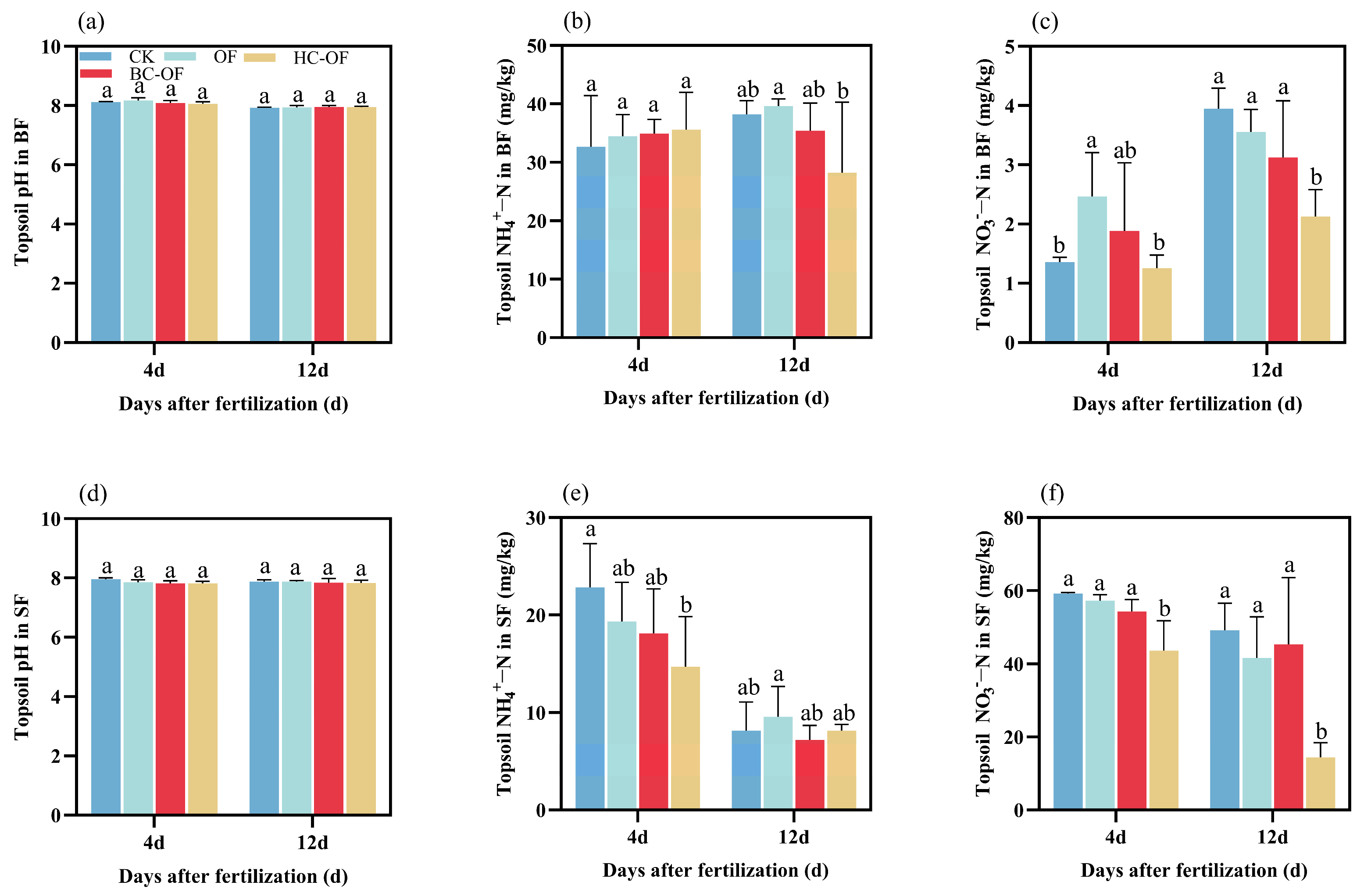
2.5. Topsoil pH, NH4+-N, and NO3−-N Concentration in the Wheat Season
2.6. Soil Fertility Characteristics
3. Discussion
3.1. Effects of Different Organic Fertilizers on Rice and Wheat Yield and NUE
3.2. Effects of Different Organic Fertilizers on NH3 Volatilization from Saline–Alkali Soil
3.3. Changes in Saline–Alkali Soil Fertilty as Impacted by Different Organic Fertilizer
4. Materials and Methods
4.1. Experimental Setup
4.1.1. Experimental Materials
4.1.2. Experimental Design and Management
4.2. Sample Collection and Analysis
4.2.1. Yield and N Use Efficiency (NUE)
4.2.2. Floodwater pH, NH4+-N, and NO3−-N Concentration
4.2.3. NH3 Volatilization
4.2.4. Soil Sampling and Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Skrzypek, G.; Dogramaci, S.; Rouillard, A.; Grierson, P.F. Groundwater seepage controls salinity in a hydrologically terminal basin of semi-arid northwest Australia. J. Hydrol. 2016, 542, 627–636. [Google Scholar] [CrossRef]
- Hopmans, J.W.; Qureshi, A.S.; Kisekka, I.; Munns, R.; Grattan, S.R.; Rengasamy, P.; Taleisnik, E. Critical knowledge gaps and research priorities in global soil salinity. Adv. Agron. 2021, 169, 1–191. [Google Scholar]
- Li-ping, L.; Xiao-hua, L.; Hong-bo, S.; Zhao-Pu, L.; Ya, T.; Quan-suo, Z.; Jun-qin, Z. Ameliorants improve saline–alkaline soils on a large scale in northern Jiangsu Province, China. Ecol. Eng. 2015, 81, 328–334. [Google Scholar] [CrossRef]
- Hassani, A.; Azapagic, A.; Shokri, N. Predicting long-term dynamics of soil salinity and sodicity on a global scale. Proc. Natl. Acad. Sci. USA 2020, 117, 33017–33027. [Google Scholar] [CrossRef]
- Yu, S.; Li, J.; Yingxin, L.; Hongbin, W.; Deliang, L.; Chunjie, T. Compositional and functional succession of soil bacterial communities during long-term rice cultivation on saline-alkali soils: Insights derived from a new perspective of the core bacterial taxa. Pedosphere 2024, 13, 641–654. [Google Scholar]
- Sahab, S.; Suhani, I.; Srivastava, V.; Chauhan, P.S.; Singh, R.P.; Prasad, V. Potential risk assessment of soil salinity to agroecosystem sustainability: Current status and management strategies. Sci. Total Environ. 2021, 764, 144164. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, C.; Liu, X.; Lu, Y.; Wang, Y. Saline-alkali soil applied with vermicompost and humic acid fertilizer improved macroaggregate microstructure to enhance salt leaching and inhibit nitrogen losses. Appl. Soil Ecol. 2020, 156, 103705. [Google Scholar] [CrossRef]
- Ren, T.; Fan, P.; Zuo, W.; Liao, Z.; Wang, F.; Wei, Y.; Liu, G. Biochar-amended fertilizer under drip irrigation: More conducive to improving soil carbon pool and promoting nitrogen utilization. Ecol. Indic. 2023, 154, 110583. [Google Scholar] [CrossRef]
- Rasool, M.; Akhter, A.; Soja, G.; Haider, M.S. Role of biochar, compost and plant growth promoting rhizobacteria in the management of tomato early blight disease. Sci. Rep. 2021, 11, 6092. [Google Scholar] [CrossRef]
- Feng, H.L.; Xu, C.S.; He, H.H.; Zeng, Q.; Liu, G.S. Effect of biochar on soil enzyme activity & the bacterial community and its mechanism. Environ. Sci. 2021, 42, 422–432. [Google Scholar]
- Chivenge, P.; Vanlauwe, B.; Gentile, R.; Wangechi, H.; Mugendi, D.; Van Kessel, C.; Six, J. Organic and mineral input management to enhance crop productivity in Central Kenya. Agron. J. 2009, 101, 1266–1275. [Google Scholar] [CrossRef]
- Ibrahim, A.; Abaidoo, R.C.; Fatondji, D.; Opoku, A. Hill placement of manure and fertilizer micro-dosing improves yield and water use efficiency in the Sahelian low input millet-based cropping system. Field Crops Res. 2015, 7, 29–36. [Google Scholar] [CrossRef]
- Bento, L.R.; Castro, A.J.R.; Moreira, A.B.; Ferreira, O.P.; Bisinoti, M.C.; Melo, C.A. Release of nutrients and organic carbon in different soil types from hydrochar obtained using sugarcane bagasse and vinasse. Geoderma 2019, 334, 24–32. [Google Scholar] [CrossRef]
- Bona, D.; Bertoldi, D.; Borgonovo, G.; Mazzini, S.; Ravasi, S.; Silvestri, S.; Tambone, F. Evaluating the potential of hydrochar as a soil amendment. Waste Manag. 2023, 159, 75–83. [Google Scholar] [CrossRef]
- Khosravi, A.; Zheng, H.; Liu, Q.; Hashemi, M.; Tang, Y.; Xing, B. Production and characterization of hydrochars and their application in soil improvement and environmental remediation. Chem. Eng. J. 2022, 430, 133142. [Google Scholar] [CrossRef]
- He, K.; Xu, Y.; He, G.; Zhao, X.; Wang, C.; Li, S.; Hu, R. Combined application of acidic biochar and fertilizer synergistically enhances Miscanthus productivity in coastal saline-alkaline soil. Sci. Total Environ. 2023, 893, 164811. [Google Scholar] [CrossRef]
- Mustafa, A.; Hu, X.; Abrar, M.M.; Shah, S.A.A.; Nan, S.; Saeed, Q.; Minggang, X. Long-term fertilization enhanced carbon mineralization and maize biomass through physical protection of organic carbon in fractions under continuous maize cropping. Appl. Soil Ecol. 2021, 165, 103971. [Google Scholar] [CrossRef]
- Ju, X.T.; Kou, C.L.; Christie, P.; Dou, Z.X.; Zhang, F.S. Changes in the soil environment from excessive application of fertilizers and manures to two contrasting intensive cropping systems on the North China Plain. Environ. Pollut. 2007, 145, 497–506. [Google Scholar] [CrossRef]
- Bodirsky, B.L.; Popp, A.; Lotze-Campen, H.; Dietrich, J.P.; Rolinski, S.; Weindl, I.; Stevanovic, M. Reactive nitrogen requirements to feed the world in 2050 and potential to mitigate nitrogen pollution. Nat. Commun. 2014, 5, 3858. [Google Scholar] [CrossRef]
- Akhtar, M.; Hussain, F.; Ashraf, M.Y.; Qureshi, T.M.; Akhter, J.; Awan, A.R. Influence of Salinity on Nitrogen Transformations in Soil. Commun. Soil Sci. Plant Anal. 2012, 43, 1674–1683. [Google Scholar] [CrossRef]
- Feng, Y.; He, H.; Li, D.; He, S.; Yang, B.; Xue, L.; Chu, Q. Biowaste hydrothermal carbonization aqueous product application in rice paddy: Focus on rice growth and ammonia volatilization. Chemosphere 2021, 277, 130233. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Deng, X.; Herbert, S.; Xing, B. Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma 2013, 206, 32–39. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, X.; Wang, X.; Xie, C.; Liu, J.; Cheng, Y.; Li, Y. Modified biochar affects CO2 and N2O emissions from coastal saline soil by altering soil pH and elemental stoichiometry. Sci. Total Environ. 2024, 954, 176283. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, H.; Zhang, X.; Zhang, J.; Zhou, S. Optimal straw retention strategies for low-carbon rice production: 5 year results of an in situ trial in eastern China. Agronomy 2023, 13, 1456. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Chi, Z.; Zheng, J.; Li, L.; Zhang, X.; Pan, G. Biochar provided limited benefits for rice yield and greenhouse gas mitigation six years following an amendment in a fertile rice paddy. Catena 2019, 179, 20–28. [Google Scholar] [CrossRef]
- Huang, M.; Fan, L.; Chen, J.; Jiang, L.; Zou, Y. Continuous applications of biochar to rice: Effects on nitrogen uptake and utilization. Sci. Rep. 2018, 8, 11461. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, S.; Liu, L.; Wu, L.; Ding, X. Combined organic amendments and mineral fertilizer application increase rice yield by improving soil structure, P availability and root growth in saline-alkaline soil. Soil Tillage Res. 2021, 212, 105060. [Google Scholar] [CrossRef]
- Selvarajh, G.; Ch’ng, H.Y. Enhancing Soil Nitrogen Availability and Rice Growth by Using Urea Fertilizer Amended with Rice Straw Biochar. Agronomy 2021, 11, 1352. [Google Scholar] [CrossRef]
- Selvarajh, G.; Ch’ng, H.Y.; Zain, N.M.; Wei, L.S.; Liew, J.Y.; Azmin, S.N.H.M.; Damrongrak, I. Enriched rice husk biochar superior to commercial biochar in ameliorating ammonia loss from urea fertilizer and improving plant uptake. Heliyon 2024, 10, e32080. [Google Scholar] [CrossRef]
- Chew, J.; Joseph, S.; Chen, G.; Zhang, Y.; Zhu, L.; Liu, M.; Fan, X. Biochar-based fertiliser enhances nutrient uptake and transport in rice seedlings. Sci. Total Environ. 2022, 826, 154174. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, J.; Lee, X.; Chen, Y.; Gao, W.; Pan, W.; Tang, Y. Effects of biochar-based fertilizers on nutrient leaching in a tobacco-planting soil. Acta Geochim. 2019, 38, 1–7. [Google Scholar] [CrossRef]
- Shi, Z.Q.; She, D.L.; Chen, X.Y.; Xia, Y.Q. Effects of salinity on soil ammonia volatilization and denitrification rates. J. Ecol. Rural Environ. 2021, 37, 1458–1464. [Google Scholar]
- Shuai, H.; Guodong, W.; Lei, Z. The Effect of Interaction between Biochar and Nitrogen Fertilizer on Ammonia Volatilization in Salinized Soil. J. Irrig. Drain. 2023, 42, 87. (In Chinese) [Google Scholar]
- Kastner, J.R.; Miller, J.; Das, K.C. Pyrolysis conditions and ozone oxidation effects on ammonia adsorption in biomass generated chars. J. Hazard. Mater. 2009, 164, 1420–1427. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, X.; Zhou, S.; Shang, H.; Luo, J.; Tsang, D.C. Hydrothermal carbonization for hydrochar production and its application. Biochar Biomass Waste 2019, 19, 275–294. [Google Scholar]
- Xie, W.M.; Li, S.J.; Shi, W.M.; Zhang, H.L.; Fang, F.; Wang, G.X.; Zhang, L.M. Quantitatively ranking the influencing factors of ammonia volatilization from paddy soils by grey relational entropy. Environ. Sci. Pollut Res. 2020, 27, 2319–2327. [Google Scholar] [CrossRef]
- Wang, B.; Li, R.; Wan, Y.; Li, Y.E.; Cai, W.; Guo, C.; Wilkes, A. Air warming and CO2 enrichment cause more ammonia volatilization from rice paddies: An OTC field study. Sci. Total Environ. 2021, 752, 142071. [Google Scholar] [CrossRef]
- Abulaiti, A.; She, D.; Zhang, W.; Xia, Y. Regulation of denitrification/ammonia volatilization by periphyton in paddy fields and its promise in rice yield promotion. J. Sci. Food Agric. 2023, 103, 4119–4130. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.M.; Zhao, C.W.; Sun, X.; Wang, Z.C. Reclamation of saline-sodic soil properties and improvement of rice (Oriza sativa L.) growth and yield using desulfurized gypsum in the west of Songnen Plain, northeast China. Geoderma 2012, 187, 24–30. [Google Scholar] [CrossRef]
- Francis, D.D.; Vigil, M.F.; Mosier, A.R. Gaseous losses of nitrogen other than through denitrification. Nitrogen Agric. Syst. 2008, 49, 255–279. [Google Scholar]
- Feng, Y.; Han, L.; Li, D.; Sun, M.; Wang, X.; Xue, L.; Xing, B. Presence of microplastics alone and co-existence with hydrochar unexpectedly mitigate ammonia volatilization from rice paddy soil and affect structure of soil microbiome. J. Hazard. Mater. 2022, 422, 126831. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Chen, L.; Shutes, B.; Yan, B.; Zhang, F.; Zhu, H. Nitrogen migration and transformation in a saline-alkali paddy ecosystem with application of different nitrogen fertilizers. Env. Sci. Pollut Res. 2023, 30, 51665–51678. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, S.; Banik, C.; Laird, D.A.; Smith, R.; Brown, R.C. Enhancing biochar as scaffolding for slow release of nitrogen fertilizer. ACS Sustain. Chem. Eng. 2021, 9, 8222–8231. [Google Scholar] [CrossRef]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; Gasser, M.O.; MacDonald, J.D.; Pelster, D.E.; Bertrand, N. NH3 volatilization, soil concentration and soil pH following subsurface banding of urea at increasing rates. Can. J. Soil Sci. 2013, 93, 261–268. [Google Scholar] [CrossRef]
- Rehman, M.Z.; Batool, Z.; Ayub, M.A.; Hussaini, K.M.; Murtaza, G.; Usman, M.; Ali, S. Effect of acidified biochar on bioaccumulation of cadmium (Cd) and rice growth in contaminated soil. Environ. Technol. Innov. 2020, 19, 101015. [Google Scholar] [CrossRef]
- Stevenson, F.J. Genesis, composition, and reactions. In Humus Chemistry; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Feng, Y.; Du, H.; Wulandari, T.; Poinern, G.E.J.; Jiang, Z.T.; Fawcett, D.; Yang, L. Hydrochar amendments stimulate soil nitrous oxide emission by increasing production of hydroxyl radicals and shifting nitrogen functional genes in the short term: A culture experiment. Chemosphere 2022, 302, 134771. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, J.M.; Paneque, M.; Miller, A.Z.; Knicker, H. Relating physical and chemical properties of four different biochars and their application rate to biomass production of Lolium perenne on a Calcic Cambisol during a pot experiment of 79 days. Sci. Total Environ. 2014, 499, 175–184. [Google Scholar] [CrossRef]
- Melo, T.M.; Bottlinger, M.; Schulz, E.; Leandro, W.M.; de Aguiar Filho, A.M.; Wang, H.; Rinklebe, J. Plant and soil responses to hydrothermally converted sewage sludge (sewchar). Chemosphere 2018, 206, 338–348. [Google Scholar] [CrossRef]
- Yu, S.; Feng, Y.; Xue, L.; Sun, H.; Han, L.; Yang, L.; Chu, Q. Biowaste to treasure: Application of microbial-aged hydrochar in rice paddy could improve nitrogen use efficiency and rice grain free amino acids. J. Clean. Prod. 2019, 240, 118180. [Google Scholar] [CrossRef]
- Chu, Q.; Xue, L.; Singh, B.P.; Yu, S.; Müller, K.; Wang, H.; Yang, L. Sewage sludge-derived hydrochar that inhibits ammonia volatilization, improves soil nitrogen retention and rice nitrogen utilization. Chemosphere 2020, 245, 125558. [Google Scholar] [CrossRef]
- Liu, K.; Ran, Q.; Li, F.; Shaheen, S.M.; Wang, H.; Rinklebe, J.; Fang, L. Carbon-based strategy enables sustainable remediation of paddy soils in harmony with carbon neutrality. Carbon Res. 2022, 1, 12. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Q.; Zheng, H.; Li, M.; Liu, Y.; Wang, X.; Xing, B. Biochar as a sustainable tool for improving the health of salt-affected soils. Soil Environ. Health 2023, 1, 100033. [Google Scholar] [CrossRef]
- Liang, F.; Li, B.; Vogt, R.D.; Mulder, J.; Song, H.; Chen, J.; Guo, J. Straw return exacerbates soil acidification in major Chinese croplands. Resour. Conserv. Recycl. 2023, 198, 107176. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, W.; Zhang, F.; Shi, Y.; Wang, Y.; Zhang, X.; Xu, R. Exchangeable acidity characteristics of farmland black soil in northeast China. Geoderma Reg. 2024, 38, e00852. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, H.; Xue, L.; Liu, Y.; Gao, Q.; Lu, K.; Yang, L. Biochar applied at an appropriate rate can avoid increasing NH3 volatilization dramatically in rice paddy soil. Chemosphere 2017, 168, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Tang, Q.; Xie, W.; Yu, J.; Wang, L.; Wang, B. Application of products derived from pyrolysis and hydrothermal carbonization as conditioners for aerobic composting produced multiple beneficial effects: Evaluation amended on 10-ton pilot scale trials. Chem. Eng. J. 2025, 505, 159793. [Google Scholar] [CrossRef]
- Huang, W.; Sun, H.; Sun, X.; Gong, X.; Bian, R.; Wang, Y.; Feng, Y. Co-amendment of dicyandiamide with waste carbonization products into composting: Enhanced fertility, reduced gas emission and increased economic benefits. J. Clean. Prod. 2024, 471, 143379. [Google Scholar] [CrossRef]
- Zhang, W.; Han, B.; Wille, U.; Butterly, C.; He, J.; Chen, D. Surface modification of coal tailings by thermal air oxidation for ammonia capture. J. Clean. Prod. 2022, 362, 132525. [Google Scholar] [CrossRef]



| Crop Season | Treatment | Grain Yield (g/pot) | Spike Number | Kernels per Spike | Thousand-Kernel Weight (g) | Annual Yield |
|---|---|---|---|---|---|---|
| Rice | CK | 77.29 ± 1.81 a | 28.50 ± 1.12 a | 105.98 ± 5.35 a | 26.62 ± 0.59 a | 110.13 ± 2.01 a |
| OF | 82.97 ± 4.48 a | 31.25 ± 1.47 a | 104.46 ± 4.77 a | 25.38 ± 0.60 a | 113.24 ± 4.31 a | |
| BC-OF | 86.17 ± 4.88 a | 30.75 ± 1.30 a | 109.44 ± 3.73 a | 25.75 ± 1.14 a | 118.26 ± 1.39 a | |
| HC-OF | 87.18 ± 3.17 a | 30.75 ± 2.17 a | 115.53 ± 4.94 a | 26.27 ± 0.63 a | 122.45 ± 4.52 a | |
| Wheat | CK | 29.73 ± 1.29 b | 35.25 ± 2.68 a | 20.57 ± 1.79 a | 41.25 ± 1.28 a | |
| OF | 30.26 ± 0.76 b | 35.50 ± 2.69 a | 22.20 ± 2.23 a | 38.76 ± 1.58 a | ||
| BC-OF | 32.09 ± 1.26 ab | 35.25 ± 2.68 a | 22.72 ± 1.87 a | 40.36 ± 2.33 a | ||
| HC-OF | 35.27 ± 1.46 a | 38.50 ± 2.60 a | 23.69 ± 1.66 a | 38.77 ± 0.92 a |
| Treatment | pH | EC (μs/cm) | TN (g/kg) | NH4+-N (mg/kg) | NO3−-N (mg/kg) | AP (mg/kg) | AK (mg/kg) | SOM (g/kg) |
|---|---|---|---|---|---|---|---|---|
| CK | 7.88 ± 0.05 a | 959 ± 37.50 a | 0.53 ± 0.01 c | 1.43 ± 0.45 b | 28.30 ± 3.14 a | 5.32 ± 0.27 a | 243.20 ± 3.47 a | 9.27 ± 0.80 a |
| OF | 7.78 ± 0.05 ab | 1077.50 ± 11.50 a | 0.55 ± 0.02 bc | 0.47 ± 0.12 b | 30.04 ± 2.51 a | 5.68 ± 0.43 a | 283.67 ± 2.89 a | 9.40 ± 0.86 a |
| BC-OF | 7.80 ± 0.08 ab | 926.25 ± 25.00 a | 0.57 ± 0.03 ab | 4.70 ± 0.08 a | 31.86 ± 2.50 a | 5.43 ± 0.51 a | 270.66 ± 5.10 a | 9.11 ± 0.32 a |
| HC-OF | 7.75 ± 0.08 b | 1185.25 ± 14.50 a | 0.59 ± 0.01 a | 4.14 ± 0.25 a | 35.25 ± 1.46 a | 5.32 ± 0.87 a | 259.86 ± 7.65 a | 10.30 ± 0.51 a |
| Treatment | pH | EC (ms/cm) | TN (g/kg) | AP (mg/kg) | AK (g/kg) | OM (g/kg) |
|---|---|---|---|---|---|---|
| OF | 7.29 | 3.75 | 19.28 | 69.96 | 1.13 | 427.28 |
| BC-OF | 8.34 | 3.90 | 19.29 | 28.98 | 1.99 | 419.67 |
| HC-OF | 7.62 | 4.18 | 23.21 | 113.96 | 1.27 | 439.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Huang, W.; Feng, Y.; Ma, M.; Sun, H. Effects of Biochar- and Hydrochar-Amended Organic Fertilizer on Crop Production, NH3 Loss, and Fertility of Coastal Saline–Alkali Soil. Plants 2025, 14, 3616. https://doi.org/10.3390/plants14233616
Liu C, Huang W, Feng Y, Ma M, Sun H. Effects of Biochar- and Hydrochar-Amended Organic Fertilizer on Crop Production, NH3 Loss, and Fertility of Coastal Saline–Alkali Soil. Plants. 2025; 14(23):3616. https://doi.org/10.3390/plants14233616
Chicago/Turabian StyleLiu, Chang, Wang Huang, Yanfang Feng, Meng Ma, and Haijun Sun. 2025. "Effects of Biochar- and Hydrochar-Amended Organic Fertilizer on Crop Production, NH3 Loss, and Fertility of Coastal Saline–Alkali Soil" Plants 14, no. 23: 3616. https://doi.org/10.3390/plants14233616
APA StyleLiu, C., Huang, W., Feng, Y., Ma, M., & Sun, H. (2025). Effects of Biochar- and Hydrochar-Amended Organic Fertilizer on Crop Production, NH3 Loss, and Fertility of Coastal Saline–Alkali Soil. Plants, 14(23), 3616. https://doi.org/10.3390/plants14233616





