Recent Insights into the Molecular Mechanisms of Salt Tolerance in Melon (Cucumis melo L.)
Abstract
1. Introduction
2. Salt Stress Damage in Melon
2.1. Germination and Seedling Growth Inhibition
2.2. Fruit Quality and Yield Reduction
2.3. Physiological Stress
2.3.1. Osmotic Stress
2.3.2. Ionic Toxicity and Nutrient Imbalance
2.3.3. Oxidative Damage
3. Molecular Mechanisms of Salt Tolerance in Melon
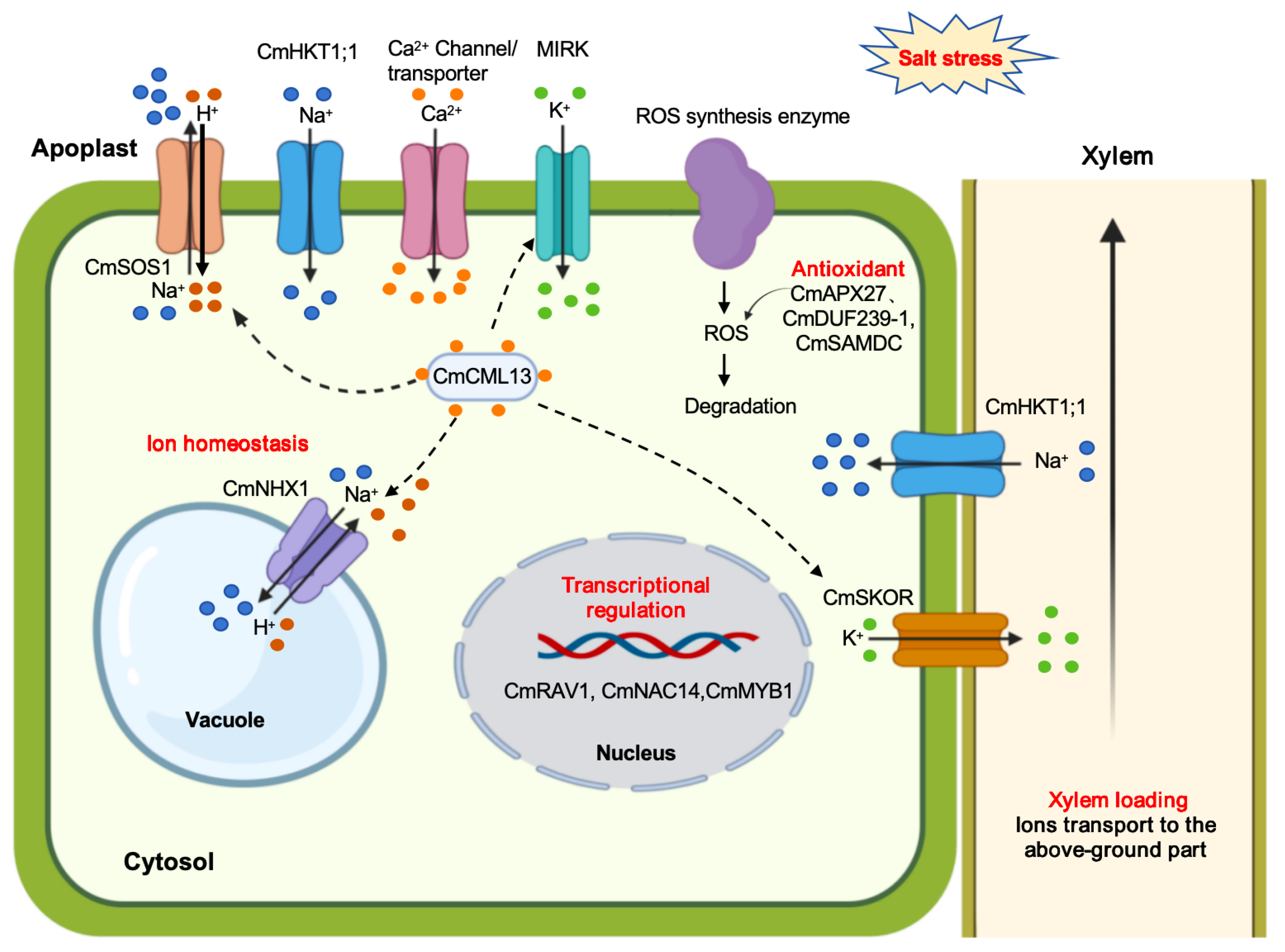
3.1. Ion Homeostasis Regulation
3.2. Antioxidant Regulation
| Pathway | Gene | Function | Reference |
|---|---|---|---|
| Ion transport regulation | MIRK | MIRK mediates K+ uptake and maintains a favorable K+/Na+ balance under saline conditions. | [29,41] |
| CmNHX1 | CmNHX1, a vacuolar Na+/H+ antiporter, is mainly expressed in roots, stems, and leaves. Overexpression of CmNHX1 in ATX3 yeast enhances salt tolerance. | [36] | |
| CmHKT1;1 | CmHKT1;1 retrieves Na+ from the xylem to prevent excessive shoot accumulation and is strongly induced by salinity in tolerant melon cultivars. Overexpression in Arabidopsis enhances salt tolerance by maintaining a favorable K+/Na+ balance. | [37] | |
| CmSKOR | CmSKOR encodes a Shaker-type outward-rectifying K+ channel that mediates K+ efflux from root stelar cells to the xylem, thereby regulating K+ allocation to aerial tissues. | [40] | |
| CmCML13 | CmCML13 encodes a calmodulin-like (CML) protein, enhances salt tolerance in transgenic Arabidopsis by significantly reducing shoot Na+ content, independent of the HKT1 pathway. | [44] | |
| Transcriptional regulation | CmRAV1 | CmRAV1 encodes a nuclear transcription factor with AP2 and B3 domains, is strongly induced by NaCl, especially in roots and flowers. Its overexpression in Arabidopsis enhances salt tolerance, improving germination and maintaining root growth under saline conditions. | [56] |
| CmNAC14 | CmNAC14 acts as a negative regulator of salt stress, with its overexpression in Arabidopsis enhancing sensitivity. | [57] | |
| CmMYB1 | CmMYB1 respond rapidly to salt stress in early transcriptional reprogramming and negatively regulate salt stress. | [58] | |
| Antioxidant regulation | CmAPX27 | CmAPX27 encodes an ascorbate peroxidase, enhances APX activity and strengthens ROS scavenging, | [50] |
| CmDUF239-1 | CmDUF239-1 plays a dual role in promoting salt tolerance by regulating antioxidant defenses and ion transport. | [26,55] | |
| CmSAMDC | CmSAMDC regulates spermidine and spermine biosynthesis, Overexpression of CmSAMDC in Arabidopsis enhances salt tolerance by reducing malondialdehyde (MDA) accumulation. | [54] | |
| CmLEA-S | CmLEA-S, a Late Embryogenesis Abundant (LEA) protein, protects cells by stabilizing membranes, scavenging ROS, and enhancing antioxidant enzyme activities. | [59] | |
| Others regulation | CmUBC | Encodes an E2 ubiquitin-conjugating enzyme, constitutively expressed throughout diverse tissues and transcriptional induced by salinity. | [60] |
| CmKCS | CmKCS5, CmKCS6, CmKCS10, and CmKCS12 exhibit pronounced transcriptional upregulation under salt stress, likely bolstering melon membrane integrity. | [61] | |
| CmTPR | Several CmTPR genes are upregulated under salt stress, which is critical for maintaining protein stability and regulating stress-related pathways. | [62] |
3.3. Transcriptional Regulation
3.4. Other Molecular Pathways Associated with Salt Tolerance in Melon
4. Conclusions and Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Nguyen, P.D.T.; Lao, T.D.; Le, T.A.H.; Nguyen, N.H. Abiotic stress responses in melon (Cucumis melo): Emerging underlying molecular mechanisms and biotechnological advances to cope with the issue. Ann. Appl. Biol. 2024, 185, 4–10. [Google Scholar] [CrossRef]
- Chevilly, S.; Dolz-Edo, L.; Martínez-Sánchez, G.; Morcillo, L.; Vilagrosa, A.; López-Nicolás, J.M.; Blanca, J.; Yenush, L.; Mulet, J.M. Distinctive traits for drought and salt stress tolerance in melon (Cucumis melo L.). Front. Plant Sci. 2021, 12, 777060. [Google Scholar] [CrossRef]
- Chen, C.; Yu, W.; Xu, X.; Wang, Y.; Wang, B.; Xu, S.; Lan, Q.; Wang, Y. Research advancements in salt tolerance of Cucurbitaceae: From salt response to molecular mechanisms. Int. J. Mol. Sci. 2024, 25, 9051. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Gao, C.; Gao, Y.; Wang, C.; Jiao, Z.; Xu, A.; Dong, Y.; Sun, J. Investigating salt tolerance in melon during germination and early seedling stages. Horticulturae 2025, 11, 397. [Google Scholar] [CrossRef]
- Sivritepe, N.; Sivritepe, H.; Eris, A. The effects of NaCl priming on salt tolerance in melon seedlings grown under saline conditions. Sci. Hortic. 2003, 97, 229–237. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ response mechanisms to salinity stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Liu, L.; Xia, W.; Li, H.; Zeng, H.; Wei, B.; Han, S.; Yin, C. Salinity inhibits rice seed germination by reducing α-amylase activity via decreased bioactive gibberellin content. Front. Plant Sci. 2018, 9, 275. [Google Scholar] [CrossRef]
- Xiong, M.; Xu, J.; Zhou, Z.; Peng, B.; Shen, Y.; Shen, H.; Xu, X.; Li, C.; Deng, L.; Feng, G. Salinity inhibits seed germination and embryo growth by reducing starch mobilization efficiency in barley. Plant Direct 2024, 8, e564. [Google Scholar] [CrossRef]
- Ulaş, A.; Yetisir, H. Grafting for sustainable growth performance of melon (Cucumis melo L.) under salt-stressed hydroponic condition. Eurasian J. Soil Sci. Dev. 2019, 8, 201–210. [Google Scholar]
- Ameen, H.H.H.H.; Ulas, A. Leaf physiological and root morphological characteristics contributing to salt tolerance of snake melon (Cucumis melo var. flexuosus) genotypes under hydroponics. J. Crop Health 2025, 77, 89. [Google Scholar] [CrossRef]
- Liu, T.; Amanullah, S.; Xu, H.; Gao, P.; Du, Z.; Hu, X.; Han, M.; Che, Y.; Zhang, L.; Qi, G.; et al. RNA-Seq identified putative genes conferring photosynthesis and root development of melon under salt stress. Genes 2023, 14, 1728. [Google Scholar] [CrossRef]
- Botía, P.; Navarro, J.M.; Cerdá, A.; Martínez, V. Yield and fruit quality of two melon cultivars irrigated with saline water at different stages of development. Eur. J. Agron. 2005, 23, 243–253. [Google Scholar] [CrossRef]
- Badr, M.A.; Abou Hussein, S.D. Yield and Fruit Quality of Drip-irrigated Cantaloupe under Salt Stress Conditions in an Arid Environment. Aust. J. Basic Appl. Sci. 2008, 2, 141–148. [Google Scholar]
- Rabab, S.; Francesco, O.; Giorgio, G. Ionic partitioning and stomatal regulation: Dissecting functional elements of the genotypic basis of salt stress adaptation in grafted melon. Plant Signal. Behav. 2013, 8, e27334. [Google Scholar]
- Ibrarullah; Rahman, H.U. Effect of salinity on growth and yield of muskmelon (Cucumis melo L.) genotypes. Int. J. Bot. Stud. 2020, 5, 581–585. [Google Scholar]
- Silva, F.H.A.; Morais, P.L.D.; Morais, M.A.S.; Gonzalez, V.R.; Dias, N.S. Post-harvest quality of melon accessions subjected to salinity. Braz. J. Biol. 2024, 84, e276161. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Pinheiro, D.T.; da Silva, A.L.; da Silva, L.J.; Sekita, M.C.; Dias, D.C.F.S. Germination and antioxidant action in melon seeds exposed to salt stress. Pesqui. Agropecuária Trop. 2016, 46, 336–342. [Google Scholar] [CrossRef]
- Kuşvuran, S. Effects of drought and salt stresses on growth, stomatal conductance, leaf water and osmotic potentials of melon genotypes (Cucumis melo L.). Afr. J. Agric. Res. 2012, 7, 775–781. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress tolerance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Guo, S.; Shu, S.; Sun, J.; Tezuka, T. Effects of proline on photosynthesis and root reactive oxygen species (ROS) metabolism in two melon cultivars (Cucumis melo L.) under NaCl stress. Afr. J. Biotechnol. 2011, 10, 18381–18390. [Google Scholar] [CrossRef]
- An, P.; Inanaga, S.; Lux, A.; Li, X.J.; Ali, M.E.K.; Matsui, T.; Sugimoto, Y. Effects of salinity and relative humidity on two melon cultivars differing in salt tolerance. Biol. Plant. 2002, 45, 409–415. [Google Scholar] [CrossRef]
- Xiong, M.; Zhang, X.; Shabala, S.; Shabala, L.; Chen, Y.; Xiang, C.; Nawaz, M.A.; Bie, Z.; Wu, H.; Yi, H.; et al. Evaluation of salt tolerance and contributing ionic mechanism in nine Hami melon landraces in Xinjiang, China. Sci. Hortic. 2018, 237, 277–286. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, Z.; Meng, L.; Li, Y.; Peng, Y. CmDUF239-1 improves the salt tolerance of grafted melon by enhancing antioxidant capacity and K+/Na+ homeostasis. Plants 2025, 14, 2670. [Google Scholar] [CrossRef]
- Akrami, M.; Arzani, A.; Majnoun, Z. Leaf ion content, yield and fruit quality of field-grown melon under saline conditions. Exp. Agric. 2019, 55, 707–722. [Google Scholar] [CrossRef]
- Kuşvuran, S. Ion regulation in different organs of melon (Cucumis melo) genotypes under salt stress. Int. J. Agric. Biol. 2012, 14, 141–144. [Google Scholar]
- Zhang, Y.D.; Véry, A.A.; Wang, L.M.; Deng, Y.W.; Sentenac, H.; Huang, D.F. A K+ channel from salt-tolerant melon inhibited by Na+. New Phytol. 2011, 189, 856–868. [Google Scholar] [CrossRef]
- Tavanti, T.R.; Melo, A.A.R.; Moreira, L.D.K.; Sanchez, D.E.J.; Silva, R.D.S.; Silva, R.M.D.; Reis, A.R.D. Micronutrient fertilization enhances ROS scavenging system for alleviation of abiotic stresses in plants. Plant Physiol. Biochem. 2021, 160, 386–396. [Google Scholar] [CrossRef]
- Sarabi, B.; Bolandnazar, S.; Ghaderi, N.; Ghashghaie, J. Genotypic differences in physiological and biochemical responses to salinity stress in melon (Cucumis melo L.) plants: Prospects for selection of salt tolerant landraces. Plant Physiol. Biochem. 2017, 119, 294–311. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Zhang, L.; Wang, J.; You, Y. Influence of selenium on growth, lipid peroxidation and antioxidative enzyme activity in melon (Cucumis melo L.) seedlings under salt stress. Acta Soc. Bot. Pol. 2013, 82, 193–197. [Google Scholar] [CrossRef]
- Ji, H.; Pardo, J.M.; Batelli, G.; Van Oosten, M.J.; Bressan, R.A.; Li, X. The Salt Overly Sensitive (SOS) pathway: Established and emerging roles. Mol. Plant 2013, 6, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Petrov, V.; Yun, D.J.; Gechev, T. Revisiting plant salt tolerance: Novel components of the SOS pathway. Trends Plant Sci. 2023, 28, 1060–1069. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.D.; Gonzalez Perez, P.; Deng, Y.W.; Li, Z.Z.; Huang, D.F. Isolation and characterization of a vacuolar Na+/H+ antiporter gene from Cucumis melo L. Afr. J. Biotechnol. 2011, 10, 1752–1759. [Google Scholar]
- Apse, M.P.; Aharon, G.S.; Snedden, W.A.; Blumwald, E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 1999, 285, 1256–1258. [Google Scholar] [CrossRef]
- Gao, L.; Yang, S.; Wei, S.; Huang, D.; Zhang, Y. Supportive role of the Na+ transporter CmHKT1;1 from Cucumis melo in transgenic Arabidopsis salt tolerance through improved K+/Na+ balance. Plant Mol. Biol. 2020, 103, 561–580. [Google Scholar] [CrossRef]
- Ren, Z.; Gao, J.; Li, L.; Cai, X.; Huang, W.; Chao, D.; Zhu, M.; Wang, Z.; Luan, S.; Lin, H. A rice quantitative trait locus for salt tolerance encodes a sodium transporter (OsHKT1;5). Nat. Genet. 2005, 37, 1141–1146. [Google Scholar] [CrossRef]
- Davenport, R.J.; Muñoz-Mayor, A.; Jha, D.; Essah, P.A.; Rus, A.; Tester, M. The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant Cell Environ. 2007, 30, 497–507. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, L.; Gao, L.; Véry, A.; Sentenac, H.; Zhang, Y. Constitutive expression of CmSKOR, an outward K+ channel gene from melon, in Arabidopsis thaliana involved in saline tolerance. Plant Sci. 2018, 274, 492–502. [Google Scholar] [CrossRef]
- Wang, L.; Wei, S.; Chen, J.; Zhang, Y.; Huang, D. Regulation of the inward rectifying K+ channel MIRK and ion distribution in two melon cultivars (Cucumis melo L.) under NaCl salinity stress. Acta Physiol. Plant. 2013, 35, 2789–2800. [Google Scholar] [CrossRef]
- Gierth, M.; Mäser, P.; Schroeder, J.I. The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol. 2005, 137, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, H.; Leng, X.; Zhang, X.; Xiao, B.; Liu, H.; Xue, D.; Wang, Y.; Wu, C.; Wang, W. Genome-wide identification and expression analysis of the CmHAK gene family in melon (Cucumis melo L.). Horticulturae 2023, 9, 1138. [Google Scholar] [CrossRef]
- Yang, S.; Xiong, X.; Arif, S.; Gao, L.; Zhao, L.; Shah, I.H.; Zhang, Y. A calmodulin-like CmCML13 from Cucumis melo improved transgenic Arabidopsis salt tolerance through reduced shoot Na+, and also improved drought resistance. Plant Physiol. Biochem. 2020, 155, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Yang, S.L.; Xiong, X.; Zhao, L.N.; Zhang, Y.D. Expression of CmCBL1 and physiological response in melon (Cucumis melo L.) seedlings under NaCl stress. J. Shanghai Jiaotong Univ. Agric. Sci. 2018, 37, 187–194. [Google Scholar]
- Xiong, X.; Zhao, L.; Yang, S.; Samiah, A.; Zhang, Y. Genome-wide identification of CmCIPK family and its expression analysis under abiotic stress in melon. Acta Agric. Zhejiangensis 2021, 33, 1625–1639. [Google Scholar]
- Miyauchi, H.; Moriyama, S.; Kusakizako, T.; Kumazaki, K.; Nakane, T.; Yamashita, K.; Hirata, K.; Dohmae, N.; Nishizawa, T.; Ito, K.; et al. Structural basis for xenobiotic extrusion by eukaryotic MATE transporter. Nat. Commun. 2017, 8, 1633. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, F.G.; Tang, R.J.; Yu, Y.; Song, J.; Wang, Y.; Li, L.; Luan, S. Two tonoplast MATE proteins function as turgor-regulating chloride channels in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E2036–E2045. [Google Scholar] [CrossRef]
- Shah, I.H.; Manzoor, M.A.; Sabir, I.A.; Ashraf, M.; Haq, F.; Arif, S.; Abdullah, M.; Niu, Q.; Zhang, Y. Genome-wide identification and comparative analysis of MATE gene family in Cucurbitaceae species and their regulatory role in melon (Cucumis melo) under salt stress. Hortic. Environ. Biotechnol. 2022, 63, 595–612. [Google Scholar] [CrossRef]
- Song, J.; Zhu, Z.; Zhang, T.; Meng, X.; Zhang, W.; Gao, P. Genome-wide identification, evolutionary analysis, and functional studies of APX genes in melon (Cucumis melo L.). Int. J. Mol. Sci. 2023, 24, 17571. [Google Scholar] [CrossRef]
- Zhang, G.; Ding, Q.; Wei, B. Genome-wide identification of superoxide dismutase gene families and their expression patterns under low-temperature, salt and osmotic stresses in watermelon and melon. 3 Biotech 2021, 11, 194. [Google Scholar] [CrossRef]
- Wi, S.J.; Kim, W.T.; Park, K.Y. Overexpression of carnation S-adenosylmethionine decarboxylase gene generates a broad-spectrum tolerance to abiotic stresses in transgenic tobacco plants. Plant Cell Rep. 2006, 25, 1111–1121. [Google Scholar] [CrossRef]
- Peremarti, A.; Bassie, L.; Christou, P.; Capell, T. Spermine facilitates recovery from drought but does not confer drought tolerance in transgenic rice plants expressing Datura stramonium S-adenosylmethionine decarboxylase. Plant Mol. Biol. 2009, 70, 253–264. [Google Scholar] [CrossRef]
- Liu, C.M.; Yang, N.; Wen, D.; Lu, M.M.; Ming, T.T. Salt tolerance analysis of transgenic Arabidopsis thaliana plants with heterologous expression of CmSAMDC gene. Acta Agric. Jiangxi 2019, 31, 50–54. [Google Scholar]
- Li, Y.; Tan, Z.; Liu, Y.; Peng, Y.; Liu, C. Root-specific overexpression of the CmDUF239-1 gene enhances heat tolerance in melon seedlings by upregulating antioxidant enzymes activities, proline content, and expression of heat shock protein-related genes. Horticulturae 2025, 11, 1198. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Liu, B.; Yang, S.; Xiong, X.; Hassani, D.; Zhang, Y. CmRAV1 shows differential expression in two melon (Cucumis melo L.) cultivars and enhances salt tolerance in transgenic Arabidopsis plants. Acta Biochim. Biophys. Sin. 2019, 51, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Gao, L.; Zhang, Y.; Zhang, F.; Yang, X.; Huang, D. Genome-wide investigation of the NAC transcription factor family in melon (Cucumis melo L.) and their expression analysis under salt stress. Plant Cell Rep. 2016, 35, 1827–1839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Dai, Z.; Yuan, Y.; Wei, S. CmMYB1 gene clone from Cucumis melo and its functional roles under salt stress. Plant Breed. 2021, 140, 1123–1135. [Google Scholar] [CrossRef]
- Aduse Poku, S.; Nkachukwu Chukwurah, P.; Aung, H.H.; Nakamura, I. Over-expression of a melon Y3SK2-type LEA gene confers drought and salt tolerance in transgenic tobacco plants. Plants 2020, 9, 1749. [Google Scholar] [CrossRef]
- Vierstra, R.D. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol. 2009, 10, 385–397. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M.; Tang, X.; Yang, X.; Zhang, Z.; Wu, J. Genome-wide identification of β-Ketoacyl CoA synthase gene family in melon (Cucumis melo L.) and its expression analysis in autotoxicity, saline-alkali, and microplastic exposure environments. Curr. Issues Mol. Biol. 2025, 47, 195. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Meng, Y.; Ding, F.; Yang, K.; Wang, C.; Zhang, H.; Jin, H. Comparative analysis of TPR gene family in Cucurbitaceae and expression profiling under abiotic stress in Cucumis melo L. Horticulturae 2024, 10, 83. Horticulturae 2024, 10, 83. [Google Scholar] [CrossRef]
- Behera, T.K.; Krishna, R.; Ansari, W.A.; Aamir, M.; Kumar, P.; Kashyap, S.P.; Pandey, S.; Kole, C. Approaches involved in the vegetable crops salt stress tolerance improvement: Present status and way ahead. Front. Plant Sci. 2022, 12, 787292. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, M.C.; Patir, M.G. Molecular characterization, 3D model analysis, and expression pattern of the CmUBC gene encoding the melon ubiquitin-conjugating enzyme under drought and salt stress conditions. Biochem. Genet. 2014, 52, 90–105. [Google Scholar] [CrossRef]
- Batsale, M.; Bahammou, D.; Fouillen, L.; Mongrand, S.; Joubès, J.; Domergue, F. Biosynthesis and functions of very-long-chain fatty acids in the responses of plants to abiotic and biotic stresses. Cells 2021, 10, 1284. [Google Scholar] [CrossRef]
- Yang, W.; Ling, Y.; Li, M.; Zhang, X.; Liu, B. Screening and identification of saline-tolerant germplasm in melon. Agriculture 2023, 13, 2051. [Google Scholar] [CrossRef]
- Adıgüzel, P.; Nyirahabimana, F.; Shimira, F.; Solmaz, İ.; Taşkın, H. Applied biotechnological approaches for reducing yield gap in melon grown under saline and drought stresses: An overview. J. Soil Sci. Plant Nutr. 2022, 23, 139–151. [Google Scholar] [CrossRef]
- Sharma, N.; Deol, J.K.; Kaur, G.; Kaur, A.; Sharma, S.P.; Sarao, N.K. Exploring the role of advanced genomics in melon breeding: A review. Plant Mol. Biol. Report. 2025, 43, 978–1005. [Google Scholar] [CrossRef]
- Chovelon, V.; Restier, V.; Giovinazzo, N.; Dogimont, C.; Aarrouf, J. Histological study of organogenesis in Cucumis melo L. after genetic transformation: Why is it difficult to obtain transgenic plants? Plant Cell Rep. 2011, 30, 2001–2011. [Google Scholar] [CrossRef]
- Wan, L.; Wang, Z.; Zhang, X.; Zeng, H.; Ren, J.; Zhang, N.; Sun, Y.; Mi, T. Optimised Agrobacterium-Mediated Transformation and Application of Developmental Regulators Improve Regeneration Efficiency in Melons. Genes 2023, 14, 1432. [Google Scholar] [CrossRef]
- Garcia-Mas, J.; Benjak, A.; Sanseverino, W.; Bourgeois, M.; Mir, G.; González, V.M.; Hénaff, E.; Câmara, F.; Cozzuto, L.; Lowy, E.; et al. The genome of melon (Cucumis melo L.). Proc. Natl. Acad. Sci. USA. 2012, 109, 11872–11877. [Google Scholar] [CrossRef]
- Feng, J.; Wang, N.; Li, Y.; Wang, H.; Zhang, W.; Wang, H.; Chai, S. Recent Progress in Genetic Transformation and Gene Editing Technology in Cucurbit Crops. Agronomy 2023, 13, 755. [Google Scholar] [CrossRef]
- Li, X.; Cao, C.; Liu, Y.; Bolaños-Villegas, P.; Wang, J.; Zhou, R.; Hou, J.; Li, Q.; Mao, W.; Wang, P.; et al. Enhancing Genetic Transformation Efficiency of Melon (Cucumis melo L.) through an Extended Sucrose-Removal Co-Culture. Plant Cell Rep. 2025, 44, 123. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, Y.; Yang, J.; Xu, D.; Chen, Q.; Xin, K.; Chen, X.; Tang, J.; Chen, J.; Ma, Z. Recent Insights into the Molecular Mechanisms of Salt Tolerance in Melon (Cucumis melo L.). Plants 2025, 14, 3598. https://doi.org/10.3390/plants14233598
Jing Y, Yang J, Xu D, Chen Q, Xin K, Chen X, Tang J, Chen J, Ma Z. Recent Insights into the Molecular Mechanisms of Salt Tolerance in Melon (Cucumis melo L.). Plants. 2025; 14(23):3598. https://doi.org/10.3390/plants14233598
Chicago/Turabian StyleJing, Yanping, Jihai Yang, Dingfan Xu, Qiufeiyang Chen, Kexing Xin, Xunfeng Chen, Jun Tang, Jian Chen, and Zhihu Ma. 2025. "Recent Insights into the Molecular Mechanisms of Salt Tolerance in Melon (Cucumis melo L.)" Plants 14, no. 23: 3598. https://doi.org/10.3390/plants14233598
APA StyleJing, Y., Yang, J., Xu, D., Chen, Q., Xin, K., Chen, X., Tang, J., Chen, J., & Ma, Z. (2025). Recent Insights into the Molecular Mechanisms of Salt Tolerance in Melon (Cucumis melo L.). Plants, 14(23), 3598. https://doi.org/10.3390/plants14233598








